Abstract
Aims/Introduction: Although the improvement of postprandial hyperglycemia by an alpha‐glucosidase inhibitor (α‐GI) has been associated with a risk reduction of cardiovascular events, the relationship between postprandial hyperglycemia and arterial stiffness has not been well understood. We therefore examined whether ameliorating the postprandial state by α‐GI leads to an improvement in arterial stiffness.
Materials and Methods: A total of 22 patients with type 2 diabetes mellitus were treated with acarbose. Cardio‐ankle vascular index (CAVI) as the arterial stiffness was measured by using a VaSera CAVI instrument before and 12 months after acarbose treatment. Serum high‐sensitivity C‐reactive protein (hs‐CRP), pentraxin‐3 (PTX3) and matrix metalloproteinase (MMP) ‐2, ‐9 were measured at the same time points. Furthermore, circulating peripheral blood mononuclear cells were examined for the frequencies of CD14 positive cells expressing membrane type‐1 MMP (MT1‐MMP) at the single cell level using flow cytometry.
Results: After acarbose treatment, postprandial glucose and glycosylated hemoglobin (HbA1c) were significantly decreased. Serum levels of hs‐CRP, PTX3, MMP‐2 and MMP‐9 were significantly decreased. CAVI showed a significant reduction, although the changes were not significant in blood pressure and heart rate. MT1‐MMP expression was significantly decreased by acarbose treatment. In multivariate analysis, improvement of blood glucose, decrease of PTX3 levels and MT1‐MMP expression were independent predictors of beneficial change in CAVI.
Conclusions: The present study showed that the beneficial effects of acarbose on arterial stiffness are mediated by an improvement of postprandial hyperglycemia and vascular remodeling markers. In conclusion, acarbose treatment might reduce the risk of cardiovascular diseases by altering the arterial stiffness in postprandial hyperglycemic status. (J Diabetes Invest, doi:10.1111/j.2040‐1124.2010.00079.x, 2010)
Keywords: Postprandial hyperglycemia, Arterial stiffness, Matrix metalloproteinases
Introduction
Diabetic patients are thought to be at higher risk for ischemic heart diseases, such as myocardial infarction and angina pectoris, than non‐diabetic patients.1 The aims of medical treatment of diabetes are to maintain the same quality of life and to ensure the same life expectancy as healthy individuals. In particular, it is important to prevent complications and the progression of the disease. Microangiopathy, such as retinopathy and nephropathy, is correlated with long‐term chronic hyperglycemia.2 In contrast, patients with impaired glucose tolerance (IGT), a stage just before the diagnosis of diabetes, particularly those with postprandial hyperglycemia, have a higher risk of developing macrovascular events, such as acute coronary syndrome. Because the correlation between postprandial hyperglycemia and an increased risk of macrovascular events has been shown by the Diabetes Epidemiology: Collaborative analysis of Diagnostic criteria in Europe (DECODE) study3 and the Funagata Diabetes Study,4 postprandial blood glucose levels are newly considered to play an important role in the management of diabetes mellitus, in addition of conventional parameters, such as fasting blood glucose and glycosylated hemoglobin (HbA1c) levels. This mechanism might involve the unstable arteriosclerotic plaque associated with postprandial hyperglycemia, such as impaired vascular endothelial function, increased oxidative stress and increased insulin resistance. It is also reported that apoptosis of human umbilical vein endothelial cells were more markedly enhanced by a transient, intermittent hyperglycemic condition (i.e. postprandial hyperglycemic condition) than a persistent hyperglycemic condition, suggesting that management of transient hyperglycemia helps inhibit atherosclerosis progression.5 The STOP‐NIDDM study involving individuals with IGT6 and the Meta‐analysis of Risk Improvement Under Acarbose (MeRIA) study involving type 2 diabetic patients7 have shown that the alpha‐glucosidase inhibitor (α‐GI), acarbose, used in the treatment of postprandial hyperglycemia is helpful in reducing the incidence of major cardiovascular events, in particular, acute coronary syndrome. Among vascular remodeling markers and pro‐inflammatory cytokines, matrix metalloproteinases (MMP), high sensitivity C‐reactive protein (hs‐CRP) are considered as important factors involved in plaque destabilization.8 It has been reported that high glucose conditions alter MMP expression in atherosclerosis related cells9,10 and serum hs‐CRP levels.11
The progression of large‐artery atherosclerosis is correlated with glycemic control in patients with diabetes mellitus and is deeply involved in the prognosis of the disease. To evaluate arteriosclerotic risk factors, carotid artery echography is used for the evaluation of atherosclerotic characterization, such as intima‐media complex thickness and plaque properties, and pulse wave velocity (PWV) is used for the evaluation of arterial wall stiffness or arterial stiffness.12 PWV of the aorta (elastic artery) independently reflects risk markers for the occurrence of organ damage and cardiovascular disease, and a measure of arterial stiffness reflects arterial elasticity or stiffness, allowing early evaluation of arteriosclerosis.13 Although diabetes increases arterial stiffness, independently of aging and hypertension, and this arterial stiffness is highly associated with a post‐challenge hyperglycemic spike, but not fasting glucose level,14 its etiology and pathology have not yet been fully elucidated and it remains to be determined how the control of postprandial hyperglycemia might influence arterial stiffness.
The present study was designed to determine how acarbose used in the treatment of postprandial hyperglycemia influenced arterial stiffness in patients with type 2 diabetes mellitus.
Materials and methods
The inclusion criteria for the present study were: both male and female outpatients aged 20 years and older with type 2 diabetes mellitus, occasional or 2‐h postprandial blood glucose levels of 200 mg/dL or higher, and HbA1c levels between 6.5 and 8.0%. There were 22 patients in the present study. Four of the patients (18%) were pre‐treated with another α‐GI (voglibose) and stopped voglibose before acarbose starting. The other 18 patients were treated with no anti‐diabetic drugs. The following types of patients were excluded from the study: (i) patients receiving oral anti‐diabetic drugs other than α‐GI; (ii) those treated with insulin; (iii) those with a history of severe ketosis, diabetic coma or precoma, serious hepatic or renal dysfunction, severe infection or severe injury and perioperative patients; (iv) those with a history of sensitivity to acarbose; (v) those who are pregnant or are likely to be pregnant; and (vi) those judged by the attending physician to be unable to be included in the study. After the purpose and method of the present study were explained to the patients, they provided informed consent for the participation in this study.
Measurement parameters were: blood glucose levels, HbA1c levels, hs‐CRP, pentraxin 3 (PTX3), MMP‐2, MMP‐9, tissue inhibitor of matrix metalloproteinase (TIMP)‐1 and TIMP‐2. An immunological latex turbidimetry method was used for the measurement of hs‐CRP. MMP‐2, MMP‐9, TIMP‐1 and TIMP‐2 were quantified using an enzyme‐linked immunosorbent assay (ELISA) kit (Daiichi Fine Chemical, Toyama, Japan). The expression of membrane type‐1 matrix metalloproteinase (MT1‐MMP) in peripheral blood mononuclear cells (PBMNC) was determined on a FACScan flow cytometer (Becton Dickinson, Franklin Lakes, NJ, USA) using leukocytes from the heparinized whole blood. MT1‐MMP expression was defined as the proportion of MT1‐MMP‐positive cells in CD14‐positive cells (Figure 1). Blood samples for measurements of all previous listed values were obtained from the cubital vein 2 h after breakfast or lunch.
Figure 1.
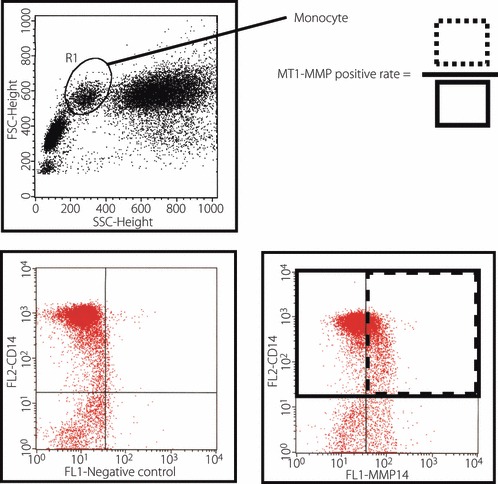
Membrane type‐1 matrix metalloproteinase (MT1‐MMP) expression on peripheral blood mononuclear cells. Flow cytometry analysis was carried out on the whole blood samples. Monocyte phenotype was determined by flow cytometry with gating on CD14 positive cells. Surface expression of MT1‐MMP was estimated by the frequencies of CD14 positive cells expressing MT1‐MMP at the single cell level. FL2, fluorescence 2; FSC, forward scatter.
After a 10‐min rest in the supine position, the cardio‐ankle vascular index (CAVI), a parameter of arterial stiffness, was measured by VaSera VS‐1000 (Fukuda Denshi, Tokyo, Japan), a device for measuring blood pressure and pulse waves. Eating, drinking and smoking just before the tests were forbidden.
Acarbose was given at an initial dose of 50 mg three times daily just before meals to avoid intestinal side‐effects. The dose was increased to 100 mg three times daily after the tolerability was confirmed. In principle, concomitant use of other anti‐diabetic drugs was not allowed. As for other drugs, the dosing regimen was maintained in principle. The duration of acarbose therapy was 12 months. Serological tests were carried out, and CAVI was measured at baseline and after the completion of the treatment.
Statistical Analysis
All measured values were expressed as the mean ± standard deviation. A paired t‐test was used to compare the difference in each parameter at baseline and after the completion of the treatment. Univariate and multivariate analysis of linear regression were carried out to identify the correlation between the change in CAVI and the changes in blood glucose, HbA1c, hs‐CRP, PTX3, MMP‐2, MMP‐9, TIMP‐1, TIMP‐2 and MT1‐MMP. A P‐value <0.05 was considered statistically significant.
Results
Patients’ Clinical Characteristics
A total of 22 patients with type 2 diabetes (mean age, 67 ± 8 years; 68% male) were studied. The mean duration of diabetes mellitus was 5.9 ± 10 years, and cardiovascular risk factors included hypertension (45%), dyperlipidemia (27%) and smoking (18%; Table 1). All patients designated to the acarbose‐treatment group continued taking 100 mg acarbose three times a day during the trial after an initial low dose of acarbose (150 mg daily) for a few weeks.
Table 1. Changes of patients’ data.
| Acarbose | P‐value | ||
|---|---|---|---|
| Before | 12 months later | ||
| n (male/female) | 22 (15/7) | ||
| Age (years) | 67 ± 8 | ||
| Duration of DM (years) | 5.9 ± 10 | ||
| Coronary risk factor | |||
| Hypertension, n (%) | 10 (45) | ||
| Dyslipidemia, n (%) | 6 (27) | ||
| Smoking history, n (%) | 4 (18) | ||
| Medication | |||
| Statin, n (%) | 5 (23) | ||
| ARB/ACE‐I, n (%) | 7 (32) | ||
| β‐blocker, n (%) | 2 (9) | ||
| Creatinine, mg/dL | 0.94 ± 0.21 | 0.98 ± 0.26 | 0.41 |
| BMI (kg/m2) | 23.4 ± 6.2 | 23.3 ± 6.7 | 0.52 |
| Blood pressure (mmHg) | |||
| Systolic | 134 ± 4 | 132 ± 8 | 0.43 |
| Diastolic | 74 ± 8 | 74 ± 5 | 0.27 |
| Heart rate (bpm) | 66 ± 8 | 65 ± 9 | 0.61 |
| Postprandial glucose (mg/dL) | 203 ± 42 | 141 ± 22 | 0.017 |
| HbA1c (%) | 7.6 ± 0.5 | 6.5 ± 0.4 | 0.011 |
| LDL cholesterol (mg/dL) | 127 ± 24 | 122 ± 29 | 0.35 |
| HDL cholesterol (mg/dL) | 45 ± 18 | 51 ± 14 | 0.42 |
| Triglyceride (mg/dL) | 154 ± 42 | 148 ± 35 | 0.23 |
ARB, angiotensin‐II receptor blocker; ACE‐I, angiotensin converting enzyme inhibitor; BMI, body mass index, DM, diabetes mellitus; HbA1c, glycosylated hemoglobin; HDL, high‐density lipoprotein; LDL, low‐density lipoprotein.
Changes in Glucose Parameters After Acarbose Treatment
After treatment of acarbose for 12 months, 2‐h postprandial glucose levels and HbA1c were significantly decreased (from 210 ± 23 to 141 ± 38 mg/dL; P = 0.0018 and from 7.6 ± 0.5 to 6.5 ± 0.4%; P = 0.011, respectively; Table 2).
Table 2. Changes of gelatinolytic activity and inflammatory cytokines data.
| Acarbose | P‐value | ||
|---|---|---|---|
| Before | 12 months later | ||
| MMP‐2, ng/mL (CV%) | 837 ± 118 (11) | 731 ± 89 (12) | 0.024 |
| TIMP‐2, ng/mL (CV%) | 82 ± 11 (13) | 85 ± 18 (21) | 0.32 |
| MMP‐9, ng/mL (CV%) | 36.3 ± 8.1 (22) | 28.1 ± 6.2 (22) | 0.048 |
| TIMP‐1, ng/mL (CV%) | 133 ± 13 (10) | 153 ± 37 (24) | 0.16 |
| hs‐CRP, mg/L (CV%) | 2.21 ± 0.86 (39) | 1.49 ± 0.64 (43) | 0.042 |
| Pentraxin‐3, ng/mL (CV%) | 2.47 ± 0.31 (13) | 1.90 ± 0.21 (11) | 0.0013 |
hs‐CRP, high‐sensitivity C‐reactive protein; MMP, matrix metalloproteinase; TIMP, tissue inhibitor of matrix metalloproteinase.
Acarbose Treatment Reduces Gelatinolytic Activity and Inflammatory Cytokines
Some of the patients had gastrointestinal symptoms, such as flatulence or abdominal distension, during the first few weeks of therapy, but those were tolerable in all patients. There were no hypoglycemic events or liver dysfunction in any cases during the study. The changes in serum levels of gelatinolytic activity, CRP and PTX3 are shown in Table 1. After 12 months of acarbose treatment, MMP‐2 and MMP‐9 were significantly decreased (from 837 ± 118 to 731 ± 89 ng/mL; P = 0.024 and from 36.3 ± 8.1 to 28.1 ± 6.2 ng/mL; P = 0.048, respectively), but there were no significant changes in TIMP‐1 and TIMP‐2 levels (Table 3). Similarly, hs‐CRP and pentraxin‐3 levels were significantly decreased (from 2.21 ± 0.86 to 1.49 ± 0.64 mg/L; P = 0.042 and from 2.47 ± 0.31 to 1.90 ± 0.21 ng/mL; P = 0.0012, respectively; Table 3). Furthermore, we evaluated the expression of MT1‐MMP on PBMNC from patients and found a significant decrease after acarbose treatments (from 34.0 ± 3.6 to 27.4 ± 4.1%; P = 0.043; Figure 2a).
Table 3. Predictors of change in cardio‐ankle vascular index.
| P‐value | ||
|---|---|---|
| Univariate | Multivariate | |
| Age | 0.57 | |
| Sex | 0.84 | |
| Baseline BMI | 0.51 | |
| Δ Blood glucose | 0.0082 | 0.026 |
| Δ HbA1c | 0.12 | |
| Δ hs‐CRP | 0.036 | 0.28 |
| Δ Pentraxin‐3 | 0.0011 | 0.0068 |
| Δ MMP‐2 | 0.018 | 0.42 |
| Δ TIMP‐2 | 0.52 | |
| Δ MMP‐9 | 0.012 | 0.15 |
| Δ TIMP‐1 | 0.48 | |
| Δ MT1‐MMP | 0.010 | 0.041 |
BMI, body mass index; HbA1c, glycosylated hemoglobin; hs‐CRP, high‐sensitivity C‐reactive protein; MMP, matrix metalloproteinase; MT1‐MMP, membrane type‐1 matrix metalloproteinase; TIMP, tissue inhibitor of matrix metalloproteinase.
Figure 2.
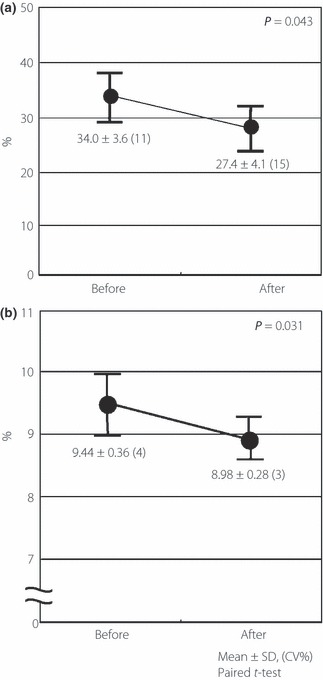
Effects of acarbose on Membrane type‐1 matrix metalloproteinase (MT1‐MMP) expression and arterial stiffness. (a) Circulating peripheral blood mononuclear cells (PBMNC) from patients with type 2 diabetes mellitus were examined for the frequencies of CD14 positive cells expressing MT1‐MMP at the single cell level using flow cytometry. The expression of MT1‐MMP on PBMNC was significantly decreased by acarbose treatments. (b) Cardio‐ankle vascular index (CAVI) was measured as the arterial stiffness. Value of CAVI was significantly reduced after acarbose treatment (from 9.44 ± 0.36 to 8.98 ± 0.28; P = 0.031), although the changes were not significant in blood pressure and heart rate.
Effects of Acarbose on Change of Arterial Stiffness (CAVI)
As in Figure 2b, a significant reduction in CAVI was shown (from 9.44 ± 0.36 to 8.98 ± 0.28; P = 0.031), although the changes were not significant in blood pressure and heart rate (Figure 2b).
The univariate predictors of changes in CAVI are listed in Table 3. A significant association was found in change of blood glucose, PTX3, MMP‐2, MMP‐9 and MT1‐MMP. Furthermore, with a multivariate procedure, improvement of blood glucose, decrease of PTX3 levels and MT1‐MMP expression were independent predictors of beneficial change in CAVI (Table 3).
Discussion
In the present study, acarbose used in the treatment of postprandial hyperglycemia improved arterial stiffness in patients with type 2 diabetes mellitus. PWV of the aorta (elastic artery) is known to independently reflect risk factors, such as arterial elasticity and stiffness, for the occurrence of organ damage and cardiovascular disease, which allows the progression of arteriosclerosis to be evaluated early.13 Cruickshank et al.15 reported that there is a correlation between impaired vascular function and mortality in diabetic patients, and aortic PWV is markedly higher in diabetic patients than in healthy subjects. There are other reports stating that a transient increase in blood glucose levels increases PWV in patients with type 1 diabetes and that transient hyperglycemia during an oral glucose tolerance test is associated with higher CAVI values, suggesting that high blood glucose levels aggravated vascular function.14,16 The mechanism by which the hyperglycemic condition aggravates vascular function might involve impaired endothelial function, aggravation of inflammatory markers and hypercoagulation/hyperfibrinolysis. This mechanism also explains how postprandial hyperglycemia is involved in the occurrence of cardiovascular events, suggesting that the control of postprandial hyperglycemia greatly contributes to the reduction of these events. In the present study, patients with postprandial hyperglycemia associated with higher values of CAVI (an index of arterial stiffness) were treated with acarbose, and their postprandial hyperglycemia was well controlled, thereby improving arterial stiffness. The improvement of postprandial hyperglycemia was also associated with a decrease in the serum levels of inflammatory markers, such as hs‐CRP and PTX3, and a decrease in the serum levels of MMP‐2 and MMP‐9, which play a role in vascular remodeling and plaque instability.17 The results of the present study supported the findings that acarbose therapy is useful for the management of postprandial hyperglycemia in patients with type 2 diabetes mellitus, contributing to the reduction of blood levels of pro‐inflammatory cytokines and the improvement of risk factors for arteriosclerosis, and ultimately improving arterial stiffness.
In the present study, acarbose resulted in the improvement of arterial stiffness. Because acarbose given orally is rarely absorbed from the gastrointestinal system, it is hard to imagine that acarbose would directly affect arterial stiffness. With a multivariate procedure, change of blood glucose and PTX3 were independent predictors of improvement of arterial stiffness after acarbose treatment (Table 3). These improvements of blood glucose and PTX3 induced by acarbose treatments probably contribute to the improvement of arterial stiffness. Because we have no data about other α‐GI, it is far from convincing that acarbose can decrease the inflammation directly. It at least partly might be the additional effects of acarbose beyond glucose‐lowering. Kato et al.18 reported that acarbose has more beneficial effects on endothelial function beyond the improvement in glycaemic control, rather than the glinide drugs. Furthermore, previous reports have shown that acarbose‐induced improvement of metabolic status might reduce blood pressure,19 and acarbose treatment delays progression of intima‐media thickness of the common carotid arteries compared with placebo, even after a reduction of glucose20.
An in vivo study using macrophages showed that a chronic hyperglycemic condition promotes the production of MMP as a result of chronic inflammation.10 However, such an increased production of MMP was reportedly to be due to a transient increase in blood glucose levels, but not due to a chronic hyperglycemic condition, suggesting the correlation between a transient increase in blood glucose levels and the occurrence of cardiovascular events.21 The present study showed that acarbose therapy not only improved postprandial hyperglycemia, but also reduced serum levels of MMP in the clinical setting. Expression of adhesion molecules associated with transient hyperglycemia reportedly plays an important role in upregulating the production and activity of MMP. In addition, MT1‐MMP expression on the PBMNC has been investigated. There are studies investigating the correlation between MT1‐MMP expression and unstable atherosclerotic plaques in humans and knockout mice.22,23 We have previously shown that macrophages accumulate in the shoulder region prone to plaque disruption in human coronary atherosclerotic plaques and MT1‐MMP is expressed in the macrophages,24 and that strong expression of MT1‐MMP is induced in PBMNC by pro‐inflammatory cytokines or oxidized low‐density lipoprotein (ox‐LDL).25 The present study investigated monocyte surface expression of MT1‐MMP and showed for the first time that such MT1‐MMP expression is directly involved in plaque disruption. A recent study showed, based on detailed optical coherence tomographic findings of unstable coronary plaques, that monocytes are attached to the surface of the unstable coronary plaques.26 MT‐MMP has a central role in the MMP activation cascade locally around the cells where MT‐MMP is expressed, activating MMP‐2. It is therefore speculated that the degradation of the extracellular matrix is enhanced on the surface of the plaque to which monocytes are attached. More specifically, MMP‐2 is a type IV collagenase, an extracellular matrix degrading enzyme induced by atherogenic cytokines,27,28 leading to an unstable fibrous cap covering the plaque surface.29 Thus, determining MT1‐MMP expression levels on the monocyte surface is useful for the direct evaluation of unstable plaque. In the present study, acarbose was effective in reducing such MT1‐MMP expression. The results of the present study suggested that this drug is useful in stabilizing plaques, particularly when it is used in the treatment of patients with postprandial hyperglycemia. In the STOP‐NIDDM study and the MeRIA7 study evaluating the association between the control of postprandial hyperglycemia and the occurrence of cardiovascular events, acarbose significantly reduced the incidence of myocardial infarction as a result of unstable plaques. Our data provide support for the mechanism.
The present study showed that acarbose, used in the treatment of postprandial hyperglycemia in patients with type 2 diabetes mellitus, contributes to the reduction of the blood levels of pro‐inflammatory cytokines as well as the improvement of risk factors for the occurrence of arteriosclerosis.
Acknowledgements
The authors would like to thank Hiromi Nishimura and Yumie Yasuzaki for secretarial assistance. We attest to the fact that none of our financial relationships or commercial associations create a potential conflict of interest relative to this manuscript.
References
- 1.Pyörälä K, Laakso M, Uusitupa M. Diabetes and atherosclerosis: an epidemiologic view. Diabetes Metab Rev 1987; 3: 463–524 [DOI] [PubMed] [Google Scholar]
- 2.Ramlo‐Halsted BA, Edelman SV. The natural history of Type 2 diabetes: practical points to consider in developing prevention and treatment strategies. Clin Diabetes 2000; 18: 80–85 [Google Scholar]
- 3.The DECODE study group . Glucose tolerance and mortality: comparison of WHO and American Diabetes Association diagnostic criteria. European Diabetes Epidemiology Group. Diabetes Epidemiology: Collaborative analysis Of Diagnostic criteria in Europe. Lancet 1999; 354: 617–621 [PubMed] [Google Scholar]
- 4.Tominaga M, Eguchi H, Manaka H, et al. Impaired glucose tolerance is a risk factor for cardiovascular disease, but not impaired fasting glucose. The Funagata Diabetes Study. Diabetes Care 1999; 22: 920–924 [DOI] [PubMed] [Google Scholar]
- 5.Azuma K, Kawamori R, Toyofuku Y, et al. Repetitive fluctuations in blood glucose enhance monocyte adhesion to the endothelium of rat thoracic aorta. Arterioscler Thromb Vasc Biol 2006; 26: 2275–2280 [DOI] [PubMed] [Google Scholar]
- 6.Chiasson JL, Josse RG, Gomis R, et al. Acarbose treatment and the risk of cardiovascular disease and hypertension in patients with impaired glucose tolerance: the STOP‐NIDDM trial. JAMA 2003; 290: 486–494 [DOI] [PubMed] [Google Scholar]
- 7.Hanefeld M, Cagatay M, Petrowitsch T, et al. Acarbose reduces the risk for myocardial infarction in type 2 diabetic patients: meta‐analysis of seven long‐term studies. Eur Heart J 2004; 25: 10–16 [DOI] [PubMed] [Google Scholar]
- 8.Nabata A, Kuroki M, Ueba H, et al. C‐reactive protein induces endothelial cell apoptosis and matrix metalloproteinase‐9 production in human mononuclear cells: implications for the destabilization of atherosclerotic plaque. Atherosclerosis 2008; 196: 129–135 [DOI] [PubMed] [Google Scholar]
- 9.Death AK, Fisher EJ, McGrath KC, et al. High glucose alters matrix metalloproteinase expression in two key vascular cells: potential impact onatherosclerosis in diabetes. Atherosclerosis 2003; 168: 263–269 [DOI] [PubMed] [Google Scholar]
- 10.Nareika A, Sundararaj KP, Im YB, et al. High glucose and interferon gamma synergistically stimulate MMP‐1 expression in U937 macrophages by increasing transcription factor STAT1 activity. Atherosclerosis 2009; 202: 363–371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang YH, Chen SY, Wang TD, et al. The relationships among serum glucose, albumin concentrations and carotid atherosclerosis in men with spinal cord injury. Atherosclerosis 2009; 206: 528–534 [DOI] [PubMed] [Google Scholar]
- 12.Safar ME, Levy BI, Struijker‐Boudier H. Current perspectives on arterial stiffness and pulse pressure in hypertension and cardiovascular diseases. Circulation 2003; 107: 2864–2869 [DOI] [PubMed] [Google Scholar]
- 13.Laurent S, Boutouyrie P, Asmar R, et al. Aortic stiffness is an independent predictor of all‐cause and cardiovascular mortality in hypertensive patients. Hypertension 2001; 37: 1236–1241 [DOI] [PubMed] [Google Scholar]
- 14.Huang CL, Chen MF, Jeng JS, et al. Postchallenge hyperglycaemic spike associate with arterial stiffness. Int J Clin Pract 2007; 61: 397–402 [DOI] [PubMed] [Google Scholar]
- 15.Cruickshank K, Riste L, Anderson SG, et al. Aortic pulse‐wave velocity and its relationship to mortality in diabetes and glucose intolerance. An integrated index of vascular function? Circulation 1999; 106: 2085–2090 [DOI] [PubMed] [Google Scholar]
- 16.Gordin D, Rönnback M, Forsblom C, et al. Acute hyperglycaemia rapidly increases arterial stiffness in young patients with type 1 diabetes. Diabetologia 2007; 50: 1808–1814 [DOI] [PubMed] [Google Scholar]
- 17.Hingorani AD, Shah T, Casas JP, et al. C‐reactive protein and coronary heart disease: predictive test or therapeutic target? Clin Chem 2009; 55: 239–255 [DOI] [PubMed] [Google Scholar]
- 18.Kato T, Inoue T, Node K. Postprandial endothelial dysfunction in subjects with new‐onset type 2 diabetes: an acarbose and nateglinide comparative study. Cardiovasc Diabetol 2010; 9: 12–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rosenbaum P, Peres RB, Zanella MT, et al. Improved glycemic control by acarbose therapy in hypertensive diabetic patients: effects on blood pressure and hormonal parameters. Braz J Med Biol Res 2002; 35: 877–884 [DOI] [PubMed] [Google Scholar]
- 20.Hanefeld M, Chiasson JL, Koehler C, et al. Acarbose slows progression of intima‐media thickness of the carotid arteries in subjects with impaired glucose tolerance. Stroke 2004; 35: 1073–1078 [DOI] [PubMed] [Google Scholar]
- 21.Sun J, Xu Y, Dai Z, et al. Intermittent high glucose enhances proliferation of vascular smooth muscle cells by upregulating osteopontin. Mol Cell Endocrinol 2009; 313: 64–69 [DOI] [PubMed] [Google Scholar]
- 22.Rajavashisth TB, Xu XP, Jovinge S, et al. Membrane type 1 matrix metalloproteinase expression in human atherosclerotic plaques: evidence for activation by proinflammatory mediators. Circulation 1999; 99: 3103–3109 [DOI] [PubMed] [Google Scholar]
- 23.Sakamoto T, Seiki M. Cytoplasmic tail of MT1‐MMP regulates macrophage motility independently from its protease activity. Genes Cells 2009; 14: 617–626 [DOI] [PubMed] [Google Scholar]
- 24.Uzui H, Harpf A, Liu M, et al. Increased expression of membrane type 3‐matrix metalloproteinase in human atherosclerotic plaque: role of activated macrophages and inflammatory cytokines. Circulation 2002; 106: 3024–3030 [DOI] [PubMed] [Google Scholar]
- 25.Uzui H, Nakano A, Mitsuke Y, et al. Membrane Type 1 Matrix Metalloproteinase Expression On Peripheral Blood Mononuclear Cells After Acute Myocardial Infarction. Circulation, 2007; 116: II_523 [Google Scholar]
- 26.Kubo T, Akasaka T. Recent advances in intracoronary imaging techniques: focus on optical coherence tomography. Expert Rev Med Devices 2008; 5: 691–697 [DOI] [PubMed] [Google Scholar]
- 27.Uzui H, Lee JD, Shimizu H, et al. The role of protein‐tyrosine phosphorylation and gelatinase production in the migration and proliferation of smooth muscle cells. Atherosclerosis 2000; 149: 51–59 [DOI] [PubMed] [Google Scholar]
- 28.Yue H, Lee JD, Shimizu H, et al. Effects of magnesium on the production of extracellular matrix metalloproteinases in cultured rat vascular smooth muscle cells. Atherosclerosis 2003; 166: 271–277 [DOI] [PubMed] [Google Scholar]
- 29.Newby AC. Metalloproteinases and vulnerable atherosclerotic plaques. Trends Cardiovasc Med 2007; 17: 253–258 [DOI] [PMC free article] [PubMed] [Google Scholar]


