Abstract
One of the main challenges to malaria elimination is the resilience of vectors, such as Anopheles arabiensis, that evade lethal exposure to insecticidal control measures or express resistance to their active ingredients. This study investigated a novel technology for population control that sterilizes mosquitoes using pyriproxyfen, a juvenile hormone analogue. Females of An. arabiensis were released in a semifield system divided into four equal sections, and each section had a mud hut sheltering a tethered cow providing a blood source for mosquitoes. In all sections, the inner mud hut walls and roofs were lined with black cotton cloth. In one-half of the sections, the cloth was dusted with pyriproxyfen. An overwhelming 96% reduction in adult production was achieved in pyriproxyfen-treated sections compared with control sections. This unprecedented level of control can be exploited to design new vector control strategies that particularly target existing behaviorally resilient and insecticide-resistant populations.
Introduction
Current frontline malaria vector control interventions, such as long-lasting insecticide-treated nets (LLINs) and indoor residual spraying (IRS), have contributed greatly to the recent successes in malaria control.1 However, these tools are more effective against vector species that primarily feed indoors on humans and rest indoors. They are less effective against outdoor feeding and resting mosquitoes. Anopheles arabiensis, currently mediating most of the residual malaria transmission in east Africa,2,3 is not optimally controlled by LLINs and IRS, because it commonly feeds outdoors on humans or cattle, rests outdoors, and can enter but then rapidly exit houses containing these products without exposure to lethal doses of their active ingredients (AIs).3,4
Another challenge to malaria vector control is the development of resistance in malaria vectors against all classes of insecticides currently used for LLINs and IRS, particularly pyrethroids, the most widely used and the only class approved for use in bednets.5
Pyriproxyfen (PPF) is a juvenile hormone analogue that traditionally has been used in aquatic habitats to prevent mosquito larvae and pupae from developing into adults. However, it can also sterilize adult mosquitoes on contact.6–8 This study builds from our previous work performed in laboratory conditions showing that An. arabiensis mosquitoes were particularly vulnerable to sterilization immediately after blood feeding.8 Adult mosquitoes can also transfer PPF from resting sites to breeding sites to interfere with immature development.9,10 Here, we show, for the first time, an operationally practicable means of controlling a free-flying captive population of An. arabiensis using PPF.
Materials and Methods
This study was carried out in southern Tanzania inside a semifield system (SFS) with walls consisting of netting only, and therefore, the microclimate inside it closely resembled the natural environment outside of it.11 The SFS was divided into four equal sections, with a space volume of approximately 360 m3 each. In each section, a mud hut sheltering a tethered cow, eight clay pots, and four plastic basins with soil and water were designed to provide blood, resting, and oviposition sites for mosquitoes (Figure 1). In all sections, the inner mud hut walls and roofs were lined with black cotton cloth, and in one-half of the sections, the cloth was dusted with PPF powder (0.6–0.8 g AI/m2). In total, 5,000 unfed 3 to 9-day-old insectary-reared An. arabiensis females, previously caged with equivalent numbers of males, were released per section, with a cow to provide blood for the first 3 days only. Mosquitoes used in the experiments were starved 6 hours before release. Therefore, they fed on the cow, and after 3 days, a solution of 6% glucose was set up at multiple locations inside the SFS for sugar feeding. These mosquitoes remained in the SFS to complete their gonotrophic cycle. All pupae that subsequently developed from the aquatic habitats were removed, counted, and reared in small cages to monitor the numbers of emerging adults and therefore, the impact of PPF exposure on the mosquitoes' ability to produce viable offspring. Seven days after larvae were observed in the habitats, 150 mL water were collected from every habitat using a glass beaker to determine whether PPF had been transferred to these habitats by contaminated mosquitoes during oviposition.12 To assess the presence of PPF in each beaker, larval bioassays were conducted using second and third instar larvae from the insectary. Twenty An. arabiensis larvae were introduced in each beaker and monitored daily until all larvae and pupae had either died or developed and emerged to adults. Five replicates each of the control and treatment were completed in three separate experiments in the following setup. In the first experiment, two replicates (treatment and control) were run (5,000 × 4 = 20,000 mosquitoes); in the second experiment, two replicates (treatment and control) were run (5,000 × 4 = 20,000 mosquitoes), and in the third experiment, one replicate (treatment and control) was run (5,000 × 2 = 10,000 mosquitoes), making a total of 50,000 mosquitoes reared and released.
Figure 1.
Semifield system (SFS) setup. (A) SFS with (B) mud huts built inside each section to shelter a cow and (C) breeding habitats. (D) Mud huts were lined with black cloth and dusted with PPF in treatment sections.
All statistical analyses were conducted in R v2.12.213 (R Development Core Team, University of Auckland, Auckland, New Zealand) using the lme4 package for generalized linear mixed effects models.14 To determine any differences in the numbers of pupae or adults produced between treated and control sections, a generalized linear mixed effects model with a Poisson distribution and a log link function for count data was performed. The treatment group (control or PPF) was classified as a fixed effect, whereas SFS section nested within experiment was put in as a random effect as per the experimental design. A visual inspection of the plots of error versus fitted values distribution was used to determine the best model fit. The model was then tested with each nested parameter separately to determine the underlying variation. SFS section was found to count for a lot of variation and therefore, required the full nested model to be retained. The differences in pupal emergence rates in both SFS habitats and the bioassays experiments were compared by fitting a generalized linear mixed effects model with binomial error structure and logit link function for proportion data. The data were fitted to a model including treatment as a fixed effect and breeding habitat nested within SFS section nested within experiment as a random effect as per the experimental design. Visual inspection of the plots of error versus fitted values distribution was used to determine the best model fit. Model reduction was conducted by removing nested parameters one by one; however, the full nested model was retained.
Results
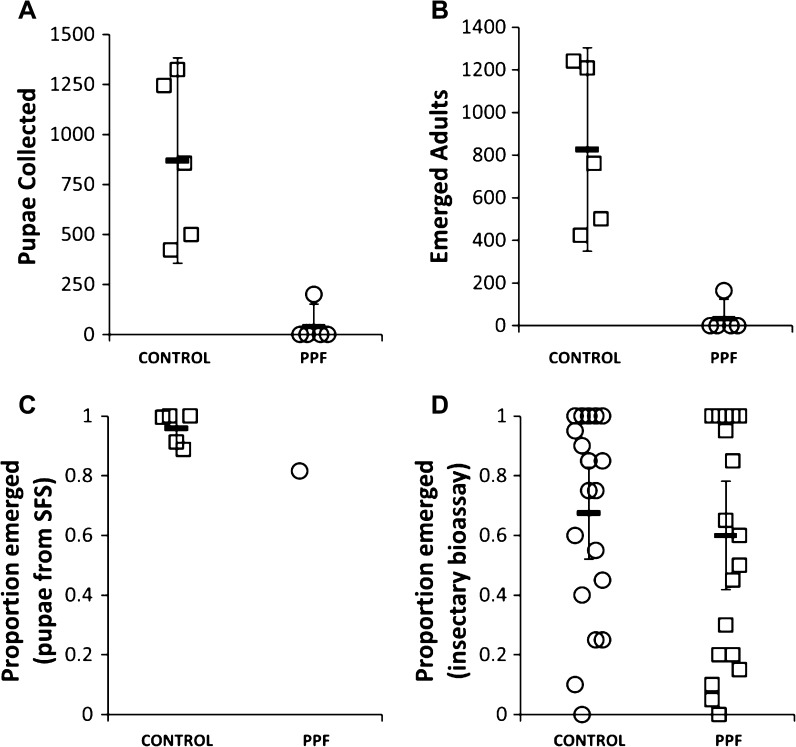
Experiments lasted between 11 and 16 days from release of adult mosquitoes to collection of the last pupae in the breeding habitats. An overwhelming 95% reduction in pupal production and 96% reduction in adult production were achieved in PPF-treated sections compared with control sections (Figure 2A and B). In four of five replicates, exposure to this juvenile hormone analogue completely sterilized all mosquitoes; not a single pupa or new adult was seen. The few adults emerging from a PPF-treated section in the fifth replicate probably resulted from mosquitoes that had been contaminated with PPF but were not completely sterilized and managed to lay eggs. The pupae collected in the PPF-treated section showed a significantly lower emergence rate (82%; 164/201) compared with the control (95%; 4,132/4,349; χ2 [1] = 65.6, P < 0.001) (Figure 2C). This result suggested possible PPF autodissemination to the breeding habitats by contaminated mosquitoes. However, bioassays with insectary larvae reared in water from the control and PPF-treated habitats showed similar emergence rates (Figure 2D). A similar pattern has been observed in recent studies (Lwetoijera DW and others, unpublished data), where PPF activity is more pronounced in breeding habitats with organic material than water samples kept in glass beakers.
Figure 2.
Impact of PPF on mosquito emergence. Number of (A) pupae produced and (B) adults emerging from control and treated sections and the proportion of adult emergence in (C) SFS and (D) insectary bioassays.
Discussion
The striking level of sterilization seen in this key malaria vector reveals an exciting new opportunity for malaria vector control. This technology is a practical, novel technology for population control that sterilizes mosquitoes rather than killing them. It offers the chance to develop new tools that are not compromised by existing resistance mechanisms. New paradigms in vector control are in great demand, especially for vectors such as An. arabiensis4,15 and other anophelines16 that exhibit flexibility in feeding and resting indoors and outdoors and minimize their contact with conventional adulticides applied indoors. The findings reported here have limitations given that the experiments were conducted within an enclosed environment on insectary-reared mosquitoes that had never been subjected to insecticide pressure. However, this technology can be readily adapted in natural conditions to assess its impact on wild populations of An. arabiensis.
Treating walls and roof linings with PPF comprehensively sterilizes captive populations of free-flying An. arabiensis, making it a powerful control tool and an easy complement to LLINs and IRS. PPF-treated materials could be deployed outdoors in areas where mosquitoes rest or transit, such as areas where people gather in the early hours of the evening and inside and outside of cattle sheds. These treated materials could also be specifically designed to attract resting mosquitoes. Similar substrates are already exploited for the delivery of conventional insecticides.17 Our prototype uses a safe and registered insecticide class that has yet to be deployed against adult malaria vectors. Alternatives to conventional adulticides are desperately needed. The physiological resistance to pyrethroids, recently characterized in populations of An. arabiensis from Zanzibar, precipitated the substitution of pyrethroids for a less cost-effective carbamate compound with a history of resistance development in malaria vectors.18,19 No resistance to PPF has been reported in mosquitoes (J. Invest and others, unpublished data), and no cross-resistance has been observed between PPF and other classes of insecticides of public health interest. PPF could be applied in combination, mosaics, or rotations with current insecticides to mitigate the emergence of resistance.5 It is remarkably stable in the shade and available in a variety of commercial formulations that fit this new application.
The indication that the few mosquitoes that managed to lay eggs from the PPF-treated section also transferred PPF to their breeding habitats and significantly reduced subsequent mosquito emergence is a welcome development. The autodissemination of PPF by adult mosquitoes has been already observed in Aedes species,9,10 and we are working to prove the same phenomenon in malaria vectors.
ACKNOWLEDGMENTS
The authors thank Katharina Kreppel for help with statistical analyses and George Corliss for reviewing the final draft of the manuscript. We are grateful to Peter Masabho, Andrew Kafwenji, Monica Mpingwa, and Hassan Mtambala for help with running the experiments and mosquito rearing. The authors thank two anonymous reviewers who helped improve this manuscript. Permission to publish was obtained from the National Institute for Medical Research (reference number NIMR/HQ/P.12 VOL.XIV/03).
Footnotes
Financial support: This work was supported by Bill and Melinda Gates Foundation Grant OPP52644.
Authors' addresses: Dickson W. Lwetoijera, Caroline Harris, Gerry F. Killeen, Stefan Dongus, and Silas Majambere, Environmental Health and Ecological Sciences, Ifakara Health Institute, Dar es Salaam, Tanzania, and Liverpool School of Tropical Medicine, Liverpool, United Kingdom, E-mails: dwilson@ihi.or.tz, charris@ihi.or.tz, gkilleen@ihi.or.tz, sdongus@ih.or.tz, and smajambere@ihi.or.tz. Samson S. Kiware, Environmental Health and Ecological Sciences, Ifakara Health Institute, Dar es Salaam, Tanzania, and Marquette University, Milwaukee, WI, E-mail: skiware@ihi.or.tz. Gregor J. Devine, QIMR Berghofer Institute of Medical Research, Brisbane, Australia, E-mail: greg.devine@qimrberghofer.edu.au.
References
- 1.World Health Organization World Malaria Report. 2012. http://www.who.int/malaria/publications/world_malaria_report_2012/en/ Available at. Accessed July 24, 2013.
- 2.Bayoh MN, Mathias DK, Odiere MR, Mutuku FM, Kamau L, Gimnig JE, Vulule JM, Hawley WA, Hamel MJ, Walker ED. Anopheles gambiae: historical population decline associated with regional distribution of insecticide-treated bed nets in western Nyanza Province, Kenya. Malar J. 2010;9:62. doi: 10.1186/1475-2875-9-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Russell TL, Govella NJ, Azizi S, Drakeley CJ, Kachur SP, Killeen GF. Increased proportions of outdoor feeding among residual malaria vector populations following increased use of insecticide-treated nets in rural Tanzania. Malar J. 2011;10:80. doi: 10.1186/1475-2875-10-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Okumu FO, Mbeyela E, Lingamba G, Moore J, Ntamatungiro AJ, Kavishe DR, Kenward MG, Turner E, Lorenz LM, Moore SJ. Comparative field evaluation of combinations of long-lasting insecticide treated nets and indoor residual spraying, relative to either method alone, for malaria prevention in an area where the main vector is Anopheles arabiensis. Parasit Vectors. 2013;6:46. doi: 10.1186/1756-3305-6-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.World Health Organization Global Plan for Insecticide Resistance Management in Malaria Vectors. 2012. http://www.who.int/malaria/publications/atoz/gpirm/en/ Available at. Accessed July 24, 2013.
- 6.Ohba SY, Ohashi K, Pujiyati E, Higa Y, Kawada H, Mito N, Takagi M. The effect of pyriproxyfen as a “population growth regulator” against Aedes albopictus under semi-field conditions. PLoS One. 2013;8:e67045. doi: 10.1371/journal.pone.0067045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ohashi K, Nakada K, Ishiwatari T, Miyaguchi J, Shono Y, Lucas JR, Mito N. Efficacy of pyriproxyfen-treated nets in sterilizing and shortening the longevity of Anopheles gambiae (Diptera: Culicidae) J Med Entomol. 2012;49:1052–1058. doi: 10.1603/me12006. [DOI] [PubMed] [Google Scholar]
- 8.Harris C, Lwetoijera DW, Dongus S, Matowo NS, Lorenz LM, Devine GJ, Majambere S. Sterilizing effects of pyriproxyfen on Anopheles arabiensis and its potential use in malaria control. Parasit Vectors. 2013;6:144. doi: 10.1186/1756-3305-6-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Devine GJ, Zamora Perea E, Killeen GF, Stancil JD, Clark SJ, Morrison AC. Using adult mosquitoes to transfer insecticides to Aedes aegypti larval habitats. Proc Natl Acad Sci USA. 2009;106:11530–11534. doi: 10.1073/pnas.0901369106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Caputo B, Ienco A, Cianci D, Pombi M, Petrarca V, Baseggio A, Devine GJ, della Torre A. The “auto-dissemination” approach: a novel concept to fight Aedes albopictus in urban areas. PLoS Negl Trop Dis. 2012;6:e1793. doi: 10.1371/journal.pntd.0001793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ferguson HM, Ng'habi KR, Walder T, Kadungula D, Moore SJ, Lyimo I, Russell TL, Urassa H, Mshinda H, Killeen GF, Knols BG. Establishment of a large semi-field system for experimental study of African malaria vector ecology and control in Tanzania. Malar J. 2008;7:158. doi: 10.1186/1475-2875-7-158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Devine GJ, Killeen GF. The potential of a new larviciding method for the control of malaria vectors. Malar J. 2010;9:142. doi: 10.1186/1475-2875-9-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.R Core Team . A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2013. [Google Scholar]
- 14.Bates D, Maechler M, Bolker B. Linear Mixed-Effects Models Using S4 Classes. 2013. http://cran.r-project.org/web/packages/lme4/index.html Available at. Accessed January 13, 2014.
- 15.Kitau J, Oxborough RM, Tungu PK, Matowo J, Malima RC, Magesa SM, Bruce J, Mosha FW, Rowland MW. Species shifts in the Anopheles gambiae complex: do LLINs successfully control Anopheles arabiensis? PLoS One. 2012;7:e31481. doi: 10.1371/journal.pone.0031481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Elliott R. The influence of vector behavior upon malaria transmission. Am J Trop Med Hyg. 1972;21:755–763. doi: 10.4269/ajtmh.1972.21.755. [DOI] [PubMed] [Google Scholar]
- 17.Messenger LA, Miller NP, Adeogun AO, Awolola TS, Rowland M. The development of insecticide-treated durable wall lining for malaria control: insights from rural and urban populations in Angola and Nigeria. Malar J. 2012;11:332. doi: 10.1186/1475-2875-11-332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haji KA, Khatib BO, Smith S, Ali AS, Devine GJ, Coetzee M, Majambere S. Challenges for malaria elimination in Zanzibar: pyrethroid resistance in malaria vectors and poor performance of long-lasting insecticide nets. Parasit Vectors. 2013;6:82. doi: 10.1186/1756-3305-6-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Okoye PN, Brooke BD, Koekemoer LL, Hunt RH, Coetzee M. Characterisation of DDT, pyrethroid and carbamate resistance in Anopheles funestus from Obuasi, Ghana. Trans R Soc Trop Med Hyg. 2008;102:591–598. doi: 10.1016/j.trstmh.2008.02.022. [DOI] [PubMed] [Google Scholar]