Abstract
Protective immunity to cholera is serogroup specific, and serogrouping is defined by the O-specific polysaccharide (OSP) of lipopolysaccharide (LPS). We characterized OSP-specific immune responses in adult recipients of an oral killed cholera vaccine (OCV WC-rBS) and compared these with responses in patients with cholera caused by Vibrio cholerae O1 Ogawa. Although vaccinees developed plasma immunoglobulin G (IgG), IgM, IgA antibody and antibody secreting cell (ASC, marker of mucosal response) to Ogawa OSP and LPS 7 days after vaccination, responses were significantly lower than that which occurred after cholera. Similarly, patients recovering from cholera had detectable IgA, IgM, and IgG memory B cell (MBC) responses against OSP and LPS on Day 30 and Day 90, whereas vaccinees only developed IgG responses to OSP 30 days after the second immunization. The markedly lower ASC and MBC responses to OSP and LPS observed among vaccinees might explain, in part, the lower protection of an OCV compared with natural infection.
Introduction
Cholera is a dehydrating diarrheal illness of humans caused by toxigenic strains of Vibrio cholerae O1. Vibrio cholerae can be divided into over 200 serogroups, but only serogroups O1 and O139 organisms have been known to cause epidemic cholera. Globally, almost all cholera is currently caused by V. cholerae O1 organisms.1 Cholera affects an estimated 3–5 million people annually, resulting in over 100,000 deaths globally.2,3 Based on genotypic and phenotypic differences, the O1 serogroup can be divided into classical and El Tor biotypes and into Ogawa and Inaba serotypes.4 Inaba differs from the Ogawa serotype only by the absence of a 2-O-methyl group in the non-reducing terminal sugar of the O-specific polysaccharide (OSP) component of the lipopolysaccharide (LPS).5–7 The prevalent serotype often fluctuates during cholera outbreaks, switching between Ogawa and Inaba.8
Two types of oral cholera vaccines, consisting of whole cell, killed organisms, are currently World Health Organization (WHO)-prequalified and commercially available internationally: WC-rBS-Dukoral (Crucell, Sweden), which includes both Inaba and Ogawa serotypes of V. cholerae O1 of both El Tor and classical biotypes, admixed with recombinant cholera toxin B subunit (rCtxB); and Shanchol (Shantha Biotechnics-Sanofi, India), which includes four V. cholerae O1 strains and one O139 strain, but without any rCtxB supplement.9–13 In large-scale, randomized controlled field trials, these vaccines (or their prototypes) were found to be safe and immunogenic and conferred ∼60–80% efficacy in preventing cholera in adults and older children.11,14,15 However, the efficacy of WC-rBS is lower and of shorter duration in young children.3 In contrast, clinical cholera caused by wild-type infection generally leads to more robust and durable protection that may last for 3–7 years in both young and older individuals.14,16,17 In 2010, the WHO recommended that choleravaccine should play a larger role in limiting cholera disease burden.3
A number of immune responses have been characterized during cholera. Vibrio cholerae is a non-invasive mucosal pathogen and assessment of mucosal immunity has often included assessment of gut-activated ASC that transiently migrate in the systemic circulation before re-homing to mucosal tissue.35,40 Memory B and T cells responses have also been assessed and correlated with longevity of responses to infection and vaccination.28,31,32,37,42
Of importance, protection against cholera is serogroup specific. Infection with V. cholerae O1 provides no cross-protection from cholera caused by V. cholerae O139, and vice versa.18–20 Serogroup specificity is largely determined by the OSP portion of LPS, with OSP being connected to lipid A in LPS through a core oligosaccharide.21 Lipid A and core are similar across V. cholerae serogroups.22–25 Despite this, OSP responses during wild-type disease or after vaccinaton have only recently begun to be characterized.26,27 Here, we extend this analysis to adult recipients of an oral killed cholera vaccine, WC-rBS (Dukoral), in Dhaka, Bangladesh, and compare responses after vaccination with those induced in adult patients with cholera caused by V. cholerae O1 Ogawa, the serotype circulating in Dhaka during the study period.
Materials and Methods
Study population.
Participants, both vaccinees and patients, were adults 18–45 years of age. Table 1 describes the age and number of study participants. Thirty-two cholera patients between December 2006 and December 2012 were randomly selected from the International Center for Diarrheal Disease Research, Bangladesh (icddr,b) hospital with severe acute watery diarrhea and with stool cultures positive for V. cholerae O1. These individuals were enrolled and followed as were the vaccinees for 1 year. Before patient enrollment, stool samples were plated on taurocholate-tellurite-gelatin agar and gelatin agar (Difco, Detroit, MI) overnight at 37°C. Suspected colonies were identified by slide agglutination by using monoclonal antibodies against V. cholerae O1.20 We also analyzed the stool for other enteric pathogens, i.e., enterotoxigenic Escherichia coli,29 Salmonella, Shigella, and Campylobacter spp.,30 and tested stool by direct microscopy for cyst and vegetative forms of parasites and ova of helminths. Only patients positive for V. cholerae O1 and negative for other previous infection were enrolled in this study.
Table 1.
Age and numbers of cholera patients and vaccinees enrolled in this study
| Specimen tested | Vibrio cholerae O1 serotype | No. of patients or vaccinees | Median age in yr (25th, 75th percentile) | |
|---|---|---|---|---|
| Vaccinees | ASC* | 15 | 27 (23, 35) | |
| Plasma† | 33 | 32 (26, 39) | ||
| MBC-CS† | 24 | 33 (28, 40) | ||
| Patients | ASC‡ | Ogawa | 12 | 31 (20, 40) |
| Plasma§ | Ogawa | 17 | 29 (26, 33) | |
| MBC-CS§ | Ogawa | 14 | 36 (29, 44) |
Specimens were analyzed on Day 0 before vaccination and on follow-up Days 7, 21 (7 days after 2nd dose of vaccine), and 42.
Analyzed on Days 0, 3, 17 (3 days after 2nd dose of vaccine), 42, 90, 180, 270, 360.
Analyzed on Days 2, 7, 30.
Analyzed on Days 2, 7, 30, 90, 180, 270, 360.
ASC = antibody-secreting cell assay; MBC-CS = memory B cell assay using culture supernatant.
We obtained 10 mL of venous blood at Day 0 (before vaccination), 3 days after ingestion of each vaccine dose (Day 3 and Day 17), and again at Days 42, 90, 180, 270, and 360 from the vaccinee cohort. From patients, we obtained blood samples on the second day after hospitalization and then at Days 7, 30, 90, 180, 270, and 360. All the cholera patients were treated with intravenous fluid resuscitation and with doxycycline, ciprofloxacin, or azithromycin.26,31,32 Stored plasma samples were analyzed for anti-OSP, LPS, and vibriocidal antibody responses.
In addition, 15 vaccinees who fulfilled similar criteria were enrolled from September 2011 to June 2012 to assess ASC responses to Ogawa-OSP, LPS, and CtxB in fresh cells recovered from venous blood on Days 0, 7 (7 days after the first dose of vaccine), 21 (7 days after the second dose of vaccine), and 42, with responses compared with samples obtained from 15 patients at Days 2, 7, and 30 with V. cholerae O1 Ogawa infection. The Institutional Review Boards of the icddr,b and the Massachusetts General Hospital approved this study. All study participants gave informed written consent for study enrollment.
Antigen preparation.
We used LPS, OSP, and rCtxB as antigens to measure immunological responses. Vibrio cholerae LPS was prepared as previously described.21 Briefly, LPS from V. cholerae O1, Ogawa (strain X-25049) was obtained by the standard hot phenol-water extraction procedure followed by protein denaturation and enzymatic treatment (proteinase K, DNase I, and RNase A), followed by ultracentrifugation (100,000 × g for 3 hours).21,26 The OSP was recovered by acid hydrolysis of LPS as previously described, generating OSP attached to core oligosaccharide (OSPc).21,26,27 The OSPc:BSA (bovine serum albumin) conjugates were also generated as previously described.21,26,27 To facilitate binding of OSP to immunological plates, and to permit display of OSP in a sun-burst pattern consistent with single point attachment similar to that occurring on wild-type V. cholerae, we assessed immune responses targeting OSP using OSPc:BSA (henceforth referred to as OSP in the below described immunologic assays). To assess responses targeting CtxB, we used recombinant antigen supplied by Professor A. M. Svennerholm, Gothenburg University, Sweden.
Enzyme-linked immunosorbent assays (ELISAs) for OSP and LPS-specific Immunoglobulin A (IgA), IgG, and IgM antibodies in plasma.
We quantified anti-OSP and anti-LPS IgA, IgG, and IgM responses in plasma using a standard ELISA as previously described.20,21,26,27 Briefly, we coated 96-well polystyrene plates (Nunc F, USA) with V. cholerae O1 Ogawa OSPc:BSA (1 μg/mL) dissolved in carbonate buffer (pH 9.6), and Ogawa LPS (2.5 μg/mL) dissolved in phosphate buffered saline (PBS) (pH 7.2–7.4).20,28,33 To each well, we added 100 μL of plasma (diluted 1:50 in 0.1% BSA in PBS-0.05% Tween), and after incubation and washing, detected antigen-specific antibodies in the sample using horseradish peroxidase-conjugated rabbit anti-human IgA, IgG, and IgM (Jackson Immunoresearch, West Grove, PA, 1:1,000 dilution) as secondary antibodies. After incubation at 37°C for 90 minutes, the plates were washed and developed with ortho-phenylene diamine (Sigma, St. Louis, MO) in 0.1 M sodium citrate buffer (pH 4.5) and 0.012% hydrogen peroxide by reading the plates kinetically for 5 min at 14-second intervals.26,28,31,33 The maximum slope for an optical density change of 0.2 U was reported as milli-optical density units per minute (mOD/min). We normalized data to ELISA units by calculating the ratio of the mOD/min of the test sample to that of a standard of pooled convalescent phase plasma that was included on each plate. The pooled plasma was prepared from samples obtained from patients with V. cholerae O1 Ogawa (N = 5) or V. cholerae O1 Inaba (N = 5) infection.
Vibriocidal antibody assay in plasma.
Vibriocidal antibody responses in plasma samples were measured using a standard laboratory protocol described previously, with guinea pig complement and the homologous serotype of V. cholerae O1 Ogawa (X-25049) as the target organism for measurement in patients.26,28,31,34 The same strain was used to measure the vibriocidal responses in vaccinees. The vibriocidal titer was defined as the reciprocal of the highest plasma dilution resulting in more than 50% reduction of the optical density associated with V. cholerae O1 growth compared with that of positive control wells without plasma.32
Quantification of circulating IgA, IgG, and IgM ASC to OSP, LPS, and CtxB.
Ogawa-OSP, -LPS, and CtxB-specific ASC responses were measured by enzyme-linked immunosorbent spot (ELISPOT) following a standard protocol.26,31,32,35,37 Briefly, nitrocellulose-bottomed plates (Millipore, Bedford, MA) were coated with Ogawa OSPc:BSA (10 μg/mL), Ogawa-LPS (25 μg/mL), affinity-purified goat anti-human Ig (5 μg/mL; Jackson Immunology Research, West Grove, PA) in PBS (pH 7.3–7.4), monosialotetrahexosylganglioside (GM1 ganglioside, 3 nM/mL), or keyhole limpet hemocyanin (KLH, Pierce Biotechnology, Rockford, IL, 2.5 μg/mL), and were incubated overnight at 4°C.20 Before blocking, 100 μL of rCtxB (2.5 μg/mL)/well was added to the GM1-coated plates, and the plates were incubated for 1 hour at 37°C. All plates were then blocked for 2 hours at 37°C with RPMI 1640 that included 10% fetal bovine serum before use. Peripheral blood mononuclear cells (PBMCs) were harvested as previously described by centrifugation of whole blood samples diluted two times in PBS (pH 7.2–7.4) on Ficoll-Isopaque (Pharmacia, Piscataway, NJ).32,35 A total of 5 × 105 PBMCs/well were added to the OSPc:BSA, LPS, and CtxB-coated plates, whereas 1 × 105 PBMCs/starting well were added to the total Ig-coated plates and serially diluted. After incubating the plates at 37°C for 3 hours, plates were washed and IgG, IgM, and IgA ASCs were detected using horseradish peroxidase-conjugated mouse anti-human IgM (Hybridoma Reagent Laboratory, Baltimore, MD), and horseradish peroxidase-conjugated goat anti-human IgA and alkaline phosphatase-conjugated IgG (Southern Biotech, Birmingham, AL), diluted 1:500. After overnight incubation at 4°C, wells with the IgG conjugate were developed with 5-bromo-4-chloro-3-indolyl-phosphate–nitroblue tetrazolium and wells with the IgA and IgM conjugates were developed with 3-amino-9-ethylcarbazole. The ASC were independently quantified by two individuals using a stereomicroscope (Leica WILD M3Z). The KLH-coated plates were used as a negative control. The number of antigen-specific IgG, IgM, and IgA ASC were expressed per 106 PBMCs.
ELISAs for OSP-, LPS- and CtxB-specific IgA, IgG, and IgM antibodies in supernatants of memory B-cell culture.
We stimulated PBMCs with a polyclonal B cell mitogen mixture optimized to stimulate antigen independent proliferation and differentiation of memory B cells (MBCs) into ASCs as previously described31,32,38,39; supernatants of these MBC cultures were then assayed for anti-OSP, anti-LPS, and anti-CtxB IgA, IgG, and IgM responses, using a standard ELISA protocol.33 In brief, PBMCs were cultured in a medium that consisted of RPMI-1640, 10% fetal bovine serum, 200 U/mL penicillin, 200 μg/mL streptomycin, 2 mM L-glutamine, 50 μM β-mercaptoethanol, and a mixture of three B-cell mitogens; 6 μg/mL of CpG oligonucleotide (Operon, Huntsville, AL); a 1/100,000 dilution of crude pokeweed mitogen extract; and a 1/10,000 dilution of fixed Staphylococcus aureus Cowan (Sigma). One-half million PBMCs per well were placed in 24-well cell culture plates (BD Biosciences, San Jose, CA) containing 1 mL of this medium. As a negative control, PBMCs were also placed into wells containing this culture medium without mitogens. Plates were incubated at 37°C in a 5% CO2 incubator. After 5 to 6 days, contents of culture wells were collected and centrifuged. All stimulated or all unstimulated culture supernatants from a given patient on a given day of study were pooled together, mixed with a protease inhibitor cocktail,31 and frozen at −70°C for subsequent use in ELISA assays as described previously.
Statistical analyses.
We compared the magnitude of acute to convalescent phase responses using the Wilcoxon Signed Rank test. The Mann-Whitney U test was used to compare between the immune responses to OSP and LPS or between vaccinees and patients. We used Pearson correlation analysis to check the correlation coefficient, and linear regression to draw a line graph. All reported P values were two-tailed, with a cutoff of P ≤ 0.05 considered a threshold for statistical significance. Analysis and figure preparation were performed using Graphpad Prism 5.0 (GraphPad Software, Inc., La Jolla, CA) and SPSS 14 (SPSS Inc., Chicago, IL).
Results
Comparison of IgA, IgG, and IgM antibody responses in plasma to V. cholerae O1 OSP and LPS antigens in vaccinees and patients.
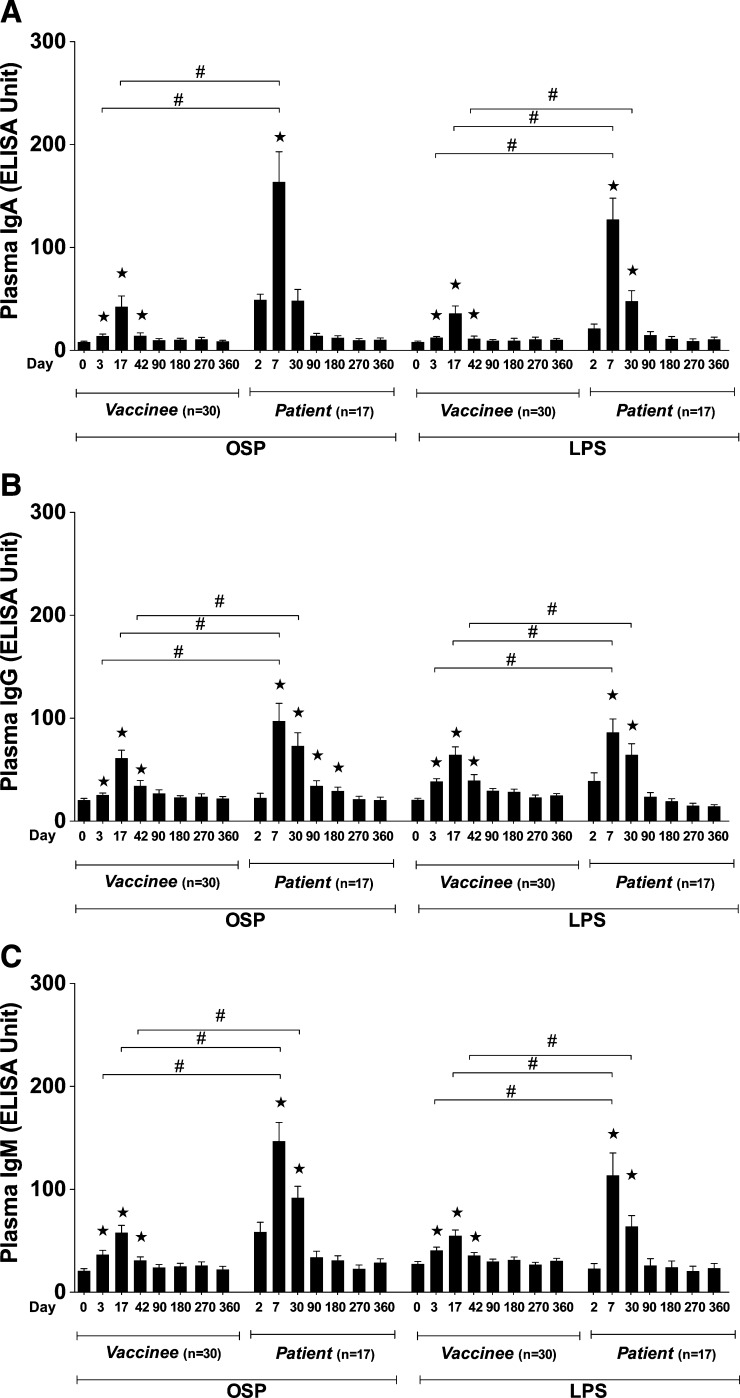
We administered two doses of oral cholera vaccine WC-rBS (Dukoral) separated by 2 weeks to 30 healthy volunteers between October 2008 and June 2010 and followed these individuals for a year.28 We found significant IgA, IgG, and IgM antibody responses to Ogawa OSP and LPS in vaccinees, starting 3 days after the first dose of vaccine (Figure 1). These responses remained significantly elevated compared with baseline until 1 month after the second dose of vaccine (Day 42; Figure 1A–C). When plasma IgA, IgG, and IgM antibody responses to Ogawa OSP and LPS in vaccinees were directly compared with those in adult patients infected with V. cholerae O1 Ogawa, we found that the responses in vaccinees at comparable time points were significantly lower than those following natural infection (Figure 1A–C). The antibody responses in plasma to OSP in vaccinees over 1 year were correlated with responses to LPS on the same days (IgA, 0.61, IgG, R = 0.60, IgM, 0.70, P < 0.01, respectively), as we had found previously in adult patients with cholera.26
Figure 1.
Mean normalized immunoglobulin A (IgA), IgG, and IgM responses in plasma of vaccinees and patients to Ogawa OSPc:BSA (OSP, O-specific polysaccharide) and lipopolysaccharide (LPS). Asterisks indicate a statistically significant difference (P ≤ 0.05, two-tailed) from baseline (Day 0 or Day 2) levels within a particular antigen group and “#” indicates a statistically significant difference (P ≤ 0.05) between similar days in vaccinees and patients.
Comparison of plasma antibody responses to OSP and LPS with vibriocidal antibody responses in vaccinees.
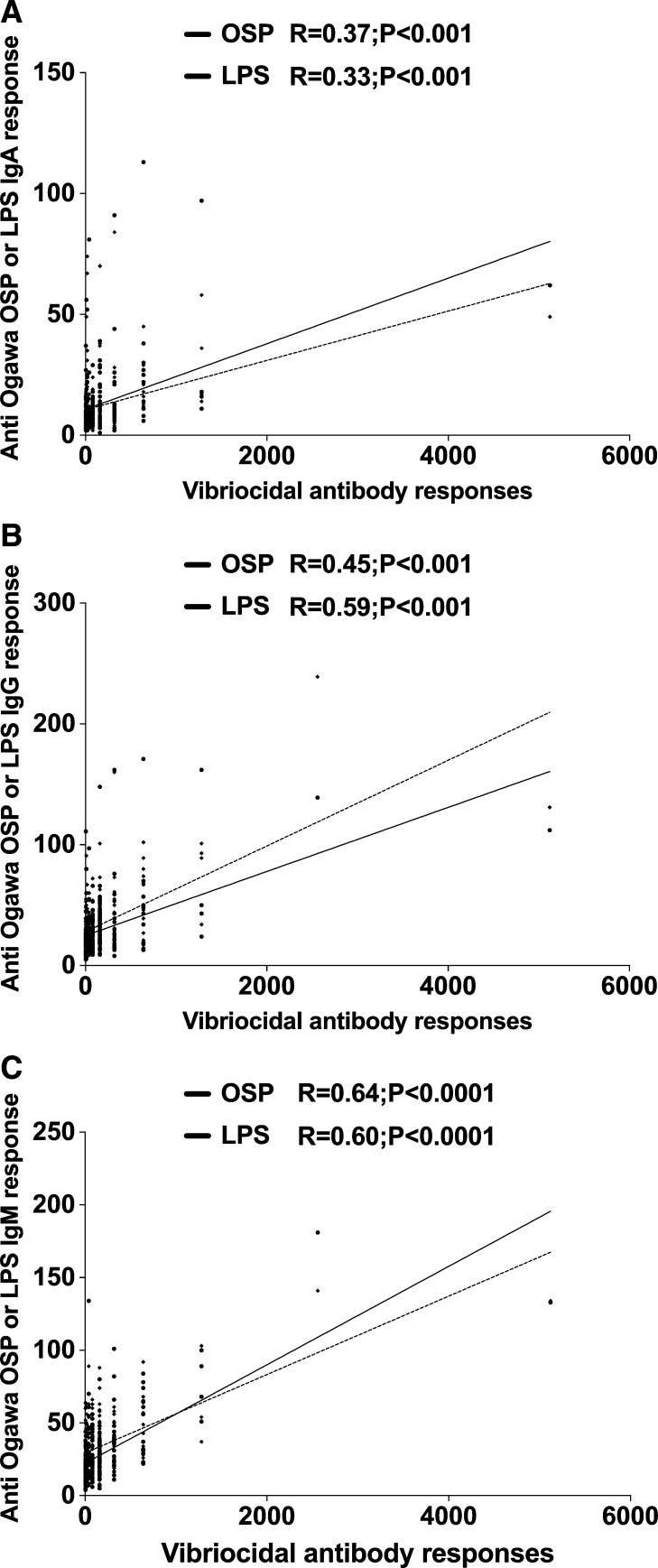
We compared vibriocidal antibody responses in vaccinees with plasma IgM, IgG, and IgA responses to Ogawa OSP and LPS (Figure 2A–C). We found that the IgM responses to Ogawa OSP and LPS in vaccinees best correlated with vibriocidal responses on the same day (R = 0.64, R = 0.60, P < 0.001, respectively) (Figure 2C). Ogawa OSP and LPS responses in the IgG isotype were less strongly correlated with vibriocidal antibody responses (R = 0.50, R = 0.59, P < 0.001, respectively) (Figure 2B). There was a fairly poor, although significant, correlation between anti-OSP and anti-LPS responses in the IgA isotype and vibriocidal responses (R = 0.37, R = 0.33, P < 0.001, respectively; Figure 2A).
Figure 2.
Correlation between vibriocidal antibody responses and plasma immunoglobulin A (IgA), IgG, or IgM antibody responses to OSPc:BSA (OSP) and lipopolysaccharide (LPS) in adult vaccinees. The lines indicate the correlations between the different responses to OSP and LPS, and the vibriocidal antibody response.
Circulating IgA, IgG, and IgM antibody secreting cells after vaccination or infection.
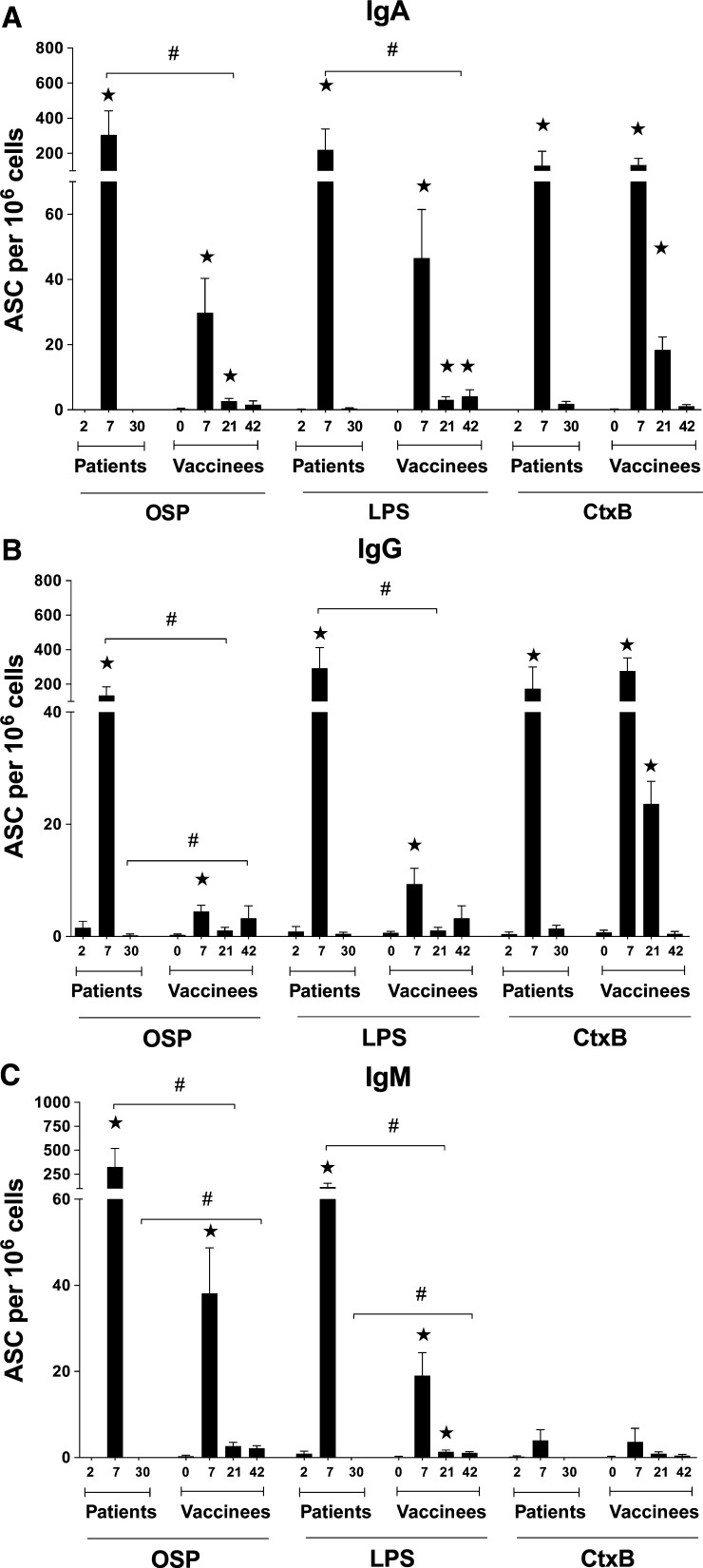
We assessed IgA, IgG, and IgM ASC responses to Ogawa OSP, LPS, and CtxB in peripheral blood of 15 vaccinees at four time points: Day 0 before vaccination (baseline), Day 7 after each vaccine dose (Day 7 and Day 21 after first dose of vaccine), and at Day 42 after the first vaccination day (Figure 3A–C). The ASC assay quantifies the gut associated lymphoid tissue-activated ASCs as they transiently circulate in the blood before returning to mucosal effector sites; this response therefore is considered to reflect a recent mucosal exposure35,40 and may be used as a proxy measure for mucosal immunity.41 We found the ASC responses to both OSP and LPS in vaccinees were comparable, and peaked on Day 7 after the first dose of vaccine, returning to baseline by 1 month after intake of the second dose. Notably, however, the second dose of vaccine did not stimulate a similar ASC response as the first dose of vaccine. In comparing ASC responses after vaccination with those following infection, the responses to OSP and to LPS were significantly higher in the patients than in the vaccinees. Responses to CtxB (which is present in high amounts in the oral vaccine) did not differ significantly between infected patients and vaccinees. Of note, neither patients nor vaccinees generated significant IgM ASC specific for CtxB after infection or vaccination.26,33
Figure 3.
Antibody secreting cell (ASC) responses to Ogawa OSPc:BSA (OSP, O-specific polysaccharide), lipopolysaccharide (LPS), and CtxB in patients and vaccinees. Mean circulating antigen-specific immunoglobulin A (IgA), IgG, and IgM ASC responses to Ogawa OSP, LPS, and CtxB with standard error bars (Figure 3A–C, respectively). Asterisks indicate a statistically significant difference (P ≤ 0.05) from baseline (Day 0 or Day 2) and “#” indicates a statistically significant difference (P ≤ 0.05) between similar days in vaccinees and patients.
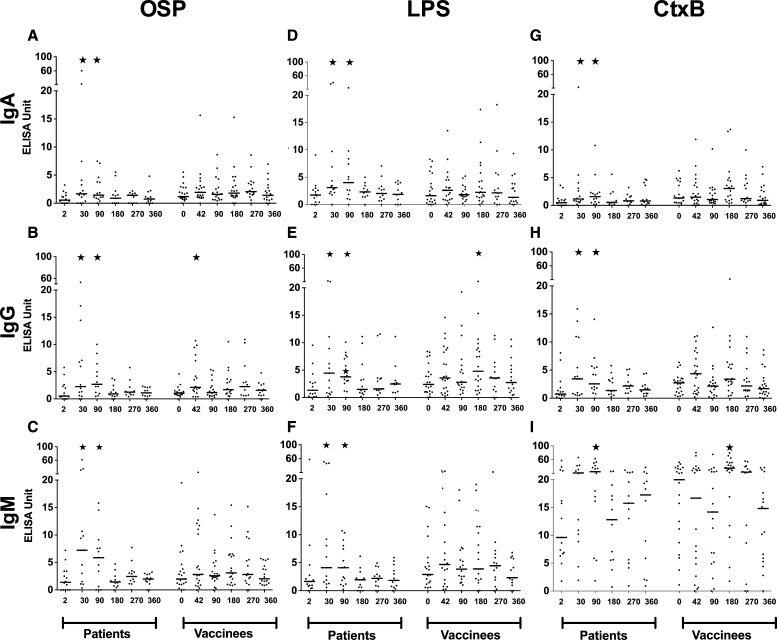
Development of memory B-cell responses as assessed in memory B-cell culture supernatants.
Supernatants from MBC cultures were available from 14 patients and 22 vaccinees at Days 2, 30 (patients) or 42 (vaccinees), as well as on Days 90, 180, 270, and 360. Antigen-specific (OSP, LPS, and rCtxB) ELISAs were performed on these supernatants for antigen-specific IgA, IgG, and IgM responses. Although patients developed significantly elevated OSP-, LPS- and rCtxB-specific memory B-cell responses in the IgA, IgG, and IgM isotypes on Day 30 and Day 90 that waned by Day 180, these responses were largely absent from vaccinees (Figure 4A–I). In vaccinees within the first 90 days after immunization, only an anti-OSP IgG response was detectable at Day 42 (30 days after the second immunization).
Figure 4.
Antigen-specific B-cell memory responses of immunoglobulin A (IgA), IgG, and IgM isotypes in cholera patients and vaccinees, determined from enzyme-linked immunosorbent assay (ELISA) measurements of culture supernatant of memory B cells(MBCs). Asterisks indicate a statistically significant difference (P ≤ 0.05) from baseline (Day 0 or Day 2) levels within a particular antigen group.
Discussion
This study characterizes immune response to the Ogawa O-specific polysaccharide of LPS in adult recipients of an oral killed cholera vaccine, and compares responses to those that occur after natural infection in adult cholera patients in Bangladesh. These results add to our understanding of immunologic events that follow cholera disease or vaccination.28,31,33,42,44
The vibriocidal response, a complement-dependent serum bactericidal antibody response of patients who have previously had cholera, is an indirect marker of protection.45,46 Studies on cholera patients and vaccinees implicate cholera LPS-specific immune responses as playing a role in mediating protective immunity to cholera, and suggest that LPS, or an antigenic component of LPS, i.e., OSP, might be an attractive target for vaccine design.26,32,37,47,48 However, the analysis of immune responses to V. cholerae LPS is complicated by the heterogeneous nature of standard LPS preparations. In a previous study, we used mass spectrometry to analyze a V. cholerae O1 LPS preparation and found that the sample contained over 600 V. cholerae proteins in addition to polysaccharide and lipid components.26 As the OSP of V. cholerae LPS defines serogroup specificity, this polysaccharide may contribute significantly to the observed and specific protection afforded by anti-LPS antibodies.
The vaccinees in our study showed very similar plasma IgG, IgM, and IgA antibody responses to either OSP or LPS from the homologous Ogawa serotype. The plasma antibody responses to OSP and LPS increased significantly at convalescence compared with those at the acute phase, and strongly correlated with each other for all three antibody isotypes over the year of follow-up. These plasma antibody responses after oral cholera vaccination also correlated well with the vibriocidal antibody levels at comparable time points, particularly the IgM antibodies to OSP or LPS. We found a similar trend after natural infection with V. cholerae26; the data suggest that responses against LPS, and more specifically OSP, may mediate the observed vibriocidal antibody responses. In support of this, in a previous study we showed that the vibriocidal response could be largely adsorbed away in a concentration-dependent manner by addition of OSP.26
Immune responses at the gut surface play a critical role in mediating protection from cholera, and previously infected patients may have an anamnestic immune response mediated by mucosal lymphocytes.28,37 Gut homing ASCs in the blood shortly after infection are considered a marker of a subsequent mucosal response.20,37 In this study, we found that circulating levels of ASCs specific for OSP and LPS in the IgG, IgM, and IgA isotypes in vaccinees increased similarly after 7 days, and that these ASC responses then returned to baseline levels by Day 42. Of note, there was no detectable ASC response after administration of the second dose of WC-rBS, suggesting a possible lack of mucosal boosting in this population from a highly cholera-endemic area. The number of ASCs specific to LPS and to CtxB was consistent with previous studies in vaccinees.20,26,32,33,36 However, the OSP- or LPS-specific ASC responses seen following natural infection were markedly higher than those in vaccinees (Figure 3).
The total number of MBC-derived ASCs can be detected directly for a given isotype by ELISPOT at the end of 5–6 days of in vitro culture.28,31,32,49 The supernatants of these MBC cultures can be used to assess both the number of ASCs produced during in vitro culture and the amount of isotype-specific antibodies secreted by these ASCs.33 In previous studies, our group has shown antibodies specific to LPS and CtxB in supernatants of MBC cultures up to 3 months after infection.33 In this current study, we analyzed MBC responses against OSP, LPS, and CtxB in culture supernatants from both patients and vaccinees during 1 year of follow-up. We found evidence of MBCs to OSP, LPS and CtxB out to 3 months after infection however little evidence of development of MBC responses after vaccination. In a previous study, we had found detectable memory B-cells by ELISPOT assay out to one year after infection by V. cholerae.28,32 Our results suggest that the MBC assay using culture supernatant may be less sensitive than assessing the MBC response by ELISPOT, perhaps caused by the infrequency of antigen-specific MBCs in in vitro culture and the resultant low levels of antibodies in culture supernatants. This may explain the difference in our previous study showing evidence of antigen-specific MBCs in the circulation out to 1 year after infection using the ELISPOT assay, but out to just 3 months after infection when using an ELISA of culture supernatants of MBCs. However, the most important observation in this current study is that vaccinees produce less detectable MBC responses to major cholera antigens (LPS, OSP, and CtxB), and reduced magnitudes of IgA, IgG, and IgM plasma antibody and ASC responses compared with responses in patients recovering from wild-type cholera.
Our study has limitations. We focused our analysis on anti-Ogawa responses because that was the predominant serotype of V. cholerae O1 in Dhaka at the time of the study. However, our earlier observations suggest similar responses to OSP and LPS after both Ogawa and Inaba wild-type infection,26 therefore we anticipate that our results would be similar if done at a time of predominantly circulating Inaba V. cholerae infection. Second, our study focused on vaccinees who received WC-rBS (Dukoral), and a newer generation oral cholera vaccine, Shanchol, has been WHO-prequalified and is now part of the WHO vaccine stockpile portfolio. Shanchol is bivalent for V. cholerae O1 and O139 and is not supplemented by rCtxB. Preliminary reports suggest that protection against cholera after vaccination with Shanchol may be longer in duration than that afforded by immunization with Dukoral (WC-rBS). We however, do not have results of the MBC responses in participants given Shanchol and are not able to compare the longevity of responses with that seen in response to Dukoral. Third, our vaccinees whom we followed up for 1 year, did not have plasma or MBC culture supernatant at Day 7 or Day 21 similar to that of Day 7 in our patient group.28 Our study also did not include assessment of memory B responses by the more sensitive ELISPOT assay. Despite these limitations, our findings that there is a markedly lower response targeting OSP, especially of mucosal and MBC immune responses, after immunization with an oral killed cholera vaccine compared with natural infection, may help explain differences in protective immunity produced by vaccination compared with natural infection, and could lead to development of improved cholera vaccination strategies.
Disclaimer: Potential conflicts of interest. All authors: no conflicts.
Footnotes
Financial support: This research was supported by the icddr,b, by the Intramural Research Program of the National Institutes of Health, NIDDK, and extramural grants from the National Institutes of Health, including the National Institute of Allergy and Infectious Diseases (U01 AI058935 [S.B.C., E.T.R.], R03 AI063079 [F.Q.], U01 AI077883 and AI106878 [E.T.R.]), K08 AI089721 [R.C.C.], K08AI100923 [D.T.L.]) and the Fogarty International Center, Training Grant in Vaccine Development and Public Health (TW005572 [T.U., M.M.A., R.R., and F.Q.]), Career Development Awards (K01 TW07409 [J.B.H.] and K01 TW07144 [R.C.L.], and a Fogarty International Clinical Research Scholars Award (R24 TW007988 [T.U., R.A.J.]), as well as by Swedish Sida grant INT-ICDDR,B-HN-01-AV (F.Q.), a Physician Scientist Early Career Award from the Howard Hughes Medical Institute (R.C.L.), a Postdoctoral Fellowship in Tropical Infectious Diseases from the American Society for Tropical Medicine and Hygiene - Burroughs Wellcome Fund (D.T.L.), and a Thrasher Research Fund Early Career Award (D.T.L.).
Authors' addresses: Taher Uddin, Amena Aktar, Russell A. Johnson, M. Arifur Rahman, Sadia Afrin, Aklima Akter, Mohammad Murshid Alam, Fahima Chowdhury, Ashraful I. Khan, and Firdausi Qadri, International Centre For Diarrhoeal Disease Research, Bangladesh (icddr,b) - Immunology Laboratory, Dhaka, Bangladesh, E-mails: taher_imm@icddrb.org, bio_amn015@yahoo.com, russell.a.johnson@gmail.com, marifur@icddrb.org, sadia_afrin89@yahoo.com, aklima17@gmail.com, shafiul@icddrb.org, fchowdhury@icddrb.org, ashrafk@icddrb.org, and fqadri@icddrb.org. Peng Xu and Pavol Kováč, National Institutes of Health - NIDDK, LBC, Bethesda, MD, E-mails: xup3@mail.nih.gov and Kovac@mail.nih.gov. Daniel T. Leung, Meagan K. Bufano, Yanan Yu, and Ying Wu-Freeman, Massachusetts General Hospital - Infectious Diseases, Boston, MA, E-mails: dleung@partners.org, MBUFANO@partners.org, YYVAUGHN@PARTNERS.ORG, and YWUFREEMAN@PARTNERS.ORG. Atiqur Rahman, University of Dhaka - Department of Biochemistry and Molecular Biology, Dhaka, Bangladesh, E-mail: atique303@gmail.com. Rasheduzzaman Rashu, International Centre For Diarrhoeal Disease Research, Bangladesh (icddr,b) - Immunology Laboratory, Dhaka, Bangladesh, and Massachusetts General Hospital - Infectious Diseases, Boston, MA, E-mail: rashedimm@yahoo.com. Jason B. Harris, Department of Pediatrics, Harvard Medical School, Boston, MA, and Division of Infectious Diseases, Massachusetts General Hospital, Boston, MA, E-mail: jbharris@partners.org. Regina C. LaRocque and Richelle C. Charles, Division of Infectious Diseases, Massachusetts General Hospital, Boston, MA, and Department of Medicine, Harvard Medical School, Boston, MA, E-mails: RCLAROCQUE@PARTNERS.ORG and RCCHARLES@PARTNERS.ORG. Stephen Calderwood, Division of Infectious Diseases, Massachusetts General Hospital, Boston, MA, Department of Medicine, Harvard Medical School, Boston, MA, and Department of Microbiology and Immunobiology, Harvard Medical School, Boston, MA, E-mail: SCALDERWOOD@PARTNERS.ORG. Edward T. Ryan, Division of Infectious Diseases, Massachusetts General Hospital, Boston, MA, Department of Medicine, Harvard Medical School, Boston, MA, and Department of Immunology and Infectious Diseases, Harvard School of Public Health, Boston, MA, E-mail: ETRYAN@PARTNERS.ORG.
References
- 1.Harris JB, LaRocque RC, Qadri F, Ryan ET, Calderwood SB. Cholera. Lancet. 2012;379:2466–2476. doi: 10.1016/S0140-6736(12)60436-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zuckerman JN, Rombo L, Fisch A. The true burden and risk of cholera: implications for prevention and control. Lancet Infect Dis. 2007;7:521–530. doi: 10.1016/S1473-3099(07)70138-X. [DOI] [PubMed] [Google Scholar]
- 3.Anonymous Cholera vaccines: WHO position paper-recommendations. Vaccine. 2010;28:4687–4688. doi: 10.1016/j.vaccine.2010.05.008. [DOI] [PubMed] [Google Scholar]
- 4.Stroeher UH, Karageorgos LE, Morona R, Manning PA. Serotype conversion in Vibrio cholerae O1. Proc Natl Acad Sci USA. 1992;89:2566–2570. doi: 10.1073/pnas.89.7.2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hisatsune K, Kondo S, Isshiki Y, Iguchi T, Haishima Y. Occurrence of 2-O-methyl-N-(3-deoxy-L-glycero-tetronyl)-D-perosamine (4-amino-4,6-dideoxy-D-manno-pyranose) in lipopolysaccharide from Ogawa but not from Inaba O forms of O1 Vibrio cholerae. Biochem Biophys Res Commun. 1993;190:302–307. doi: 10.1006/bbrc.1993.1046. [DOI] [PubMed] [Google Scholar]
- 6.Wang J, Villeneuve S, Zhang J, Lei P, Miller CE, Lafaye P, Nato F, Szu SC, Karpas A, Bystricky S, Robbins JB, Kovac P, Fournier JM, Glaudemans CP. On the antigenic determinants of the lipopolysaccharides of Vibrio cholerae O:1, serotypes Ogawa and Inaba. J Biol Chem. 1998;273:2777–2783. doi: 10.1074/jbc.273.5.2777. [DOI] [PubMed] [Google Scholar]
- 7.Ito T, Higuchi T, Hirobe M, Hiramatsu K, Yokota T. Identification of a novel sugar, 4-amino-4,6-dideoxy-2-O-methylmannose in the lipopolysaccharide of Vibrio cholerae O1 serotype Ogawa. Carbohydr Res. 1994;256:113–128. doi: 10.1016/0008-6215(94)84231-0. [DOI] [PubMed] [Google Scholar]
- 8.Harris AM, Chowdhury F, Begum YA, Khan AI, Faruque AS, Svennerholm AM, Harris JB, Ryan ET, Cravioto A, Calderwood SB, Qadri F. Shifting prevalence of major diarrheal pathogens in patients seeking hospital care during floods in 1998, 2004, and 2007 in Dhaka, Bangladesh. Am J Trop Med Hyg. 2008;79:708–714. [PMC free article] [PubMed] [Google Scholar]
- 9.Anh DD, Canh do G, Lopez AL, Thiem VD, Long PT, Son NH, Deen J, von Seidlein L, Carbis R, Han SH, Shin SH, Attridge S, Holmgren J, Clemens J. Safety and immunogenicity of a reformulated Vietnamese bivalent killed, whole-cell, oral cholera vaccine in adults. Vaccine. 2007;25:1149–1155. doi: 10.1016/j.vaccine.2006.09.049. [DOI] [PubMed] [Google Scholar]
- 10.Mahalanabis D, Lopez AL, Sur D, Deen J, Manna B, Kanungo S, von Seidlein L, Carbis R, Han SH, Shin SH, Attridge S, Rao R, Holmgren J, Clemens J, Bhattacharya SK. A randomized, placebo-controlled trial of the bivalent killed, whole-cell, oral cholera vaccine in adults and children in a cholera endemic area in Kolkata, India. PLoS ONE. 2008;3:e2323. doi: 10.1371/journal.pone.0002323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saha A, Chowdhury MI, Khanam F, Bhuiyan MS, Chowdhury F, Khan AI, Khan IA, Clemens J, Ali M, Cravioto A, Qadri F. Safety and immunogenicity study of a killed bivalent (O1 and O139) whole-cell oral cholera vaccine Shanchol, in Bangladeshi adults and children as young as 1 year of age. Vaccine. 2011;29:8285–8292. doi: 10.1016/j.vaccine.2011.08.108. [DOI] [PubMed] [Google Scholar]
- 12.Sur D, Lopez AL, Kanungo S, Paisley A, Manna B, Ali M, Niyogi SK, Park JK, Sarkar B, Puri MK, Kim DR, Deen JL, Holmgren J, Carbis R, Rao R, Nguyen TV, Donner A, Ganguly NK, Nair GB, Bhattacharya SK, Clemens JD. Efficacy and safety of a modified killed-whole-cell oral cholera vaccine in India: an interim analysis of a cluster-randomized, double-blind, placebo-controlled trial. Lancet. 2009;374:1694–1702. doi: 10.1016/S0140-6736(09)61297-6. [DOI] [PubMed] [Google Scholar]
- 13.Kanungo S, Paisley A, Lopez AL, Bhattacharya M, Manna B, Kim DR, Han SH, Attridge S, Carbis R, Rao R, Holmgren J, Clemens JD, Sur D. Immune responses following one and two doses of the reformulated, bivalent, killed, whole-cell, oral cholera vaccine among adults and children in Kolkata, India: a randomized, placebo-controlled trial. Vaccine. 2009;27:6887–6893. doi: 10.1016/j.vaccine.2009.09.008. [DOI] [PubMed] [Google Scholar]
- 14.Clemens JD, van Loon F, Sack DA, Rao MR, Ahmed F, Chakrabort YJ, Kay BA, Khan MR, Yunus MD, Harris JR, Clemens JD, Rao MR, Svennerholm A-M, Holmgren J. Biotype as determinant of natural immunizing effect of cholera. Lancet. 1991;337:883–884. doi: 10.1016/0140-6736(91)90207-6. [DOI] [PubMed] [Google Scholar]
- 15.Sur D, Kanungo S, Sah B, Manna B, Ali M, Paisley AM, Niyogi SK, Park JK, Sarkar B, Puri MK, Kim DR, Deen JL, Holmgren J, Carbis R, Rao R, Nguyen TV, Han SH, Attridge S, Donner A, Ganguly NK, Bhattacharya SK, Nair GB, Clemens JD, Lopez AL. Efficacy of a low-cost, inactivated whole-cell oral cholera vaccine: results from 3 years of follow-up of a randomized, controlled trial. PLoS Negl Trop Dis. 2011;5:e1289. doi: 10.1371/journal.pntd.0001289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ali M, Emch M, Park JK, Yunus M, Clemens J. Natural cholera infection-derived immunity in an endemic setting. J Infect Dis. 2011;204:912–918. doi: 10.1093/infdis/jir416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Levine MM, Black RE, Clements ML, Cisneros L, Nalin DR, Young CR. Duration of infection-derived immunity to cholera. J Infect Dis. 1981;143:818–820. doi: 10.1093/infdis/143.6.818. [DOI] [PubMed] [Google Scholar]
- 18.Albert MJ, Alam K, Rahman AS, Huda S, Sack RB. Lack of cross-protection against diarrhea due to Vibrio cholerae O1 after oral immunization of rabbits with V. cholerae O139 Bengal. J Infect Dis. 1994;169:709–710. doi: 10.1093/infdis/169.3.709. [DOI] [PubMed] [Google Scholar]
- 19.Waldor MK, Colwell R, Mekalanos JJ. The Vibrio cholerae O139 serogroup antigen includes an O-antigen capsule and lipopolysaccharide virulence determinants. Proc Natl Acad Sci USA. 1994;91:11388–11392. doi: 10.1073/pnas.91.24.11388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Qadri F, Wenneras C, Albert MJ, Hossain J, Mannoor K, Begum YA, Mohi G, Salam MA, Sack RB, Svennerholm AM. Comparison of immune responses in patients infected with Vibrio cholerae O139 and O1. Infect Immun. 1997;65:3571–3576. doi: 10.1128/iai.65.9.3571-3576.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xu P, Alam MM, Kalsy A, Charles RC, Calderwood SB, Qadri F, Ryan ET, Kovac P. Simple, direct conjugation of bacterial O-SP-core antigens to proteins: development of cholera conjugate vaccines. Bioconjug Chem. 2011;22:2179–2185. doi: 10.1021/bc2001984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cox AD, Brisson JR, Varma V, Perry MB. Structural analysis of the lipopolysaccharide from Vibrio cholerae O139. Carbohydr Res. 1996;290:43–58. doi: 10.1016/0008-6215(96)00135-8. [DOI] [PubMed] [Google Scholar]
- 23.Cox AD, Perry MB. Structural analysis of the O-antigen-core region of the lipopolysaccharide from Vibrio cholerae O139. Carbohydr Res. 1996;290:59–65. doi: 10.1016/0008-6215(96)00131-0. [DOI] [PubMed] [Google Scholar]
- 24.Vinogradov EV, Bock K, Holst O, Brade H. The structure of the lipid A-core region of the lipopolysaccharides from Vibrio cholerae O1 smooth strain 569B (Inaba) and rough mutant strain 95R (Ogawa) Eur J Biochem. 1995;233:152–158. doi: 10.1111/j.1432-1033.1995.152_1.x. [DOI] [PubMed] [Google Scholar]
- 25.Hankins JV, Madsen JA, Giles DK, Brodbelt JS, Trent MS. Amino acid addition to Vibrio cholerae LPS establishes a link between surface remodeling in Gram-positive and Gram-negative bacteria. Proc Natl Acad Sci USA. 2012;109:8722–8727. doi: 10.1073/pnas.1201313109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Johnson RA, Uddin T, Aktar A, Mohasin M, Alam MM, Chowdhury F, Harris JB, LaRocque RC, Bufano MK, Yu Y, Wu-Freeman Y, Leung DT, Sarracino D, Krastins B, Charles RC, Xu P, Kovac P, Calderwood SB, Qadri F, Ryan ET. Comparison of immune responses to the O-specific polysaccharide and lipopolysaccharide of Vibrio cholerae O1 in Bangladeshi adult patients with cholera. Clin Vaccine Immunol. 2012;19:1712–1721. doi: 10.1128/CVI.00321-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leung DT, Uddin T, Xu P, Aktar A, Johnson RA, Rahman MA, Alam MM, Bufano MK, Eckhoff G, Wu-Freeman Y, Yu Y, Sultana T, Khanam F, Saha A, Chowdhury F, Khan AI, Charles RC, Larocque RC, Harris JB, Calderwood SB, Kovac P, Qadri F, Ryan ET. Immune responses to the O-specific polysaccharide antigen in children receiving a killed oral cholera vaccine compared to responses following natural cholera infection in Bangladesh. Clin Vaccine Immunol. 2013;20:780. doi: 10.1128/CVI.00035-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alam MM, Riyadh MA, Fatema K, Rahman MA, Akhtar N, Ahmed T, Chowdhury MI, Chowdhury F, Calderwood SB, Harris JB, Ryan ET, Qadri F. Antigen-specific memory B-cell responses in Bangladeshi adults after one- or two-dose oral killed cholera vaccination and comparison with responses in patients with naturally acquired cholera. Clin Vaccine Immunol. 2011;18:844–850. doi: 10.1128/CVI.00562-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Qadri F, Asaduzzaman M, Wenneras C, Mohi G, Albert MJ, Abdus Salam M, Sack RB, Jertborn M, McGhee JR, Sack DA, Holmgren J. Enterotoxin-specific immunoglobulin E responses in humans after infection or vaccination with diarrhea-causing enteropathogens. Infect Immun. 2000;68:6077–6081. doi: 10.1128/iai.68.10.6077-6081.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Qadri F, Raqib R, Ahmed F, Rahman T, Wenneras C, Das SK, Alam NH, Mathan MM, Svennerholm AM. Increased levels of inflammatory mediators in children and adults infected with Vibrio cholerae O1 and O139. Clin Diagn Lab Immunol. 2002;9:221–229. doi: 10.1128/CDLI.9.2.221-229.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jayasekera CR, Harris JB, Bhuiyan S, Chowdhury F, Khan AI, Faruque AS, Larocque RC, Ryan ET, Ahmed R, Qadri F, Calderwood SB. Cholera toxin-specific memory B cell responses are induced in patients with dehydrating diarrhea caused by Vibrio cholerae O1. J Infect Dis. 2008;198:1055–1061. doi: 10.1086/591500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Harris AM, Bhuiyan MS, Chowdhury F, Khan AI, Hossain A, Kendall EA, Rahman A, LaRocque RC, Wrammert J, Ryan ET, Qadri F, Calderwood SB, Harris JB. Antigen-specific memory B-cell responses to Vibrio cholerae O1 infection in Bangladesh. Infect Immun. 2009;77:3850–3856. doi: 10.1128/IAI.00369-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kendall EA, Tarique AA, Hossain A, Alam MM, Arifuzzaman M, Akhtar N, Chowdhury F, Khan AI, Larocque RC, Harris JB, Ryan ET, Qadri F, Calderwood SB. Development of immunoglobulin M memory to both a T-cell-independent and a T-cell-dependent antigen following infection with Vibrio cholerae O1 in Bangladesh. Infect Immun. 2009;78:253–259. doi: 10.1128/IAI.00868-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Qadri F, Mohi G, Hossain J, Azim T, Khan AM, Salam MA, Sack RB, Albert MJ, Svennerholm AM. Comparison of the vibriocidal antibody response in cholera due to Vibrio cholerae O139 Bengal with the response in cholera due to Vibrio cholerae O1. Clin Diagn Lab Immunol. 1995;2:685–688. doi: 10.1128/cdli.2.6.685-688.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Qadri F, Ryan ET, Faruque AS, Ahmed F, Khan AI, Islam MM, Akramuzzaman SM, Sack DA, Calderwood SB. Antigen-specific immunoglobulin A antibodies secreted from circulating B cells are an effective marker for recent local immune responses in patients with cholera: comparison to antibody-secreting cell responses and other immunological markers. Infect Immun. 2003;71:4808–4814. doi: 10.1128/IAI.71.8.4808-4814.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shamsuzzaman S, Ahmed T, Mannoor K, Begum YA, Bardhan PK, Sack RB, Sack DA, Svennerholm AM, Holmgren J, Qadri F. Robust gut associated vaccine-specific antibody-secreting cell responses are detected at the mucosal surface of Bangladeshi subjects after immunization with an oral killed bivalent V. cholerae O1/O139 whole cell cholera vaccine: comparison with other mucosal and systemic responses. Vaccine. 2009;27:1386–1392. doi: 10.1016/j.vaccine.2008.12.041. [DOI] [PubMed] [Google Scholar]
- 37.Uddin T, Harris JB, Bhuiyan TR, Shirin T, Uddin MI, Khan AI, Chowdhury F, LaRocque RC, Alam NH, Ryan ET, Calderwood SB, Qadri F. Mucosal immunologic responses in cholera patients in Bangladesh. Clin Vaccine Immunol. 2011;18:506–512. doi: 10.1128/CVI.00481-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Crotty S, Aubert RD, Glidewell J, Ahmed R. Tracking human antigen-specific memory B cells: a sensitive and generalized ELISPOT system. J Immunol Methods. 2004;286:111–122. doi: 10.1016/j.jim.2003.12.015. [DOI] [PubMed] [Google Scholar]
- 39.Crotty S, Felgner P, Davies H, Glidewell J, Villarreal L, Ahmed R. Cutting edge: long-term B cell memory in humans after smallpox vaccination. J Immunol. 2003;171:4969–4973. doi: 10.4049/jimmunol.171.10.4969. [DOI] [PubMed] [Google Scholar]
- 40.Qadri F, Jonson G, Begum YA, Wenneras C, Albert MJ, Salam MA, Svennerholm AM. Immune response to the mannose-sensitive hemagglutinin in patients with cholera due to Vibrio cholerae O1 and O0139. Clin Diagn Lab Immunol. 1997;4:429–434. doi: 10.1128/cdli.4.4.429-434.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Czerkinsky C, Prince SJ, Michalek SM, Jackson S, Russell MW, Moldoveanu Z, McGhee JR, Mestecky J. IgA antibody-producing cells in peripheral blood after antigen ingestion: evidence for a common mucosal immune system in humans. Proc Natl Acad Sci USA. 1987;84:2449–2453. doi: 10.1073/pnas.84.8.2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Koelle K, Rodo X, Pascual M, Yunus M, Mostafa G. Refractory periods and climate forcing in cholera dynamics. Nature. 2005;436:696–700. doi: 10.1038/nature03820. [DOI] [PubMed] [Google Scholar]
- 43.Patel SM, Rahman MA, Mohasin M, Riyadh MA, Leung DT, Alam MM, Chowdhury F, Khan AI, Weil AA, Aktar A, Nazim M, LaRocque RC, Ryan ET, Calderwood SB, Qadri F, Harris JB. Memory B cell responses to Vibrio cholerae O1 lipopolysaccharide are associated with protection against infection from household contacts of patients with cholera in Bangladesh. Clin Vaccine Immunol. 2012;19:842–848. doi: 10.1128/CVI.00037-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Leung DT, Rahman MA, Mohasin M, Patel SM, Aktar A, Khanam F, Uddin T, Riyadh MA, Saha A, Alam MM, Chowdhury F, Khan AI, Charles R, LaRocque R, Harris JB, Calderwood SB, Qadri F, Ryan ET. Memory B cell and other immune responses in children receiving two doses of an oral killed cholera vaccine compared to responses following natural cholera infection in Bangladesh. Clin Vaccine Immunol. 2012;19:690–698. doi: 10.1128/CVI.05615-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Glass RI, Svennerholm AM, Khan MR, Huda S, Huq MI, Holmgren J. Seroepidemiological studies of El Tor cholera in Bangladesh: association of serum antibody levels with protection. J Infect Dis. 1985;151:236–242. doi: 10.1093/infdis/151.2.236. [DOI] [PubMed] [Google Scholar]
- 46.Saha D, LaRocque RC, Khan AI, Harris JB, Begum YA, Akramuzzaman SM, Faruque AS, Ryan ET, Qadri F, Calderwood SB. Incomplete correlation of serum vibriocidal antibody titer with protection from Vibrio cholerae infection in urban Bangladesh. J Infect Dis. 2004;189:2318–2322. doi: 10.1086/421275. [DOI] [PubMed] [Google Scholar]
- 47.Losonsky GA, Yunyongying J, Lim V, Reymann M, Lim YL, Wasserman SS, Levine MM. Factors influencing secondary vibriocidal immune responses: relevance for understanding immunity to cholera. Infect Immun. 1996;64:10–15. doi: 10.1128/iai.64.1.10-15.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Harris JB, LaRocque RC, Chowdhury F, Khan AI, Logvinenko T, Faruque AS, Ryan ET, Qadri F, Calderwood SB. Susceptibility to Vibrio cholerae infection in a cohort of household contacts of patients with cholera in Bangladesh. PLoS Negl Trop Dis. 2008;2:e221. doi: 10.1371/journal.pntd.0000221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Leung DT, Rahman MA, Mohasin M, Riyadh MA, Patel SM, Alam MM, Chowdhury F, Khan AI, Kalivoda EJ, Aktar A, Bhuiyan MS, LaRocque RC, Harris JB, Calderwood SB, Qadri F, Ryan ET. Comparison of memory B cell, antibody-secreting cell, and plasma antibody responses in young children, older children, and adults with infection caused by Vibrio cholerae O1 El Tor Ogawa in Bangladesh. Clin Vaccine Immunol. 2011;18:1317–1325. doi: 10.1128/CVI.05124-11. [DOI] [PMC free article] [PubMed] [Google Scholar]