Abstract
We surveyed Peace Corps Medical Officers (PCMOs) to determine the frequency of and responses to possible rabies exposures of U.S. Peace Corps volunteers (PCVs). Surveys were sent to 56 PCMOs serving in countries with moderate or high rabies vaccine recommendations from the U.S. Centers for Disease Control and Prevention (CDC), of which 38 (68%) responded. Thirty-seven PCMOs reported that, of 4,982 PCVs, 140 (3%) experienced possible rabies exposures. Of these, 125 (89%) had previously received rabies vaccination, 129 (92%) presented with adequately cleansed wounds, and 106 (76%) were deemed to require and were given post-exposure prophylaxis (PEP). Of 35 respondents, 30 (86%) reported that rabies vaccine was always accessible to PCVs in their country within 24 hours. Overall, the Peace Corps is successful at preventing and treating possible rabies exposures. However, this study identified a few gaps in policy implementation. The Peace Corps should continue and strengthen efforts to provide education, preexposure vaccination, and PEP to PCVs.
Introduction
Rabies is a disease caused by zoonotic neurotropic viruses (family Rhabdoviridae, genus Lyssavirus), which are most often transmitted to humans through the bite of an infected mammal. In the absence of treatment, human rabies is almost universally fatal. However, the disease is highly preventable with appropriate and timely postexposure prophylaxis (PEP), which includes a thorough washing of the wound, a series of rabies vaccine doses, and, for previously unvaccinated individuals, administration of rabies immune globulin (RIG). Individuals who have previously received rabies vaccine require two booster doses of vaccine, whereas those who have not received rabies vaccine in the past require four doses of vaccine. Despite the effectiveness of PEP in preventing human rabies, more than 55,000 people die each year from rabies globally; 95% of these deaths occur in Asia and Africa.1,2
Rabies in travelers.
Although a definitive rate of possible rabies exposures in travelers has not been calculated, a range of 16–200/100,000 travelers has been reported.2 A recent study of PEP records estimated that 0.4% (range 0.01–2.3%) of travelers receive a possible rabies-contaminated bite per month of residence in a rabies-endemic country.3 A study of travelers to Southeast Asia found that, each month, 1.11 of 100 travelers received a possible rabies-contaminated bite and 3.12 of 100 travelers received a possible rabies-contaminated lick.4 Travelers are at highest risk for rabies exposure in countries with endemic canine rabies; travelers to these countries may consider preexposure vaccination.1
Rabies preexposure vaccination recommendations for United States travelers are made by using methodology from the U.S. Centers for Disease Control and Prevention's (CDC) Travelers' Health and Poxvirus and Rabies Branches (Unpublished data, CDC). These recommendations, which are listed on the individual country destination pages on the CDC Travelers' Health website,5 are based on a combination of the country's canine rabies endemicity level and availability of PEP. Four recommendation strength levels are used: strong, moderate, weak, or no recommendation listed (Table 1). Countries with strong recommendation strengths usually have endemic canine rabies and/or limited access to RIG. The CDC recommends preexposure vaccination for travelers to these countries, particularly if planned activities may result in contact with mammals. Countries with moderate recommendation strengths usually have both endemic canine rabies and stable supplies of appropriate RIG and rabies vaccine. For such countries, preexposure vaccination is recommended only for travelers with occupational risks. For countries with either moderate or strong vaccine recommendations, long-term travelers or those staying in remote areas are advised to receive preexposure vaccination.
Table 1.
Rabies vaccination recommendation strengths categorized by the U.S. Centers for Disease Control and Prevention (CDC) Travelers' Health Branch*
| Recommendation strength | Description |
|---|---|
| None | Country page shows no information on rabies vaccinations. |
| Weak | Rabies vaccination is only recommended for travelers involved in any activities that might bring them into direct contact with bats. These travelers include wildlife professionals, researchers, veterinarians, or adventure travelers visiting areas where bats are commonly found. |
| Moderate | Rabies vaccination is only recommended for travelers with significant occupational risks, such as veterinarians, and for long-term travelers and expatriates living in areas with a significant risk of exposure. Travelers involved in any activities that might bring them into direct contact with bats, carnivores, and other mammals, such as wildlife professionals, researchers, veterinarians, or adventure travelers visiting areas where bats, carnivores, and other mammals are commonly found |
| Strong | Rabies vaccination is recommended for travelers spending a lot of time outdoors, especially in rural areas, involved in activities such as bicycling, camping, or hiking. Also recommended for travelers with significant occupational risks, such as veterinarians, and for long-term travelers and expatriates living in areas with a significant risk of exposure. Children are considered at higher risk because they tend to play with animals, may receive more severe bites, or may not report bites. |
In the United States, rabies preexposure vaccination recommendations for travelers are made by using methodology from the CDC Travelers' Health and Poxvirus and Rabies Branches (Unpublished data, CDC). These recommendations are listed on the individual country destination pages on the CDC Travelers' Health website (www.cdc.gov/travel)
United States Peace Corps.
Peace Corps volunteers (PCVs) often serve in remote rural areas, live among the local population, and work with local people on projects related to education, health, economic development, environment, youth, and agriculture. As of January, 2013, there were 8,073 PCVs serving in 76 countries. Many PCVs were serving in Africa (43%), Latin America (21%), Eastern Europe/Central Asia (15%), or Asia (10%).6 A previous estimate found the rate of rabies PEP for PCVs in rabies-endemic areas to be 43.6/1,000 PCVs per year.7
Peace Corps' policy is to routinely provide preexposure rabies vaccination with vaccines approved for use by the U.S. Food and Drug Administration (FDA) to all PCVs upon their arrival in host countries with CDC strong or moderate rabies vaccination recommendations. Preexposure vaccination is not routinely provided to PCVs in countries with no or weak recommendations, because risk of rabies exposure in these countries is thought to be low. Upon the PCV's initial arrival in the country, Peace Corps Medical Officers (PCMOs) provide a minimum of 20 hours of health-related training and basic medical supplies to the PCVs, including supplies for and information about how to treat wounds from animal bites. This training varies by country, but the rabies prevention component consists of teaching PCVs about country risk level, avoiding or minimizing possible exposures, and proper wound care. The PCVs are advised to immediately report any exposures to their PCMO. The PCMOs are physicians, nurse practitioners, and physician assistants contracted by Peace Corps to provide primary health care to PCVs overseas. When PCVs experience possible rabies exposures or have any other medical problem that cannot be handled at their primary work site, Peace Corps' policy is to pay for treatment, including the use of FDA-approved RIG or rabies vaccine, by a regional health care clinic staffed by at least one PCMO.8 When a local supply does not exist, Peace Corps will also pay to express ship FDA-approved RIG or rabies vaccines for PCVs.
We sought to 1) assess the frequency of possible rabies exposures, clinic procedures, and availability of rabies PEP for PCVs seen by PCMOs in 2011; and 2) describe the accessibility and type of RIG and rabies vaccine available to non-PCV, United States travelers according to PCMOs, as they likely have knowledge about the local availability of rabies biologics in the country where they are stationed.
Methods
We developed a secure, web-based survey and distributed survey invitation e-mails to PCMOs in each of the countries with PCMO clinics. In countries with more than one PCMO, only one was asked to respond to the survey. In cases where a single PCMO was in charge of multiple countries, that PCMO was asked to respond on behalf of all of these countries. One reminder survey invitation e-mail was sent to encourage participation of nonresponders. The survey was determined to be a non-research activity by CDC Human Subjects Advisors.
The survey contained ∼20 questions, although the exact question count varied, as follow-up questions were asked only if specific responses were provided for certain questions. Questions pertained to the frequency of and protocols for possible rabies exposures in PCVs, the accessibility and types of RIG and rabies vaccine used for PCVs, and the PCMO's personal opinion on the accessibility of RIG and rabies vaccine for non-PCV travelers in the country. Respondents were asked to provide data for the year 2011.
The PCMOs responding from countries with no or weak vaccination recommendations were excluded from analyses. Results were compiled by region so that individual respondents could not be identified; all identifying information was deleted before analysis. The region classifications used were those previously used by CDC Travelers' Health Branch.9 Total PCMO visits per year were calculated by multiplying average visits per month by 12. The number of clinic visits per PCV and percent of total clinic visits due to possible rabies exposure were calculated using only responses from PCMOs who provided an average number of clinic visits per month. We did not adjust the data for unit or item non-response, and our analyses are based on unweighted data. Data were analyzed by using SAS 9.3 (SAS Institute, Cary, NC).
Results
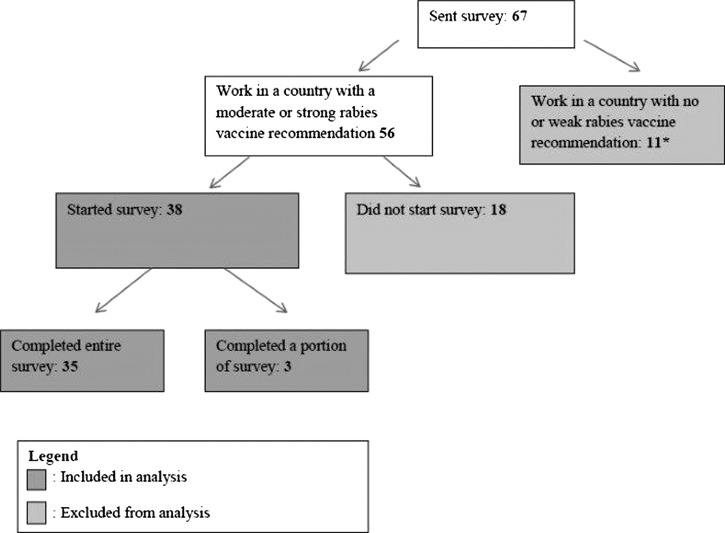
Surveys were sent to one PCMO in each of 67 countries, 56 (84%) of which were countries with moderate or strong rabies recommendations. Each PCMO treated PCVs in a single country, with one exception. One responding PCMO treated PCVs in eight countries, one country of which had a moderate or strong rabies recommendation. Because not all of the PCVs treated by this PCMO served in moderate or strong recommendation countries, this response was excluded from analysis. Of the 56 eligible PCMOs, 38 (68%) PCMOs started the survey, and 35 (63%) finished it (Figure 1).
Figure 1.
Survey responses from Peace Corps Medical Officers (PCMOs) included in analysis, 2011. (*This includes one PCMO who treated Peace Corps volunteers in 8 countries, including 1 country with a moderate or strong rabies recommendation.)
Possible rabies exposures among PCVs in 2011.
There were a total of 5,071 PCVs stationed in the 38 respondent countries. In the 33 countries that provided data on PCMO clinic visits, there were 4,597 PCVs. These PCVs made 26,856 visits for various health reasons at these clinics, averaging 5.8 visits per PCV during 2011. In the 37 countries that provided data on possible rabies exposures, 140 of 4,982 (2.8%) PCVs presented to PCMO clinics with possible rabies exposures. The region with the highest proportion of PCVs presenting to PCMO clinics with possible rabies exposures was the Mexico, Central America, and Caribbean region (5.7% of PCVs) and the regions with the lowest proportions were Southern Africa (1.2%) and North Africa/Middle East (1.2%). In the 33 countries that provided data on clinic visits, 0.5% of PCMO clinic visits were due to possible rabies exposures. The percentage of PCMO clinic visits due to possible rabies exposures was highest in the West, Central, and East Africa (0.9% of all visits) region and lowest in Tropical South America (0.3%) (Table 2).
Table 2.
Possible rabies exposures as percent of Peace Corps volunteers (PCVs) and percent of visits in 2011, by region
| West, Central, and East Africa | Eastern Europe and Northern Asia | Tropical South America | Mexico, Central America, and Caribbean | East and Southeast Asia | North Africa and Middle East | Southern Africa | Indian Ocean | Total | |
|---|---|---|---|---|---|---|---|---|---|
| Total PCVs in 33 countries that provided a number for clinic visits | 1,672 | 797 | 678 | 582 | 301 | 184 | 250 | 133 | 4,597 |
| Total clinic visits | 4,140 | 5,160 | 7,296 | 7,344 | 1,140 | 456 | 600 | 720 | 26,856 |
| Clinic visits per PCV* | 2.5 | 6.5 | 10.8 | 12.6 | 3.8 | 2.5 | 2.4 | 5.4 | 5.8 |
| Total PCVs in 37 countries that provided a number of possible rabies exposures | 1,928 | 797 | 737 | 582 | 301 | 254 | 250 | 133 | 4,982 |
| PCVs with possible rabies exposures | 44 | 21 | 24 | 33 | 8 | 3 | 3 | 4 | 140 |
| Percent of PCVs in country who presented with a possible rabies exposure | 2.3% | 2.6% | 3.3% | 5.7% | 2.7% | 1.2% | 1.2% | 3.0% | 2.8% |
| Percent of total clinic visits due to possible rabies exposures† | 0.9% | 0.4% | 0.3% | 0.5% | 0.7% | 0.7% | 0.5% | 0.6% | 0.5% |
This number was calculated using the total Peace Corps volunteers (PCVs) in 33 countries.
This number was calculated using possible rabies exposures from the 33 countries. Seven possible rabies exposures from West, Central, and East Africa were removed from the calculation.
Of the 140 PCVs who presented to PCMO clinics with possible rabies exposures, 125 (89%) had received preexposure vaccination, 129 (92%) presented with adequately cleansed wounds, and 106 (76%) were deemed to require and received PEP (Table 3). Specifically, of the 33 possible exposures in the Mexico, Central America, and Caribbean region, 17 (52%) were given PEP and 26 (79%) presented with adequately cleansed wounds. Seven (33%) of the 21 PCVs with possible rabies exposures in Eastern Europe and Northern Asia had received preexposure vaccination.
Table 3.
Characteristics of possible rabies exposures among Peace Corps volunteers (PCVs) in 2011, by region
| West, Central, and East Africa | Eastern Europe and Northern Asia | Tropical South America | Mexico, Central America, and Caribbean | East and Southeast Asia | North Africa and Middle East | Southern Africa | Indian Ocean | Total | |
|---|---|---|---|---|---|---|---|---|---|
| Number of possible rabies exposures | 44 | 21 | 24 | 33 | 8 | 3 | 3 | 4 | 140 |
| Had previous rabies vaccine, n (%) | 44 (100) | 7 (33) | 24 (100) | 33 (100) | 8 (100) | 2 (67) | 3 (100) | 4 (100) | 125 (89) |
| Presented with an adequately cleansed wound, n (%) | 44 (100) | 21 (100) | 23 (96) | 26 (79) | 7 (88) | 2 (67) | 2 (67) | 4 (100) | 129 (92) |
| Deemed to require and given postexposure prophylaxis, n (%) | 39 (89) | 17 (81) | 17 (71) | 17 (52) | 8 (100) | 3 (100) | 3 (100) | 2 (50) | 106 (76) |
Accessibility, types, and procedures of rabies vaccine for PCVs in 2011.
Thirty of 35 (86%) responding PCMOs replied that rabies vaccine was always accessible to PCVs within 24 hours of a possible exposure (Table 4); the five respondents who did not reply that rabies vaccine was always available to PCVs listed reasons including “the cost is too high,” “there are difficulties in the cold chain for preservation,” “in general, there is not enough supply,” “rabies is not a risk to PCVs in my country,” and “it takes too much time to receive from our supplier.” No single reason was mentioned by more than one PCMO.
Table 4.
Accessibility, type, and administration schedule of rabies vaccine when needed for postexposure prophylaxis (PEP) of Peace Corps volunteers (PCVs) in 2011, by region
| West, Central, and East Africa | Eastern Europe and Northern Asia | Tropical South America | Mexico, Central America, and Caribbean | East and Southeast Asia | North Africa and Middle East | Southern Africa | Indian Ocean | Total | |
|---|---|---|---|---|---|---|---|---|---|
| In your clinic in 2011, how often was rabies vaccine accessible* when it was needed for PCVs? | |||||||||
| Number of responses | 12 | 6 | 4 | 5 | 3 | 2 | 2 | 1 | 35 |
| Never, n (%) | 0 (0) | 0 (0) | 1 (25) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (2.9) |
| Seldom, n (%) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (50) | 0 (0) | 0 (0) | 1 (2.9) |
| Sometimes, n (%) | 1 (8.3) | 0 (0) | 0 (0) | 1 (20) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 2 (5.7) |
| Often, n (%) | 0 (0) | 0 (0) | 0 (0) | 1 (20) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (2.9) |
| Always, n (%) | 11 (92) | 6 (100) | 3 (75) | 3 (60) | 3 (100) | 1 (50) | 2 (100) | 1 (100) | 30 (86) |
| In your clinic during 2011, what type of rabies vaccine was used as a part of PEP for rabies? | |||||||||
| Number of responses | 12 | 6 | 3 | 6 | 3 | 2 | 2 | 1 | 35 |
| Human diploid cell, n (%) | 3 (25) | 2 (33) | 0 (0) | 1 (17) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 6 (17) |
| Purified chick embryo cell, n (%) | 0 (0) | 1 (17) | 0 (0) | 0 (0) | 1 (33) | 0 (0) | 0 (0) | 0 (0) | 2 (6) |
| Vero cell, n (%) | 9 (75) | 2 (33) | 3 (100) | 3 (50) | 2 (67) | 2 (100) | 2 (100) | 1 (100) | 24 (69) |
| Other, n (%) | 0 (0) | 0 (0) | 0 (0) | 1 (17) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (2.9) |
| I don't know, n (%) | 0 (0) | 1 (17) | 0 (0) | 1 (17) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 2 (5.7) |
| In your clinic during 2011, which rabies vaccine administration schedule did you typically prescribe for PEP? | |||||||||
| Number of responses | 12 | 6 | 3 | 5 | 3 | 2 | 2 | 1 | 34 |
| 4-dose intramuscular, n (%) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (33) | 0 (0) | 0 (0) | 0 (0) | 1 (2.9) |
| 5-dose intramuscular, n (%) | 0 (0) | 4 (67) | 0 (0) | 0 (0) | 0 (0) | 1 (50) | 0 (0) | 0 (0) | 5 (15) |
| 2-dose intramuscular, n (%) | 11 (92) | 2 (33) | 3 (100) | 5 (100) | 2 (67) | 1 (50) | 2 (100) | 1 (100) | 27 (79) |
| 3-dose intramuscular, n (%) | 1 (8.3) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (2.9) |
“Accessible” was defined as available within 24 hours.
The most common type of rabies vaccine that PCMOs reported using for PCVs was Vero cell (69%) (Table 4). Other vaccines used for PCVs included human diploid cell (17%) and purified chick embryo cell (6%). No respondents reported using tissue culture vaccines.
Of the 34 respondents who reported that rabies vaccine was available in the country where they were stationed, 27 (79%) reported using a 2-dose intramuscular vaccine schedule for PCVs receiving PEP for rabies. Other reported vaccine schedules included 5-dose intramuscular (15%), 4-dose intramuscular (2.9%), and 3-dose intramuscular (2.9%) (Table 4). Of the six respondents who reported vaccine schedules other than 2-dose intramuscular, 4 (67%) were from the Eastern Europe and Northern Asia regions.
Accessibility, types, and procedures of rabies biologics for non-PCV travelers in 2011.
Of 35 PCMO respondents, nine (26%) believed that RIG was never accessible within 24 hours for non-PCV travelers in the countries where they were stationed (Table 5). An additional nine (26%) believed that RIG was seldom accessible for non-PCV travelers within 24 hours. The most common reported reasons that RIG was not available were “the cost is too high” (56%), “there is not enough supply” (32%), and “there are difficulties in the cold chain for preservation” (27%). The most commonly reported type of RIG that respondents thought was used for travelers in their country was human RIG (39%). Three respondents also reported that equine RIG was used (12%) (Table 5).
Table 5.
Opinion of Peace Corps Medical Officers regarding accessibility and type of rabies immune globulin (RIG), and the accessibility and type of rabies vaccine for postexposure prophylaxis (PEP) of non-Peace Corps volunteer travelers in their country of station, 2011
| West, Central, and East Africa | Eastern Europe and Northern Asia | Tropical South America | Mexico, Central America, and Caribbean | East and Southeast Asia | North Africa and Middle East | Southern Africa | Indian Ocean | Total | |
|---|---|---|---|---|---|---|---|---|---|
| In the country where you were stationed in 2011, how often was RIG accessible* when it was needed for travelers? | |||||||||
| Number of responses | 12 | 6 | 4 | 5 | 3 | 2 | 2 | 1 | 35 |
| Never, n (%) | 4 (33) | 1 (17) | 3 (75) | 0 (0) | 0 (0) | 0 (0) | 1 (50) | 0 (0) | 9 (26) |
| Seldom, n (%) | 2 (17) | 2 (33) | 0 (0) | 2 (40) | 1 (33) | 1 (50) | 1 (50) | 0 (0) | 9 (26) |
| Sometimes, n (%) | 2 (17) | 2 (33) | 1 (25) | 2 (40) | 1 (33) | 1 (50) | 0 (0) | 1 (100) | 10 (29) |
| Often, n (%) | 4 (33) | 1 (17) | 0 (0) | 0 (0) | 1 (33) | 0 (0) | 0 (0) | 0 (0) | 6 (17) |
| Always, n (%) | 0 (0) | 0 (0) | 0 (0) | 1 (20) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (2.8) |
| In the country in which you were stationed during 2011, what type of RIG was used for rabies PEP? | |||||||||
| Number of responses | 8 | 5 | 1 | 5 | 3 | 2 | 1 | 1 | 28 |
| Human RIG, n (%) | 3 (38) | 2 (40) | 1 (100) | 2 (40) | 2 (67) | 0 (0) | 0 (0) | 0 (0) | 10 (39) |
| Equine RIG, n (%) | 0 (0) | 1 (20) | 0 (0) | 0 (0) | 2 (67) | 0 (0) | 0 (0) | 0 (0) | 3 (12) |
| Other, n (%) | 1 (13) | 0 (0) | 0 (0) | 2 (40) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 3 (12) |
| I don't know, n (%) | 4 (50) | 3 (60) | 0 (0) | 1 (20) | 0 (0) | 2 (100) | 1 (100) | 1 (100) | 12 (46) |
| In the country where you were stationed in 2011, how often was rabies vaccine accessible* when it was needed for travelers? | |||||||||
| Number of responses | 12 | 6 | 4 | 5 | 3 | 2 | 2 | 1 | 35 |
| Never, n (%) | 0 (0) | 0 (0) | 1 (25) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (2.9) |
| Seldom, n (%) | 2 (17) | 1 (17) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 3 (8.6) |
| Sometimes, n (%) | 3 (25) | 0 (0) | 1 (25) | 2 (40) | 0 (0) | 1 (50) | 0 (0) | 1 (100) | 8 (23) |
| Often, n (%) | 4 (33) | 5 (83) | 1 (25) | 2 (40) | 3 (100) | 0 (0) | 0 (0) | 0 (0) | 15 (43) |
| Always, n (%) | 3 (25) | 0 (0) | 1 (25) | 1 (20) | 0 (0) | 1 (50) | 2 (100) | 0 (0) | 8 (23) |
| In the country where you were stationed in 2011, what type of rabies vaccine was used as a part of PEP for rabies? | |||||||||
| Number of responses | 12 | 6 | 3 | 5 | 3 | 2 | 2 | 1 | 34 |
| Vero cell, n (%) | 7 (58) | 3 (50) | 3 (100) | 3 (60) | 2 (67) | 2 (100) | 2 (100) | 1 (100) | 23 (68) |
| Human diploid cell, n (%) | 3 (25) | 1 (17) | 0 (0) | 2 (40) | 1 (33) | 0 (0) | 0 (0) | 0 (0) | 7 (21) |
| Purified chick embryo cell, n (%) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 2 (67) | 0 (0) | 0 (0) | 0 (0) | 2 (5.9) |
| Other, n (%) | 1 (8.3) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (2.9) |
| Don't know, n (%) | 1 (8.3) | 3 (50) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 4 (12) |
“Accessible” was defined as available within 24 hours.
Eight (23%) of 35 PCMO respondents thought that rabies vaccine was always accessible to travelers within 24 hours of a possible exposure (Table 5). An additional 15 (43%) thought that rabies vaccine was often accessible. Only one (2.9%), a respondent in Tropical South America, thought that rabies vaccine was never accessible to travelers. The most common reported reasons that the vaccine was not always available included “the cost is too high” (48%) and “there are difficulties in the cold chain for preservation” (33%). When asked what type of vaccine was used for PEP for travelers in their country, 23 (68%) indicated that Vero cell was used, 7 (21%) human diploid cell, 2 (6%) purified chick embryo cell, and 4 (12%) did not know.
Discussion
We found that 2.8% of PCVs in countries with high and moderate rabies vaccine recommendations experienced a possible rabies exposure in 2011. In addition, we found several strengths in the knowledge of and responses to possible rabies exposures among PCMOs and a few gaps in knowledge and response. Of importance, no rabies deaths among PCVs were reported by the Peace Corps in 2011 (Charles M, personal communication, January 15, 2013).
We found that, in responding countries in 2011, 2.8% of PCVs, or 0.23% of PCVs per month, presented with possible rabies exposures. These estimates are lower than previous studies3,7; this suggests that, despite the possible differences in living conditions and activities between PCVs and other types of travelers, PCVs may not have any increased risk of possible rabies exposure compared with other travelers to rabies-endemic countries. Conversely, this finding may also reflect the PCVs' focused education regarding prevention of possible rabies exposures.
Most respondents replied that rabies vaccine was always accessible to PCVs within 24 hours, and most respondents also reported using a 2-dose intramuscular schedule of Vero cell vaccine for PEP in PCVs. The 2-dose intramuscular vaccine schedule is consistent with the CDC and World Health Organization (WHO) recommendations for PEP for previously vaccinated individuals. Because PCVs in all countries with moderate and strong rabies recommendations should be vaccinated for rabies before service, this vaccination schedule is appropriate.
Nonetheless, five PCMOs reported that rabies vaccine was not always accessible to PCVs within 24 hours. Because it is the Peace Corps' policy to supply rabies vaccines to PCVs who require PEP, a gap in knowledge may exist among a minority of PCMOs. In addition, six PCMOs reported that they used vaccine schedules other than 2-dose intramuscular for PCVs that required PEP. Four of these PCMOs were in the Eastern Europe and Northern Asia region; in this region, only a third of PCVs presenting with possible rabies exposure had prior vaccination for rabies. This combination of findings suggests that not all PCVs in the Eastern Europe and Northern Asia region were given preexposure vaccination as recommended. The most common vaccine that PCMOs reported using for PCVs who required PEP was Vero cell vaccine. Vero cell vaccine is WHO-reviewed and commonly used in many countries, but it is not approved by the FDA.
Only 20% of respondents thought that RIG was often or always accessible within 24 hours for non-PCV travelers to their countries, whereas 66% thought that rabies vaccine was often or always accessible within 24 hours. These numbers are lower than those reported in a recent survey of travel medicine practitioners worldwide. Of respondents to that survey, 91% reported that rabies vaccine was often or always available and 75% reported that RIG was always available.9 However, respondents to the survey of travel medicine practitioners differed from respondents to the survey of PCMOs in both the number of respondents from each region and the countries from each region included.
The most frequent vaccine thought to be used for travelers when they needed PEP was Vero cell vaccine; this is the same vaccine most frequently reported to be used on PCVs. In addition, three respondents reported that equine RIG, rather than human RIG, was used for travelers who required PEP. Two of these respondents were located in the East and Southeast Asia region and one was located in the Eastern Europe and Northern Asia region. Although equine RIG can be used successfully to prevent rabies, human RIG is preferable because of lower incidence of adverse events.2 These findings suggest that approved rabies vaccine may be easier to obtain in countries with strong or moderate rabies vaccine recommendations than the recommended RIG, supporting the assertion that travelers to countries with strong or moderate rabies vaccine recommendations should consider preexposure vaccination before travel.
The results of this study are subject to limitations. First, the numbers presented in this report reflect self-reported survey responses from PCMOs, rather than official records from clinics. It is unclear whether the PCMOs consulted their official records when reporting incidence of possible rabies exposures and PEP among PCVs. Furthermore, 38 of 56 eligible PCMOs who were sent the survey started the survey. Although that is a strong response rate, the responses of the 18 PCMOs who did not respond may have differed. The interpretation of results was also limited to the respondent's interpretation of the survey questions. In addition, it is possible that, because of language barriers and/or the wording of questions, some PCMOs may have misinterpreted survey questions and thus provided incorrect responses. Furthermore, the results are only for 1 year (2011). Because of limitations of the data, the results are not compared with a control group. Therefore, it is unclear how much impact the education provided by the Peace Corps had on the incidence or handling of possible rabies exposures among PCVs.
The PCVs and other travelers to countries with strong and moderate rabies vaccine recommendations are at risk for possible rabies exposure. The Peace Corps mitigates risks associated with possible rabies exposures by providing education, preexposure vaccination, and PEP to PCVs. Further investigation into the success of this program could provide a model for other organizations to follow in the prevention and treatment of possible rabies exposures, whereas the identified gaps will help the Peace Corps improve its own education and care delivery to PCVs.
The opinions of PCMOs regarding availability of rabies biologics for non-PCV travelers to their countries suggest that non-PCV travelers to countries with strong or moderate rabies recommendations are in need of education and preexposure vaccination. A combination of preexposure vaccination and bite-avoidance education would help to prevent rabies in situations where travelers are at risk for exposure and rabies biologics, particularly RIG, may not be available.
ACKNOWLEDGMENTS
We thank the Peace Corps Medical Officers for their participation. We also thank Ava Navin and Clive Brown for their review of the manuscript.
Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention (CDC).
Footnotes
Authors' addresses: Kira Harvey, Emily S. Jentes, Katherine J. Johnson, Mark J. Sotir, and Gary W. Brunette, Division of Global Migration and Quarantine, Centers for Disease Control and Prevention, Atlanta, GA, E-mails: jii3@cdc.gov, ejentes@cdc.gov, kate.j.johnson@gmail.com, mps6@cdc.gov, and fvd3@cdc.gov. Myrna Charles, Chief, Epidemiology and Surveillance, Office of Medical Services, Peace Corps Headquarters, Washington, DC, E-mail: myrna.charles@redcross.org. Brett Petersen and Jesse D. Blanton, Division of High-Consequence Pathogens and Pathology, Centers for Disease Control and Prevention, Atlanta, GA, E-mails: Ige3@cdc.gov and Asi5@cdc.gov. Mark J. Lamias, Office of the Director, National Center for Emerging and Zoonotic Infectious Diseases, Centers for Disease Control and Prevention, Atlanta, GA, E-mail: Bnz6@cdc.gov.
References
- 1.WHO . WHO Expert Consultation on Rabies: First Report. Volume 931. Geneva: WHO; 2004. WHO Technical Report Series. [Google Scholar]
- 2.Rupprecht CE, Shlim DR. Rabies. In: Brunette GW, Kozarsky PE, Magill AJ, Whatley AD, editors. Health Information for International Travel. Vol. 2012. New York: Oxford University Press; 2012. pp. 272–278. 2012. [Google Scholar]
- 3.Gautret P, Parola P. Rabies vaccination for international travelers. Vaccine. 2012;30:126–133. doi: 10.1016/j.vaccine.2011.11.007. [DOI] [PubMed] [Google Scholar]
- 4.Piyaphanee W, Kittitrakul C, Lawpoosri S, Gautret P, Kashino W, Chaoenpong W, Ponam T, Sibunruang S, Phumratanaprapin W, Tantawichien T. Risk of potentially rabid animal exposure among foreign travelers in Southeast Asia. PLoS Negl Trop Dis. 2012;6:e1852. doi: 10.1371/journal.pntd.0001852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention Travelers' Health Destinations. 2013. www.cdc.gov/travel/destinations/list Available at. Accessed February 8, 2013.
- 6.Peace Corps Fast Facts. 2012. http://www.peacecorps.gov/about/fastfacts/ Available at. Accessed February 8, 2013.
- 7.Bernard KW, Fishbein DB. Pre-exposure rabies prophylaxis for travelers: are the benefits worth the cost? Vaccine. 1991;9:833–836. doi: 10.1016/0264-410x(91)90221-q. [DOI] [PubMed] [Google Scholar]
- 8.Peace Corps What about Safety? 2012. http://www.peacecorps.gov/learn/safety/ Available at. Accessed February 8, 2013.
- 9.Jentes ES, Blanton JD, Johnson KJ, Petersen BW, Lamias MJ, Robertson K, Franka R, Briggs D, Costa P, Lai I, Quarry D, Rupprecht CE, Marano N, Brunette GW. The global availability of rabies immune globulin and rabies vaccine in clinics providing direct care to travelers. J Travel Med. 2013;20:148–158. doi: 10.1111/jtm.12024. [DOI] [PubMed] [Google Scholar]