Abstract
Aims/Introduction: Duloxetine has been suggested to exert analgesic effects by activating the descending inhibitory system through inhibition of serotonin (5‐HT) and noradrenaline (NA) reuptake. This randomized controlled trial investigated the efficacy and safety of duloxetine in Japanese patients with diabetic neuropathic pain (DNP).
Materials and Methods: Duloxetine 40 or 60 mg/day or placebo was given orally once daily for 12 weeks. The primary efficacy measure was weekly mean 24‐h average pain severity score on the 11‐point Numerical Rating Scale.
Results: At 12 weeks vs baseline, the 24‐h average pain score (adjusted mean ± SE) was significantly improved in the combined duloxetine (−2.47 ± 0.18) and duloxetine 40 mg (−2.41 ± 0.21) and 60 mg groups (−2.53 ± 0.21) as compared with the placebo group (−1.61 ± 0.18). Duloxetine also exerted significant improvements over the placebo in nearly all secondary outcome measures including 24‐h worst pain, night pain, Brief Pain Inventory (BPI) pain scores, Patient’s Global Impression of Improvement (PGI‐I) and health outcome measures, namely, various BPI interference scores. The incidence of adverse events (AE) was higher in the duloxetine groups than in the placebo group (duloxetine overall, 84.8%; duloxetine 40 mg, 84.7%; duloxetine 60 mg, 84.9%; placebo, 73.7%). Most AE were mild or moderate in severity, and resolved or relieved. There were no clinically significant safety concerns.
Conclusions: Duloxetine 40 or 60 mg/day showed superiority over the placebo at reducing pain scores in patients with DNP. Duloxetine is safe, efficacious and clinically useful in the management of DNP. This trial was registered with ClinicalTrials.gov (no. NCT‐00552175). (J Diabetes Invest, doi: 10.1111/j.2040‐1124.2010.00073.x, 2010)
Keywords: Diabetic neuropathic pain, Duloxetine, Serotonin and noradrenaline reuptake inhibitor
Introduction
Recently, the number of diabetic patients in Japan has increased. It is now thought to amount to 8.9 million, or 22.1 million when including incipient diabetic individuals1. Among three major complications of diabetes mellitus, diabetic neuropathy seems to have the highest incidence, with 36.7% of diabetic patients reported to be suffering from this condition2.
Diabetic neuropathic pain (DNP) is characterized by the symptomatic nature of an aching, burning, tingling or stabbing sensation3. DNP not only is often increased at night and affects sleep4, but also interferes with daily life, leading to deterioration of quality of life and a depressive state in severe cases5.
Epalrestat and mexiletine hydrochloride are approved and widely used in Japan for the indication of DNP. Drugs listed as therapeutic options for DNP in Evidence‐based Practice Guideline for the Treatment of Diabetes in Japan6 include epalrestat, mexiletine hydrochloride, antidepressants, anticonvulsants, non‐steroidal anti‐inflammatory drugs (NSAIDs) and sustained‐release oxycodone. NSAIDs might be efficacious against mild DNP, but not against moderate and severe forms. Tricyclic antidepressants, certain anticonvulsants and opioid analgesics are recommended for the treatment DNP, but might be limited by side effects7.
Serotonin (5‐HT) and noradrenaline (NA) have been implicated in the modulation of intrinsic analgesic mechanisms through descending inhibitory neurons in the brain and spinal cord8–11. An imbalance in these neurotransmitter mechanisms might contribute to central sensitization and hyperexcitability, thereby leading to persistent pain in DNP12. Current evidence suggests that antidepressants that have been shown to have analgesic effects in pain conditions exert such analgesic effects independent of improvement in mood or anxiety13,14. Instead, potentiation of 5‐HT and NA activity in the central nervous system (CNS) through inhibition of their reuptake has been suggested as a probable mechanism of the analgesic action of antidepressants against neuropathic pain15,16.
Duloxetine hydrochloride is a selective and potent 5‐HT and NA reuptake inhibitor (SNRI)17 that has been shown to be effective in animal models of persistent and neuropathic pain18. Recently, duloxetine at doses of 60 mg/day given once or twice daily (120 mg/day) has been shown to be efficacious for the relief of pain in patients with DNP in randomized, double‐blind, placebo‐controlled clinical trials19–21. Based on this evidence, duloxetine was approved for the indication of DNP by the FDA in 2004. Presently, duloxetine has been made available in 90 countries as a therapeutic drug for DNP, and is recommended for this purpose by a number of USA and European guidelines22–25.
In Japan, duloxetine has not yet been authorized as an approved therapeutic drug for DNP, although it is indicated for major depressive disorders. Tolerability and safety of duloxetine at dosages ≤60 mg/day have been confirmed in Japanese subjects during a phase I study26. In a double‐blind, placebo‐controlled phase II study of duloxetine ≤60 mg/day in Japanese patients with DNP, there were no safety concerns (unpublished data). Furthermore, data from phase II studies carried out in Japan and abroad19–21 suggest that duloxetine ≥40 mg/day might improve DNP in Japanese patients.
The objective of the present study was to verify the superiority of once‐daily oral dosing with the SNRI duloxetine 40 and 60 mg/day combined vs placebo therapy using as primary end‐point weekly mean change of 24‐h average pain score on the 11‐point Numerical Rating Scale27.
Materials and Methods
Patients
Patients, men and women aged 20–<80 years, had to have sustained pain for ≥6 months as a result of distal symmetric polyneuropathy caused by type 1 or type 2 diabetes mellitus. Pain was assessed on the local site of the foot, leg or hand with reference to the symptoms of an aching, burning, tingling or stabbing sensation and allodynia. Diagnosis of neuropathy was based on a revised version of the abbreviated diagnostic criteria for distal symmetric polyneuropathy proposed by the Diabetic Neuropathy Study Group in Japan (revised in 2002)28. The other main criteria for selection of subjects included glycated hemoglobin (HbA1c) ≤9.4% at screening, fluctuation of HbA1c≤1.0% at 42–70 days before screening, and the weekly mean of the 24‐h average pain score rated by the 11‐point (0–10) Numerical Rating Scale27 collected from patient diaries over 7 days before initiation of the study drug administration being ≥4, that is, indicative of moderate or severe pain.
Main exclusion criteria were patients with psychiatric diseases, such as mania, bipolar disorder, depression, anxiety disorders and eating disorders, or patients with history of these diseases that needed any pharmacotherapy during the past year. Patients were also excluded if they had a complication that might affect assessment of DNP, such as neurological disorders unrelated to diabetic neuropathy, a skin condition in the area of the neuropathy that could alter sensation and other painful conditions. Patients were allowed to take a maximum daily dose of 1.5 g of acetaminophen, but no other drugs and therapies for DNP were allowed. Apart from insulin dose level, changes to existing diabetes treatments were prohibited.
Before randomization, an assigning table was prepared using Create Key Code 3.3. Patients were randomly assigned to duloxetine 40 or 60 mg or placebo groups in a 1:1:2 ratio by stochastic minimization allocation taking into account the following four factors: (i) weekly mean of 24‐h average pain score at baseline < or ≥6; (ii) duration of diabetic neuropathy < or ≥2 years; (iii) type 1 or type 2 diabetes mellitus; and (iv) each study center.
Study Design
Enrolment for the present study, which was carried out at 73 centers in Japan, began in December 2007 and ended in March 2009. This was a multicenter, randomized, double‐blind, placebo‐controlled, group‐comparison phase III study in patients with DNP. The primary objective was to evaluate the efficacy of oral duloxetine 40 or 60 mg/day once daily vs placebo against DNP.
A screening period for 1–2 weeks during which entry criteria were evaluated without study medication was followed by a 13‐week treatment period when subjects were treated with duloxetine 40 or 60 mg/day or placebo then a 1‐week post‐treatment period without study medication. Considering the safety of patients, a gradually titrating phase for the first 1–2 weeks initiating with 20 mg/day and increasing the dose at 20‐mg weekly increments was set during the treatment period. There was also a 1‐week tapering phase. Patients were seen at biweekly visits for the first 4 weeks of treatment then every 4 weeks thereafter.
The present study conforms to the principles of the Declaration of Helsinki and the study protocol was approved by the Institutional Review Board at each center. All patients provided written informed consent before participating in any study‐related procedures.
Efficacy Evaluation
The primary efficacy measure for the present study was the reduction of the weekly mean of the 24‐h average pain score as measured by the 11‐point (from 0 = no pain to 10 = worst possible pain) Numerical Rating Scale recorded in a diary by patients each day. Secondary efficacy measures were pain severity for 24‐h worst pain and night pain as measured by the 11‐point Numerical Rating Scale, Patient’s Global Impression of Improvement (PGI‐I) Scale29 recorded at weeks 2, 4, 8 and 12, and severity and interference portions of Brief Pain Inventory (BPI)30 recorded at randomization and weeks 2, 4, 8 and 12.
Safety Evaluation
Safety measures included spontaneously reported adverse events (AE), concomitant medications, bodyweight, vital signs (such as sitting blood pressure and heart rate) and ECG being recorded at each visit. Laboratory parameters including hematology, blood chemistry, HbA1c, lipids and urinalysis were generally recorded every 4 weeks. The value for HbA1c (%) was estimated as a National Glycohemoglobin Standardization Program (NGSP) equivalent value (%) calculated by the formula HbA1c (%) = HbA1c (JDS) (%) + 0.4%, considering the relational expression of HbA1c (JDS) (%) measured by the previous Japanese standard substance and measurement methods and HbA1c (NGSP)31. For patients who discontinued the study, the aforementioned assessments were collected at their last visit.
Statistical Analysis
The present study was planned to enrol 300 patients (150 for the placebo group and 75 each for the duloxetine 40 and 60 mg/day groups). The study would have ≥80% power to detect a difference between the combined duloxetine group and placebo group by t‐test when the effect size of the combined duloxetine group to placebo group was taken as 0.33 and the level of significance as one‐sided 0.025.
Efficacy analysis was carried out using data on all randomized patients with at least one post‐baseline assessment. Efficacy analysis of the primary end‐point was made by comparing the combined duloxetine group vs placebo group with regard to change of weekly mean of the 24‐h average pain score from baseline to week 12 by incorporating all the weekly mean changes obtained at each point of the post‐baseline measurements into a mixed‐effects model repeated measures32. Secondary end‐points were made by comparing the duloxetine 40 and 60 mg groups vs placebo group with regard to change of weekly mean of the 24‐h average pain score analyzed by mixed‐effects model repeated measures. Response rate defined as the percentage of patients who achieved 30 or 50% reduction of 24‐h average pain score in the combined duloxetine and duloxetine 40 and 60 mg groups was compared vs the placebo by Fisher’s exact test.
Safety was analyzed in all patients who took at least one dose of the test drugs. With regard to the incidence of each AE and each adverse drug reaction (ADR), the combined duloxetine group was compared with the placebo group by Fisher’s exact test. Severity, causal relationship to study drugs, and outcome of AE and ADR were summarized by treatment group.
Results
Patient Disposition
The disposition of patients enrolled in the study is shown in Figure 1. Of 448 screened patients, 339 patients (40 mg, n = 86; 60 mg, n = 86; placebo, n = 167) were randomized to the study treatment. The patient population subjected to efficacy and safety analyses consisted of 338 patients, excluding one patient in the 40 mg group who did not receive the study drug and was not assessed.
Figure 1.
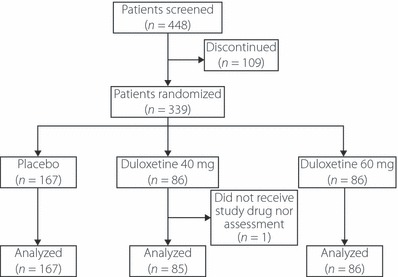
Patients’ disposition.
Demographics
Patients’ demographics and baseline characteristics are shown in Table 1. Approximately 76% of the patients were men; the mean age was 60.8 years. Most patients (95%) had type 2 diabetes mellitus; the duration of the disease was >10 years in the majority. HbA1c ranged from 7.04% in the placebo group to 7.25% in the 40 mg group. Mean duration of diabetic neuropathy was 4.3 years. Weekly mean 24‐h average pain score at baseline was 5.78. There was no significant difference of patient demographics and baseline characteristics among treatment groups.
Table 1. Patient demographics and clinical characteristics at baseline.
| Combined duloxetine (n = 171) | 40 mg (n = 85) | 60 mg (n = 86) | Placebo (n = 167) | Total (n = 338) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | n | % | |
| Sex | ||||||||||
| Male | 127 | 74.3 | 65 | 76.5 | 62 | 72.1 | 129 | 77.2 | 256 | 75.7 |
| Female | 44 | 25.7 | 20 | 23.5 | 24 | 27.9 | 38 | 22.8 | 82 | 24.3 |
| Age (years) | ||||||||||
| 20–<30 | 1 | 0.6 | – | 0.0 | 1 | 1.2 | 1 | 0.6 | 2 | 0.6 |
| 30–<40 | 8 | 4.7 | 2 | 2.4 | 6 | 7.0 | 4 | 2.4 | 12 | 3.6 |
| 40–<50 | 16 | 9.4 | 4 | 4.7 | 12 | 14.0 | 12 | 7.2 | 28 | 8.3 |
| 50–<65 | 72 | 42.1 | 44 | 51.8 | 28 | 32.6 | 90 | 53.9 | 162 | 47.9 |
| 65–<80 | 74 | 43.3 | 35 | 41.2 | 39 | 45.3 | 60 | 35.9 | 134 | 39.6 |
| Mean ± SD | 60.9 ± 10.8 | 62.1 ± 9.3 | 59.7 ± 12.1 | 60.8 ± 9.2 | 60.8 ± 10.0 | |||||
| Weight (kg) | ||||||||||
| <50 | 15 | 8.8 | 10 | 11.8 | 5 | 5.8 | 14 | 8.4 | 29 | 8.6 |
| 50–<60 | 51 | 29.8 | 30 | 35.3 | 21 | 24.4 | 53 | 31.7 | 104 | 30.8 |
| 60–<70 | 62 | 36.3 | 24 | 28.2 | 38 | 44.2 | 53 | 31.7 | 115 | 34.0 |
| ≥70 | 43 | 25.1 | 21 | 24.7 | 22 | 25.6 | 47 | 28.1 | 90 | 26.6 |
| Mean ± SD | 63.9 ± 11.9 | 62.7 ± 13.4 | 65.1 ± 10.2 | 64.5 ± 11.9 | 64.2 ± 11.9 | |||||
| 24‐h average pain score | ||||||||||
| <6 | 96 | 56.1 | 49 | 57.6 | 47 | 54.7 | 91 | 54.5 | 187 | 55.3 |
| ≥6 | 75 | 43.9 | 36 | 42.4 | 39 | 45.3 | 76 | 45.5 | 151 | 44.7 |
| Mean ± SD | 5.77 ± 1.20 | 5.79 ± 1.23 | 5.76 ± 1.17 | 5.78 ± 1.17 | 5.78 ± 1.18 | |||||
| Type of diabetes mellitus | ||||||||||
| Type 1 | 9 | 5.3 | 5 | 5.9 | 4 | 4.7 | 8 | 4.8 | 17 | 5.0 |
| Type 2 | 162 | 94.7 | 80 | 94.1 | 82 | 95.3 | 159 | 95.2 | 321 | 95.0 |
| Duration of diabetes (years) | ||||||||||
| <5 | 36 | 21.1 | 20 | 23.5 | 16 | 18.6 | 33 | 19.8 | 69 | 20.4 |
| 5–<10 | 32 | 18.7 | 18 | 21.2 | 14 | 16.3 | 32 | 19.2 | 64 | 18.9 |
| ≥10 | 103 | 60.2 | 47 | 55.3 | 56 | 65.1 | 97 | 58.1 | 200 | 59.2 |
| Unknown | – | 0.0 | – | 0.0 | – | 0.0 | 5 | 3.0 | 5 | 1.5 |
| Duration of diabetic neuropathy (years) | ||||||||||
| <2 | 53 | 31.0 | 26 | 30.6 | 27 | 31.4 | 51 | 30.5 | 104 | 30.8 |
| ≥2 | 118 | 69.0 | 59 | 69.4 | 59 | 68.6 | 116 | 69.5 | 234 | 69.2 |
| Mean ± SD | 4.4 ± 3.8 | 4.6 ± 3.9 | 4.2 ± 3.7 | 4.2 ± 4.4 | 4.3 ± 4.1 | |||||
| HbA1C (%) | ||||||||||
| Mean ± SD | 7.18 ± 0.88 | 7.25 ± 0.85 | 7.11 ± 0.90 | 7.04 ± 0.90 | – | |||||
The value for HbA1C (%) was estimated as an NGSP equivalent value (%) calculated by the formula HbA1C (%) = HbA1C (JDS) (%) + 0.4%.
Efficacy
Figure 2 shows the weekly mean change of the 24‐h average pain score from baseline to each point of measurement over 12 weeks. Change of this parameter (adjusted mean ± SE) from baseline to week 12 in the combined duloxetine, 40, 60 mg and placebo groups was −2.47 ± 0.18, −2.41 ± 0.21, −2.53 ± 0.21, −1.61 ± 0.18, respectively. Intergroup difference vs placebo for combined duloxetine was −0.87 ± 0.15 (95% confidence interval [CI], −1.17 to −0.56; P < 0.0001). On the basis of 95% CI of difference in each dose group vs placebo, the 24‐h average pain score was also judged significantly improved in the duloxetine 40 and 60 mg groups as compared with the placebo (95% CI, −1.18 to −0.43 and −1.30 to −0.56, respectively). Both the combined duloxetine and 60 mg groups showed significant decreases of the 24‐h average pain score compared with the placebo beginning at week 1, whereas this was observed in the 40 mg group beginning at week 2, and persisted in all three active treatment groups thereafter.
Figure 2.
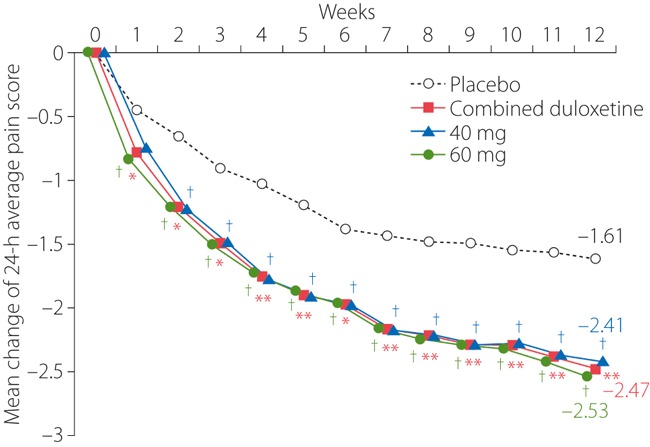
Mean weekly change of the 24‐h average pain score (repeated measures analysis). *P < 0.05 vs placebo; **P < 0.0001 vs placebo; †95% confidence interval of difference vs placebo does not include zero.
The response rate of 30% reduction of the 24‐h average pain score in the combined, 40 and 60 mg, and placebo groups was 57.3% (98/171 patients; P < 0.0001 vs placebo), 55.3% (47/85), 59.3% (51/86) and 35.3% (59/167), respectively. The response rate of 50% reduction was 39.2% (67/171 patients; P = 0.0001 vs placebo), 37.6% (32/85), 40.7% (35/86) and 19.8% (33/167), respectively.
The results of mixed‐effects model repeated measures analysis of the efficacy measures are summarized in Table 2. The combined duloxetine group produced a significantly greater improvement than the placebo for all pain measures including the 24‐h worst pain score, night pain score, and BPI pain score with respect to the worst, least, average and right now. PGI‐I score (adjusted mean ± SE) at week 12 was 2.53 ± 0.12 in the combined duloxetine group and 3.18 ± 0.12 in the placebo group. Global impression of pain improvement was significantly favorable for duloxetine vs the placebo (P < 0.0001).
Table 2. Mean change from baseline to endpoint (repeated measures analysis): efficacy and health outcome measures.
| Combined duloxetine (n = 171) | 40 mg (n = 85) | 60 mg (n = 86) | Placebo (n = 167) | |||||
|---|---|---|---|---|---|---|---|---|
| Mean baseline (SD) | Mean change (SE) | Mean baseline (SD) | Mean change (SE) | Mean baseline (SD) | Mean change (SE) | Mean baseline (SD) | Mean change (SE) | |
| Weekly mean of | ||||||||
| 24‐h average pain score | 5.77 (1.20) | −2.47 (0.18)** | 5.79 (1.23) | −2.41 (0.21)† | 5.76 (1.17) | −2.53 (0.21)† | 5.78 (1.17) | −1.61 (0.18) |
| 24‐h worst pain score | 6.58 (1.33) | −2.51 (0.19)** | 6.54 (1.33) | −2.42 (0.22)† | 6.61 (1.33) | −2.59 (0.22)† | 6.66 (1.25) | −1.55 (0.19) |
| Night pain score | 5.62 (1.59) | −2.39 (0.19)** | 5.55 (1.64) | −2.33 (0.22)† | 5.69 (1.54) | −2.45 (0.23)† | 5.50 (1.49) | −1.56 (0.19) |
| BPI pain severity | ||||||||
| Worst pain | 6.6 (1.4) | −2.59 (0.21)** | 6.5 (1.4) | −2.51 (0.25)† | 6.6 (1.5) | −2.68 (0.25)† | 6.7 (1.4) | −1.62 (0.21) |
| Least pain | 4.1 (1.7) | −1.98 (0.21)** | 4.0 (1.8) | −1.92 (0.25)† | 4.2 (1.6) | −2.04 (0.25)† | 4.1 (1.8) | −1.13 (0.21) |
| Average pain | 5.7 (1.3) | −2.54 (0.20)** | 5.6 (1.3) | −2.53 (0.23)† | 5.7 (1.3) | −2.56 (0.23)† | 5.6 (1.3) | −1.54 (0.20) |
| Pain right now | 5.2 (1.7) | −2.59 (0.22)** | 5.2 (1.8) | −2.55 (0.25)† | 5.3 (1.4) | −2.62 (0.26)† | 5.1 (1.7) | −1.67 (0.22) |
| BPI interference | ||||||||
| General activity | 4.5 (2.5) | −2.29 (0.24) | 4.5 (2.7) | −2.48 (0.29)† | 4.5 (2.4) | −2.10 (0.29) | 4.4 (2.4) | −1.88 (0.24) |
| Mood | 4.1 (2.5) | −2.28 (0.24) | 3.9 (2.5) | −2.18 (0.29) | 4.2 (2.5) | −2.39 (0.29) | 4.2 (2.4) | −1.91 (0.24) |
| Walking ability | 4.4 (2.6) | −2.31 (0.23)* | 4.4 (2.8) | −2.32 (0.28) | 4.3 (2.5) | −2.31 (0.28) | 4.0 (2.6) | −1.82 (0.23) |
| Normal work | 4.1 (2.5) | −1.86 (0.23) | 3.9 (2.6) | −1.84 (0.28) | 4.3 (2.5) | −1.90 (0.28) | 3.7 (2.7) | −1.49 (0.23) |
| Relationship with other people | 2.8 (2.5) | −1.32 (0.23)* | 2.7 (2.7) | −1.16 (0.27) | 2.9 (2.4) | −1.49 (0.27)† | 2.6 (2.5) | −0.77 (0.23) |
| Sleep | 4.2 (2.8) | −2.15 (0.24)* | 4.0 (2.8) | −2.26 (0.29)† | 4.3 (2.7) | −2.05 (0.29) | 3.9 (2.7) | −1.69 (0.24) |
| Enjoyment of life | 3.9 (2.6) | −2.15 (0.23)* | 3.7 (2.7) | −1.96 (0.28) | 4.0 (2.5) | −2.35 (0.28)† | 3.5 (2.5) | −1.59 (0.23) |
| Average of 7 interference scores | 3.99 (2.18) | −2.04 (0.20)* | 3.88 (2.25) | −2.00 (0.24) | 4.09 (2.13) | −2.08 (0.24)† | 3.75 (2.15) | −1.56 (0.20) |
| PGI‐I | – | 2.53 (0.12)** | – | 2.53 (0.14)† | – | 2.52 (0.14)† | – | 3.18 (0.12) |
BPI, Brief Pain Inventory; PGI‐I, Patient’s Global Impression of Improvement.
*P < 0.05 vs placebo; **P < 0.0001 vs placebo; †95%CI of difference vs placebo does not include zero.
Significant improvements of various individual health outcome measures were noted in the combined duloxetine group vs placebo including BPI average interference score (P = 0.0095), walking ability (P = 0.0228), relationship with other people (P = 0.0076), sleep (P = 0.0378) and enjoyment of life (P = 0.0089). However, no improvement was noted with regard to interference of general activity, mood and normal work.
Safety
A total of 46 patients (13.6%) discontinued (combined duloxetine group, n = 29 [16.9%]; 40 mg group, n = 13 [15.1%]; 60 mg group, n = 16 [18.6%]; placebo group, n = 17 [10.2%]). Among them, 30 patients (8.8%) discontinued due to AE (n = 21 [12.2%], n = 9 [10.5%], n = 12 [14.0%], and n = 9 [5.4%], respectively).
Overall, the incidence of AE was significantly (P = 0.0153) higher in the combined duloxetine group (84.8%; 145/171 patients) than in the placebo group (73.7%; 123/167 patients). The incidence of AE in the 40 and 60 mg groups was 84.7% (72/85 patients) and 84.9% (73/86 patients), respectively.
AE and ADR reported during the present study are summarized in Table 3. AE that were significantly more frequently reported in the combined duloxetine group than placebo group included somnolence (P = 0.0007), nausea (P < 0.0001) and dizziness (P = 0.0354). Most AE and ADR were mild or moderate in severity, and resolved or relieved. There was no noteworthy difference in the incidence, kind, severity and outcome of AE between the 40 and 60 mg groups.
Table 3. Incidence of adverse events reported by ≥5% patients in any group and adverse drug reactions.
| Preferred term | Adverse events | Adverse drug reactions | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Combined duloxetine (n = 171) | 40 mg (n = 85) | 60 mg (n = 86) | Placebo (n = 167) | Combined duloxetine (n = 171) | 40 mg (n = 85) | 60 mg (n = 86) | Placebo (n = 167) | |||||||||
| n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | |
| Somnolence | 37 | 21.6* | 16 | 18.8 | 21 | 24.4 | 14 | 8.4 | 37 | 21.6* | 16 | 18.8 | 21 | 24.4 | 13 | 7.8 |
| Nausea | 24 | 14.0** | 10 | 11.8 | 14 | 16.3 | 3 | 1.8 | 24 | 14.0** | 10 | 11.8 | 14 | 16.3 | 3 | 1.8 |
| Nasopharyngitis | 24 | 14.0 | 10 | 11.8 | 14 | 16.3 | 24 | 14.4 | – | 0.0 | – | 0.0 | – | 0.0 | – | 0.0 |
| AST increased | 13 | 7.6 | 5 | 5.9 | 8 | 9.3 | 6 | 3.6 | 7 | 4.1 | 3 | 3.5 | 4 | 4.7 | 4 | 2.4 |
| Constipation | 11 | 6.4 | 6 | 7.1 | 5 | 5.8 | 9 | 5.4 | 9 | 5.3 | 5 | 5.9 | 4 | 4.7 | 6 | 3.6 |
| Diarrhea | 11 | 6.4 | 4 | 4.7 | 7 | 8.1 | 6 | 3.6 | 7 | 4.1 | 4 | 4.7 | 3 | 3.5 | 3 | 1.8 |
| Dizziness | 10 | 5.8* | 6 | 7.1 | 4 | 4.7 | 2 | 1.2 | 7 | 4.1 | 4 | 4.7 | 3 | 3.5 | 2 | 1.2 |
| ALT increased | 10 | 5.8 | 5 | 5.9 | 5 | 5.8 | 6 | 3.6 | 6 | 3.5 | 3 | 3.5 | 3 | 3.5 | 4 | 2.4 |
| Malaise | 9 | 5.3 | 3 | 3.5 | 6 | 7.0 | 3 | 1.8 | 9 | 5.3 | 3 | 3.5 | 6 | 7.0 | 3 | 1.8 |
| Vomiting | 9 | 5.3 | 4 | 4.7 | 5 | 5.8 | 2 | 1.2 | 8 | 4.7* | 4 | 4.7 | 4 | 4.7 | 1 | 0.6 |
| WBC increased | 9 | 5.3 | 4 | 4.7 | 5 | 5.8 | 4 | 2.4 | 1 | 0.6 | – | 0.0 | 1 | 1.2 | 1 | 0.6 |
| GGT increased | 7 | 4.1 | 2 | 2.4 | 5 | 5.8 | 5 | 3.0 | 4 | 2.3 | 1 | 1.2 | 3 | 3.5 | 2 | 1.2 |
| LDH increased | 7 | 4.1 | 2 | 2.4 | 5 | 5.8 | 4 | 2.4 | 3 | 1.8 | 1 | 1.2 | 2 | 2.3 | 3 | 1.8 |
| CK increased | 6 | 3.5 | 6 | 7.1 | – | 0.0 | 6 | 3.6 | 1 | 0.6 | 1 | 1.2 | – | 0.0 | 3 | 1.8 |
| HbA1c increased | 6 | 3.5 | 1 | 1.2 | 5 | 5.8 | 4 | 2.4 | 4 | 2.3 | – | 0.0 | 4 | 4.7 | 3 | 1.8 |
*P < 0.05 vs placebo; **P < 0.0001 vs placebo.
ALT, alanine aminotransferase; AST, aspartate aminotransferase; CK, creatine phosphokinase; GGT, γ‐glutamyltransferase; HbA1c, glycosylated hemoglobin; LDH, lactate dehydrogenase; WBC, white blood cell count.
A total of seven serious AE were noted in five patients in the combined duloxetine group (four events in three patients in the 40 mg group and three events in two patients in the 60 mg group), whereas 18 serious AE occurred in six patients in the placebo group. No deaths occurred during the present study. Overall, no significant difference was noted between the combined duloxetine and placebo groups. Among serious AE reported, hypoglycemia (40 mg group) and self‐injurious behavior (60 mg group) were judged as ADR, although both symptoms resolved.
At 13 weeks compared with baseline, comparable and unremarkable increases of HbA1c were noted in the combined duloxetine, 40, 60 mg and placebo groups (0.06, 0.03, 0.10 and 0.10%, respectively).
Discussion
To evaluate the superiority of the SNRI duloxetine 40 and 60 mg/day once daily over the placebo, a randomized, double‐blind, 12‐week, phase III study was carried out in Japanese patients with moderate‐to‐severe DNP defined as having ≥4 on the 24‐h average pain score. As a result, duloxetine was shown to be significantly superior to the placebo in improving DNP as evaluated by a change of the 24‐h average pain score from baseline to week 12 as the primary efficacy end‐point.
Recently, Farrar et al.27,33 pooled data from placebo‐controlled studies that investigated relationships between changes of Numerical Rating Scale pain ratings and quantifiable PGI‐I scale. Their findings showed that a reduction of approximately 2 points from baseline on an 11‐point pain rating scale, equivalent to a 30% reduction on pain score from baseline, corresponds to a clinically meaningful improvement. Subjects with 30–50% reduction in the assessment scale as compared with baseline are considered to be responders in guidelines on clinical investigation of medicinal products intended for the treatment of neuropathic pain34. In the present trial, the rate of responders was significantly higher in the combined duloxetine group than the placebo group. Taking into consideration that the present study was carried out in patients with moderate or severe DNP, duloxetine might contribute favorably to the treatment of such individuals.
Excluded from participating in the present study were patients complicated with psychiatric diseases including depression. Thus, the present findings support the argument that dual inhibition of reuptake of 5‐HT and NA in the CNS contributes to the independent analgesic effect of duloxetine.
DNP is typically characterized by the manifestation of peripheral pain in the extremities, which causes unbearable distress to patients. The most important matter for those with DNP must be to lessen the pain as early as possible. Duloxetine was found to significantly improve the 24‐h average pain score vs the placebo as early as 1 week after starting treatment. Therefore, duloxetine might be useful for treating patients with DNP because of its early manifestation of an analgesic effect.
DNP is not only often increased at night and affects sleep4, but also interferes with daily life, leading to a lack of appetite and a depressive state in severe cases5,35, and eventually to the deterioration of quality of life. Another important finding in the present study was that a significant improvement in health outcome measures was noted in the combined duloxetine group over the placebo for BPI average interference score as well as BPI interference of walking ability, relationships with other people, sleep and enjoyment of life. These clinical findings suggest that duloxetine might be helpful to improve patients’ quality of life.
From the practical point of view, dosing frequency is an important clinical consideration. A systematic review of associations between dosing frequency and medication compliance showed that the latter is inversely related to the former36. The dosing frequency of epalrestat and mexiletine hydrochloride, which are widely used for the treatment of DNP in Japan, is thrice daily, whereas that of pregabalin, which is approved and used in the USA and European‐approved pregabalin, is twice daily. Because we observed a significant improvement of DNP with the once‐daily regimen, this suggests that adherence to treatment with duloxetine might be less of a clinical concern.
Incidence of AE/ADR was significantly higher in the combined duloxetine group than the placebo group, whereas that in the 40 and 60 mg groups was similar. In contrast, most AE and ADR reported in the combined duloxetine group were mild or moderate in severity and resolved or relieved. Because no clinically problematic findings were noted, it is considered that there are no safety concerns with duloxetine in the setting of DNP.
Our safety data are comparable to those observed in a previous clinical trial carried out overseas19,21, where the incidence of AE on duloxetine was 87.7% (498/568 patients); major AE were nausea (23.6%; n = 134 patients), somnolence (15.5%; n = 88), dizziness (13.4%; n = 76), constipation (11.3%; n = 64) and insomnia (10.2%; n = 58). Therefore, the type and incidence of AE in Japanese patients are similar to those in non‐Japanese patients. Furthermore, there was little difference in change of HbA1c between each duloxetine treatment group and the placebo, as was observed in studies carried out overseas; 13‐week treatment with duloxetine does not appear to adversely affect glycemic control.
In conclusion, the superiority of once‐daily dosing with the SNRI duloxetine vs placebo in improving DNP was shown in Japanese patients. Both 40 and 60 mg daily dosages of duloxetine were safe and well tolerated.
Acknowledgements
We thank the following physicians for their cooperation in this research: Yasunori Iwashima (Yoshida Hospital); Katsuyuki Yanagisawa (Sapporo City General Hospital); Kenichi Tsuchida (Manda Memorial Hospital); Takashi Sasaki (Sasaki Naika Hospital); Kenichi Kimura (Naika Kimura Kenichi Clinic); Kenichi Yamada (Kenichi Yamada Clinic); Fuminobu Okuguchi (Okuguchi Clinic of Internal Medicine); Susumu Suzuki (Ohta Nishinouchi Hospital); Hiroaki Seino (Seino Internal Medicine Clinic); Koichi Kawai (Kawai Clinic); Takeshi Osonoi (Naka Kinen Clinic); Hiroshi Ohashi (Oyama East Clinic); Takashi Kikuchi (Kikuchi Naika Clinic); Hiroyuki Asano (Asano Naika Clinic); Taro Wasada, Masayo Kawano (Saiseikai Kurihashi Hospital); Aizan Hirai (Chiba Prefectural Togane Hospital); Toru Hiyoshi (Japanese Red Cross Medical Center); Yasuhiko Iwamoto (Tokyo Women’s Medical University Hospital); Masahiro Sugawara (Sugawara Clinic); Masao Ohta (Nippon Medical School Hospital); Tamotsu Yokota (The Jikei University School of Medicine Hospital); Hiroyuki Tamura (Tokyo Kyosai Hospital); Makoto Sakurai (Sakurai Hospital); Yukiko Onishi (The Institute for Adult Diseases, Asahi Life Foundation); Sachihiko Ozawa (Minamino Clinic); Hisao Mori (Yokohama Sotetsu Bldg Clinic of Internal Medicine); Akio Ohta (St. Marianna University School of Medicine Hospital); Shiro Minami (Nippon Medical School Musashi Kosugi Hospital); Koichi Hirao (HEC Science Clinic); Masahisa Ori (Ori Clinic); Yukio Yamada (St. Marianna University School of Medicine, Yokohama City Seibu Hospital); Kumiko Hamano (Shonan Kamakura General Hospital); Tetsuo Nishikawa (Yokohama Rosai Hospital); Yasuo Terauchi, Mari Kimura (Yokohama City University Hospital); Hideaki Kaneshige (Hon‐Atsugi Medical Clinic); Ikuro Matsuba (Matsuba Clinic); Tetsuo Takuma (Takuma Saiwai Clinic); Keiko Arai (Arai Clinic); Takashi Iizuka (Asahi Internal Medicine Department Clinic); Kazuaki Yahata (Nagaoka Chuo General Hospital); Seiji Ogaku (Ogaku Clinic); Yukihiro Bando (Fukui‐ken Saiseikai Hospital); Mitsuo Imura (Yaizu City Hospital); Akira Yamauchi (Suruga Clinic); Takahisa Sano (Chubu Rosai Hospital); Masako Deguchi (Kyoto Second Red Cross Hospital); Hisataka Tegoshi, Atsushi Omoto (Kyoto First Red Cross Hospital); Kazuyoshi Nishino (Jujo Rehabilitation Hospital); Shizuo Kajiyama (Kajiyama Clinic); Shinya Makino (Osaka Gyoumeikan Hospital); Koichiro Yasuda (Saiseikai Noe Hospital); Shigeichi Shoji (Minami Osaka Hospital); Makoto Nomura (Osaka Rosai Hospital); Yasunao Yoshimasa (National Cerebral and Cardiovascular Center); Masayuki Hosoi (Osaka City General Hospital); Mika Fujimoto (Sakai Hospital Kinki University School of Medicine); Shinichiro Ohashi (Clinic Komatsu); Michiko Hayakawa (Hyogo Rehabilitation Center Hospital); Mitsuaki Kodama (Ikeda Hospital); Hiroyuki Konya (Hyogo College of Medicine Hospital); Konosuke Marui, Ryo Nagase (Okayama Rosai Hospital); Kenichi Shikata (Okayama University Hospital); Tetsuya Fukuda (The Sakakibara Heart Institute of Okayama); Fumiko Kawasaki (Kawasaki Medical School Hospital); Akihiko Nakamura (Osafune Clinic); Kiminori Yamane (Hiroshima University Hospital); Hiroyuki Nodera (Tokushima University Hospital); Tsuyoshi Oki (Omuta City General Hospital); Yasuhiro Sako (Saiseikai Fukuoka General Hospital); Shoichi Akazawa (Shinkoga Hospital); Katsumi Noda (Clinic Tenjinkita); Nobuyuki Abe (Abe Clinic); Yasuhiro Hashiguchi (Tempozan Naika Clinic). This study is financially supported by Shionogi & Co. Ltd., Eli Lilly Japan K.K., and Eli Lilly and Company. The authors have no conflict of interest to declare.
References
- 1.Ministry of Health, Labour and Welfare . Annual Report of the National Health and Nutrition Survey in 2007. Ministry of Health, Labour and Welfare, Tokyo, 2008. (Japanese). [Google Scholar]
- 2.Japan Physicians Association . Investigation into the actual conditions about diabetic neuropathy. J Jpn Phys Assoc 2001; 16: 353–381(Japanese). [Google Scholar]
- 3.Boulton AJM, Vinik AI, Arezzo JC, et al. Diabetic neuropathies. A statement by the American Diabetes Association. Diabetes Care 2005; 28: 956–962 [DOI] [PubMed] [Google Scholar]
- 4.Zelman DC, Brandenburg NA, Gore M. Sleep impairment in patients with painful diabetic peripheral neuropathy. Clin J Pain 2006; 22: 681–685 [DOI] [PubMed] [Google Scholar]
- 5.Vinik AI, Mehrabyan A. Diabetic neuropathies. Med Clin North Am 2004; 88: 947–999 [DOI] [PubMed] [Google Scholar]
- 6.The Japan Diabetes Society . Evidence‐based Practice Guideline for the Treatment of Diabetes in Japan, 2nd edn The Japan Diabetes Society, Tokyo, 2007; 93–104 (Japanese). [Google Scholar]
- 7.McQuay HJ, Tramer M, Nye BA, et al. A systematic review of antidepressants in neuropathic pain. Pain 1996; 68: 217–227 [DOI] [PubMed] [Google Scholar]
- 8.Clark FM, Proudfit HK. The projections of noradrenergic neurons in the A5 catecholamine cell group to the spinal cord in the rat: anatomical evidence that A5 neurons modulate nociception. Brain Res 1993; 616: 200–210 [DOI] [PubMed] [Google Scholar]
- 9.Basbaum AI, Fields HL. Endogenous pain control systems: brainstem spinal pathways and endorphin circuitry. Annu Rev Neurosci 1984; 7: 309–338 [DOI] [PubMed] [Google Scholar]
- 10.Fields HL, Basbaum AI. Central nervous system mechanisms of pain modulation In: Wall PD, Melzack R (eds). Textbook of Pain. Churchill Livingstone, New York, USA, 1999; 309–329 [Google Scholar]
- 11.Fields HL, Heinricher MM, Mason P. Neurotransmitters in nociceptive modulatory circuits. Annu Rev Neurosci 1991; 14: 219–245 [DOI] [PubMed] [Google Scholar]
- 12.Coderre TJ, Katz J. Peripheral and central hyperexcitability: differential signs and symptoms in persistent pain. Behav Brain Sci 1997; 20: 404–419 [DOI] [PubMed] [Google Scholar]
- 13.Max MB, Culnane M, Schafer SC, et al. Amitriptyline relieves diabetic neuropathy pain in patients with normal or depressed mood. Neurology 1987; 37: 589–596 [DOI] [PubMed] [Google Scholar]
- 14.Russell IJ, Mease PJ, Smith TR, et al. Efficacy and safety of duloxetine for treatment of fibromyalgia in patients with or without major depressive disorder: results from a 6‐month, randomized, double‐blind, placebo‐controlled, fixed‐dose trial. Pain 2008; 136: 432–444 [DOI] [PubMed] [Google Scholar]
- 15.Bryson HM, Wilde MI. Amitriptyline. A review of its pharmacological properties and therapeutic use in chronic pain states. Drugs Aging 1996; 8: 459–476 [DOI] [PubMed] [Google Scholar]
- 16.Wernicke JF, Iyengar S, Ferrer‐Garcia MD. Treatment of chronic pain with drugs that modulate central nervous system serotonin and norepinephrine. Curr Drug Ther 2007; 2: 161–167 [Google Scholar]
- 17.Wong DT, Bymaster FP. Dual serotonin and noradrenalin uptake inhibitor class of anti‐depressants – potential for greater efficacy or just hype? Prog Drug Res 2002; 58: 169–222 [DOI] [PubMed] [Google Scholar]
- 18.Iyengar S, Webster AA, Hemrick‐Luecke SK, et al. Efficacy of duloxetine, a potent and balanced serotonin–norepinephrine reuptake inhibitor in persistent pain models in rats. J Pharmacol Exp Ther 2004; 311: 576–584 [DOI] [PubMed] [Google Scholar]
- 19.Goldstein DJ, Lu Y, Detke MJ, et al. Duloxetine vs. placebo in patients with painful diabetic neuropathy. Pain 2005; 116: 109–118 [DOI] [PubMed] [Google Scholar]
- 20.Raskin J, Pritchett YL, Wang F, et al. A double‐blind, randomized multicenter trial comparing duloxetine with placebo in the management of diabetic peripheral neuropathic pain. Pain Med 2005; 6: 346–356 [DOI] [PubMed] [Google Scholar]
- 21.Wernicke JF, Pritchett YL, D’Souza DN, et al. A randomized controlled trial of duloxetine in diabetic peripheral neuropathic pain. Neurology 2006; 67: 1411–1420 [DOI] [PubMed] [Google Scholar]
- 22.Argoff CE, Backonja MM, Belgrade MJ, et al. Consensus guidelines: treatment planning and options. Diabetic peripheral neuropathic pain. Mayo Clin Proc 2006; 81(Suppl 4): 12–25 [DOI] [PubMed] [Google Scholar]
- 23.Dworkin RH, O’Connor AB, Backonja M, et al. Pharmacologic management of neuropathic pain: evidence‐based recommendations. Pain 2007; 132: 237–251 [DOI] [PubMed] [Google Scholar]
- 24.Attal N, Cruccu G, Haanpaa M, et al. EFNS guidelines on pharmacological treatment of neuropathic pain. Eur J Neurol 2006; 13: 1153–1169 [DOI] [PubMed] [Google Scholar]
- 25.Centre for Clinical Practice at National Institute for Health and Clinical Excellence . Neuropathic Pain‐the Pharmacological Management of Neuropathic Pain in Adults in Non‐Specialist Settings. National Institute for Health and Clinical Excellence, London, UK, 2010. [PubMed] [Google Scholar]
- 26.Kumagai Y. Phase I study of duloxetine – multiple dose study (60 mg once daily for 7 days). Jpn J Clin Psychopharmacol 2009; 12: 1483–1497 [Google Scholar]
- 27.Farrar JT, Young JP Jr, LaMoreaux L, et al. Clinical importance of changes in chronic pain intensity measured on an 11‐point numerical pain rating scale. Pain 2001; 94: 149–158 [DOI] [PubMed] [Google Scholar]
- 28.Diabetic Neuropathy Study Group . Abbreviated diagnostic criteria for distal symmetric polyneuropathy. Periph Nerve 2003; 14: 225 (Japanese). [Google Scholar]
- 29.Guy W. ECDEU Assessment Manual for Psychopharmacology, Revised, 1976. US Department of Health, Education, and Welfare Publication (ADM), National Institute of Mental Health, Rockville, MD, USA, 1976: 217–222 [Google Scholar]
- 30.Cleeland CS, Ryan KM. Pain assessment: global use of the Brief Pain Inventory. Ann Acad Med Singapore 1994; 23: 129–138 [PubMed] [Google Scholar]
- 31.The committee of Japan Diabetes Society on the diagnostic criteria of diabetes mellitus . Report of the Committee on the classification and diagnostic criteria of diabetes mellitus. J Jpn Diab Soc 2010; 53: 450–467(Japanese). [Google Scholar]
- 32.Mallinckrodt CH, Kaiser CJ, Watkin JG, et al. The effect of correlation structure on treatment contrasts estimated from incomplete clinical trial data with likelihood‐based repeated measures compared with last observation carried forward ANOVA. Clin Trials 2004; 1: 477–489 [DOI] [PubMed] [Google Scholar]
- 33.Farrar JT, Pritchett YL, Robinson M, et al. The clinical importance of changes in the 0–10 Numeric Rating Scale for worst, least, and average pain intensity: analyses of data from clinical trials of duloxetine in pain disorders. J Pain 2010; 11: 109–118 [DOI] [PubMed] [Google Scholar]
- 34.Committee for Medicinal Products for Human Use . Guideline on Clinical Investigation of Medicinal Products Intended for the Treatment of Neuropathic Pain. European Medicines Agency, London, UK, 2004. [Google Scholar]
- 35.Galer BS, Gianas A, Jensen MP. Painful diabetic polyneuropathy: epidemiology, pain description, and quality of life. Diabetes Res Clin Pract 2000; 47: 123–128 [DOI] [PubMed] [Google Scholar]
- 36.Claxton AJ, Cramer J, Pierce C. A systematic review of the associations between dose regimens and medication compliance. Clin Ther 2001; 23: 1296–1310 [DOI] [PubMed] [Google Scholar]


