Abstract
We assessed the effect of mass azithromycin treatment on malaria parasitemia in a trachoma trial in Niger. Twenty-four study communities received treatment during the wet, high-transmission season. Twelve of the 24 communities were randomized to receive an additional treatment during the dry, low-transmission season. Outcome measurements were conducted at the community-level in children < 1–72 months of age in May–June 2011. Parasitemia was higher in the 12 once-treated communities (29.8%, 95% confidence interval [CI] = 21.5–40.0%) than in the 12 twice-treated communities (19.5%, 95% CI = 13.0–26.5%, P = 0.03). Parasite density was higher in once-treated communities (354 parasites/μL, 95% CI = 117–528 parasites/μL) than in twice-treated communities (74 parasites/μL, 95% CI = 41–202 parasites/μL, P = 0.03). Mass distribution of azithromycin reduced malaria parasitemia 4–5 months after the intervention. The results suggest that drugs with antimalaria activity can have long-lasting impacts on malaria during periods of low transmission.
Introduction
The World Health Organization (WHO) currently recommends repeated mass oral azithromycin distributions in trachoma-endemic areas to control the ocular strains of Chlamydia that cause the disease. A cohort study and a cluster-randomized trial have showed that these distributions could reduce childhood mortality.1,2 Cohort studies have suggested that azithromycin may reduce leading causes of childhood mortality, including respiratory disease, diarrhea, and malaria.3–7 In areas with seasonal malaria transmission, repeated administration of antimalarial drugs to children during the transmission season (intermittent preventive therapy of children [IPTc])8,9 has been shown to reduce malaria transmission, although preventive therapy outside of the transmission season is not typically recommended.3 However, transmission models suggest treatment during the low-transmission season of an infectious disease may provide significant or even maximum impact.10–12 Azithromycin has antimalaria activity, although lower than first-line agents.13–15 It is not clear whether mass administration of azithromycin during the low malaria transmission season could have a long-lasting antimalaria effect.
In a large, cluster-randomized clinical trial for trachoma, we assessed whether mass oral azithromycin distributions reduced malaria parasitemia and increased hemoglobin concentration.16 We compared community-level malaria asexual parasitemia, parasite density and gametocytemia, and the hemoglobin concentration in communities randomized to a single mass treatment with azithromycin at the beginning of the rainy season to that in communities randomized to this treatment plus a second azithromycin distribution during the dry season.
Methods
Study design.
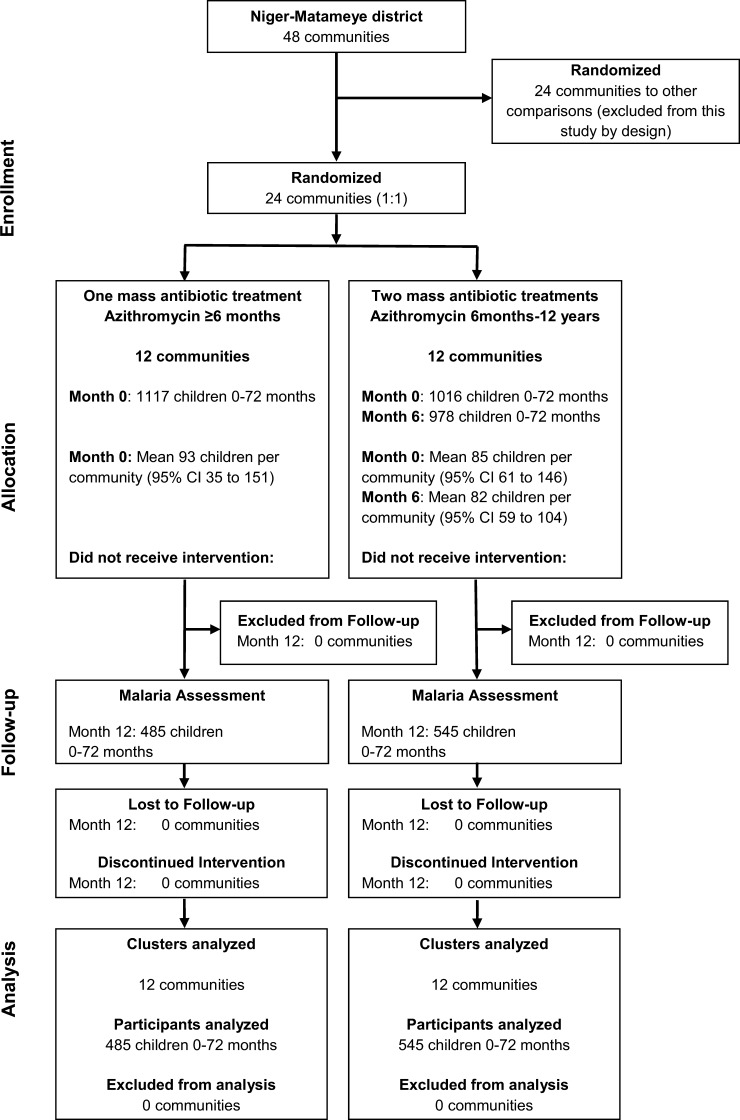
The Program for the Rapid Elimination of Trachoma (PRET) is a set of three cluster-randomized trials in Niger, Tanzania, and the Gambia.16,17 In the Niger trials, 48 grappes (government health units, termed communities in this report) were randomized into 4 arms of 12 grappes each to evaluate different treatment frequencies and treatment coverage levels (Figure 1). Inclusion criteria for the communities have been described and included total population on the last census between 250 and 600 persons and prevalence of active trachoma (trachomatous inflammation follicular, by using the WHO system)18 ≥ 10% in children < 1–72 months of age.16 We conducted a malaria assessment in 24 communities from 2 of the 4 PRET study arms during May–June 2011.
Figure 1.
Participant flow in cluster-randomized clinical trial in 24 communities in Niger.
Intervention.
Twelve communities were randomized to receive a single mass distribution of oral azithromycin to all persons ≥ 6 months of age, and 12 communities were randomized to receive a mass distribution of oral azithromycin in children 6 months to 12 years of age plus a second distribution. The treatment coverage goal was 80% of the targeted group.
The initial distribution of directly observed oral azithromycin (height-based dosing approximating 20 mg/kg19,20) was given to all eligible persons during July 3–August 9, 2010 in the 24 communities. The second mass treatment was provided only to the 12 communities randomized to receive this intervention during January 7–10, 2011. Thus, communities in the twice-treated arm were randomized to an extra mass distribution in the dry, low transmission season. During May 24–June 7, 2011, a cross-sectional survey of 50 randomly chosen children < 1–72 months of age was performed in all 24 communities. Because the selection of these 50 children was based on the 12-month census, some of these children may not have been present at the time of azithromycin distributions. If a community contained < 50 children, all eligible children were included, but note this is a community-randomized, intent-to-treat analysis of direct and indirect effects of the antibiotic.
Clinical and laboratory assessments.
Thick blood smears and blood spots of approximately 20 μL were prepared on Whatman FTA Elute cards (GE Healthcare, Piscataway, NJ) from a finger prick, after obtaining consent from a parent or guardian. Blood spots were air-dried and stored at room temperature in sealed plastic bags containing desiccant. The thick blood smears were stained with 3% Giemsa and read with a light microscope by two experienced microscopists at the Zinder Hospital in Niger. The microscopists were masked to community treatment assignment and to the readings of the other microscopist. The malaria case definition was based on the presence or absence of Plasmodium parasites on microscopy slides. Parasite densities were determined from thick blood smears by counting the number of asexual parasites per 200 leukocytes and assuming a leukocyte count of 8,000/μL.21 Parasites were considered present if both microscopists observed them. Because prevalence was low, gametocytes were considered present if either microscopist observed them.
To determine Plasmodium species, one thick blood smear–positive case was randomly chosen from each of the 24 study communities and tested for Plasmodium DNA by polymerase chain reaction (PCR). A 3-mm punch was prepared from a blood spot (Harris Micro-Punch; Ted Pella, Inc., Redding, CA), and DNA was extracted and eluted in 30 μL of sterile water, according to the FTA card manufacturer's instructions. Between each sample, the micro-punch was cleaned with bleach, rinsed with ethanol, and dried. A blank punch from a clean FTA card was taken between each sample as a negative control to test for cross-contamination.
For species determination, a portion of the cytochrome b region was amplified by using nested PCR.22 The PCRs were performed in 50-μL reactions using the TopTaq DNA polymerase kit (QIAGEN, Valencia, CA) and 5 μL of the extracted DNA (first round) or the amplicon (nested reaction). The PCR products were confirmed after electrophoresis on a 2% agarose gel. Products from samples positive for the 815-basepair cytochrome b fragment were purified by using the QIAquick PCR Purification Kit (QIAGEN) and then sequenced in both directions with nested primers (Sequetech, Mountain View, CA). Sequences were trimmed, edited, and aligned by using Sequencher 5.0 (Gene Codes Corp, Ann Arbor, MI) and then compared with reference sequences for P. falciparum, P. vivax, P. malariae, and P. ovale found in GenBank.23 Hemoglobin concentration was determined for each child (HemoCue AB, Ängelholm, Sweden). Repeat hemoglobin assessments were performed on every tenth case to assess reproducibility.
Randomization.
Communities in Niger are grouped by the government into integrated health centers (Center de Santé Intégrée [CSI]) for the organization and delivery of health services. To ensure similar allocation of any community-level predictors between the study arms before treatment, we performed stratified blocked randomization of communities within each CSI on clinical trachoma prevalence in children (trachomatous inflammation follicular in children < 1–5 years of age by using the WHO Simplified Grading System) as described.16,18 Communities were divided into 12 strata based on the 6 CSI and high or low prevalence of clinical trachoma within the CSI. Randomization of communities to the treatment arms and randomization of persons within communities was performed as described.16
Statistical analysis and sample size.
Azithromycin distributions and analyses were performed at the community level because the randomization unit was the community and infection in one person in a community is not independent of infection in another. To account for any clustering, we used the community prevalence of parasitemia as the unit of analysis. Because the data were reduced to a single statistic in each group, it is not possible to misrepresent the structure of the covariance matrix. The null hypothesis for the primary analysis was that the prevalence of malaria parasitemia was the same in both treatment arms. We estimated that 12 communities per arm would provide 80% power to detect a 3% absolute difference in malaria parasitemia, assuming a baseline prevalence of 10%, and using an intraclass correlation coefficient (ICC) of 0.075.24 Hypotheses were tested with the signed rank test, and the difference between arms was assessed by using the Hodges-Lehmann estimator (pseudomedian) and bootstrap (999 resamples) percentile 95% confidence intervals (CIs). The endpoints are included in all reported 95% CIs. All analyses were performed as intention-to-treat at the community level. Calculations were performed with Mathematica 9.0 (Wolfram Research, Champaign, IL), STATA 11 (StataCorp LP, College Station, TX), and the statistical package R 2.12 (R Foundation for Statistical Computing, Vienna, Austria).
Human participants and consent procedures.
Ethical approval for this study was obtained from the Committee for Human Research of the University of California, San Francisco and the Ethical Committee of the Niger Ministry of Health. Verbal consent was deemed ethical because of the high illiteracy rates in the study area and approved by the institutional review board. Consent was obtained from the community leaders before examination and from the child's parent or guardian at the time of examination. Verbal consent was documented on the registration form for each study participant before examination in the field. In addition, thumbprints were obtained for the baseline visit. The study was carried out in accordance with the Declaration of Helsinki, and registered as ClinicalTrials.gov NCT00792922.
Results
Participants and treatment coverage.
Forty-eight communities were enrolled in PRET-Niger, 24 of which were by design included in this trial.16 Twelve communities were randomized to receive a single mass treatment, and 12 were randomized to receive 2 mass treatments, with a goal of 80% antibiotic coverage to the targeted group (Figure 1).
A total of 2,133 children < 1–72 months of age were treated at baseline (1,117 in once-treated communities and 1,016 in twice-treated communities), and 1,030 were analyzed at one year (485 in once-treated communities and 545 in twice-treated communities). No communities were missing or lost to follow-up throughout the study. The mean azithromycin coverage of children < 1–72 months of age based on the 12-month census was 80.4% (95% CI = 76.8–84.1%) for the single treatment of the once-treated communities, and 74.4% (95% CI = 69.4–79.3%) and 78.7% (95% CI = 74.5–82.7%) for the baseline and six-month treatments, respectively, in the twice-treated communities. The total number of children 6–72 months of age who received treatment based on the 12-month census was 808 at baseline (394 in once-treated communities and 414 in twice-treated communities) and 409 at six months (in twice-treated communities). Baseline and demographic characteristics were comparable in communities randomized to receive one or two treatments (Table 1).
Table 1.
Baseline characteristics at time of enrollment of < 1–72-month-old children in 24 communities in Niger*
| Characteristic | Treatment arm | |
|---|---|---|
| Once-treated, n = 12 communities | Twice-treated, n = 12 communities | |
| Children per community | 124 (42–207) | 118 (88–147) |
| Age, mean months | 30.8 (29.8–31.9) | 31.9 (30.7–33.0) |
| Proportion female | 51.3% (48.2–54.4%) | 50.1% (47.1–53.1%) |
| Prevalence of clinical trachoma TF† | 26.5% (14.9–38.0%) | 24.1% (15.9–32.3%) |
| Prevalence of clinical trachoma TI† | 8.6% (4.4–12.8%) | 9.4% (4.9–14.0%) |
Values in parentheses are 95% confidence intervals.
Trachomatous inflammation follicular (TF) and trachomatous inflammation intense (TI) according to a World Health Organization simplified grading system.18
Malariometric and blood indices.
Malaria parasitemia was measured by thick blood smear in children < 1–72 months of age 4–5 months after the second azithromycin treatment. In the Plasmodium speciation analysis, 22 of the 24 random samples (92%) were positive for the Plasmodium cytochrome b region. The 24 blank control punches (negative controls) corresponding to each sample were PCR negative. Sequencing of the Plasmodium cytochrome b showed that 22 (100%) of the positive samples were P. falciparum (100%, 97.5% CI = 85–100%).
Parasitemia grades were concordant between the two microscopists in 94% of samples and had an ICC of 0.985 (95% CI = 0.967–0.995). The community-level Hodges-Lehmann estimators and 95% CIs for all measurement in the two arms are shown in Table 2. The community-level prevalence of parasitemia was 10.2% lower (95% CI = 0.5–22.7% lower) in the twice-treated communities than in the once-treated communities (P = 0.03). As sensitivity analysis, we separately repeated the analysis with grader 1 only, grader 2 only, and if either grader-observed parasites. As another sensitivity analysis, we performed logistic regression by using study arm as a fixed effect and community as a random effect. This regression showed that children who received an additional antibiotic treatment were significantly less likely to have malaria parasitemia observed on thick blood smear (odds ratio = 0.52, 95% CI = 0.29–0.95, P = 0.03). Community-level parasite density was 232 parasite/μL (95% CI = 17–455 parasites/μL) lower in the twice-treated communities than in the once-treated communities (P = 0.03). Community-level gametocytemia was 2.0% lower (95% CI = 0.0% lower inclusive to 6.2% lower) in the twice-treated communities than in the once-treated communities, although the low prevalence may have precluded demonstrating a significant difference between study arms if one exists (P = 0.29). The ICC for repeat measurement of hemoglobin was 0.960 (95% CI = 0.886–0.994). Community-level hemoglobin was 17.7% higher (95% CI = 28.6% lower to 64.6% higher) in the twice-treated communities than in the once-treated communities (P = 0.11).
Table 2.
Malariometric and blood indices in 24 communities in Niger randomized to one mass azithromycin treatment or one mass azithromycin treatment plus a second mass treatment in the dry, low-transmission season in children ≥ 6 months of age*
| Measurement | Treatment arm | P | |
|---|---|---|---|
| Once-treated, n = 12 communities | Twice-treated, n = 12 communities | ||
| Malaria parasitemia | 29.8% (21.5–40.0%) | 19.5% (13.0–26.5%) | 0.03 |
| Parasite density, parasites/μL | 354 (117–528) | 74 (41–202) | 0.03 |
| Gametocytemia | 1.5% (0–6.0%) | 0% (0–1.5%) | 0.29 |
| Hemoglobin, g/dL | 10.0 (9.8–10.2) | 10.2 (10.0–10.4) | 0.20 |
All values were determined by using the Hodges-Lehmann estimator (pseudomedian). Values in parentheses are 95% confidence intervals.
Discussion
In this cluster-randomized trial, communities randomized to receive an extra mass distribution of oral azithromycin during the low malaria transmission season had reduced malaria parasitemia and parasite density 4–5 months later than communities randomized to receive only a single distribution. We were unable to demonstrate a significant difference in gametocytemia or hemoglobin concentration associated with the extra mass treatment, although gametocytemia was lower and hemoglobin levels were higher in the twice-treated communities.
Although azithromycin has antimalaria activity, it is not as potent as many standard antimalaria agents and, as with other antibiotics for malaria (e.g., tetracyclines and clindamycin), has a slow action.25 For treatment of malaria, azithromycin has shown excellent efficacy against P. vivax and modest efficacy against P. falciparum in areas with limited immunity.15 In two individual-randomized trials, azithromycin demonstrated a range of preventive efficacy of 64–83% against falciparum malaria, but this was less than that of first-line agents.13,15 Azithromycin may nonetheless play some role in malaria control because it has a long half-life, is well tolerated, and is approved for use in children and pregnant women.26–28 Furthermore, azithromycin is distributed on such a large scale for trachoma that even a modest effect could be of interest.
The IPTc has been widely advocated for the control of malaria in Africa, and it has shown efficacy when administered during the wet, high-transmission season in areas with highly seasonal malaria transmission.9,29 Combination therapies for IPTc such as amodiaquine plus sulfadoxine-pyrimethamine or artesunate plus sulfadoxine-pyrimethamine have been effective in areas of seasonal malaria transmission.9,29,30 Emerging antimicrobial drug resistance has spread through malaria-endemic areas and has made treatment difficult.31 We did not measure resistance to azithromycin in Plasmodium because we were unable to perform cultures at our field site and biochemical assays for this have not been adequately described. To the best of our knowledge, resistance to antibiotics has not been demonstrated in malaria except to antifolates.32 Nevertheless, surveillance for resistance in malaria is important to track whenever any malaria therapy is used.
Transmission models suggest treatment during the low transmission season of an infectious disease may provide significant or even maximum impact.10,12 For malaria, this may be because circulating malaria parasites that are eliminated with mass treatment are not replaced in the absence of transmission.10 Mass drug administration before the malaria transmission season prevents parasite prevalence levels from recovering to their pretreatment levels, and this raises the probability of parasite elimination in these low transmission settings.12 Mathematical models of mass treatment of another seasonal disease (trachoma) imply that the optimal time for treatment to achieve elimination may be during the season of lowest transmission.11 Seasonal variations in transmission might be able to be exploited to maximize the impact of treatment.12
This cluster-randomized trial, designed to monitor trachoma, can be applied to secondary questions such as the effect of azithromycin distributions on malaria. Malaria indices were monitored at 12 months into the trial and provided a convenient means of comparing the impact of a single, low-transmission season mass treatment with azithromycin. To account for clustering, we used the prevalence of parasitemia in a community rather than an individual-based analysis. This design necessarily has some important limitations. It did not enable detection of clinical malaria cases or the evaluation of malaria during the subsequent rainy season. No assessment of the prevalence of malaria parasitemia at baseline was conducted. Inclusion of baseline prevalence as a covariate may have offered a more powerful study design or provided evidence that baseline measurements did not explain the observed association. However, the randomized post-test design does permit valid inference33 because the treatment assignments are stochastically independent of any other explanatory covariate (including baseline malaria parasitemia).34 Lack of baseline prevalence also makes it impossible to know whether malaria parasitemia decreased more in the twice-treated arm or simply increased less than in the once-treated arm. The parasitemia outcome was validated by two independent masked graders. However, external monitors were not used and molecular techniques were not used beyond the speciation analysis. In the twice-treated arm, only children received antibiotics, whereas in the once-treated arm, all ages were eligible. It is possible that treating adults had an indirect effect on children, and that this decreased the effect we observed with the extra dose in the twice-treated arm. When the readings of the two microscopists were discordant, we did not have a third adjudicate. For the primary analysis, we assumed positivity if both agreed. It should be noted that concordance was extremely high, and that we obtained similar results in sensitivity analyses using only one grader or assuming positivity if either grader observed parasites.
In summary, mass distribution of azithromycin provided during the dry, low-transmission season in Niger was associated with a reduced community prevalence of malaria parasitemia and parasite density 4–5 months later. Gametocytemia and hemoglobin concentration were not significantly different after an additional dry season administration of azithromycin. Mass administration of azithromycin was associated with reduced mortality in Ethiopia,1,2 although it is unclear if this finding was related to protection against malaria. Further studies could address the impact of mass administration of azithromycin on the incidence of clinical episodes of malaria, severe malaria, and other health outcomes.
ACKNOWLEDGMENTS
We thank Abdoul Razak Idry, Jacques Dadjo, Fatouma Seyni, Fassouma Habou for help with sample collection and administration of medication.
Footnotes
Financial support: This study was supported by the Bill and Melinda Gates Foundation (grant no. 48027); the National Institutes of Health (grant nos. NIH/NEI K23 EYO19881-01, NIH/NCRR/OD UCSF-CTSI, and KL2 RR024130); Research to Prevent Blindness; the Harper-Ingles Trust; and That Man May See.
Authors' addresses: Bruce D. Gaynor, Nicole E. Stoller, Sun N. Yu, Stephanie A. Chin, Jeremy D. Keenan, Travis C. Porco, and Thomas M. Lietman, F.I. Proctor Foundation, University of California, San Francisco, CA, E-mails: bruce.gaynor@ucsf.edu, nicole.stoller@ucsf.edu, sun.yu@ucsf.edu, stephanie.chin@ucsf.edu, jeremy.keenan@ucsf.edu, travis.porco@ucsf.edu, and tom.lietman@ucsf.edu. Abdou Amza and Baido Nassirou, Programme FSS/Université Abdou Moumouni de Niamey, E-mails: dr.amzaabdou@gmail.com and nasbeido@yahoo.fr. Boubacar Kadri, FSS/Université Abdou Moumouni de Niamey, Programme National de Lutte Contre la Cecité, Niamey, Niger, E-mail: boubacarkadri@gmail.com. Ousmane Lawan and Laouali Maman, Zinder Hospital, Zinder, Niger, E-mails: lawanousmane@yahoo.fr and laoualimaman2007@yahoo.fr. Sheila K. West, Dana Center for Preventive Ophthalmology, Wilmer Eye Institute, Johns Hopkins University, Baltimore, MD, E-mail: shwest@jhmi.edu. Robin L. Bailey, Department of Infectious and Tropical Diseases, London School of Hygiene and Tropical Medicine, London, UK, E-mail: robin.bailey@ucsf.edu. Philip J. Rosenthal, Institute for Global Health, Department of Medicine, University of California, San Francisco, CA, E-mail: prosenthal@medsfgh.ucsf.edu.
References
- 1.Keenan JD, Ayele B, Gebre T, Zerihun M, Zhou Z, House JI, Gaynor BD, Porco TC, Emerson PM, Lietman TM. Childhood mortality in a cohort treated with mass azithromycin for trachoma. Clin Infect Dis. 2011;52:883–888. doi: 10.1093/cid/cir069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Porco TC, Gebre T, Ayele B, House J, Keenan J, Zhou Z, Hong KC, Stoller N, Ray KJ, Emerson P, Gaynor BD, Lietman TM. Effect of mass distribution of azithromycin for trachoma control on overall mortality in Ethiopian children: a randomized trial. JAMA. 2009;302:962–968. doi: 10.1001/jama.2009.1266. [DOI] [PubMed] [Google Scholar]
- 3.Sadiq ST, Glasgow KW, Drakeley CJ, Muller O, Greenwood BM, Mabey DC, Bailey RL. Effects of azithromycin on malariometric indices in The Gambia. Lancet. 1995;346:881–882. doi: 10.1016/s0140-6736(95)92712-3. [DOI] [PubMed] [Google Scholar]
- 4.Whitty CJ, Glasgow KW, Sadiq ST, Mabey DC, Bailey R. Impact of community-based mass treatment for trachoma with oral azithromycin on general morbidity in Gambian children. Pediatr Infect Dis J. 1999;18:955–958. doi: 10.1097/00006454-199911000-00003. [DOI] [PubMed] [Google Scholar]
- 5.Fry AM, Jha HC, Lietman TM, Chaudhary JS, Bhatta RC, Elliott J, Hyde T, Schuchat A, Gaynor B, Dowell SF. Adverse and beneficial secondary effects of mass treatment with azithromycin to eliminate blindness due to trachoma in Nepal. Clin Infect Dis. 2002;35:395–402. doi: 10.1086/341414. [DOI] [PubMed] [Google Scholar]
- 6.Coles CL, Seidman JC, Levens J, Mkocha H, Munoz B, West S. Association of mass treatment with azithromycin in trachoma-endemic communities with short-term reduced risk of diarrhea in young children. Am J Trop Med Hyg. 2011;85:691–696. doi: 10.4269/ajtmh.2011.11-0046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lode H, Borner K, Koeppe P, Schaberg T. Azithromycin–review of key chemical, pharmacokinetic and microbiological features. J Antimicrob Chemother. 1996;37((Suppl C)):1–8. doi: 10.1093/jac/37.suppl_c.1. [DOI] [PubMed] [Google Scholar]
- 8.Ahorlu CK, Koram KA, Seakey AK, Weiss MG. Effectiveness of combined intermittent preventive treatment for children and timely home treatment for malaria control. Malar J. 2009;8:292. doi: 10.1186/1475-2875-8-292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cisse B, Sokhna C, Boulanger D, Milet J, Bâ el H, Richardson K, Hallett R, Sutherland C, Simondon K, Simondon F, Alexander N, Gaye O, Targett G, Lines J, Greenwood B, Trape JF. Seasonal intermittent preventive treatment with artesunate and sulfadoxine-pyrimethamine for prevention of malaria in Senegalese children: a randomised, placebo-controlled, double-blind trial. Lancet. 2006;367:659–667. doi: 10.1016/S0140-6736(06)68264-0. [DOI] [PubMed] [Google Scholar]
- 10.Gu W, Killeen GF, Mbogo CM, Regens JL, Githure JI, Beier JC. An individual-based model of Plasmodium falciparum malaria transmission on the coast of Kenya. Trans R Soc Trop Med Hyg. 2003;97:43–50. doi: 10.1016/s0035-9203(03)90018-6. [DOI] [PubMed] [Google Scholar]
- 11.Lee DC, Chidambaram JD, Porco TC, Lietman TM. Seasonal effects in the elimination of trachoma. Am J Trop Med Hyg. 2005;72:468–470. [PubMed] [Google Scholar]
- 12.Okell LC, Griffin JT, Kleinschmidt I, Hollingsworth TD, Churcher TS, White MJ, Bousema T, Drakeley CJ, Ghani AC. The potential contribution of mass treatment to the control of Plasmodium falciparum malaria. PLoS ONE. 2011;6:e20179. doi: 10.1371/journal.pone.0020179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Andersen SL, Oloo AJ, Gordon DM, Ragama OB, Aleman GM, Berman JD, Tang DB, Dunne MW, Shanks GD. Successful double-blinded, randomized, placebo-controlled field trial of azithromycin and doxycycline as prophylaxis for malaria in western Kenya. Clin Infect Dis. 1998;26:146–150. doi: 10.1086/516281. [DOI] [PubMed] [Google Scholar]
- 14.Chico RM, Pittrof R, Greenwood B, Chandramohan D. Azithromycin-chloroquine and the intermittent preventive treatment of malaria in pregnancy. Malar J. 2008;7:255. doi: 10.1186/1475-2875-7-255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Taylor WR, Richie TL, Fryauff DJ, Picarima H, Ohrt C, Tang D, Braitman D, Murphy GS, Widjaja H, Tjitra E, Ganjar A, Jones TR, Basri H, Berman J. Malaria prophylaxis using azithromycin: a double-blind, placebo-controlled trial in Irian Jaya, Indonesia. Clin Infect Dis. 1999;28:74–81. doi: 10.1086/515071. [DOI] [PubMed] [Google Scholar]
- 16.Amza A, Kadri B, Nassirou B, Stoller NE, Yu SN, Zhou Z, Chin S, West SK, Bailey RL, Mabey DC, Keenan JD, Porco TC, Lietman TM, Gaynor BD, PRET Partnership Community risk factors for ocular Chlamydia infection in Niger: pre-treatment results from a cluster-randomized trachoma trial. PLoS Negl Trop Dis. 2012;6:e1586. doi: 10.1371/journal.pntd.0001586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stare D, Harding-Esch E, Munoz B, Bailey R, Mabey D, Holland M, Gaydos C, West S. Design and baseline data of a randomized trial to evaluate coverage and frequency of mass treatment with azithromycin: the Partnership for Rapid Elimination of Trachoma (PRET) in Tanzania and The Gambia. Ophthalmic Epidemiol. 2011;18:20–29. doi: 10.3109/09286586.2010.545500. [DOI] [PubMed] [Google Scholar]
- 18.Thylefors B, Dawson CR, Jones BR, West SK, Taylor HR. A simple system for the assessment of trachoma and its complications. Bull World Health Organ. 1987;65:477–483. [PMC free article] [PubMed] [Google Scholar]
- 19.Basilion EV, Kilima PM, Mecaskey JW. Simplification and improvement of height-based azithromycin treatment for paediatric trachoma. Trans R Soc Trop Med Hyg. 2005;99:6–12. doi: 10.1016/j.trstmh.2004.01.014. [DOI] [PubMed] [Google Scholar]
- 20.Munoz B, Solomon AW, Zingeser J, Barwick R, Burton M, Bailey R, Mabey D, Foster A, West SK. Antibiotic dosage in trachoma control programs: height as a surrogate for weight in children. Invest Ophthalmol Vis Sci. 2003;44:1464–1469. doi: 10.1167/iovs.02-0234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.World Health Organization . WHO Basic Malaria Microscopy. Geneva: World Health Organization; 2001. [Google Scholar]
- 22.Steenkeste N, Incardona S, Chy S, Duval L, Ekala MT, Lim P, Hewitt S, Sochantha T, Socheat D, Rogier C, Mercereau-Puijalon O, Fandeur T, Ariey F. Towards high-throughput molecular detection of Plasmodium: new approaches and molecular markers. Malar J. 2009;8:86. doi: 10.1186/1475-2875-8-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Benson DA, Karsch-Mizrachi I, Lipman DJ, Ostell J, Wheeler DL. GenBank. Nucleic Acids Res. 2005;33:D34–D38. doi: 10.1093/nar/gki063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Meremikwu M, Donegan S, Esu E. Chemoprophylaxis and intermittent treatment for preventing malaria in children. Cochrane Libr. 2009 doi: 10.1002/14651858.CD003756.pub3. [DOI] [PubMed] [Google Scholar]
- 25.Dahl EL, Rosenthal PJ. Multiple antibiotics exert delayed effects against the Plasmodium falciparum apicoplast. Antimicrob Agents Chemother. 2007;51:3485–3490. doi: 10.1128/AAC.00527-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Girard AE, Girard D, English AR, Gootz TD, Cimochowski CR, Faiella JA, Haskell SL, Retsema JA. Pharmacokinetic and in vivo studies with azithromycin (CP-62,993), a new macrolide with an extended half-life and excellent tissue distribution. Antimicrob Agents Chemother. 1987;31:1948–1954. doi: 10.1128/aac.31.12.1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Taylor WR, Richie TL, Fryauff DJ, Ohrt C, Picarima H, Tang D, Murphy GS, Widjaja H, Braitman D, Tjitra E, Ganjar A, Jones TR, Basri H, Berman J. Tolerability of azithromycin as malaria prophylaxis in adults in northeast papua, indonesia. Antimicrob Agents Chemother. 2003;47:2199–2203. doi: 10.1128/AAC.47.7.2199-2203.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ward SA, Sevene EJ, Hastings IM, Nosten F, McGready R. Antimalarial drugs and pregnancy: safety, pharmacokinetics, and pharmacovigilance. Lancet Infect Dis. 2007;7:136–144. doi: 10.1016/S1473-3099(07)70025-7. [DOI] [PubMed] [Google Scholar]
- 29.Konate AT, Yaro JB, Ouedraogo AZ, Diarra A, Gansane A, Soulama I, Kangoye DT, Kabore Y, Ouedraogo E, Ouedraogo A, Tiono AB, Ouedraogo IN, Chandramohan D, Cousens S, Milligan PJ, Sirima SB, Greenwood B, Diallo DA. Intermittent preventive treatment of malaria provides substantial protection against malaria in children already protected by an insecticide-treated bednet in Burkina Faso: a randomised, double-blind, placebo-controlled trial. PLoS Med. 2011;8:e1000408. doi: 10.1371/journal.pmed.1000408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wilson AL. A systematic review and meta-analysis of the efficacy and safety of intermittent preventive treatment of malaria in children (IPTc) PLoS ONE. 2011;6:e16976. doi: 10.1371/journal.pone.0016976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Naidoo I, Roper C. Following the path of most resistance: dhps K540E dispersal in African Plasmodium falciparum. Trends Parasitol. 2010;26:447–456. doi: 10.1016/j.pt.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 32.Roper C, Pearce R, Bredenkamp B, Gumede J, Drakeley C, Mosha F, Chandramohan D, Sharp B. Antifolate antimalarial resistance in southeast Africa: a population-based analysis. Lancet. 2003;361:1174–1181. doi: 10.1016/S0140-6736(03)12951-0. [DOI] [PubMed] [Google Scholar]
- 33.Bonate P. Analysis of Pretest-Postest Designs. Boca Raton, FL: Chapman and Hall/CRC; 2000. [Google Scholar]
- 34.Fisher RA. The Design of Experiments. New York: Hafner Press; 1971. [Google Scholar]