Abstract
In many high-risk populations, access to tuberculosis (TB) diagnosis and treatment is limited and pockets of high prevalence persist. We estimated the cost-effectiveness of an extensive active case finding program in areas of Cambodia where TB notifications and household poverty rates are highest and access to care is restricted. Thirty operational health districts with high TB incidence and household poverty were randomized into intervention and control groups. In intervention operational health districts, all household and symptomatic neighborhood contacts of registered TB patients of the past two years were encouraged to attend screening at mobile centers. In control districts, routine passive case finding activities continued. The program screened more than 35,000 household and neighborhood contacts and identified 810 bacteriologically confirmed cases. The cost-effectiveness analysis estimated that in these cases the reduction in mortality from 14% to 2% would result in a cost per daily adjusted life year averted of $330, suggesting that active case finding was highly cost-effective.
Introduction
Cambodia and other high-burden countries have achieved considerable gains in tuberculosis control in recent years and the Millennium Development Goal of halving the global prevalence has already been reached.1 There is concern, however, about the slowing rate of reduction, partly because of hard-to-reach populations maintaining reservoirs of infection. This has led to a growing interest in active case finding (ACF) programs that aim to identify persons with tuberculosis (TB) who might otherwise continue to infect others for a long infectious period before seeking care, if they do so at all. The programs can also offer better diagnostic capacities than those in routine services, particularly for asymptomatic and sputum smear–negative patients. Such programs have been in place in Cambodia since 2005 but evidence to support their effectiveness and cost-effectiveness is lacking.2
The primary benefit of ACF has been stated in terms of reduction of further TB transmission, but there is little evidence for the economic case when considering direct benefits for the detected cases.3 This is an important distinction that affects funders' incentives to support such programs. For donors and government organizations whose remit is specifically TB control, programs can be evaluated in terms of cost per case detected or per case averted. If, however, these programs compete for non-TB specific funding, the costs and benefits of TB control would be more comparable with other interventions by considering direct health benefits in the detected cases.
In 2012, a large ACF program targeting household and symptomatic neighborhood contacts of known TB cases was initiated in areas of high prevalence and household poverty and where access to care was restricted, covering more than one-third of the population of Cambodia. In the first phase (2012), ACF activities took place in 15 operational districts (ODs), and 15 other ODs were used as controls where routine passive case finding (PCF) continued by using microscopy. In the second phase, the evaluation and control ODs were switched. The purpose of this study was to evaluate the cost-effectiveness of the first phase of the program and estimate the cost per disability adjusted life year (DALY) averted in bacteriologically confirmed cases detected in the ACF program.
Methods
Program description.
The health system in Cambodia is divided into 77 ODs that each serve 100,000–200,000 persons. These ODs were ranked by TB case notification rates and poverty/access-barrier scores. Thirty of the ODs with the highest rankings were randomized into intervention and control arms. In 2009, the intervention and control ODs had an almost identical case notification rate of 175 and 179 cases per 100,000.4,5 Active case finding was carried out during February–December 2012 in the intervention ODs alongside routine PCF, and the other 15 ODs continued with only routine PCF.
In the intervention ODs, community volunteers and health workers visited households of smear-positive case-patients registered for treatment in the previous two years to motivate them if they still had any TB symptoms, their household contacts irrespective of symptoms, and neighborhood contacts if they had any TB symptoms to visit the ACF sites set up in the nearest health centers on specific days for screening. Persons attending the sessions were screened for symptoms by clinicians from the National Center for TB and Leprosy Control and had a chest radiograph taken. Persons with clinical symptoms and/or chest radiographic findings consistent with TB were tested by using the GeneXpert MTB/RIF assay (Xpert, Aliso Viejo, CA). The decision to initiate treatment was made by clinician discretion. From this point, TB cases were managed by routine health services. In most instances the ACF activities took place in the health centers, which resulted in immediate initiation of treatment and low initial default rates.
Calculation of program economic costs.
The perspective of the economic analysis is that of the healthcare system and is based on the program budget with the following modifications to adjust for their economic value as opposed to financial costs. First, pre-existing capital equipment including vehicles and radiograph machines that were not included in the budget were introduced. Second, capital costs were annualized with useful life estimates obtained from World Health Organization tables (www.who.int/choice/costs/prices_t4/en/index.html). Estimates for Cambodia were not available. Therefore, values for Thailand were used instead and a 3% discount rate was applied.6 Third, training costs were annualized assuming they are effective for three years. Fourth, monitoring and evaluation specific costs were removed. Fifth, salaries that were covered through government or other funders were introduced. Sixth, standard government salaries were applied for all personnel. Seventh, program costs were obtained in 2012 U.S. dollars and are reported in the same denomination.
Cost-effectiveness model description and assumptions.
The cost-effectiveness analysis compares costs and health outcomes for persons with bacteriologically confirmed TB diagnosed in the ACF program with the modeled outcomes for these persons in the absence of ACF activities. The differences in costs and outcomes are used to calculate the incremental cost-effectiveness ratio for the ACF program running alongside PCF compared with PCF alone.
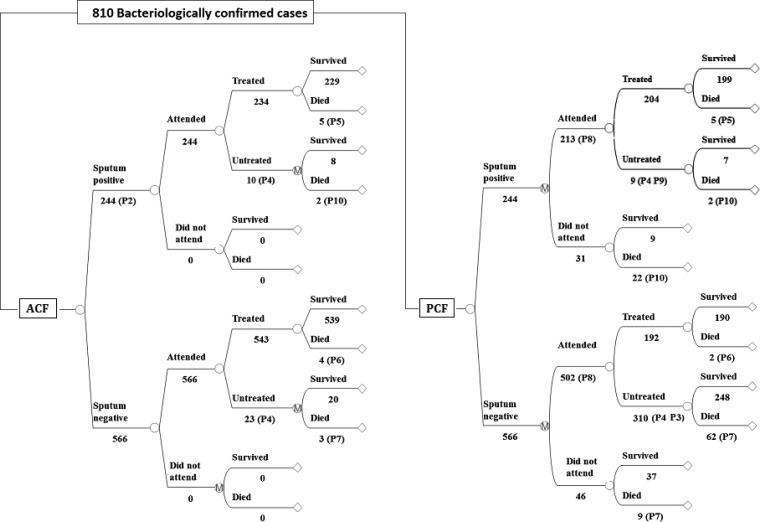
A decision tree was used to compare the two scenarios. In the ACF arm, mortality rates were applied to the cases detected and treated in the ACF program by sputum status. The progression for defaulters in the ACF arm and for all patients in the PCF arm was predicted by using a Markov model in which patients can cure naturally, die untreated, or seek treatment at a clinic in each of the subsequent six-month cycles over a period of five years. The probability of seeking care in the first cycle is high (74%, based on the relative reported incidence in the control and evaluation ODs) and decreases linearly in each cycle. Patients who are detected and treated in the PCF arm face the same mortality rates for treated TB as those in ACF. A simplified outline of the model is shown in Figure 1, and details of the parameter estimates used in the model are shown in Table 1.
Figure 1.
Model outline for active tuberculosis case finding in Cambodia. The empty circular nodes represent single chance events and those encircling an M denote a Markov process where patients can transit between subsequent states. The values shown are for this simplified illustration of the model and do not account for human immunodeficiency virus status for which adjustments are made as detailed in Table 1, for the recurrence of possible attendance for passive case finding (PCF) over the model cycles, and are based on point estimates rather than the probability distributions used in the actual model that imply some variability in outcomes. ACF = active case finding.
Table 1.
Parameter values used in the model for active tuberculosis case finding in Cambodia*
| Parameter | Model input | Best | Low | High | Reference | Comments, distributions, and impact |
|---|---|---|---|---|---|---|
| Epidemiology and treatment outcomes | ||||||
| HIV positivity in TB cases | P1 | 5.1% | 4.8% | 5.3% | 1 | |
| Sputum positivity of all bacteriologically confirmed cases | P2 | 30% | 28% | 37% | 2,10 | Beta distribution (α = 90, β = 210) |
| Mortality rate in untreated SS+ HIV− cases | P10 | 70% | Beta distribution (α = 210, β = 90) | |||
| Mortality rate in untreated SS− HIV− cases | P7 | 20% | Beta distribution (α = 60, β = 240) | |||
| Mortality rate in treated SS+ HIV− cases | P5 | 2.2% | 2 | Calculated using reported mortality of 1.8% in PCF and 0.8% in ACF in Cambodia | ||
| Mortality rate in SS−, HIV− cases in PCF (unadjusted for sputum smear status) | P6 | 0.7% | 2 | As above | ||
| Mortality rate for TB HIV+ (%) | Sensitivity analysis | 9.2 | 3.7 | 14.7 | 13 | |
| Diagnostic accuracies | ||||||
| Probability SS+ diagnosed in PCF (%) | P9 | 86 | 64 | 100 | 25 | |
| Probability SS− diagnosed in PCF (%) | P3 | 40 | 10 | 60 | Assumption | |
| Default rate (%) | P4 | 4 | 0 | 30 | 2,10 | |
| GeneXpert MTB/RIF assay sensitivity | ||||||
| Culture positive, smear positive (%) | 98 | 97 | 99 | 11,12 | ||
| Culture positive, smear negative (%) | 76 | 72 | 80 | |||
| Scenario | ||||||
| Proportion of sputum-positive cases detected via PCF in control arm | P8 | 74% | Random samples from the ratio of reported cases in control/evaluation ODs in 2012 | |||
| Cost estimates | ||||||
| ACF program cost (US$) | 363,257 | Primary data | ||||
| Cost of community DOTS in Cambodia (US$) | Cost of treatment | 250 | 200 | 300 | 7 | Varied ± 20% with negligible effect |
| Cost of sputum smear in PCF (US$) | 2.5 | 1 | 5 | 2,25 | Triangular and negligible effect | |
| Cost of outpatient PCF visits (US$) | 1.6 | WHO-CHOICE | ||||
| DALY determinants | ||||||
| TB SS+ HIV− disability weight | 0.33 | 0.22 | 0.45 | 26 | ||
| TB SS− HIV− TB disability weight | 0.19 | 22 | ||||
| TB HIV+ disability weight | 0.39 | 0.26 | 0.54 | 22,26 | ||
| Life expectancy for surviving patients (discounted) | 16 years | Age-specific life expectancy from WHO tables for patients in the ACF program | ||||
HIV = human immunodeficiency virus; TB = tuberculosis; SS = sputum smear; PCF = passive case finding; ACF = active case finding; ODs = operational districts; DOTS = directly observed treatment, short course; WHO-CHOICE = World Health Organization–Choosing Interventions that Are Cost Effective; DALY = daily-adjusted life year.
The costs included in the model are those of the ACF program, the cost of attending PCF (comprised of the cost of diagnostic tests and an estimate of the overhead costs of outpatient visits in Cambodia [www.who.int/choice/country/country_specific/en/index.htm]; costs were adjusted to 2012 U.S. dollars by using the consumer price index available at www.bls.gov/data/inflation_calculator.htm), and direct medical cost for subsequent TB treatment in community directly observed treatment, short course (DOTS).7 The incremental benefits of the program are a product of the following three factors: 1) detection of a small proportion of patients who would not otherwise access routine services and face higher mortality rates for untreated disease; 2) higher sensitivity in diagnosing smear-negative patients and to a lesser extent smear-positive patients who would attend PCF services but may not be correctly diagnosed as having TB; and 3) shorter duration of illness before treatment. Health benefits were measured by using disability adjusted life years (DALYs). These values are comprised of the duration of illness in untreated cases and the number of life-years lost in deaths because of TB.
The following assumptions were used to extrapolate from the probability of correct diagnosis and treatment to long-term health outcomes, mostly implying conservative estimates for the incremental benefits of ACF. First, the probability of patients with TB attending PCF for their symptoms in the absence of the ACF program is 74% in the first year (equivalent to the ratio of incidence of reported cases in the control population over the evaluation population) and decreases linearly per six-month cycle to 15% in year 5. Second, as the average age in case-patients detected in ACF in Cambodia has been shown to be higher than that in patients attending PCF,2 DALYs are estimated by using primary data for patients' ages and estimating their age specific life expectancy from World Health Organization tables (therefore assuming that changes in TB mortality do not affect overall mortality) and discounted at 3% without age modulation. Third, no differentiation by drug susceptibility was made because of low prevalence of multidrug-resistant TB (MDR-TB) in this setting (1.4% of all cases detected in the ACF program were resistant to rifampicin). Fourth, it is assumed conservatively that in the absence of treatment, smear-negative cases do not convert to smear-positive cases positivity and their mortality rates remain comparatively low.8 Fifth, it is assumed that in the absence of treatment the average duration to natural cure or death is three years.9
The proportion of smear-positive and smear-negative cases of bacteriologically positive cases was derived from a recent TB prevalence survey by using solid culture as the gold standard.10 This survey had shown a smear-negative to smear-positive ratio of approximately 2:1. This ratio was adjusted by the sensitivity of the GeneXpert MTB/RIF assay in detecting each of these types (76% for smear-negative cases and 98% for smear-positive cases11,12). A previous study of ACF activities in Cambodia found slightly higher mortality rates in PCF cases than in ACF cases.2 We used these data to extrapolate the respective mortality rates for treated smear-positive and smear-negative TB, which resulted in beta distributions with a mean of 2.2% and 0.7% respectively, and with considerable overlap in the two distributions. These estimates are lower than those generally reported.13 Therefore, we considered higher values in the sensitivity analyses.
The prevalence of human immunodeficiency virus (HIV) in TB cases is estimated to be 5.1%.1 Parameter values for this group assumed a higher mortality rate in treated and untreated case-patients, and the number of DALYs per case treated was adjusted downwards by 40% and 30% for smear-positive and smear-negative cases because of the lower life expectancy in surviving patients.14
The costs and health benefits for confirmed cases are summarized as an incremental cost-effectiveness ratio (ICER). This value is compared with the Cambodia 2012 gross domestic product (GDP) per capita (http://data.worldbank.org/indicator/NY.GDP.PCAP.CD) of U.S. $900 as the primary threshold below which an ICER is considered cost-effective. The results were generated by using a probabilistic sensitivity analysis, whereby model parameters are assigned probability distributions to express the uncertainty surrounding them. Using a Monte-Carlo simulation, we sampled these distributions 10,000 times to generate means for the strategies' costs, outcomes and ICERs. In addition, one-way sensitivity analyses were conducted to explore the individual effects and threshold values for key parameters. Program characteristics, including costs, number of patients screened, and the proportion of bacteriologically confirmed cases detected, were also explored to determine the configurations of these parameters for which the program is likely to be cost-effective. The model was designed by using TreeAge Pro 2013 (TreeAge Pro Inc., Williamston, MA).
Results
Program costs and direct outcomes.
Of 35,005 persons who were screened, 3,649 were tested with the GeneXpert MTB/RIF assay, of whom 810 (2.3%) were bacteriologically confirmed TB cases at the health facilities where ACF was conducted. Another 1,179 case-patients (3.4%) without bacteriologic confirmation were treated on the basis of chest radiographic findings and clinical symptoms, for which the costs and benefits were not included in the analysis.
The total economic cost of the program was estimated to be $363,257 and are divided by category in Table 2. Of the total costs of the screening program, 72% were variable costs, determined by the number of patients screened, and the remaining 28% were fixed capital and salary costs. The cost of treating the 810 bacteriologically confirmed cases with community DOTS would be approximately $200,000 without discounting for defaulters.7
Table 2.
Cost elements of the tuberculosis active case finding program in Cambodia
| Category | Economic cost | % Of total cost |
|---|---|---|
| Fixed costs | ||
| Training | $9,500 | 3 |
| Salaries for core staff | $24,500 | 7 |
| Capital equipment | $64,302 | 18 |
| Variable costs | ||
| Consumables | $93,029 | 26 |
| Per diems | $138,792 | 38 |
| Transport | $18,000 | 5 |
| Enablers | $15,133 | 4 |
| Total economic cost | $363,257 | 100 |
Modeled costs, health outcomes, and cost-effectiveness.
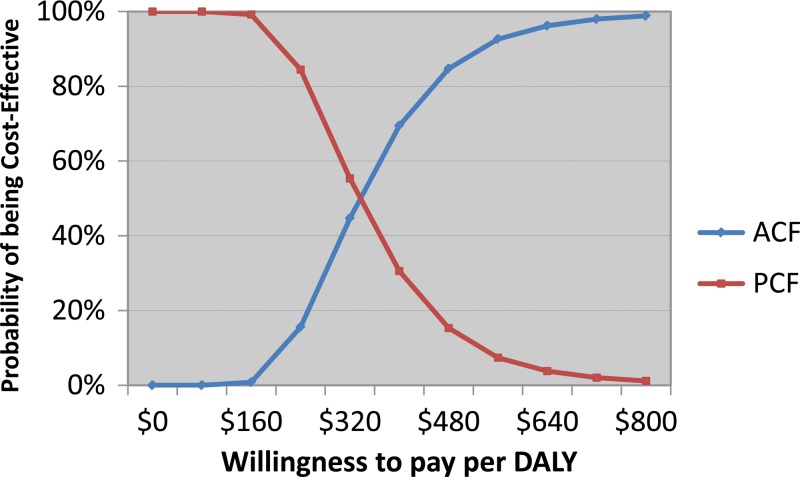
The total modeled costs were $535,000 for the ACF arm and $54,000 for the PCF arm, of which approximately 97% were the costs for DOTS and the remainder were costs for diagnosis and outpatient overheads. The modeled mortality rates for the cohort of bacteriologically confirmed cases were 2% in the ACF arm and 14% in the PCF arm. When converted to DALYs, ACF averted 1,480 DALYs, or 1.8 DALYs per patient. The cost-effectiveness of the program as compared with a scenario of PCF alone was estimated to be $330 per DALY averted, or $5,300 per death averted. When we accounted for all parameter uncertainties entered in the model, the cost-effectiveness acceptability curve (Figure 2) suggested that there is a > 90% probability of the ACF activities being cost-effective if policy makers are willing to pay more than $600 per DALY averted (Figure 3).
Figure 2.
Cost-effectiveness acceptability curve for active case finding (ACF) compared with passive case finding (PCF) in Cambodia The horizontal axis reflects how much policy makers are willing to pay per daily adjusted life year (DALY) averted. At the point estimate for the incremental cost-effectiveness ratio, the probability of ACF being cost-effective is 50%, and at a willingness to pay more than $600, the probability exceeds 90%.
Figure 3.
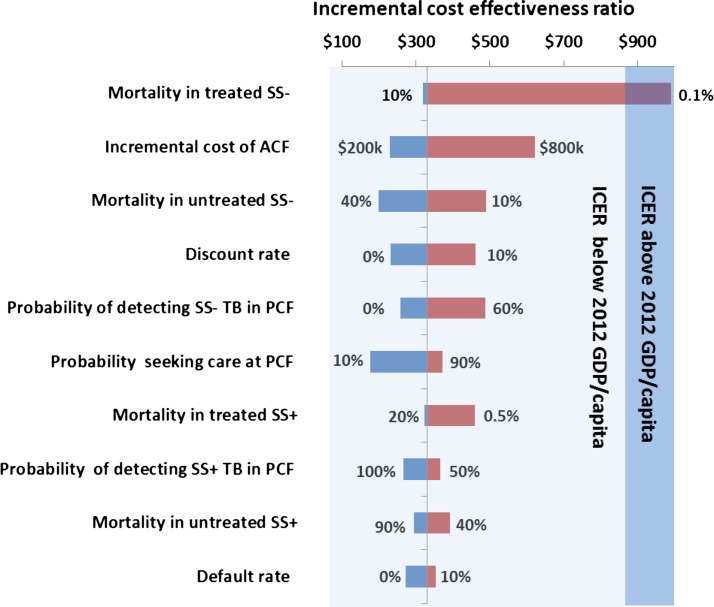
Impact of key parameters on the cost-effectiveness of the program for active tuberculosis case finding in Cambodia. SS = sputum smear; ACF = active case finding; TB = tuberculosis; PCF = passive case finding; ICER = incremental cost-effectiveness ratio; GDP = gross domestic product.
Testing individual parameters in isolation showed that only one of them increased the ICER above the GDP/capita threshold when varied from their baseline estimates within plausible ranges, as shown in Figure 4. The ICER increased above the GDP threshold only when the mortality in treated smear-negative TB cases was decreased below 0.1%.
Figure 4.
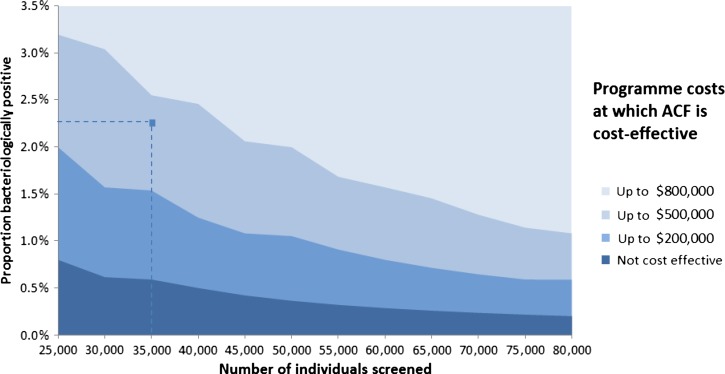
Configurations of program costs (starting with a minimum cost of $100,000), number of persons attending active case finding (ACF) sessions for screening, and proportion of persons screened who are identified as bacteriologically confirmed cases, where ACF is likely to be cost-effective, in Cambodia. At a cost of $360,000 (excluding treatment costs) and having screened 35,000 persons with a 2.3% bacteriologically confirmed TB cases, the ACF program is cost-effective given the model assumptions and parameter estimates.
Parameters describing the program characteristics, including the program costs, the number of patients screened, and the proportion of bacteriologically confirmed cases detected (a product of true prevalence and diagnostic sensitivity), were varied. This showed ACF programs being cost-effective even where the proportion of bacteriologically confirmed cases detected was as low as 0.5%, providing the overall cost can be reduced via, for instance, more efficient diagnosis and treatment algorithms.
Discussion
This analysis suggests that the ACF program in Cambodia is highly cost-effective and has an ICER that is well below the GDP per capita. The incremental benefit of ACF was driven by the modeled excess mortality in the absence of the ACF program. The model estimate of 14% mortality in this population approximates the national reported average1 and is probably an underestimate for this cohort because these are all bacteriologically confirmed cases and the context is a population with highest household poverty rates and the poorest access to care. The reduction to 2% mortality in the ACF arm is strongly supported by the high treatment completion rates found in the ACF and DOTS programs in Cambodia and the relatively low prevalence of HIV and MDR-TB.
These estimates are conservative because of several methods choices. First, health benefits associated with lower transmission are considered a positive externality. It has been estimated that each additional treated case in ACF will avoid 0.1–0.5 future transmissible cases, depending on whether the treated case-patient would have attended PCF.15 Assuming an intermediate value of 0.3 would nearly double the number of DALYs averted, or equivalently lower the ICER by almost half. Second, it was assumed that smear-negative cases do not convert to smear-positive cases and maintain lower mortality rates when untreated. In reality, it is likely that some of these case-patients face higher mortality, with or without converting to smear-positive status,16 and not all of these case-patients are likely to access care (the estimate of 20% may already partly account for such conversions). Third, one of the primary advantages of the GeneXpert MTB/RIF assay is the ability to identify the presence of MDR-TB. Although the prevalence of MDR-TB was low (1.4% rifampicin resistant), poor outcome in these patients could have increased the difference in DALYs between the two model arms by up to 10% if patients were to be treated with effective drugs. However, the costs for these treatments are high, which could imply much higher average treatment costs for all patients and thus a higher ICER. Fourth, sensitivity of the GeneXpert MTB/RIF assay has been shown to be imperfect,17 and the model did not account for the benefits of treatment of bacteriologically unconfirmed cases, many of whom could have been true TB cases.
Whether ACF activities are cost-effective is context specific, but this analysis demonstrates that ACF programs can be cost-effective across a wide range of program costs, numbers of patients screened, and proportions identified as TB cases. Within plausible ranges for these parameters, proportions identified as TB cases is the most influential. It is a product of the underlying prevalence of TB and the programs' sensitivity in detecting these cases. Although the prevalence of bacteriologically positive cases was 2.3%, this prevalence masked a large difference between all household contacts, among whom the prevalence was 0.5%, and symptomatic neighborhood contacts, among whom the prevalence was 3%. Even the lower prevalence of 0.5% in neighborhood contacts was relatively high compared with that of the general population in many settings. As shown in Figure 4, this prevalence could have a profound effect on cost-effectiveness of programs and requires careful consideration because screening household contacts irrespective of symptoms might not be cost-effective. Further explorations of optimal algorithms that are adaptable to different epidemiologic and socioeconomic contexts are required to ensure that programs run efficiently; a web-based tool is now available for policy makers to identify the most efficient algorithm in specific settings.3,18,19
Few high-quality studies have been conducted to evaluate the cost-effectiveness of ACF programs.20 Kranzer and others evaluated an ACF program in South Africa and found that the cost per case detected was higher than that estimated in our study.21 This finding is primarily caused by the relatively higher costs per person screened, which was approximately five times that of the program in Cambodia. Most other economic evaluations of ACF have modeled the costs and outcomes without the benefit of actual program data. A model-based evaluation of ACF in Kampala, Uganda, found that ACF was cost-effective and had a cost per quality adjusted life year of approximately $50–$500, depending on the targeted age groups.22 Baltussen and others found that the cost per DALY averted of treating smear-positive TB cases in Southeast Asia was approximately $7 and the incremental benefits of treating smear-negative patients was $51, both of which are considered cost-effective.23
There are several limitations to this analysis. First, it can be argued that framing the analysis in terms of direct health benefits is not relevant in situations in which funding is vertically directed at reducing transmission and ultimately targeting long-term elimination. In such contexts, the cost per case detected and per DALY averted will increase exponentially with decreasing prevalence, and use of this framework would soon suggest that the programs are not cost-effective, whereas in fact the societal benefits of TB elimination could far exceed the benefits of treating a small proportion of remaining individual cases. As in the rest of Cambodia and consistent with global trends, the incidence of reported cases decreased in the evaluation and control ODs before and during the intervention. Therefore, using direct health benefits as measures of effectiveness is a conservative framework and one that enables greater comparability with other health interventions. It also circumvents the extensive uncertainties surrounding the quality of diagnosis and reporting of TB cases that could weaken the analysis. In 2011, in the year preceding the ACF program, the incidence of reported cases in the intervention ODs diverged from the control ones (Figure 5). Assuming that the higher number of cases detected across the evaluation ODs was caused by ACF activities might have overestimated the impact of the program.
Figure 5.
Reported cases per 100,000 in the evaluation and control populations during 2009–2012 for active tuberculosis case finding in Cambodia. Q = quarter.
Second, costs and benefits of treating bacteriologically unconfirmed but clinically diagnosed TB cases (using chest radiographic findings of active TB lesions) were not included in the analysis. False-positive diagnoses for some patients can impose high medical and household costs associated with DOTS, as well as possible subjection of persons without TB to stigmatization and discrimination.3 Conversely, the sensitivity of the GeneXpert MTB/RIF assay is imperfect and this assay is not yet used for children in whom sputum sample collection is often not feasible. Costs and benefits of treating these unconfirmed TB cases were omitted.
Third, this analysis did not consider the affordability of the program. National TB programs are under-resourced in all high-burden countries1 and if funding for the ACF programs is competing with other TB control interventions, their relative costs and effects need to be compared head-to-head to determine optimal allocation of resources.24 Demonstrating the cost-effectiveness of programs in terms of direct health benefits could strengthen the justification for their funding when competing with other non-TB related interventions across the health sector.
Active case finding programs in Cambodia and similar epidemiologic and socioeconomic settings can be cost-effective even when considering direct health benefits to the patients alone, and irrespective of the impact on further transmission. Appropriate targeting of the program is critical and asymptomatic household contacts might not be a prudent choice as compared with symptomatic household and neighborhood contacts.
ACKNOWLEDGMENTS
We thank the staff members of the National TB Program, Cambodia, for conducting the ACF program and Susie Dunachie and Chris Pollard (Mahidol Oxford Tropical Medicine Research Unit) for assistance in structuring the analysis.
Footnotes
Note: The World Health Organization holds the copyright to this article and has granted the publisher permission for the reproduction of this article.
Financial support: This study was supported by funds from the TB REACH project of Stop TB Partnership, which has multi-year grant support from the Canadian International Development Agency.
Authors' addresses: Rajendra P. Yadav, World Health Organization, Representative Office in Cambodia, Phnom Penh, Cambodia, E-mail: yadavr@wpro.who.int. Nobuyuki Nishikiori, World Health Organization, Regional Office for the Western Pacific, Manila, The Philippines, E-mail: nobu.nishikiori@gmail.com. Peou Satha and Mao T. Eang, Ministry Of Health, Cambodia, National Centre for Tuberculosis and Leprosy Control, Phnom Penh, Cambodia, E-mails: peousatha@yahoo.com and mao@online.com.kh. Yoel Lubell, Mahidol–Oxford Tropical Medicine Research Unit, Faculty of Tropical Medicine, Mahidol University, Bangkok, Thailand, E-mail: yoel@tropmedres.ac.
References
- 1.World Health Organization . Global Tubercuolsis Report 2012. Geneva: World Health Organization; 2012. [Google Scholar]
- 2.Eang MT, Satha P, Yadav RP, Morishita F, Nishikiori N, van-Maaren P, Weezenbeek CL. Early detection of tuberculosis through community-based active case finding in Cambodia. BMC Public Health. 2012;12:469. doi: 10.1186/1471-2458-12-469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lonnroth K, Corbett E, Golub J, Godfrey-Faussett P, Uplekar M, Weil D, Raviglione M. Systematic screening for active tuberculosis: rationale, definitions and key considerations. Int J Tuberc Lung Dis. 2013;17:289–298. doi: 10.5588/ijtld.12.0797. [DOI] [PubMed] [Google Scholar]
- 4.National Institute of Statistics . General Population Census of Cambodia 2008. Phnom Penh, Cambodia: National Institute of Statistics; 2008. [Google Scholar]
- 5.National Center for Tuberculosis and Leprosy Control . Annual Report for 2009. Phnom Penh, Cambodia: CENAT; 2010. [Google Scholar]
- 6.World Health Organization . WHO Guide to Cost-effectiveness Analysis. Geneva: World Health Organization; 2003. [Google Scholar]
- 7.Pichenda K, Nakamura K, Morita A, Kizuki M, Seino K, Takano T. Non-hospital DOT and early diagnosis of tuberculosis reduce costs while achieving treatment success. Int J Tuberc Lung Dis. 2012;16:828–834. doi: 10.5588/ijtld.11.0688. [DOI] [PubMed] [Google Scholar]
- 8.Frieden T. Toman's Tuberculosis Case Detection, Treatment and Monitoring: Questions and Answers. Geneva: World Health Organization; 2004. [Google Scholar]
- 9.Tiemersma EW, van der Werf MJ, Borgdorff MW, Williams BG, Nagelkerke NJ. Natural history of tuberculosis: duration and fatality of untreated pulmonary tuberculosis in HIV negative patients: a systematic review. PLoS ONE. 2011;6:e17601. doi: 10.1371/journal.pone.0017601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.National Tuberculosis Control Program . Second National Tuberculosis Prevalence Survey Cambodia, 2011. Phnom Penh, Cambodia: CENAT; 2012. [Google Scholar]
- 11.Boehme CC, Nicol MP, Nabeta P, Michael JS, Gotuzzo E, Tahirli R, Gler MT, Blakemore R, Worodria W, Gray C, Huang L, Caceres T, Mehdiyev R, Raymond L, Whitelaw A, Sagadevan K, Alexander H, Albert H, Cobelens F, Cox H, Alland D, Perkins MD. Feasibility, diagnostic accuracy, and effectiveness of decentralised use of the Xpert MTB/RIF test for diagnosis of tuberculosis and multidrug resistance: a multicentre implementation study. Lancet. 2011;377:1495–1505. doi: 10.1016/S0140-6736(11)60438-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Steingart K, Sohn H, Schiller I, Kloda LA, Boehme CC, Pai M, Dendukuri N. Xpert® MTB/RIF assay for pulmonary tuberculosis and rifampicin resistance in adults. Cochrane Database Syst Rev. 2012:CD009593. doi: 10.1002/14651858.CD009593.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Straetemans M, Glaziou P, Bierrenbach AL, Sismanidis C, van der Werf MJ. Assessing tuberculosis case fatality ratio: a meta-analysis. PLoS ONE. 2011;6:e20755. doi: 10.1371/journal.pone.0020755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vassall A, van Kampen S, Sohn H, Michael JS, John KR, den Boon S, Davis JL, Whitelaw A, Nicol MP, Gler MT, Khaliqov A, Zamudio C, Perkins MD, Boehme CC, Cobelens F. Rapid diagnosis of tuberculosis with the Xpert MTB/RIF assay in high burden countries: a cost-effectiveness analysis. PLoS Med. 2011;8:e1001120. doi: 10.1371/journal.pmed.1001120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Borgdorff MW, Floyd K, Broekmans JF. Interventions to reduce tuberculosis mortality and transmission in low- and middle-income countries. Bull World Health Organ. 2002;80:217–227. [PMC free article] [PubMed] [Google Scholar]
- 16.Mello FC, Bastos LG, Soares SL, Rezende VM, Conde MB, Chaisson RE, Kritski AL, Ruffino-Netto A, Werneck GL. Predicting smear negative pulmonary tuberculosis with classification trees and logistic regression: a cross-sectional study. BMC Public Health. 2006;6:43. doi: 10.1186/1471-2458-6-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vadwai V, Boehme C, Nabeta P, Shetty A, Alland D, Rodrigues C. Xpert MTB/RIF: a new pillar in diagnosis of extrapulmonary tuberculosis? J Clin Microbiol. 2011;49:2540–2545. doi: 10.1128/JCM.02319-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van't Hoog AH, Meme HK, Laserson KF, Agaya JA, Muchiri BG, Githui WA, Odeny LO, Marston BJ, Borgdorff MW. Screening strategies for tuberculosis prevalence surveys: the value of chest radiography and symptoms. PLoS ONE. 2012;7:e38691. doi: 10.1371/journal.pone.0038691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nishikiori N, Van Weezenbeek C. Target prioritization and strategy selection for active case-finding of pulmonary tuberculosis: a tool to support country-level project planning. BMC Public Health. 2013;13:97. doi: 10.1186/1471-2458-13-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fox GJ, Dobler CC, Marks GB. Active case finding in contacts of people with tuberculosis. Cochrane Database Syst Rev. 2011:CD008477. doi: 10.1002/14651858.CD008477.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kranzer K, Lawn SD, Meyer-Rath G, Vassall A, Raditlhalo E, Govindasamy D, van Schaik N, Wood R, Bekker LG. Feasibility, yield, and cost of active tuberculosis case finding linked to a mobile HIV service in Cape Town, South Africa: a cross-sectional study. PLoS Med. 2012;9:e1001281. doi: 10.1371/journal.pmed.1001281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mupere E, Schiltz NK, Mulogo E, Katamba A, Nabbuye-Sekandi J, Singer ME. Effectiveness of active case-finding strategies in tuberculosis control in Kampala, Uganda. Int J Tuberc Lung Dis. 2013;17:207–213. doi: 10.5588/ijtld.12.0160. [DOI] [PubMed] [Google Scholar]
- 23.Baltussen R, Floyd K, Dye C. Cost effectiveness analysis of strategies for tuberculosis control in developing countries. BMJ. 2005;331:1364. doi: 10.1136/bmj.38645.660093.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dowdy DW. Economic analyses of diagnostics for tuberculosis: what's the point? Expert Rev Pharmacoecon Outcomes Res. 2012;12:137–139. doi: 10.1586/erp.12.5. [DOI] [PubMed] [Google Scholar]
- 25.Dowdy DW, O'Brien MA, Bishai D. Cost-effectiveness of novel diagnostic tools for the diagnosis of tuberculosis. Int J Tuberc Lung Dis. 2008;12:1021–1029. [PubMed] [Google Scholar]
- 26.Salomon JA, Vos T, Hogan DR, Gagnon M, Naghavi M, Mokdad A, Begum N, Shah R, Karyana M, Kosen S, Farje MR, Moncada G, Dutta A, Sazawal S, Dyer A, Seiler J, Aboyans V, Baker L, Baxter A, Benjamin EJ, Bhalla K, Bin Abdulhak A, Blyth A, Bourne R, Braithwaite T, Brooks P, Brugha TS, Bryan-Hancock C, Buchbinder R, Burney P, Calabria B, Chen H, Chugh SS, Cooley R, Criqui MH, Cross M, Dabhadkar KC, Dahodwala N, Davis A, Degenhardt L, Díaz-Torné C, Dorsey ER, Driscoll T, Edmond K, Elbaz A, Ezzati M, Feigin V, Ferri CP, Flaxman AD, Flood L, Fransen M, Fuse K, Gabbe BJ, Gillum RF, Haagsma J, Harrison JE, Havmoeller R, Hay RJ, Hel-Baqui A, Hoek HW, Hoffman H, Hogeland E, Hoy D, Jarvis D, Karthikeyan G, Knowlton LM, Lathlean T, Leasher JL, Lim SS, Lipschultz SE, Lopez AD, Lozano R, Lyons R, Malekzadeh R, Marcenes W, March L, Margolis DL, McGill N, McGrath J, Mensah GA, Meyer AC, Michaud C, Moran A, Mori R, Murdoch ME, Naldi L, Newton CR, Norman R, Omer SB, Osborne R, Pearce N, Perez-Ruiz F, Perico N, Pesudovs K, Phillips D, Pourmalek F, Rehm JT, Prince M, Remuzzi G, Richardson K, Room R, Saha S, Sampson U, Sanchez-Riera L, Segui-Gomez M, Shahraz S, Shibuya K, Singh D, Sliwa K, Smith E, Soeriomataram I, Steiner T, Stolk WA, Stovner LJ, Sudfeld C, Taylor HR, Tleyjeh IM, van der Werf MJ, Watson WL, Weatherall DJ, Weintraub R, Weisskopf MG, Whiteford H, Wilkinson JD, Woolf AD, Zheng ZJ, Murray CJ, Jonas JB. Common values in assessing health outcomes from disease and injury: disability weights measurement study for the Global Burden of Disease Study 2010. Lancet. 2012;380:2129–2143. doi: 10.1016/S0140-6736(12)61680-8. [DOI] [PMC free article] [PubMed] [Google Scholar]