Abstract
Locally manufactured sodium hypochlorite (chlorine) solution has been sold in Zimbabwe since 2010. During October 1, 2011–April 30, 2012, 4,181 suspected and 52 confirmed cases of typhoid fever were identified in Harare. In response to this outbreak, chlorine tablets were distributed. To evaluate household water treatment uptake, we conducted a survey and water quality testing in 458 randomly selected households in two suburbs most affected by the outbreak. Although 75% of households were aware of chlorine solution and 85% received chlorine tablets, only 18% had reportedly treated stored water and had the recommended protective level of free chlorine residuals. Water treatment was more common among households that reported water treatment before the outbreak, and those that received free tablets during the outbreak (P < 0.01), but was not associated with chlorine solution awareness or use before the outbreak (P > 0.05). Outbreak response did not build on pre-existing prevention programs.
Introduction
Salmonella enterica serovar Typhi (Typhi) is a Gram-negative bacterium that causes typhoid fever—a systemic illness of varying severity characterized by fever, headache, cough, abdominal pain, and other gastrointestinal symptoms. Humans are the only host reservoir of Typhi. Infection occurs by the fecal–oral route, most frequently in populations without access to safe drinking water or sanitation.1 An estimated 13.5 million episodes of typhoid fever occurred worldwide in 2010, and sub-Saharan Africa had the highest estimated regional incidence rate of 724.6 cases/100,000 person-years.2
Economic decline, population growth, and deterioration in operations and maintenance of municipal water and sanitation systems have exposed residents of Harare, the capital city of Zimbabwe with a population of 2 million, to enteric diseases.3–5 A cholera outbreak that began in Chitungwiza in August 2008 spread quickly to the rest of the country and reached Harare City by October; ultimately, Harare reported 19,550 cholera cases and the national total reached over 98,500 cases and 4,200 deaths by June 2009.6
Household water treatment and safe storage (HWTS) with sodium hypochlorite (chlorine) solution is a proven, low-cost intervention that improves the microbiological quality of drinking water in developing countries and significantly reduces morbidity caused by waterborne diseases.7–10 Household water chlorination has also been used in emergency response, including responses to waterborne disease outbreaks, with variable success.10,11 Following the massive cholera outbreak in 2008–2009 in Zimbabwe, the U.S. Agency for International Development's Office of U.S. Foreign Disaster Assistance (USAID/OFDA) supported Population Services International (PSI) to establish local production and social marketing of WaterGuard—1.25% sodium hypochlorite solution for household water treatment—throughout Zimbabwe to reduce the risk of subsequent waterborne disease outbreaks. Approximately 170,000 bottles of WaterGuard were sold or distributed during September 2010–September 2011 (PSI, unpublished data). Each bottle of WaterGuard treats 1,000 L, providing 20 L of treated water per day for 50 days. In 2011–2012, the estimated retail price of each bottle ranged from US$ 0.30–0.50.
In October 2011, a cluster of suspected typhoid fever cases was reported to the City of Harare Health Services Department (CHHSD) from the Dzivaresekwa suburb, home to an estimated 97,000 people (City of Harare, unpublished data). A case-control study conducted by CHHSD in November suggested water was the source of the ongoing outbreak (Muti and others, unpublished data), and water samples from multiple public boreholes and shallow wells yielded Escherichia coli, an indicator of fecal contamination.12 In response to the outbreak, CHHSD and the water, sanitation, and hygiene (WASH) cluster, composed of non-governmental organizations (NGOs) and international agencies, conducted interventions starting in October 2011. The interventions included health and hygiene educational campaigns and distribution of household HWTS products. Although monthly reported cases decreased from 566 in November to 456 in December, 1,389 cases were reported in January 2012, notably in the neighboring suburb Kuwadzana with an estimated population of 129,000 (City of Harare, unpublished data).
The Zimbabwe Ministry of Health and Child Welfare (MOHCW) and CHHSD requested assistance from the U.S. Centers for Disease Control and Prevention (CDC) to describe the epidemiology of the outbreak and the response activities, the use of the HWTS products among residents of the affected suburbs, and factors that influenced HWTS uptake. Concurrently, USAID/OFDA requested CDC to evaluate the impact of WaterGuard on HWTS uptake during the outbreak. A small number of Typhi isolates were available for additional characterization in the laboratory and results were compared with those from previous outbreaks of typhoid fever in sub-Saharan Africa.
Methods
Epidemiology of the outbreak.
Line lists of all suspected and confirmed cases seen at all 52 health centers in Harare were maintained at the CHHSD. Suspected cases were defined as fever of ≥ 3 days duration and either malaise, headache, vomiting, diarrhea, constipation, or cough in a person who lived in or visited Harare since October 1, 2011. Confirmed cases met the suspected case definition and had Typhi isolated from the blood, stool, or urine culture. Early in the outbreak, contact tracing for all suspected cases was conducted, including home visits. Blood, stool, and urine specimens were collected from all suspected cases from Harare suburbs designated as high risk. As the etiology of the outbreak was confirmed and the antimicrobial susceptibility patterns were established, only every fifth suspected case and later every tenth suspected case was cultured for Typhi. The data presented in this work were obtained from the CHHSD on May 2, 2012.
Isolate characterization.
CDC provided technical and material support to diagnostic laboratories involved in the outbreak response in Zimbabwe. Additionally, CDC conducted pulsed-field gel electrophoresis (PFGE) characterization and antimicrobial susceptibility testing (AST) on eight Typhi isolates from the outbreak, and three Typhi isolates from an outbreak that occurred in Mabvuku, a suburb of Harare, in 2009 (Figure 1). Standard PFGE methods were used and patterns were analyzed using BioNumerics version 5.0 software (Applied Maths, Sint-Martens-Latem, Belgium).13 The AST was performed using broth microdilution (Sensititire, Trek Diagnostics part of Thermo Scientific, Cleveland, OH) according to manufacturer's instructions and interpreted using Clinical and Laboratory Standards Institute criteria when available.14
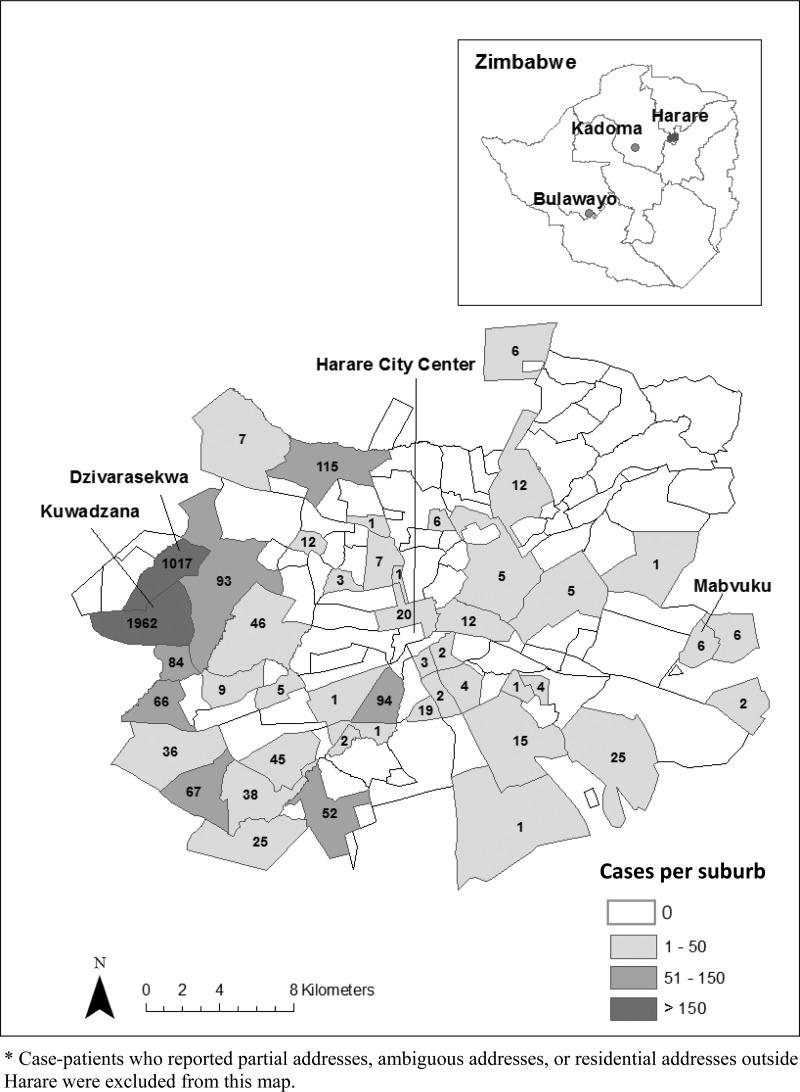
Figure 1.
Map of Harare, Zimbabwe, showing total number of confirmed and suspected cases of typhoid fever by suburbs, October 1, 2011–April 30, 2012 (N = 4,002*).
Evaluation of household-level intervention and water treatment uptake.
Stakeholder interviews and a cross-sectional survey in Kuwadzana and Dzivaresekwa, selected for the large number of cases reported from these two suburbs, were conducted in March 2012.
Stakeholder interviews.
Details of household-level response activities conducted by the WASH cluster were collected through interviews with several WASH cluster members responsible for activity coordination and MOHCW and CHHSD officials.
Population and sample.
Sample size calculations for similar studies suggested a sample size of 200 per program or community was suitable for identifying household-level differences between HWTS users and non-users.10 Using maps to create a sampling frame, 250 housing units (to account for non-responses) were randomly selected from each of the two suburbs, Kuwadzana and Dzivaresekwa. A housing unit was defined as a single housing structure. A household was defined as a group of individuals who shared one cooking pot. If the housing unit selected contained multiple households, one was randomly selected for inclusion in the study sample. One respondent from each household, who was a resident of the selected household for ≥ 6 months and was responsible for water and childcare in the household at the time of the survey, was selected for the survey.
Survey procedures.
Face-to-face interviews were conducted by trained native speakers of the dominant local language (Shona) using a structured questionnaire. The questionnaire included questions on household demographics, knowledge about typhoid fever, water sources, and HWTS use before and during the typhoid fever outbreak, HWTS products and typhoid prevention messages received during the outbreak, and ability and motivational factors around HWTS. Responses to items comprising ability and motivation behavioral constructs (self-efficacy, beliefs, social support, and perceived threat severity and susceptibility) were graded on a five-point Likert scale from strongly agree to strongly disagree. The questionnaire was translated into Shona and back-translated to English to verify translation accuracy and pre-tested in another Harare suburb. Samples of reportedly treated water stored in the household were tested for free chlorine residuals (FCR) using the Hach Color Wheel test kit (Hach Company, Loveland, CO). All completed survey forms were entered into Access 2010 (Microsoft Corp., Redmond, WA) and Techneos Entryware version 6.1 (Techneos Systems Inc., Vancouver, BC, Canada) databases.
Analysis of survey data.
Data were analyzed using SAS version 9.3 (SAS Institute Inc., Cary, NC) and STATA version 11 (Stata Corp., College Station, TX). Frequencies of survey respondents and household characteristics for each suburb are reported. McNemar's test was used to compare reported HWTS behaviors before and during the outbreak. Data on ability and motivational factors reported by a respondent per household were prepared and analyzed following the steps outlined previously: a dichotomous variable was created for each behavioral construct using a composite score of items and graded as low or negative if the average score was ≤ 3.0 and high or positive if > 3.0.15–17 The HWTS outcomes were measured as reporting water treatment during the outbreak; having reportedly treated stored water in the household on the day of the unannounced visit; and having reportedly treated stored water with FCR ≥ 0.2 mg/L on the day of unannounced visit. The χ2 test P values are reported for factors associated with HWTS outcomes.
Ethics.
The Zimbabwe MOHCW and CHHSD gave permission to conduct these activities as they constituted public health response and program evaluation. Verbal informed consent for participation in the survey was obtained from each respondent.
Results
Epidemiology of the outbreak.
From October 1, 2011 to April 30, 2012, a total of 4,181 suspected and 52 confirmed cases of typhoid fever were identified in Harare (Figures 1 and 2). Patient median age was 15 years (range: < 1–95 years); 54% were female. Two patients died and 1,788 (43%) were hospitalized. Suspected cases were reported predominantly in the high-density suburbs of Kuwadzana (1,965) and Dzivaresekwa (1,017). Two hundred and seven patients treated in Harare reported home addresses in other provinces; however, limited surveillance and laboratory capacity outside Harare made it impossible to confirm whether the outbreak spread to other areas.
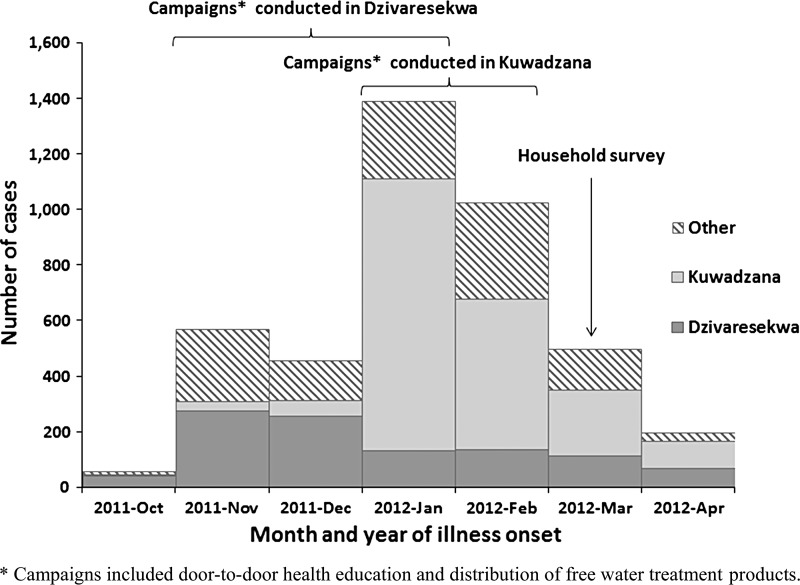
Figure 2.
Total number of confirmed and probable cases of typhoid fever in Harare, Zimbabwe, by suburb and month of illness onset, and the timing of typhoid fever prevention campaigns and the survey, October 1, 2011–April 30, 2012 (N = 4,181).
Isolate characterization.
Three Typhi isolates from a 2009 typhoid fever outbreak in Mabvuku and eight Typhi isolates from the 2011–12 outbreak collected before December 2011 in Dzivaresekwa were analyzed at CDC. Ten of 11 isolates shared an indistinguishable XbaI pattern previously seen in isolates from Malawi and Tanzania; a single isolate from 2011 had a different XbaI pattern. The three isolates from 2009 and two of the 2011 isolates also shared an indistinguishable BlnI pattern; two other BlnI patterns were seen among the remaining six isolates from 2011. None of these BlnI patterns had been seen previously in the PulseNet database, the molecular subtyping database of foodborne disease-causing bacteria. All eleven isolates were resistant to ampicillin, chloramphenicol, streptomycin, sulfisoxazole, and trimethoprim-sulfamethoxazole; a single isolate from 2011 showed intermediate resistance to amoxicillin/clavulanic acid. The isolates were susceptible to ciprofloxacin and ceftriaxone.
Evaluation of household-level intervention and water treatment uptake.
Household-level intervention.
In Dzivaresekwa, between October 2011 and January 2012, NGOs distributed information, education, and communication (IEC) materials and sufficient chlorine tablets to treat 20 L of water every day per household for 3 months to 12,000 households. Narrow-necked jerry cans18 and soap were also distributed to 3,500 households, and WaterMaker brand flocculant/disinfectant sachets (Control Chemicals (Pty) Ltd., Johannesburg, South Africa) were provided to some households. In November and December 2011, different NGOs distributed a 3-month supply of chlorine tablets and conducted door-to-door health education to 17,000 households in Dzivaresekwa. Several new boreholes were also sunk. In Kuwadzana, NGOs conducted door-to-door health education at 30,500 households and distributed IEC materials and a 3-month supply of chlorine tablets to 22,000 households in January and February 2012. Church leader training, awareness campaigns at church congregations, and educational drama sessions were also conducted. At least three different types of chlorine tablets were distributed in these responses: Oasis brand 67-mg tablets meant for 8–10 L of water (Hydrachem Ltd., West Sussex, UK), Aquatabs brand 67-mg tablets meant for 8–10 L of water, and Aquatabs brand 167-mg tablets meant for 20–25 L of water (Medentech, Wexford, Ireland). These doses are recommended for emergencies, such as disease outbreaks, to achieve a chlorine dosage of 4–5 mg/L, when the chlorine demand in water is expected to be higher. Written materials that were handed out during the distribution noted to use “1 tablet for 20 L of water,” without specifying the tablet size. Households that received 67-mg tablets were verbally instructed to use 2 tablets for 20 L by volunteers. Households were instructed to treat water from all sources daily.
Although WaterGuard was not distributed during the outbreak, residents of both suburbs received WaterGuard flyers with the free products, and were encouraged to buy WaterGuard to continue HWTS after finishing the supply of free products that were distributed in the emergency. The reasons responders did not distribute WaterGuard included: WaterGuard was considered by WASH cluster partners as a “permanent” or “maintenance” product, and not an emergency product; some NGOs had stocks of chlorine tablets available for distribution and had promoted the distribution of tablets for this and previous emergencies; WaterGuard solution bottles are bulkier and heavier, thus harder to distribute than tablets using the door-to-door distribution mechanism; and, PSI was promoting WaterGuard through social marketing and did not want free product distribution to affect the success of the longer-term social marketing effort.
Household survey respondent characteristics.
The median age of the survey respondents for 458 households in Kuwadzana and Dzivaresekwa was 31 years; 85% were women (Table 1). Over 80% had at least a secondary education, and over 80% of households had a mobile phone, a television, or both. The median monthly household income was US$ 225.
Table 1.
Socio-demographic characteristics and typhoid fever knowledge of survey respondents and households, Dzivaresekwa and Kuwadzana suburbs, Harare, Zimbabwe, March 2012*
| Kuwadzana | Dzivaresekwa | Overall | ||||
|---|---|---|---|---|---|---|
| N | n (%) | N | n (%) | N | n (%) | |
| Sex | 226 | 231 | 457 | |||
| Female | 192 (85) | 198 (86) | 390 (85) | |||
| Age in years | ||||||
| Median age (range) | 32 (16–85) | 31 (16–70) | 31 (16–85) | |||
| Highest level of education | 227 | 231 | 458 | |||
| None | 5 (2) | 5 (2) | 10 (2) | |||
| Primary | 34 (15) | 40 (17) | 74 (16) | |||
| Secondary | 166 (73) | 174 (75) | 340 (74) | |||
| More than secondary | 22 (10) | 12 (5) | 34 (7) | |||
| Religion | 214 | 229 | 443 | |||
| Pentecostal | 87 (41) | 101 (44) | 188 (42) | |||
| Protestant | 48 (22) | 54 (24) | 102 (23) | |||
| Apostolic Sector | 44 (21) | 30 (13) | 74 (17) | |||
| Other | 35 (16) | 44 (19) | 79 (18) | |||
| Household ownership of communication devices | ||||||
| Radio | 227 | 109 (48) | 229 | 109 (48) | 456 | 218 (48) |
| Television | 227 | 185 (82) | 230 | 188 (82) | 457 | 373 (82) |
| Mobile telephone | 227 | 207 (91) | 230 | 218 (95) | 457 | 425 (93) |
| Household monthly income (2012 US$)† | 129 | 121 | 250 | |||
| Median | 250 | 200 | 225 | |||
| Range | 0–2,000 | 0–2,000 | 0–2,000 | |||
| Have heard of typhoid fever | 227 | 225 (99) | 231 | 229 (99) | 458 | 454 (99) |
| Beliefs about cause of typhoid | 207 | 205 | 412 | |||
| Drinking contaminated water | 154 (74) | 163 (80) | 317 (77) | |||
| Eating contaminated food | 104 (50) | 122 (60) | 226 (55) | |||
| Poor hand hygiene | 104 (50) | 88 (43) | 192 (47) | |||
| Spirits/curse | 1 (< 1) | 1 (< 1) | 2 (< 1) | |||
| Typhoid fever treatment | 221 | 228 | 449 | |||
| Go to clinic/hospital | 216 (98) | 225 (99) | 441 (98) | |||
| Home remedy | 82 (37) | 76 (33) | 158 (35) | |||
| Go to traditional/herbal/faith healer | 2 (1) | 1 (< 1) | 3 (1) | |||
Percentages may not sum to 100% because of rounding.
US$ is a legal currency in Zimbabwe.
Typhoid fever knowledge.
Over 99% of respondents had heard of Typhoid fever (Table 1). Drinking contaminated water was most commonly reported as the cause of typhoid fever, followed by eating contaminated food, and poor hand hygiene: only one person in each suburb reported spirits or curse as the cause of typhoid fever. In another question on typhoid fever treatment, 98% of respondents reported they would go to a clinic or a hospital and only three respondents reported they would go to a traditional, herbal, or faith healer.
Source of household water.
Before the outbreak, the primary source of drinking water for 75% of households was municipal tap water, whereas remaining households primarily drank water from boreholes (Table 2). Borehole water was also the main alternative source of water in these two suburbs when disruption of the municipal supply occurred. During the outbreak, the reported source of drinking water did not change in Kuwadzana, although the number of households reporting borehole water as the primary source increased in Dzivaresekwa. Only 30% of respondents believed their current water was safe to drink.
Table 2.
Source of household water, WaterGuard knowledge, intervention coverage, and household water treatment among survey respondents and households, Dzivaresekwa and Kuwadzana suburbs, Harare, Zimbabwe, March 2012*
| Kuwadzana | Dzivaresekwa | Overall | ||||
|---|---|---|---|---|---|---|
| N | n (%) | N | n (%) | N | n (%) | |
| Primary source of water before outbreak | 220 | 222 | 442 | |||
| Municipal tap water | 164 (75) | 169 (76) | 333 (75) | |||
| Borehole | 55 (25) | 50 (23) | 105 (24) | |||
| Well | 1 (< 1) | 3 (1) | 4 (1) | |||
| Primary source of water during outbreak | 224 | 228 | 452 | |||
| Municipal tap water | 164 (73) | 80 (35) | 244 (54) | |||
| Borehole | 57 (25) | 146 (64) | 203 (45) | |||
| Well/Rain water | 3 (2) | 2 (1) | 5 (1) | |||
| Believe that current source of water is safe to drink | 224 | 62 (28) | 227 | 72 (32) | 451 | 134 (30) |
| WaterGuard knowledge before outbreak | ||||||
| Saw advertisements | 222 | 173 (78) | 225 | 161 (72) | 447 | 334 (74) |
| Saw bottles in stores | 223 | 139 (62) | 227 | 116 (51) | 450 | 255 (57) |
| Purchased | 224 | 42 (19) | 225 | 44 (20) | 449 | 86 (19) |
| Received any products during campaigns | 224 | 181 (81) | 224 | 198 (88) | 448 | 379 (85) |
| Received information on typhoid fever prevention | 223 | 200 (90) | 229 | 205 (90) | 452 | 405 (90) |
| If received tablets, have used them | 176 | 157 (89) | 196 | 177 (90) | 372 | 334 (90) |
| Reported using any household water treatment | 227 | 231 | 458 | |||
| Before outbreak | 159 (70) | 177 (77) | 336 (73) | |||
| During outbreak | 179 (79) | 201 (87) | 380 (83) | |||
| Reported using WaterGuard | 227 | 231 | 458 | |||
| Before outbreak | 29 (13) | 27 (12) | 56 (12) | |||
| During outbreak | 29 (13) | 34 (15) | 63 (14) | |||
| Commonly reported reasons for not using WaterGuard during outbreak | 187 | 195 | 382 | |||
| Too expensive/cannot afford | 28 (15) | 40 (21) | 68 (18) | |||
| Never heard of WaterGuard | 23 (12) | 31 (16) | 54 (14) | |||
| Have free products | 16 (9) | 32 (16) | 48 (13) | |||
| Do not know how to use WaterGuard | 17 (9) | 24 (12) | 41 (11) | |||
For some items, N may vary by small numbers because of non-response.
Intervention coverage and water treatment behavior.
Before the outbreak, 74% of respondents had seen WaterGuard advertisements, 57% had seen WaterGuard in stores, and 19% had purchased a bottle (Table 2). Overall, 81% of households in Kuwadzana, and 88% of households in Dzivaresekwa reported receiving at least one water treatment product, predominantly chlorine tablets: 90% of households that received chlorine tablets reported using them. Seventy-three percent of households reported use of any water treatment before the outbreak, consisting mostly of boiling or chlorine tablets. The percentage of households that reported using water treatment increased during the outbreak to 83%, the majority using chlorine tablets (P < 0.01). Approximately 12% of households reported using WaterGuard before the outbreak, and there was no change in its usage during the outbreak (P > 0.05). The commonly reported reasons for not using WaterGuard during the outbreak included too expensive, did not know what WaterGuard is, had free products, and did not know how to use WaterGuard. Of 458 households surveyed, 346 (76%) had stored water on the day of the unannounced visit; 97% were using covered containers for water storage (Figure 3). Only 143 households (31% of all households and 41% of households with stored water) reported their stored water was treated. Samples from 81 (18% of all households and 59% of households with reportedly treated water that consented to testing) had the recommended FCR of 0.2–2.0 mg/L; 17 (4% of all households and 12% of households with reportedly treated water that consented testing) had FCR > 2.0 mg/L. Of 339 households with chlorine tablets available for observation, 242 (71%) had Aquatabs brand 67-mg tablets only, 81 (24%) had Aquatabs brand 167-mg tablets only, 8 (2%) had both Aquatabs brand 67-mg and 167-mg tablets, and 8 (2%) had Oasis brand 67-mg or 167-mg tablets. When asked about the number of tablets per volume of water used for water treatment (dosage), of 301 respondents, 182 (60%) reported the recommended dosage during emergency; 95 (32%) reported using one 67-mg tablet for 20–25 L of water, which is the recommended dosage for non-emergency; and the remaining 24 (8%) reported various incorrect dosages. Of the 63 respondents who reported using WaterGuard during outbreak, 45 (71%) reported also using tablets, though the timing of each product use was not collected.
Figure 3.
Presence and free chlorine residual (FCR) of stored drinking water on the day of unannounced visit, Dzivaresekwa and Kuwadzana suburbs, Harare, Zimbabwe, March 2012.
Ability and motivation behavioral factors.
Most respondents agreed that diarrhea could kill people and 77% considered typhoid a serious problem caused by drinking poor quality water (Table 3). Fifty-six percent of respondents believed that many people in the neighborhood were still succumbing to typhoid fever at the time of the survey. About 75% of respondents reportedly felt that their families are more vulnerable to get typhoid if they did not treat water with chlorine products. At least 85% of respondents reported they felt able to effectively practice HWTS. Approximately 86% received social support from family on water treatment, and 68% reported talking among friends about water treatment. Two-thirds of respondents believed that water treatment products made water taste bad, whereas 51% were not sure if water treatment products were harmful.
Table 3.
Ability and motivation behavioral factors associated with typhoid fever outbreak and household water treatment among survey respondents, Dzivaresekwa and Kuwadzana suburbs, Harare, Zimbabwe, March 2012 (N = 457*)
| Construct‡ | Response n (%)† | ||
|---|---|---|---|
| Agree | Not sure | Disagree | |
| Threat severity: an individual's view about the extent to which typhoid fever may harm him or her family | |||
| Typhoid is a serious problem in our community | 354 (78) | 49 (11) | 53 (12) |
| Diarrheal diseases can kill | 437 (96) | 11 (2) | 6 (1) |
| In this community, people suffer from typhoid caused by poor water quality | 350 (77) | 73 (16) | 34 (7) |
| In my community, many people continue to get typhoid | 255 (56) | 118 (26) | 82 (18) |
| Threat susceptibility: an individual's view about the chances that typhoid fever may harm his or her family | |||
| My family members are more vulnerable to get typhoid if I do not treat water with chlorine product(s) | 345 (75) | 49 (11) | 63 (14) |
| The risk of family members getting typhoid seems to be increasing | 296 (65) | 53 (12) | 107 (23) |
| Whenever my family members drink water, I think about their risk of getting typhoid | 274 (60) | 48 (11) | 135 (29) |
| Self-efficacy: a person's feeling about ability to effectively practice household water treatment | |||
| I am able to use water treatment product(s) to improve water quality | 407 (89) | 9 (2) | 39 (9) |
| I am confident that I can effectively treat water with water treatment products | 387 (85) | 23 (5) | 47 (10) |
| I am able to persuade my partner/family members to treat water to improve the quality of drinking water | 412 (90) | 23 (5) | 22 (5) |
| Social support: the help an individual receives that may be emotional, tangible, or informational | |||
| My friends encourage me to treat water all year around | 337 (74) | 41 (9) | 79 (17) |
| My family members support my decision to treat water | 390 (86) | 36 (8) | 30 (7) |
| My friends talk a lot about water treatment | 310 (68) | 45 (10) | 102 (22) |
| Beliefs: an individual's views about household water treatment | |||
| Treating water makes the water taste bad | 303 (67) | 39 (9) | 113 (25) |
| Water treatment products contain chemicals that are not good for health | 94 (21) | 231 (51) | 132 (29) |
| Water treatment products do not kill all the germs in water | 130 (29) | 171 (38) | 154 (34) |
For some items, N may vary by small numbers because of non-response. Percentages may not sum to 100% because of rounding.
Likert scale was collapsed into three categories to summarize the results.
Constructs are ideas produced by combining simple statements (items) that try to explain certain behavior.
Factors associated with water treatment.
Reported water treatment during the outbreak, having reportedly treated stored water at home, and having reportedly treated stored water with FCR ≥ 0.2 mg/L were more common among households that reported water treatment before the outbreak, and those that received either typhoid prevention messages or any free products during the outbreak (P < 0.01). Older respondents (≥ 31 years) were also more likely than younger respondents to have reportedly treated stored water (P < 0.01) and to have reportedly treated stored water with FCR ≥ 0.2 mg/L (P < 0.01), although they were not more likely to report any water treatment during the outbreak (Table 4). Respondents' sex, religion, education level, household income, or knowledge of correct dosage (tablet per water volume) were not associated with the three outcome metrics, nor was knowledge and usage of WaterGuard before the outbreak (P > 0.05). Households whose primary water source was borehole water during the outbreak were less likely to have reportedly treated stored water (P < 0.01) or have reportedly treated stored water with FCR ≥ 0.2 mg/L (P = 0.01). In the analysis of ability and motivation behavioral factors, respondent's high self-efficacy about water treatment, high perceived susceptibility to typhoid fever, and good family support in water treatment were associated with each of the three outcome metrics (P < 0.05) (Table 5). Reported diarrhea (defined as three or more loose bowel movements in 24 hours) among household members in the 2 weeks before the survey, and reported typhoid fever among household members since October 2011 were not associated with the outcome metrics (P > 0.05).
Table 4.
Factors associated with household water treatment, Dzivaresekwa and Kuwadzana suburbs, Harare, Zimbabwe, March 2012
| Reported water treatment since outbreak began | Had reportedly treated stored water | Had reportedly treated stored water with FCR ≥ 0.2 mg/L | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Yes (N = 380)* | No (N = 78)* | P | Yes (N = 143)* | No (N = 315)* | P | Yes (N = 98)* | No (N = 360)* | P | |
| Respondent's age ≥ 31 years, n (%) | 197 (52) | 42 (54) | 0.75 | 91 (64) | 148 (47) | < 0.01 | 64 (65) | 175 (49) | < 0.01 |
| Reported water treatment before outbreak began, n (%) | 306 (82) | 30 (43) | < 0.01 | 120 (86) | 216 (72) | < 0.01 | 82 (87) | 254 (73) | < 0.01 |
| Primary source of water is borehole, n (%) | 174 (46) | 37 (47) | 0.84 | 51 (36) | 160 (51) | < 0.01 | 28 (29) | 183 (51) | < 0.01 |
| Received typhoid prevention message, n (%) | 351 (93) | 54 (72) | < 0.01 | 138 (97) | 267 (86) | < 0.01 | 94 (97) | 311 (88) | < 0.01 |
| Received products, n (%) | 343 (92) | 36 (47) | < 0.01 | 131 (95) | 248 (80) | < 0.01 | 92 (96) | 287 (82) | < 0.01 |
For some items, N may vary by small numbers because of non-response.
Values that are statistically significant at the α = 0.05 level are indicated in bold.
Table 5.
Ability and motivation factors for household water treatment and their association with household water treatment behavior, Dzivaresekwa and Kuwadzana suburbs, Harare, Zimbabwe, March 2012
| Reported water treatment since outbreak began | Had reportedly treated stored water | Had reportedly treated stored water with FCR ≥ 0.2 mg/L | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Yes (N = 380)* | No (N = 78)* | P | Yes (N = 143)* | No (N = 315)* | P | Yes (N = 98)* | No (N = 360)* | P | |
| High self-efficacy, n (%) | 354 (94) | 57 (73) | < 0.01 | 139 (98) | 272 (86) | < 0.01 | 96 (99) | 315 (88) | < 0.01 |
| High threat susceptibility, n (%) | 269 (71) | 45 (58) | 0.02 | 116 (81) | 198 (63) | < 0.01 | 82 (84) | 232 (65) | < 0.01 |
| Good social support, n (%) | 298 (79) | 50 (64) | < 0.01 | 120 (84) | 228 (73) | 0.01 | 83 (85) | 265 (74) | 0.03 |
| Positive beliefs, n (%) | 125 (33) | 23 (29) | 0.51 | 50 (36) | 98 (31) | 0.36 | 35 (36) | 113 (32) | 0.37 |
| High threat severity, n (%) | 340 (91) | 66 (86) | 0.17 | 128 (91) | 278 (90) | 0.72 | 90 (93) | 316 (89) | 0.31 |
For some items, N may vary by small numbers because of non-response.
Values that are statistically significant at the α = 0.05 level are indicated in bold.
Discussion
Large outbreaks of typhoid fever, like the one described in this report, have become increasingly common in sub-Saharan Africa.19 Although some outbreaks have occurred in rural areas, others have struck residents of national capitals, such as Kinshasa and Lusaka, and the disease has become endemic in some parts of Nairobi (Clark and others, unpublished data).20–22 The PFGE patterns of strains from the Harare outbreak in 2011–12 were indistinguishable from or highly similar (1 or 2 bands different) to those from an earlier outbreak in Harare and to strains from Malawi and Tanzania, suggesting persistence of these strains over time in Zimbabwe and dispersion across southern and eastern Africa. The recent evidence of the magnitude of epidemic and endemic typhoid fever in sub-Saharan African countries highlights the continued importance of typhoid fever prevention and control in this region. Baseline knowledge of typhoid fever and disease transmission in an urban, educated population such as seen in Harare, Zimbabwe potentially differ from that of rural, less educated populations: prevention efforts and health education materials must be tailored to pre-existing local knowledge and conditions.
One potential prevention strategy is increasing community education and access to products to improve drinking water quality and household hygiene. Previous research in emergency contexts has documented that higher uptake of HWTS is associated with distribution of an effective HWTS method, including the necessary supplies and training, to households with contaminated water who were familiar with the HWTS method before the emergency.10 The launch of WaterGuard following the 2008–09 cholera outbreak in Zimbabwe raised awareness of household water treatment and provided Harare residents with long-term access to a product marketed for that purpose. However, although we documented high awareness of WaterGuard and high reported coverage of the educational and HWTS product distribution campaigns in the two suburbs most affected by the outbreak, reported treatment on the day of an unannounced visit was low, FCR levels in reportedly treated water were often inadequate, and the knowledge of chlorine tablet dosage (tablets per volume of water) was frequently inaccurate, which suggested confusion about correct use during emergencies. Although we did not observe the direct impact of WaterGuard in this evaluation of HWTS during the outbreak, we found that households that used any HWTS method, including WaterGuard, before the outbreak were more likely to report and practice water treatment during the outbreak, as were households that had received typhoid prevention messages or any free product for water treatment during the outbreak. Households that used boreholes as a primary water source were less likely to use HWTS during the outbreak, possibly caused by a false sense of safety.
In focus group discussions among residents of the two suburbs and volunteers who conducted educational campaigns and product distribution, discussion participants emphasized that water treatment alone does not alleviate typhoid fever in their communities, and that local government should collect waste more frequently to keep the environment clean, and conduct continuous awareness campaigns on waste disposal methods (Chizororo and others, unpublished data). Community members also voiced resistance to buying WaterGuard because of the cost and because they did not like the taste of water treated at home, suggesting the need for improved messaging. Retailers who order WaterGuard directly from suppliers charge ≤ US$ 0.50 per bottle of WaterGuard which should be affordable for most households. Although HWTS is low-cost and effective when used properly, to definitively reduce the risk of typhoid fever, cholera, and other waterborne diseases in Harare, long-term improvements in the provision of piped, treated, potable water will be needed.
We recommended that, until such time as affected populations have access to centralized, treated water sources, implementers continue to emphasize the need to treat water from all sources daily through social mobilization activities. We recommended that government officials and WASH cluster partners that conduct response activities be better coordinated to ensure distribution of standardized products and usage instructions. In particular, HWTS products should be of the same type and dosage to minimize confusion among recipients. Larger-scale distribution of standardized safe water storage containers might also improve correct use of water treatment products and maintenance of adequate FCR levels in treated water. Self-efficacy in using water treatment products, perceiving one's family to be susceptible to typhoid fever, and having good social support to treat water, were among the key behavioral determinants of HWTS use during this typhoid fever outbreak. These findings concur with other studies on HWTS use in Africa.8,23 Campaigns should emphasize equipping individuals with knowledge and skills to effectively use HWTS products to improve water quality and safety. In emergency contexts, messages should also highlight the health implications of typhoid fever and the risk of getting waterborne diseases to families and communities as prior research has found that water disinfectant sales increased in Zambia during a cholera outbreak and rainy season, when the perceived risk of cholera was high.24 However, in the development context, threat of disease is insufficient to motivate behavior changes, as a study in Guatemala found that the health impact of reduced diarrheal illnesses was not a motivator for chlorinating water.25 In a review of behavior change literature on HWTS options, Figueroa suggests that no single approach to the promotion of water treatment is likely to be sufficient to sustain the practice, and that addressing all of the factors—social, cultural, economic, demographic, political, and ecological—that facilitate or inhibit behavior change is needed to achieve sustained behavior change.26 One promising approach to develop social support for water treatment is the formation of social clubs where members discuss these issues and encourage one another.27
The limitations of the household survey included difficulty establishing the temporal relationship of perceptions or knowledge and reported behavior because of the cross-sectional survey design, the use of FCR as a proxy for microbiologically safe water, and a sample size insufficient for multivariate regression analysis or to detect health impact from water treatment. Two suburbs most affected by the outbreak were selected for the survey: the data may not be generalizable to other populations. Furthermore, the survey was conducted 5 months after the outbreak onset and the number of new cases had declined in the two suburbs. The small number of Typhi isolates collected early in the outbreak for PFGE subtyping and AST analysis limits how these data can be interpreted. It is not known if these isolates are representative of the outbreak as a whole, and therefore they may underestimate the strain diversity.
Since 2009, USAID/OFDA has supported PSI in local production and social marketing of WaterGuard as a disaster risk reduction measure in Zimbabwe. However, WaterGuard solution bottles were not distributed during the outbreak response. Based upon the findings and observations, recommendations were made to distribute WaterGuard in future emergencies. Welthungerhilfe, PSI, and Kadoma City Health Department (KCHD) successfully conducted a pilot study in Kadoma to distribute WaterGuard using vouchers in July 2012 as part of the public health emergency preparedness and response activity. Within 48 hours of outbreak notification to the WASH cluster, nearly 2,000 vouchers were distributed and 65% were redeemed, suggesting high user motivation and acceptance of the product (Welthungerhilfe, PSI, and KCHD, unpublished data). The pilot study also showed that the cost of distributing locally produced WaterGuard using voucher system was US$ 0.50 per bottle, which treats 1,000 L of water. This is 42% less expensive than distributing imported chlorine tablets per liter of water treated, which is estimated to cost US$ 0.86 for 1,000 L of water at emergency dosage. A partnership between the emergency response community and retailers that sell WaterGuard was identified as critical to ensure sustained stocking by retailers for daily use and access to the product during emergencies. In December 2012, 9 months after the investigation, 3,500 WaterGuard bottles were requested by an NGO to distribute to suspected typhoid fever patients through treatment centers in several Harare suburbs. The results presented herein highlight both the challenges and promise of linking disaster prevention and emergency programs.
ACKNOWLEDGMENTS
We thank the research assistants and survey participants for their contributions to the evaluation.
Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention, the U.S. Agency for International Development, and the U.S. Government.
Footnotes
Financial support: This investigation was supported by the U.S. Centers for Disease Control and Prevention Division of Global Disease Detection and Emergency Response, the U.S. Agency for International Development's Office of U.S. Foreign Disaster Assistance, the United Nations Children's Fund-Zimbabwe, Welthungerhilfe-Zimbabwe, and Population Services International-Zimbabwe.
Authors' addresses: Maho Imanishi and Rachel B. Slayton, Division of Foodborne, Waterborne, and Environmental Diseases, Centers for Disease Control and Prevention, Atlanta, GA, and Epidemic Intelligence Service, Scientific Education and Professional Development Program Office, Centers for Disease Control and Prevention, Atlanta, GA, E-mails: m.imanishi@gmail.com and via3@cdc.gov. Tracy Ayers, Molly M. Freeman, and Eric Mintz, Division of Foodborne, Waterborne, and Environmental Diseases, Centers for Disease Control and Prevention, Atlanta, GA, E-mails: eyk6@cdc.gov, evy7@cdc.gov, and emintz@cdc.gov. Patience F. Kweza and Lazarus R. Kuonza, Field Epidemiology and Laboratory Training Program, National Institute for Communicable Diseases, Johannesburg, South Africa, E-mails: Patiencek@nicd.ac.za and LazarusK@nicd.ac.za. Tanaka Urayai, Population Services International-Zimbabwe, Harare, Zimbabwe, E-mail: turayai@psi-zim.co.zw. Odrie Ziro, Welthungerhilfe-Zimbabwe, Harare, Zimbabwe, E-mail: Odrie.Ziro@welthungerhilfe.de. Wellington Mushayi and Monica Francis-Chizororo, Collaborating Centre for Operational Research and Evaluation, United Nations Children's Funds-Zimbabwe, Harare, Zimbabwe, E-mails: wmushayi@unicef.org and mchizororo@unicef.org. Emmaculate Govore, Clemence Duri, and Prosper Chonzi, City of Harare Health Services Department, Harare, Zimbabwe, E-mails: echotogovore@yahoo.com, kireduri@gmail.com, and chonziprosper@yahoo.com. Sekesai Mtapuri-Zinyowera, National Microbiology Reference Laboratory, Ministry of Health and Child Welfare, Harare, Zimbabwe, E-mail: zinyoweras@nmrl.org.zw. Portia Manangazira, Ministry of Health and Child Welfare, Harare, Zimbabwe, E-mail: directoredc@gmail.com. Peter H. Kilmarx, Centers for Disease Control and Prevention-Zimbabwe, Harare, Zimbabwe, and Division of Global HIV/AIDS, Centers for Disease Control and Prevention, Atlanta, GA, E-mail: pbk4@cdc.gov. Daniele Lantagne, Tufts University, Medford, MA, E-mail: daniele.lantagne@tufts.edu.
References
- 1.Levine MM. Typhoid Fever. In: Brachman PS, Abrutyn E, editors. Bacterial Infections of Humans: Epidemiology and Control. Fourth edition. New York, NY: Springer; 2009. pp. 913–937. [Google Scholar]
- 2.Buckle GC, Walker CL, Black RE. Typhoid fever and paratyphoid fever: systematic review to estimate global morbidity and mortality for 2010. J Glob Health. 2012;2:10401. doi: 10.7189/jogh.02.010401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nhapi I. The water situation in Harare, Zimbabwe: a policy and management problem. Water Policy. 2009;11:221–235. [Google Scholar]
- 4.Tanyanyiwa VI, Mutungamiri I. Residents perception on water and sanitation problems in Dzivaresekwa 1 high density suburb, Harare. Journal of Sustainable Development in Africa. 2011;13:309–342. [Google Scholar]
- 5.Zimbabwe National Statistics Agency Census 2012 Preliminary Report. 2013. http://www.zimstat.co.zw/dmdocuments/CensusPreliminary2012.pdf Available at. Accessed May 28, 2013.
- 6.World Health Organization Cholera in Zimbabwe: Epidemiological Bulletin Number 27 Week 24. 2009. http://www.who.int/hac/crises/zwe/sitreps/zimbabwe_epi_w24_7_13june2009.pdf Available at. Accessed January 31, 2014.
- 7.Quick RE, Venczel LV, Mintz ED, Soleto L, Aparicio J, Gironaz M, Hutwagner L, Greene K, Bopp C, Maloney K, Chavez D, Sobsey M, Tauxe RV. Diarrhoea prevention in Bolivia through point-of-use water treatment and safe storage: a promising new strategy. Epidemiol Infect. 1999;122:83–90. doi: 10.1017/s0950268898001782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Quick RE, Kimura A, Thevos A, Tembo M, Shamputa I, Hutwagner L, Mintz E. Diarrhea prevention through household-level water disinfection and safe storage in Zambia. Am J Trop Med Hyg. 2002;66:584–589. doi: 10.4269/ajtmh.2002.66.584. [DOI] [PubMed] [Google Scholar]
- 9.Arnold BF, Colford JM., Jr Treating water with chlorine at point-of-use to improve water quality and reduce child diarrhea in developing countries: a systematic review and meta-analysis. Am J Trop Med Hyg. 2007;76:354–364. [PubMed] [Google Scholar]
- 10.Lantagne DS, Clasen TF. Use of household water treatment and safe storage methods in acute emergency response: case study results from Nepal, Indonesia, Kenya, and Haiti. Environ Sci Technol. 2012;46:11352–11360. doi: 10.1021/es301842u. [DOI] [PubMed] [Google Scholar]
- 11.Date KPB, Nygren B, Were V, Koka V, Ayers T, Quick R. Evaluation of a rapid cholera response activity—Nyanza Province, Kenya, 2008. J Infect Dis. 2013;208((Suppl 1)):S62–S68. doi: 10.1093/infdis/jit198. [DOI] [PubMed] [Google Scholar]
- 12.Centers for Disease Control and Prevention Notes from the field: Salmonella Typhi infections associated with contaminated water—Zimbabwe, October 2011–May 2012. MMWR. 2012;61:435. [PubMed] [Google Scholar]
- 13.Ribot EM, Fair MA, Gautom R, Cameron DN, Hunter SB, Swaminathan B, Barrett TJ. Standardization of pulsed-field gel electrophoresis protocols for the subtyping of Escherichia coli O157:H7, Salmonella, and Shigella for PulseNet. Foodborne Pathog Dis. 2006;3:59–67. doi: 10.1089/fpd.2006.3.59. [DOI] [PubMed] [Google Scholar]
- 14.Clinical and Laboratory Standards Institute . Performance Standards for Antimicrobial Susceptibility Testing; Twentieth Informational Supplement. Wayne, PA: CLSI; 2013. [Google Scholar]
- 15.Patel DS, Chapman S. The Dashboard: A Tool for Social Marketing Decision Making. PSI Research Division; 2005. http://www.psi.org/sites/default/files/publication_files/dashboard-2005.pdf Concept paper. Available at. Accessed May 10, 2013. [Google Scholar]
- 16.Chapman S, Patel D. Concept Paper PSI Behavior Change Framework “Bubbles”: Proposed Revision. 2004. http://www.psi.org/sites/default/files/publication_files/behaviorchange.pdf Available at. Accessed April 18, 2013.
- 17.United States Agency for International Development Access and Behavioral Outcome Indicators for Water, Sanitation, and Hygiene. 2010. http://www.hip.watsan.net/page/4148 Available at. Accessed April 14, 2013.
- 18.Centers for Disease Control and Prevention The Safe Water System, Jerry Cans. 2012. http://www.cdc.gov/safewater/jerrycan.html Available at. Accessed October 23, 2013.
- 19.Slayton RB, Date KA, Mintz ED. Vaccination for typhoid fever in sub-Saharan Africa. Hum Vaccin Immunother. 2013;9:903–906. doi: 10.4161/hv.23007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Breiman RF, Cosmas L, Njuguna H, Audi A, Olack B, Ochieng JB, Wamola N, Bigogo GM, Awiti G, Tabu CW, Burke H, Williamson J, Oundo JO, Mintz ED, Feikin DR. Population-based incidence of typhoid fever in an urban informal settlement and a rural area in Kenya: implications for typhoid vaccine use in Africa. PLoS ONE. 2012;7:e29119. doi: 10.1371/journal.pone.0029119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lutterloh E, Likaka A, Sejvar J, Manda R, Naiene J, Monroe SS, Khaila T, Chilima B, Mallewa M, Kampondeni SD, Lowther SA, Capewell L, Date K, Townes D, Redwood Y, Schier JG, Nygren B, Tippett Barr B, Demby A, Phiri A, Lungu R, Kaphiyo J, Humphrys M, Talkington D, Joyce K, Stockman LJ, Armstrong GL, Mintz E. Multidrug-resistant typhoid fever with neurologic findings on the Malawi-Mozambique border. Clin Infect Dis. 2012;54:1100–1106. doi: 10.1093/cid/cis012. [DOI] [PubMed] [Google Scholar]
- 22.Muyembe-Tamfum JJ, Veyi J, Kaswa M, Lunguya O, Verhaegen J, Boelaert M. An outbreak of peritonitis caused by multidrug-resistant Salmonella typhi in Kinshasa, Democratic Republic of Congo. Travel Med Infect Dis. 2009;7:40–43. doi: 10.1016/j.tmaid.2008.12.006. [DOI] [PubMed] [Google Scholar]
- 23.Wood S, Foster J, Kols A. Understanding why women adopt and sustain home water treatment: insights from the Malawi antenatal care program. Soc Sci Med. 2012;75:634–642. doi: 10.1016/j.socscimed.2011.09.018. [DOI] [PubMed] [Google Scholar]
- 24.Quick R. Changing community behavior: experience from three African countries. Int J Environ Health Res. 2003;13((Suppl 1)):S115–S121. doi: 10.1080/0960312031000102877. [DOI] [PubMed] [Google Scholar]
- 25.Luby SP, Mendoza C, Keswick BH, Chiller TM, Hoekstra RM. Difficulties in bringing point-of-use water treatment to scale in rural Guatemala. Am J Trop Med Hyg. 2008;78:382–387. [PubMed] [Google Scholar]
- 26.Figueroa ME, Kincaid DL. Social, Cultural and Behavioral Correlates of Household Water Treatment and Storage. 2010. http://www.jhuccp.org/sites/all/files/Household%20Water%20Treatment%20and%20Storage%202010.pdf Available at. Accessed December 13, 2013.
- 27.Waterkeyn J, Cairncross S. Creating demand for sanitation and hygiene through community health clubs: a cost-effective intervention in two districts in Zimbabwe. Soc Sci Med. 2005;61:1958–1970. doi: 10.1016/j.socscimed.2005.04.012. [DOI] [PubMed] [Google Scholar]