Abstract
Routine serological diagnoses for leishmaniases, except in visceral cases, are performed using whole-parasite antigens. We used enzyme-linked immunosorbent assay (ELISA) to evaluate the performance of Leishmania infantum rHsp83 compared with L. major-like total promastigote antigen in the diagnosis of cutaneous (CL), mucosal (ML), and visceral leishmaniases (VL). ELISA-rHsp83 was significantly more sensitive than ELISA–L. major-like when considering either CL/ML (P = 0.041) or all leishmaniasis patients (P = 0.013). When samples from other infectious disease patients were evaluated for cross-reactivity, ELISA-rHsp83 was more specific than ELISA–L. major-like, specifically for Chagas disease samples (P < 0.001). We also evaluated the anti-rHsp83 antibody titers months after treatment and observed no significant difference in ML (P = 0.607) or CL (P = 0.205). We recommend ELISA–L. infantum-rHsp83 as a routine confirmatory serological assay for the diagnosis of Leishmania infection because of the high sensitivity, the specificity, and the insignificant cross-reactivity with other infectious diseases.
Introduction
Leishmaniases are caused by protozoa of the genus Leishmania and may present as a visceral or tegumentary disease. Typically found in tropical and subtropical areas, leishmaniases are endemic in 98 countries.1 Visceral leishmaniasis (VL) is a systemic disease that affects the mononuclear phagocyte system,2 whereas tegumentary leishmaniasis (TL) comprise localized cutaneous leishmaniasis (CL), leishmaniasis recidivans, disseminated cutaneous leishmaniasis (DL), diffuse CL (DCL), and mucosal leishmaniasis (ML); the specific manifestation is dependent on the Leishmania species and the host immune response.3
Diagnosis of leishmaniases is based on epidemiological data, clinical features, and laboratory test results, which include a parasitological examination and serological assays.4 Parasitological examinations are accurate but laborious to perform, and they have low sensitivity.5–8 However, when polymerase chain reactions (PCRs) are used for the search of Leishmania DNA, the sensitivity of the detection is considered higher. The sensitivity is even higher if the target gene is a kinetoplast DNA sequence that is present in multiple copies in Leishmania. Nevertheless, PCRs are not routinely used because of their technical complexity.4,5 Thus, assays commonly used in the serological diagnosis of VL are the indirect immunofluorescence assay (IIFA), the enzyme-linked immunosorbent assay (ELISA), and the direct agglutination test (DAT) using total Leishmania promastigote antigen.3,9,10
Serology is not a routine procedure for diagnosis of TLs, such as localized CL, because of the low sensitivity of the tests.11,12 In ML, serology may have a complementary role in diagnosis, because the sensitivity is higher.13,14 However, variable results have been achieved with serological methods; the sensitivity and specificity of such methods depend on the type, source, purity, and antigen preparation used.12,13,15–17 In addition, Leishmania species-related variations in the results have been reported.18
One of the drawbacks in most currently available serological assays is that the antigen is either a total parasite lysate or whole promastigote, whose production depends on the intricate growth of the parasite. Development of recombinant Leishmania antigen for serological diagnosis would be valuable, because the production of such an antigen would be parasite growth-independent and more standardized and uniform. For VL, identification of the rK39 antigen has been a promising diagnostic contribution. It has been used in ELISA and immunochromatographic strip tests, which have facilitated field applicability,10,19 and they are now commonly used worldwide.20
Previously, L. major Hsp6021 and L. braziliensis Hsp7022 were cloned and tested in 15 CL samples from Colombia, which were found to have a mean optical density significantly higher than the optical density of sera from healthy negative controls. These two antigens were also tested in 46 ML samples from Brazil and had 89% sensitivity. More recently, Souza and others23 evaluated seven L. infantum-derived recombinant proteins in 102 TL serum samples and found that Hsp70 was a promising antigen, with 65.0% sensitivity and 92.0% specificity. Previously, we had tested L. (L.) infantum Hsp83 using a limited number of CL and ML samples and obtained 100% reactivity.24 Interestingly, we also found no cross-reactivity with Chagas disease serum samples.24 Because the previous data showed good sensitivity and specificity in CL and ML, in this study, we investigated L. (L.) infantum Hsp83 in CL, ML, and VL samples to test its sensitivity as well as in samples from other infectious diseases (i.e., Chagas disease, blastomycosis, histoplasmosis, aspergillosis, chromomycosis, toxoplasmosis, cytomegalovirosis, malaria, and tuberculosis) to test for cross-reactivity.
In TL, anti-Leishmania antibodies level has been previously shown to drop after treatment, independent of cure or failure.18 Therefore, we also performed serology on patient samples obtained during a follow-up visit to test anti-rHsp83 antigen antibody titers.
Similar to most of the currently available total parasite-based tests, the rHsp83 antigen test is not species-specific, and therefore, our test would be beneficial for leishmaniasis diagnostics.
Materials and Methods
Antigens.
L. infantum Hsp83 was expressed in Escherichia coli M15 as a recombinant protein and purified by Ni-NTA affinity chromatography (QIAGEN GmbH, Hilden, North Rhine-Westphalia, Germany).25 The total L. major-like (MHOM/BR/71/49)26 promastigote antigen was prepared according to the work by Hoshino-Shimizu and others,27 with modifications.28 The extract of this strain was used, because the reaction is genus-specific, and it can detect leishmaniasis caused by different species of Leishmania.29,30
Patients and serum samples.
Sera from patients with CL (12) and ML (14) were obtained from patients treated at the Department of Dermatology, Faculdade de Medicina, Universidade de São Paulo. The clinical diagnosis was confirmed by parasitological and immunological methods. The lesions were examined for the presence of parasites directly and/or in biopsy specimens.31 All patients except two had a positive intradermal Montenegro test.32 The majority (86.6%) of patients was treated with pentavalent antimony (Sanofi Aventis Farmacêutica Ltda, Suzano, São Paulo, Brazil), 7.7% of patients were treated with Amphotericin B (Bristol-Myers-Squibb, São Paulo, São Paulo, Brazil), and 7.7% of patients received both treatments (Table 1). Sera from patients with VL (30) were from active VL patients in Piauí state, Northeastern Brazil, and patients had been diagnosed based on clinical presentation and positive serology and/or the presence of parasites in bone marrow. Sera from patients with other infectious diseases were collected from 79 patients: Chagas disease (N = 23), blastomycosis (N = 7), histoplasmosis (N = 6), aspergillosis (N = 5), chromomycosis (N = 7), toxoplasmosis (N = 14), cytomegalovirosis (N = 4), malaria (N = 9), and tuberculosis (N = 4). Samples from 30 healthy blood bank donors were used as controls.
Table 1.
Characteristics of 26 TL patients, including clinical form, duration of disease at baseline, number of relapses, and disease status
| Time | Relapses | Cured |
|---|---|---|
| CL | ||
| 0.8 | 0 | Yes |
| 0.4 | 1 | Yes |
| 0.3 | 1 | Yes |
| 0.5 | 2 | Yes |
| 0.3 | 0 | Yes |
| 0.2 | 1 | Yes |
| 0.8 | 0 | Yes |
| 0.3 | 0 | Yes |
| 1.0 | 0 | Yes |
| 0.3 | 1 | Yes |
| 30.0 | 1 | Yes |
| 0.6 | 0 | Yes |
| ML | ||
| 5.0 | 1 | Unknown |
| 0.5 | 1 | Yes |
| 0.7 | 1 | Yes |
| 16.0 | 0 | Yes |
| 0.3 | 0 | Yes |
| 53.0 | 0 | Yes |
| 50.0 | 1 | Unknown |
| 20.0 | 1 | Yes |
| 8.0 | 1 | Yes |
| 1.0 | 0 | Yes |
| 4.0 | 1 | Yes |
| 40.0 | 1 | Yes |
| 56.0 | 1 | Yes |
| 1.0 | 3 | Yes |
Relapses = number of relapses; time = disease duration at baseline in years; unknown = up to the collection of the third sample, there was no report of the patient being cured; yes = patient was reported as cured before collection of the third sample.
Post-therapeutic evaluation of antibodies in CL and ML.
For the serological follow-up, 12 patients with CL and 14 patients with ML were examined at three different time periods: at a baseline before the initiation of the therapy (T0) and 6 (T1), and 12 months (T2) after the onset of therapy. At T0, CL patients had a disease evolution time varying from 0.2 to 30 years (median = 0.45; interquartile 25–75% = 0.30–0.80), and ML patients had a disease evolution time ranging from 0.3 to 56 years (6.5; 1–40) (Table 1).
ELISA.
All sera were tested for immunoglobulin G (IgG) antibodies at 1:50 dilution in the ELISA using rHsp83 (ELISA-rHsp83) or whole L. major-like parasite lysate (ELISA–L. major-like) as previously described.14,24 For both antigens, the cutoff point was determined using a receiver operating characteristic (ROC) curve.33 The reactivity index (RI) was calculated for each sample by dividing the sample absorbance value by the cutoff. Samples were considered positive if the RI value was ≥ 1.
Statistical analysis.
The sensitivity and specificity of tests and the 95% confidence intervals (95% CIs) were calculated using the ROC curve. The results were compared using McNemar's test. The agreement between the ELISA results with rHsp83 or L. major-like antigen was estimated by the κ-index. Mann–Whitney rank sum test (two groups) and Friedman repeated measures analysis of variance on Ranks and Tukey test (three or more groups) were used to compare medians.
Ethical issues.
All participants gave informed consent individually before giving the blood samples. This study was approved by the Ethics Committees of the Instituto de Medicina Tropical de Sao Paulo, Universidade de São Paulo and the Hospital das Clinicas, Faculdade de Medicina, Universidade de São Paulo.
Results
Performance of ELISA-rHsp83 compared with ELISA–L. major-like.
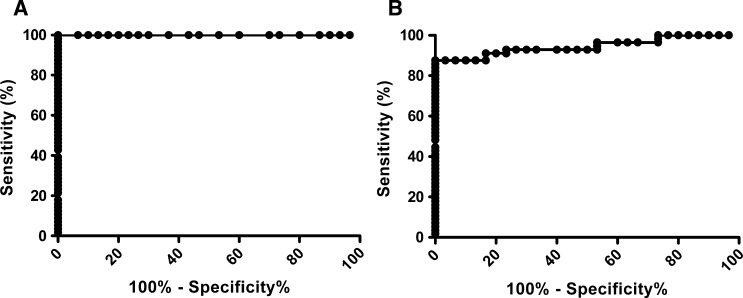
The ROC curve was generated from 56 leishmaniasis patient samples and 30 healthy blood bank samples assayed by ELISA-rHsp83 (Figure 1A) and ELISA–L. major-like (Figure 1B). To reach a maximum specificity (95% CI) of 100.0% (88.43–100.0%), cutoff values were 0.070 for ELISA-rHsp83 and 0.190 for ELISA–L. major-like.
Figure 1.
ROC curve with leishmaniasis (N = 56) and control blood bank (N = 30) samples. (A) ELISA-rHsp83 generated a cutoff value of 0.070. (B) ELISA–L. major-like generated a cutoff value of 0.190.
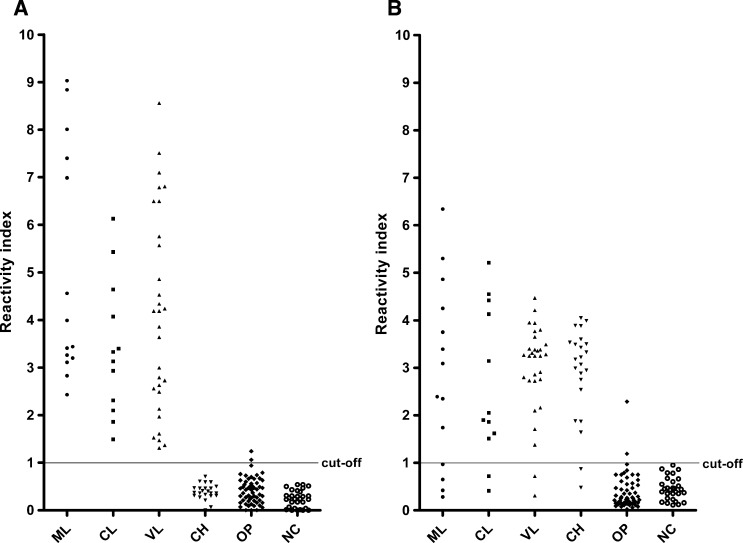
Using rHsp83 as the antigen in the ELISA assay led to a significantly higher sensitivity in detecting anti-Leishmania antibodies compared with using L. major-like antigen (P = 0.013, McNemar's test) (Table 2). Strong agreement between the evaluated antigens was observed, with κ = 0.807 (0.682–0.932). Figure 2 shows the reactivity of all tested serum samples with either antigen.
Table 2.
Sensitivity and specificity of ELISA-rHsp83 and ELISA–L. major-like
| Samples | N | ELISA-rHsp83 | ELISA–L. major-like | ||
|---|---|---|---|---|---|
| Sensitivity (%) | CI (%) | Sensitivity (%) | CI (%) | ||
| CL | 12 | 100.0 | 73.54–100.0 | 83.33 | 51.59–97.91 |
| ML | 14 | 100.0 | 76.84–100.0 | 71.43 | 41.90–91.61 |
| VL | 30 | 100.0 | 88.43–100.0 | 93.33 | 77.93–99.18 |
| Total | 56 | 100.0* | 93.62–100.0 | 87.50 | 75.93–94.82 |
| Sensitivity (%) | CI (%) | Sensitivity (%) | CI (%) | ||
| Normal control | 30 | 100.0 | 88.43–100.0 | 100.0 | 88.43–100.0 |
P = 0.013 compared with ELISA–L. major-like (McNemar's test).
Figure 2.
Reactivity index of sera from ML (N = 14), CL (N = 12), VL (N = 30), Chagas disease (CH; N = 23), other infectious diseases (OP; N = 56), and control blood bank donors (NC; N = 30) in (A) ELISA-rHsp83 and (B) ELISA–L. major-like.
ELISA-rHsp83 and ELISA–L. major-like cross-reactivity to other infectious disease samples.
We found an overall specificity (95% CI) of 97.47% (91.15–99.69%) and 70.89% (59.58–80.57%) for ELISA-rHsp83 and ELISA–L. major-like antigens (Table 3), respectively, in serum samples from patients with other infectious diseases. The difference in performance of the two antigens was significant (P < 0.001, McNemar's test).
Table 3.
Specificity (%) of ELISA-rHsp83 and ELISA–L. major-like for other infectious disease samples
| Samples | N | ELISA-rHsp83 | ELISA–L. major-like | ||
|---|---|---|---|---|---|
| Specificity (%) | CI (%) | Specificity (%) | CI (%) | ||
| Chagas disease | 23 | 100.0* | 85.18–100.0 | 8.70 | 1.07–28.04 |
| Blastomycosis | 7 | 100.0 | 59.04–100.0 | 85.71 | 42.13–99.64 |
| Histoplasmosis | 6 | 100.0 | 54.07–100.0 | 83.33 | 35.88–99.58 |
| Aspergillosis | 5 | 80.00 | 28.36–99.49 | 100.0 | 47.82–100.0 |
| Cromomycosis | 7 | 100.0 | 59.04–100.0 | 100.0 | 59.04–100.0 |
| Toxoplasmosis | 14 | 92.86 | 66.13–99.82 | 100.0 | 76.84–100.0 |
| Cytomegalovirosis | 4 | 100.0 | 39.76–100.0 | 100.0 | 39.76–100.0 |
| Malaria | 9 | 100.0 | 66.37–100.0 | 100.0 | 67.37–100.0 |
| Tuberculosis | 4 | 100.0 | 39.76–100.0 | 100.0 | 39.76–100.0 |
| Total | 79 | 97.47* | 74.97–91.90 | 70.89 | 58.25–79.47 |
P < 0.001 compared with ELISA–L. major-like (McNemar's test).
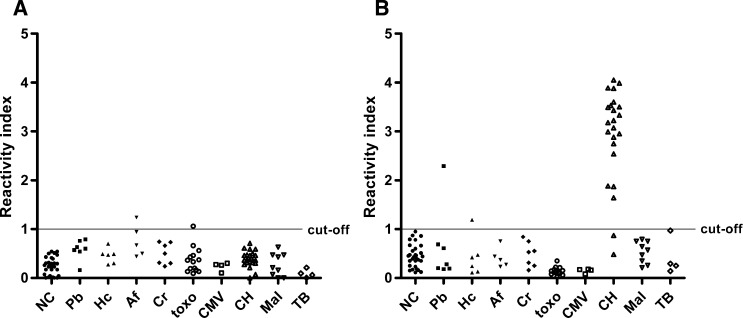
For samples from patients with Chagas disease, ELISA specificity was significantly higher with rHsp83 than L. major-like antigen (P < 0.001, McNemar's test). In the sera from patients with other infectious disease (excluding Chagas disease), both antigens had the same specificity: 96.43% (95% CI = 87.69–99.56%; P = 0.617, McNemar's test). Non-specific reactions with rHsp83 antigen were observed with samples from aspergillosis and toxoplasmosis patients. With the L. major-like antigen, 21 of 23 (91.30%) serum samples from Chagas disease patients showed cross-reactivity (Figure 3).
Figure 3.
(A) ELISA-rHsp83 and (B) ELISA–L. major-like cross-reactivity with other infectious disease samples: NC (N = 30), blastomycosis (Pb; N = 7), histoplasmosis (Hc; N = 6), aspergillosis (Af; N = 5), chromomycosis (Cr; N = 7), toxoplasmosis (toxo; N = 14), cytomegalovirosis (CMV; N = 4), CH (N = 23), malaria (Mal; N = 9), and tuberculosis (TB; N = 4).
Post-therapeutic evaluation of anti-Leishmania antibody titers in ELISA-rHsp83 in CL and ML.
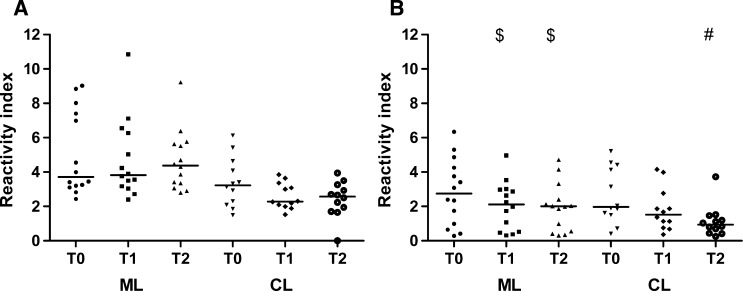
We found no significant difference when assessing the post-therapeutic antibody levels in 26 patients with TL (CL = 12 and ML = 14) using ELISA-rHsp83 (Friedman repeated measures analysis of variance on ranks: ML [P = 0.607], CL [P = 0.205]). In contrast, ELISA–L. major-like median values showed a progressive decrease; the medians were significantly higher at T0 compared with T1 and T2 in ML (P = 0.0003) and at T0 compared with T2 in CL (P = 0.0023) (Figure 4).
Figure 4.
Reactivity index for follow-up samples in ML and CL patients in (A) ELISA-rHsp83 and (B) ELISA–L. major-like. $P < 0.05 compared with T0 in ML (Friedman repeated measures analysis of variance on ranks and Tukey test). #P < 0.05 compared with T0 in CL (Friedman repeated measures analysis of variance on ranks and Tukey test).
Discussion
Early diagnosis of leishmaniases is vital to avoid permanent damage, sequelae, and even death, depending on the disease form. Although detection of the parasite confirms diagnosis, the lack of detection does not exclude the presence of the disease or infection, because parasitological methods have low sensitivity.3,7,31,34–36
In this study, we evaluated L. infantum rHsp83 as an antigen in ELISA for the diagnosis of CL, ML, and VL and compared the results with L. major-like total promastigote antigen. ELISA-rHsp83 was significantly more sensitive than ELISA–L. major-like when comparing results from all leishmaniasis patients (P = 0.013) and TL (P = 0.041). Previously, Guimarães and others16 used ELISA–L. major-like and reported low sensitivity (66.3%) and specificity (77.5%) for TL patients, whereas Barroso-Freitas and others13 obtained better results using ELISA-L. braziliensis (97.5% sensitivity and 100.0% specificity) for TL patients. Using seven L. infantum-derived recombinant proteins to test TL sera, Hsp70 was considered the most promising, with 65.0% sensitivity and 92.0% specificity.23
For VL patients, several groups have tested ELISA-L. infantum rK39 and found sensitivity/specificity values of 88.6%/92.4%37 and 100%/96%.38 ELISA-rK39 has high diagnostic and prognostic use in human immunodeficiency virus (HIV)-infected patients coinfected with L. infantum.39 Reactivity of the widely used immunochromatographic test using the rK39 antigen was shown to vary depending not only on the patients' origins but also, the product source.20
Although rK39 has been important for VL diagnosis, this antigen does not allow detection of antibodies in CL or ML.40 However, the recombinant Hsp83 antigen detects VL, CL, and ML with high sensitivity (100.0%), a great advantage over the previously mentioned antigens.
Cross-reactivity of Leishmania antigens with sera samples from geographic areas where other protozoa diseases, particularly Chagas disease, overlap with leishmaniasis is common. Total promastigote antigen is known to cross-react with sera positive for several other infectious diseases, especially Chagas disease.24,30,39 To overcome such non-specific reactions, several recombinant proteins have been tried with varying success. In this study, we obtained maximum indices with both ELISA-rHsp83 and ELISA–L. major-like for healthy blood bank samples. However, for serum samples from patients with other infectious diseases, rHsp83 was significantly more specific, particularly with respect to Chagas disease samples (P < 0.001). Souza and others23 obtained 90% specificity with rHsp70, whereas we found 100.0% specificity with rHsp83 in the Chagas disease samples. Aspergillosis and toxoplasmosis serum samples provided non-specific reactions with rHsp83, but the values were near the cutoff level and had lower positivity than has been observed in other studies.31,41
In the present study, a significant decrease in antibody levels detected by ELISA–L. major-like was observed 6 months after the onset of therapy in ML patients and 12 months after therapy onset in CL patients, independent of the therapeutic response. Using ELISA-L. amazonensis, Romero and others18 also observed a decrease in antibody levels in CL patients after treatment that was unrelated to therapeutic responses. In contrast, we did not detect a significant decrease in antibody levels post-treatment as measured by the ELISA-rHsp83. Control of cure by serological tests is a controversial subject in the literature.42 The persistence of antibodies after treatment could indicate the continued presence of viable parasites, raising the possibility of a relapse.43
We believe that L. infantum-rHsp83 is a good antigen for use in the serodiagnosis of leishmaniases. Because of its high specificity and sensitivity and insignificant cross-reactivity with other infectious diseases, we suggest the ELISA-rHsp83 as a routine confirmatory serological assay for the diagnosis of Leishmania infection.
Footnotes
Financial support: This work was supported by Fundação de Amparo à Pesquisa do Estado de São Paulo (2011/02235-0), Conselho Nacional de Pesquisa (CNPq; research fellowship; to H.G.), Instituto Nacional de Ciência e Tecnologia-CNPq-Nanotecnologia para Marcadores Integrados, and Laboratório de Investigação Médica-38/Hospital das Clínicas da Faculdade de Medicina da Universidade de São Paulo.
Authors' addresses: Beatriz Julieta Celeste, Maria Carmen Arroyo Sanchez, and Eduardo Milton Ramos-Sanchez, Instituto de Medicina Tropical de São Paulo, Universidade de São Paulo, São Paulo, Brazil, E-mails: bjcelest@usp.br, arroyo@usp.br, and eduardors22@hotmail.com. Luiz Guilherme M. Castro, Departamento de Dermatologia da Faculdade de Medicina, Universidade de São Paulo, São Paulo, Brazil, E-mail: guiga@dermamail.com.br. Francisco Assis Lima Costa, Departamento de Clínica e Cirurgia Veterinária, Centro de Ciências Agrárias, Universidade Federal do Piauí, Teresina, Piauí, Brazil, E-mail: fassisle@gmail.com. Hiro Goto, Departamento de Medicina Preventiva, Faculdade de Medicina and Instituto de Medicina Tropical de São Paulo, Universidade de São Paulo, São Paulo, Brazil, E-mail: hgoto@usp.br.
References
- 1.Pan American Health Organization Leishmaniases. Report Leishmaniases. 2013;1:1–4. [Google Scholar]
- 2.Goto H, Prianti M. Immunoactivation and immunopathogeny during active visceral leishmaniasis. Rev Inst Med Trop Sao Paulo. 2009;51:241–246. doi: 10.1590/s0036-46652009000500002. [DOI] [PubMed] [Google Scholar]
- 3.Goto H, Lauletta Lindoso JA. Cutaneous and mucocutaneous leishmaniasis. Infect Dis Clin North Am. 2012;26:293–307. doi: 10.1016/j.idc.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 4.Goto H, Lindoso JA. Current diagnosis and treatment of cutaneous and mucocutaneous leishmaniasis. Expert Rev Anti Infect Ther. 2010;8:419–433. doi: 10.1586/eri.10.19. [DOI] [PubMed] [Google Scholar]
- 5.Al-Hucheimi SN, Sultan BA, Al-Dhalimi MA. A comparative study of the diagnosis of Old World cutaneous leishmaniasis in Iraq by polymerase chain reaction and microbiologic and histopathologic methods. Int J Dermatol. 2009;48:404–408. doi: 10.1111/j.1365-4632.2009.03903.x. [DOI] [PubMed] [Google Scholar]
- 6.Schubach A, Cuzzi-Maya T, Oliveira AV, Sartori A, de Oliveira-Neto MP, Mattos MS, Araujo ML, Souza WJ, Haddad F, Perez Mde A, Pacheco RS, Momen H, Coutinho SG, de Almeida Marzochi MC, Marzochi KB, da Costa SC. Leishmanial antigens in the diagnosis of active lesions and ancient scars of American tegumentary leishmaniasis patients. Mem Inst Oswaldo Cruz. 2001;96:987–996. doi: 10.1590/s0074-02762001000700018. [DOI] [PubMed] [Google Scholar]
- 7.Sotto MN, Yamashiro-Kanashiro EH, da Matta VL, de Brito T. Cutaneous leishmaniasis of the New World: diagnostic immunopathology and antigen pathways in skin and mucosa. Acta Trop. 1989;46:121–130. doi: 10.1016/0001-706x(89)90006-5. [DOI] [PubMed] [Google Scholar]
- 8.Vega-Lopez F. Diagnosis of cutaneous leishmaniasis. Curr Opin Infect Dis. 2003;16:97–101. doi: 10.1097/00001432-200304000-00006. [DOI] [PubMed] [Google Scholar]
- 9.Romero GA, Boelaert M. Control of visceral leishmaniasis in Latin America—a systematic review. PLoS Negl Trop Dis. 2010;4:e584. doi: 10.1371/journal.pntd.0000584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Srividya G, Kulshrestha A, Singh R, Salotra P. Diagnosis of visceral leishmaniasis: developments over the last decade. Parasitol Res. 2012;110:1065–1078. doi: 10.1007/s00436-011-2680-1. [DOI] [PubMed] [Google Scholar]
- 11.Edrissian GH, Darabian P. A comparison of enzyme-linked immunosorbent assay and indirect fluorescent antibody test in the sero-diagnosis of cutaneous and visceral leishmaniasis in Iran. Trans R Soc Trop Med Hyg. 1979;73:289–292. doi: 10.1016/0035-9203(79)90084-1. [DOI] [PubMed] [Google Scholar]
- 12.Kar K. Serodiagnosis of leishmaniasis. Crit Rev Microbiol. 1995;21:123–152. doi: 10.3109/10408419509113537. [DOI] [PubMed] [Google Scholar]
- 13.Barroso-Freitas AP, Passos SR, Mouta-Confort E, Madeira MF, Schubach AO, Santos GP, Nascimento LD, Marzochi MC, Marzochi KB. Accuracy of an ELISA and indirect immunofluorescence for the laboratory diagnosis of American tegumentary leishmaniasis. Trans R Soc Trop Med Hyg. 2009;103:383–389. doi: 10.1016/j.trstmh.2008.12.019. [DOI] [PubMed] [Google Scholar]
- 14.Guimaraes MC, Celeste BJ, Franco EL, Cuce LC, Belda W., Jr Evaluation of serological diagnostic indices for mucocutaneous leishmaniasis: immunofluorescence tests and enzyme-linked immunoassays for IgG, IgM and IgA antibodies. Bull World Health Organ. 1989;67:643–648. [PMC free article] [PubMed] [Google Scholar]
- 15.el Safi SH, Evans DA. A comparison of the direct agglutination test and enzyme-linked immunosorbent assay in the sero-diagnosis of leishmaniasis in the Sudan. Trans R Soc Trop Med Hyg. 1989;83:334–337. doi: 10.1016/0035-9203(89)90493-8. [DOI] [PubMed] [Google Scholar]
- 16.Guimaraes MC, Celeste BJ, Franco EL. Diagnostic performance indices for immunofluorescent tests and enzyme immunoassays of leishmaniasis sera from northern and north-eastern Brazil. Bull World Health Organ. 1990;68:39–43. [PMC free article] [PubMed] [Google Scholar]
- 17.Zeyrek FY, Korkmaz M, Ozbel Y. Serodiagnosis of anthroponotic cutaneous leishmaniasis (ACL) caused by Leishmania tropica in Sanliurfa Province, Turkey, where ACL Is highly endemic. Clin Vaccine Immunol. 2007;14:1409–1415. doi: 10.1128/CVI.00133-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Romero GA, de la Gloria Orge Orge M, de Farias Guerra MV, Paes MG, de Oliveira Macedo V, de Carvalho EM. Antibody response in patients with cutaneous leishmaniasis infected by Leishmania (Viannia) braziliensis or Leishmania (Viannia) guyanensis in Brazil. Acta Trop. 2005;93:49–56. doi: 10.1016/j.actatropica.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 19.Srivastava P, Dayama A, Mehrotra S, Sundar S. Diagnosis of visceral leishmaniasis. Trans R Soc Trop Med Hyg. 2011;105:1–6. doi: 10.1016/j.trstmh.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cunningham J, Hasker E, Das P, El Safi S, Goto H, Mondal D, Mbuchi M, Mukhtar M, Rabello A, Rijal S, Sundar S, Wasunna M, Adams E, Menten J, Peeling R, Boelaert M. WHO/TDR Visceral Leishmaniasis Laboratory Network A global comparative evaluation of commercial immunochromatographic rapid diagnostic tests for visceral leishmaniasis. Clin Infect Dis. 2012;55:1312–1319. doi: 10.1093/cid/cis716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rey-Ladino JA, Joshi PB, Singh B, Gupta R, Reiner NE. Leishmania major: molecular cloning, sequencing, and expression of the heat shock protein 60 gene reveals unique carboxy terminal peptide sequences. Exp Parasitol. 1997;85:249–263. doi: 10.1006/expr.1996.4137. [DOI] [PubMed] [Google Scholar]
- 22.Amorim AG, Carrington M, Miles MA, Barker DC, de Almeida ML. Identification of the C-terminal region of 70 kDa heat shock protein from Leishmania (Viannia) braziliensis as a target for the humoral immune response. Cell Stress Chaperones. 1996;1:177–187. doi: 10.1379/1466-1268(1996)001<0177:iotctr>2.3.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Souza AP, Soto M, Costa JM, Boaventura VS, de Oliveira CI, Cristal JR, Barral-Netto M, Barral A. Towards a more precise serological diagnosis of human tegumentary leishmaniasis using Leishmania recombinant proteins. PLoS ONE. 2013;8:e66110. doi: 10.1371/journal.pone.0066110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Celeste BJ, Angel SO, Castro LG, Gidlund M, Goto H. Leishmania infantum heat shock protein 83 for the serodiagnosis of tegumentary leishmaniasis. Braz J Med Biol Res. 2004;37:1591–1593. doi: 10.1590/s0100-879x2004001100001. [DOI] [PubMed] [Google Scholar]
- 25.Angel SO, Requena JM, Soto M, Criado D, Alonso C. During canine leishmaniasis a protein belonging to the 83-kDa heat-shock protein family elicits a strong humoral response. Acta Trop. 1996;62:45–56. doi: 10.1016/s0001-706x(96)00020-4. [DOI] [PubMed] [Google Scholar]
- 26.Momen H, Grimaldi G, Jr, Pacheco RS, Jaffe CL, McMahon-Pratt D, Marzochi MC. Brazilian Leishmania stocks phenotypically similar to Leishmania major. Am J Trop Med Hyg. 1985;34:1076–1084. doi: 10.4269/ajtmh.1985.34.1076. [DOI] [PubMed] [Google Scholar]
- 27.Hoshino-Shimizu S, Camargo ME, Nagasse TK. A stable polysaccharide-hemagglutination reagent for the diagnosis of acute or recent Trypanosoma cruzi infections. Rev Inst Med Trop Sao Paulo. 1978;20:208–212. [PubMed] [Google Scholar]
- 28.Guimaraes MC, Celeste BJ, de Castilho EA, Mineo JR, Diniz JM. Immunoenzymatic assy (ELISA) in mucocutaneous leishmaniasis, kala-azar, and Chagas' disease: an epimastigote Trypanosoma cruzi antigen able to distinguish between anti-Trypanosoma and anti-Leishmania antibodies. Am J Trop Med Hyg. 1981;30:942–947. doi: 10.4269/ajtmh.1981.30.942. [DOI] [PubMed] [Google Scholar]
- 29.de Arruda MM, Figueiredo FB, Cardoso FA, Hiamamoto RM, Brazuna JC, de Oliveira MR, Noronha EF, Romero GA. Validity and reliability of enzyme immunoassays using Leishmania major or L. infantum antigens for the diagnosis of canine visceral leishmaniasis in Brazil. PLoS ONE. 2013;8:e69988. doi: 10.1371/journal.pone.0069988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guimaraes MC, Celeste BJ, Corrales EM, Antunes CM. Comparison on the performance of Leishmania major-like and Leishmania braziliensis braziliensis as antigen for New World leishmaniasis IgG-immunofluorescence test. Rev Inst Med Trop Sao Paulo. 1991;33:503–508. doi: 10.1590/s0036-46651991000600012. [DOI] [PubMed] [Google Scholar]
- 31.Cuba Cuba CA, Marsden PD, Barreto AC, Rocha R, Sampaio RR, Patzlaff L. Parasitologic and immunologic diagnosis of American (mucocutaneous) leishmaniasis. Bull Pan Am Health Organ. 1981;15:249–259. [PubMed] [Google Scholar]
- 32.Montenegro J. A cutis-reação na leishmaniose. An Fac Med Univ Sao Paulo. 1926;1:323–330. [Google Scholar]
- 33.Greiner M, Pfeiffer D, Smith RD. Principles and practical application of the receiver-operating characteristic analysis for diagnostic tests. Prev Vet Med. 2000;45:23–41. doi: 10.1016/s0167-5877(00)00115-x. [DOI] [PubMed] [Google Scholar]
- 34.Chappuis F, Rijal S, Soto A, Menten J, Boelaert M. A meta-analysis of the diagnostic performance of the direct agglutination test and rK39 dipstick for visceral leishmaniasis. BMJ. 2006;333:723. doi: 10.1136/bmj.38917.503056.7C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Weigle KA, de Davalos M, Heredia P, Molineros R, Saravia NG, D'Alessandro A. Diagnosis of cutaneous and mucocutaneous leishmaniasis in Colombia: a comparison of seven methods. Am J Trop Med Hyg. 1987;36:489–496. doi: 10.4269/ajtmh.1987.36.489. [DOI] [PubMed] [Google Scholar]
- 36.Marsden PD. Mucosal leishmaniasis (“espundia” Escomel, 1911) Trans R Soc Trop Med Hyg. 1986;80:859–876. doi: 10.1016/0035-9203(86)90243-9. [DOI] [PubMed] [Google Scholar]
- 37.Pedras MJ, de Gouvea Viana L, de Oliveira EJ, Rabello A. Comparative evaluation of direct agglutination test, rK39 and soluble antigen ELISA and IFAT for the diagnosis of visceral leishmaniasis. Trans R Soc Trop Med Hyg. 2008;102:172–178. doi: 10.1016/j.trstmh.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 38.Mohapatra TM, Singh DP, Sen MR, Bharti K, Sundar S. Compararative evaluation of rK9, rK26 and rK39 antigens in the serodiagnosis of Indian visceral leishmaniasis. J Infect Dev Ctries. 2010;4:114–117. doi: 10.3855/jidc.544. [DOI] [PubMed] [Google Scholar]
- 39.Houghton RL, Petrescu M, Benson DR, Skeiky YA, Scalone A, Badaro R, Reed SG, Gradoni L. A cloned antigen (recombinant K39) of Leishmania chagasi diagnostic for visceral leishmaniasis in human immunodeficiency virus type 1 patients and a prognostic indicator for monitoring patients undergoing drug therapy. J Infect Dis. 1998;177:1339–1344. doi: 10.1086/515289. [DOI] [PubMed] [Google Scholar]
- 40.Singh S, Sivakumar R. Recent advances in the diagnosis of leishmaniasis. J Postgrad Med. 2003;49:55–60. doi: 10.4103/0022-3859.927. [DOI] [PubMed] [Google Scholar]
- 41.Abramo C, Fontes CJ, Krettli AU. Cross-reactivity between antibodies in the sera of individuals with leishmaniasis, toxoplasmosis, and Chagas' disease and antigens of the blood-stage forms of Plasmodium falciparum determined by indirect immunofluorescence. Am J Trop Med Hyg. 1995;53:202–205. doi: 10.4269/ajtmh.1995.53.202. [DOI] [PubMed] [Google Scholar]
- 42.de Oliveira MR, Macedo Vde O, de Carvalho EM, Barral A, Marotti JG, Bittencourt A, de Abreu MV, Orge MLG, Lessa Hde A, Marsden PD. An evolutionary study of mucosal leishmaniasis (a 7- to 17-year follow-up) due to Leishmania (Viannia) braziliensis in Tres Bracos, Bahia. Rev Soc Bras Med Trop. 1995;28:325–332. doi: 10.1590/s0037-86821995000400004. [DOI] [PubMed] [Google Scholar]
- 43.Walton BC. Evaluation of chemotherapy of American leishmaniasis by the indirect fluorescent antibody test. Am J Trop Med Hyg. 1980;29:747–752. doi: 10.4269/ajtmh.1980.29.747. [DOI] [PubMed] [Google Scholar]