Abstract
Outbreaks of Ebola and Marburg virus diseases have recently increased in frequency in Uganda. This increase is probably caused by a combination of improved surveillance and laboratory capacity, increased contact between humans and the natural reservoir of the viruses, and fluctuations in viral load and prevalence within this reservoir. The roles of these proposed explanations must be investigated in order to guide appropriate responses to the changing epidemiological profile. Other African settings in which multiple filoviral outbreaks have occurred could also benefit from such information.
Introduction
Marburgvirus and Ebolavirus, the two genera of the Filoviridae family of virus, cause human diseases known as Marburg virus disease (Marburg) and Ebola virus disease (Ebola), respectively. They are highly lethal diseases, with case fatality ratios typically between 20% and 90%, depending on the virus species.1–3 Evidence suggests that cave- and forest-dwelling fruit bats of the Pteropodidae family (suborder Megachiroptera) are the natural reservoir of both viruses.2–7
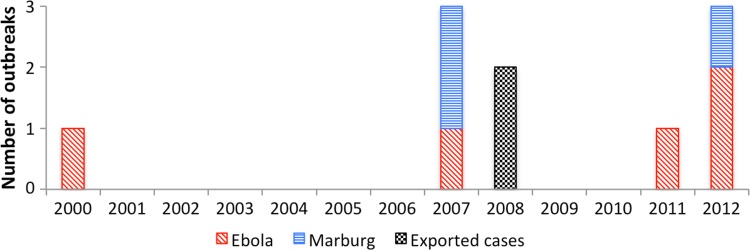
The frequency of filoviral disease outbreaks in Uganda has increased in recent years (Figure 1). Before 2007, there had only been one reported outbreak (of Ebola) in Uganda.8,9 However, since 2007, nine filoviral disease outbreaks have occurred: six of Ebola and three of Marburg.10–16 One of these outbreaks in 2007 and 2008 involved Bundibugyo ebolavirus, which was the first new species of filovirus to be described in several years, an occurrence that has been ascribed to improved surveillance capacity.11,12
Figure 1.
Timeline of outbreaks of filoviral disease known to have occurred in Uganda.
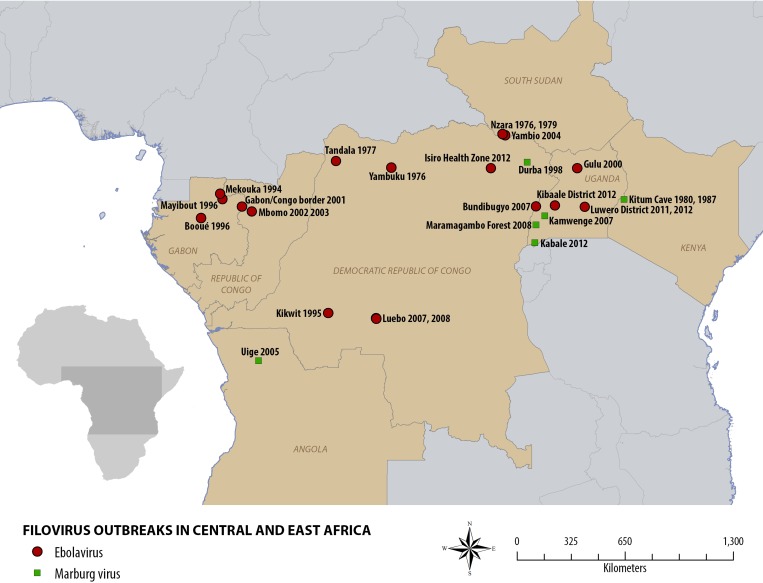
Similar patterns have been observed elsewhere: there were no reported outbreaks of Ebola globally between 1979 and 1994, but this period was followed by near simultaneous reemergence of the disease in Gabon, Cote D'Ivoire, and the Democratic Republic of the Congo (Figure 2).17,18 Similarly, there were no known cases of Marburg globally between 1987 and 1998,17 whereas three outbreaks have occurred in Uganda since 2007.
Figure 2.
Map of the known outbreaks of filoviral disease in Central and East Africa.
The reasons for the apparent increasing frequency of filoviral disease outbreaks in Uganda are unknown. We present the most likely explanations and suggest steps to further elucidate filoviral transmission dynamics and guide the healthcare response to the changing context.
Possible Explanations of Changing Epidemiological Profile
Changes to the surveillance system.
Improved sensitivity of filoviral disease surveillance in Uganda might account for the increased number of epidemics, through the detection of isolated cases or small clusters that would previously have gone undetected.
Awareness about filoviral diseases and their signs and symptoms is increasing among Ugandan health professionals, and experience is growing with each subsequent outbreak. Investment in building surveillance capacity with the World Health Organization's integrated disease surveillance and response strategy has also contributed to this. Political will and buy-in from the government of Uganda for strengthening surveillance, combined with international assistance, played a vital role in Uganda. International assistance included the establishment of a ready-to-use isolation ward by Médecins Sans Frontières in collaboration with the Ministry of Health, and the Centers for Disease Control and Prevention provided substantial technical and financial support for building capacity for the surveillance of viral hemorrhagic fevers in Uganda, particularly by improving capacity for laboratory diagnosis at the Uganda Virus Research Institute in Entebbe.
Educating communities and their health staff could result in additional improvements. Epidemics of filoviral disease frequently occur in settings with local beliefs in witchcraft and curses,19 which undermine surveillance and infection control at the peripheral level: affected individuals often seek healthcare from traditional healers first rather than at Ministry of Health-supervised facilities, during which time they remain undetected and can infect their contacts.20 This process also happens when formal healthcare providers do not suspect filoviral disease and when patients resist being admitted to isolation wards during outbreaks because of fear and stigmatization.
The improved sensitivity of surveillance activities raises the possibility that other outbreaks were not detected. Therefore, enhanced surveillance may be providing a better understanding of filoviral transmission dynamics: low-level transmission, resulting in small, self-contained clusters among close contacts, may be more common than realized but are frequently missed by formal surveillance systems.
Changes in patterns of interaction between humans and bats.
There may be increasing interaction between humans and the natural reservoir of the filoviruses, perhaps through habitat encroachment or hunting activities.21–23 Commercial mining activity began at Kitaka cave in Ibanda district in January of 2007; within 9 months, four miners had contracted Marburg.10 A subsequent study reported that approximately 5% of the estimated 100,000 bats dwelling in Kitaka cave were infected by Marburgvirus.4 Two tourists visiting Uganda who developed Marburg on return to their countries both reported having visited the same cave inhabited by bats among which Marburgvirus circulates.13,14,24
Changes in filoviral seroprevalence in the host species.
Cyclic population changes of the natural reservoir could result in changes in filoviral seroprevalence and an increased frequency of zoonotic transmission events. The seroprevalence of both Ebolavirus and Marburgvirus in various bat species in Gabon and the Republic of Congo has been reported to fluctuate over time.7 Seasonal variation in Marburgvirus seroprevalence among Rousettus aegyptiacus has also been shown, with peaks coinciding with the birthing seasons.24 The same study found a strong temporal correlation between historical epidemics of Marburg among humans and these seasonal peaks.
Possible Scenarios
Three scenarios are suggested by these explanations.
-
(1)
The increase in outbreak frequency is perceived and not real. This scenario would result from successful strengthening of the national surveillance system. We would expect to see similar increases in other filoviral-endemic countries in which capacity building has been, or will be, implemented.
-
(2)
The increase is real but linked to factors specific to Uganda. In this scenario, the public health concern is limited in geographical scope, and we would not expect to observe an increase in outbreak frequency outside of Uganda.
-
(3)
The increase is real and caused by factors that are not limited to Uganda. In this scenario, an increase in outbreak frequency outside of Uganda would be expected.
.
The most likely of these scenarios is the first, because strengthening of the Ugandan national surveillance system has demonstrably taken place in recent years. However, scenarios 2 and 3 are both plausible given the possible explanations described above, and therefore they necessitate additional investigation as to their roles in the observed increased frequency of outbreaks of filoviral disease.
Discussion
These scenarios imply different interpretations and would require different responses in those countries that experience more frequent filoviral disease outbreaks. However, the available evidence in support of any of these possible scenarios is mostly circumstantial; therefore, the roles that any, all, or none of these scenarios play in the increased frequency of filoviral disease outbreaks in Uganda must be investigated. Activities should focus on the following topics.
-
(1)
Reinforcing targeted surveillance activities, particularly around areas that have experienced multiple outbreaks and areas inhabited by bat colonies proven to be seropositive for filoviruses. Establishing in-country capacity for ongoing, sustained surveillance (with concommitant reduced reliance on external intervention) is essential for a variety of diseases, including filoviral disease, in the (rural) African context.
-
(2)
Establishment of additional ready to use isolation wards at hospitals near the most affected areas; one such ward has been established at Mbarara Hospital, close to Ibanda district. Such wards can be established and maintained with minimal investment—all that is required is a suitably sized building constructed of material that is easy to disinfect (e.g., concrete) and easy to isolate from the rest of the health facility, and a minimum of supplies (particularly personal protective equipment) to be kept in storage on site.
-
(3)
Improved in-country or regional capacity for accurate laboratory and differential diagnosis, which is underway in Uganda. The introduction of rapid testing for these diseases would also be a welcome, albeit secondary, measure.
-
(4)
Accelerated genotyping of circulating filovirus strains to determine the frequency of their introduction into the human population.
-
(5)
Seroprevalence surveys of filoviral infection in the general population of the most affected areas to estimate the prevalence of undetected disease in the at-risk population.
-
(6)
Additional studies to elucidate the ecology of filoviruses in bats. These studies include investigations of the mechanisms of natural infection and the processes of virus shedding to understand the pathways of transmission. In addition, studies of the prevalence of species-specific Ebolavirus antibodies among bat populations, similar to those studies done for Marburgvirus,10,13,24 are necessary to identify the most likely natural reservoirs, thereby defining the geographical scope of risk.
-
(7)
Ecological and serological comparisons between areas that have experienced multiple epidemics and areas that have experienced single epidemics, which may reveal factors in susceptibility to, and transmission of, filoviral disease.
These activities could help healthcare providers to adapt and respond appropriately to the new paradigm. This information is relevant not only for Uganda, but also for other African countries that have experienced outbreaks of filovirus disease during the past 45 years, particularly areas that have experienced multiple outbreaks, including Western Equatoria state in South Sudan, Durba and Kampungu in the Democratic Republic of the Congo, and the Ogooué-Ivindo region of northeastern Gabon (Figure 2) (where Marburg virus has been found to be enzootic).18,25 Lessons from the Ugandan experience, whatever they may be, must be investigated, learned, and shared.
Footnotes
Authors' addresses: Jonathan A. Polonsky, Intervention Epidemiology and Training Department, Epicentre, Paris, France, E-mail: jonathan.polonsky@epicentre.msf.org. Joseph F. Wamala, Epidemiological Surveillance Division, Ministry of Health, Kampala, Uganda, E-mail: j_wamala@yahoo.com. Hilde de Clerck, Emergency Desk, Médecins Sans Frontières Operational Centre Brussels, Brussels, Belgium, E-mail: hilde.declerck@gmail.com. Michel Van Herp, Disease Control Unit, Médecins Sans Frontières Operational Centre Brussels, Brussels, Belgium, E-mail: michel.van.herp@brussels.msf.org. Armand Sprecher, Public Health Unit, Médecins Sans Frontières Operational Centre Brussels, Brussels, Belgium, E-mail: armand.sprecher@brussels.msf.org. Klaudia Porten, Intervention Epidemiology and Training Department, Epicentre, Paris, France, E-mail: klaudia.porten@epicentre.msf.org. Trevor Shoemaker, Viral Special Pathogens Branch, US Centers for Disease Control and Prevention, Entebbe, Uganda, E-mail: tis8@cdc.gov.
References
- 1.Centers for Disease Control and Prevention Outbreak of Marburg virus hemorrhagic fever—Angola, October 1, 2004–March 29, 2005. MMWR Morb Mortal Wkly Rep. 2005;54:308–309. [PubMed] [Google Scholar]
- 2.World Health Organization Ebola Haemorrhagic Fever. 2012. http://www.who.int/mediacentre/factsheets/fs103/en/ Available at. Accessed December 24, 2012.
- 3.World Health Organization Marburg Haemorrhagic Fever. 2012. http://www.who.int/mediacentre/factsheets/fs_marburg/en/ Available at. Accessed December 24, 2012.
- 4.Towner JS, Amman BR, Sealy TK, Carroll SAR, Comer JA, Kemp A, Swanepoel R, Paddock CD, Balinandi S, Khristova ML, Formenty PBH, Albarino CG, Miller DM, Reed ZD, Kayiwa JT, Mills JN, Cannon DL, Greer PW, Byaruhanga E, Farnon EC, Atimnedi P, Okware S, Katongole-Mbidde E, Downing R, Tappero JW, Zaki SR, Ksiazek TG, Nichol ST, Rollin PE. Isolation of genetically diverse marburg viruses from Egyptian fruit bats. PLoS Pathog. 2009;5:e1000536. doi: 10.1371/journal.ppat.1000536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Leroy EM, Kumulungui B, Pourrut X, Rouquet P, Hassanin A, Yaba P, Délicat A, Paweska JT, Gonzalez J-P, Swanepoel R. Fruit bats as reservoirs of Ebola virus. Nature. 2005;438:575–576. doi: 10.1038/438575a. [DOI] [PubMed] [Google Scholar]
- 6.Leroy EM, Epelboin A, Mondonge V, Pourrut X, Gonzalez J-P, Muyembe-Tamfum J-J, Formenty P. Human Ebola outbreak resulting from direct exposure to fruit bats in Luebo, Democratic Republic of Congo, 2007. Vector Borne Zoonotic Dis. 2009;9:723–728. doi: 10.1089/vbz.2008.0167. [DOI] [PubMed] [Google Scholar]
- 7.Pourrut X, Souris M, Towner JS, Rollin PE, Nichol ST, Gonzalez J-P, Leroy E. Large serological survey showing cocirculation of Ebola and Marburg viruses in Gabonese bat populations, and a high seroprevalence of both viruses in Rousettus aegyptiacus. BMC Infect Dis. 2009;9:159. doi: 10.1186/1471-2334-9-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Okware SI, Omaswa FG, Zaramba S, Opio A, Lutwama JJ, Kamugisha J, Rwaguma EB, Kagwa P, Lamunu M. An outbreak of Ebola in Uganda. Trop Med Int Health. 2002;7:1068–1075. doi: 10.1046/j.1365-3156.2002.00944.x. [DOI] [PubMed] [Google Scholar]
- 9.Borchert M, Mutyaba I, Van Kerkhove MD, Lutwama J, Luwaga H, Bisoborwa G, Turyagaruka J, Pirard P, Ndayimirije N, Roddy P, Van Der Stuyft P, Van Kerkhove MD. Ebola haemorrhagic fever outbreak in Masindi District, Uganda: outbreak description and lessons learned. BMC Infect Dis. 2011;11:357. doi: 10.1186/1471-2334-11-357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Adjemian J, Farnon EC, Tschioko F, Wamala JF, Byaruhanga E, Bwire GS, Kansiime E, Kagirita A, Ahimbisibwe S, Katunguka F, Jeffs B, Lutwama JJ, Downing R, Tappero JW, Formenty P, Amman B, Manning C, Towner J, Nichol ST, Rollin PE. Outbreak of Marburg hemorrhagic fever among miners in Kamwenge and Ibanda Districts, Uganda, 2007. J Infect Dis. 2011;204((Suppl 3)):S796–S799. doi: 10.1093/infdis/jir312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Towner JS, Sealy TK, Khristova ML, Albariño CG, Conlan S, Reeder SA, Quan P-L, Lipkin WI, Downing R, Tappero JW, Okware S, Lutwama J, Bakamutumaho B, Kayiwa J, Comer JA, Rollin PE, Ksiazek TG, Nichol ST. Newly discovered Ebola virus associated with hemorrhagic fever outbreak in Uganda. PLoS Pathog. 2011;4:e1000212. doi: 10.1371/journal.ppat.1000212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wamala JF, Lukwago L, Malimbo M, Nguku P, Yoti Z, Musenero M, Amone J, Mbabazi W, Nanyunja M, Zaramba S, Opio A, Lutwama JJ, Talisuna AO, Okware SI. Ebola hemorrhagic fever associated with novel virus strain, Uganda, 2007–2008. Emerg Infect Dis. 2010;16:1087–1092. doi: 10.3201/eid1607.091525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Centers for Disease Control and Prevention Imported case of Marburg hemorrhagic fever—Colorado, 2008. MMWR Morb Mortal Wkly Rep. 2010;303:413–415. [PubMed] [Google Scholar]
- 14.Timen A, Koopmans MPG, Vossen ACTM, van Doornum GJJ, Günther S, van den Berkmortel F, Verduin KM, Dittrich S, Emmerich P, Osterhaus ADME, van Dissel JT, Coutinho RA. Response to imported case of Marburg hemorrhagic fever, the Netherlands. Emerg Infect Dis. 2009;15:1171–1175. doi: 10.3201/eid1508.090051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shoemaker T, MacNeil A, Balinandi S, Campbell S, Wamala JF, McMullan LK, Downing R, Lutwama J, Mbidde E, Ströher U, Rollin PE, Nichol ST. Reemerging Sudan Ebola virus disease in Uganda, 2011. Emerg Infect Dis. 2012;18:1480–1483. doi: 10.3201/eid1809.111536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Centers for Disease Control and Prevention Outbreak Postings. 2012. http://www.cdc.gov/ncidod/dvrd/spb/outbreaks/index.htm#ebola-2012 Available at. Accessed December 24, 2012.
- 17.Leroy E, Baize S, Gonzalez JP. Ebola and Marburg hemorrhagic fever viruses: update on filoviruses. Med Trop (Mars) 2011;71:111–121. [PubMed] [Google Scholar]
- 18.MacNeil A, Rollin PE. Ebola and Marburg hemorrhagic fevers: neglected tropical diseases? PLoS Negl Trop Dis. 2012;6:e1546. doi: 10.1371/journal.pntd.0001546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.World Health Organization Ebola in Uganda—Update. 2012. http://www.who.int/csr/don/2012_11_30_ebola/en/index.html Available at. Accessed March 21, 2013.
- 20.Hewlett BS, Amola RP. Cultural contexts of Ebola in northern Uganda. Emerg Infect Dis. 2003;9:1242–1248. doi: 10.3201/eid0910.020493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Monath TP. Ecology of Marburg and Ebola viruses: speculations and directions for future research. J Infect Dis. 1999;179((Suppl 1)):S127–S138. doi: 10.1086/514281. [DOI] [PubMed] [Google Scholar]
- 22.Mwavu EN, Witkowski ETF. Land-use and cover changes (1988-2002) around Budongo Forest Reserve, NW Uganda: implications for forest and woodland sustainability. Land Degradation Dev. 2008;19:606–622. [Google Scholar]
- 23.Muehlenbein MP. Parasitological analyses of the male chimpanzees (Pan troglodytes schweinfurthii) at Ngogo, Kibale National Park, Uganda. Am J Primatol. 2005;65:167–179. doi: 10.1002/ajp.20106. [DOI] [PubMed] [Google Scholar]
- 24.Amman BR, Carroll SA, Reed ZD, Sealy TK, Balinandi S, Swanepoel R, Kemp A, Erickson BR, Comer JA, Campbell S, Cannon DL, Khristova ML, Atimnedi P, Paddock CD, Crockett RJ, Flietstra TD, Warfield KL, Unfer R, Katongole-Mbidde E, Downing R, Tappero JW, Zaki SR, Rollin PE, Ksiazek TG, Nichol ST, Towner JS. Seasonal pulses of Marburg virus circulation in juvenile Rousettus aegyptiacus bats coincide with periods of increased risk of human infection. PLoS Pathog. 2012;8:e1002877. doi: 10.1371/journal.ppat.1002877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maganga GD, Bourgarel M, Ella GE, Drexler JF, Gonzalez J-P, Drosten C, Leroy EM. Is Marburg virus enzootic in Gabon? J Infect Dis. 2011;204((Suppl 3)):S800–S803. doi: 10.1093/infdis/jir358. [DOI] [PubMed] [Google Scholar]