Abstract
In Myanmar, 60% of the population consists of mothers and children, and they are the groups most vulnerable to anemia. The objectives of this study are to determine (1) the anemia prevalence among lactating women and (2) the risk factors associated with anemia. Convenience sampling was used to select three villages in two different regions (Kachin and Shan) in Myanmar. Hemoglobin and anthropometric indicators were measured for 733 lactating women. Logistic regression analyses were used to determine factors associated with anemia. The anemia prevalence rate was 60.3% in lactating women, with 20.3% of lactating women having severe anemia. Factors of malnutrition (P = 0.026), self-reported symptoms of night blindness or poor dark adaptation (P < 0.001), lack of primary education experience (P < 0.001), low family annual capita income (< 800 MMK; P < 0.001), drinking spring or river water (P < 0.001), and drinking unboiled water (P = 0.016) were associated with anemia. To promote health in lactating women, a comprehensive intervention is needed in these regions.
Introduction
Maternal and child anemia is highly prevalent in low-income countries, resulting in substantial increases in mortality and overall disease burden.1 The World Health Organization (WHO) has reported that anemia contributes to 324,000 deaths and 12,500,000 disability adjusted life-years (DALYs) in southeast Asia, and these numbers are among the highest in the world.2 Myanmar is the largest country in southeast Asia geographically, with over 70% of its population residing in rural areas.3 Over 60% of the population (55.4 million as of December of 2006) is mothers and children, who are the most vulnerable group.3 Although the government and humanitarian organizations made great efforts to promote maternal and children health, the anemia prevalence in Myanmar is still unacceptably high (around 70% in children and 60% in women).4–6 Multiple factors contribute to anemia, including inherited conditions, infectious diseases, micronutrient deficiencies (such as iron, folate, and vitamin B12), and compromised environment factors.7,8
The post-partum period is conventionally thought to be the time of lowest anemia risk, because iron status is expected to dramatically improve after delivery because of the lower iron requirement with the birth of the infant and reduced blood losses by amenorrhea.9 However, recent studies have pointed out that post-partum anemia is far more common than previously thought.10–12 Also, women in poverty are at higher risk of anemia, which suggests that lactating women in Myanmar might face a threat of anemia.12 To date, the anemia prevalence rate and its associated risk factors to lactating women in Myanmar are still unknown. In this study, lactating women in two rural villages located at Kachin and one village located at Shan (all located in the eastern part of Myanmar) were investigated. According to the health report from the Myanmar government in 2010, Shan region has the lowest antenatal care coverage rate in the country (53.1%),13 whereas Karchin has the highest prevalence of infectious diseases.13 This study was intended to determine the prevalence of anemia in lactating women in rural Myanmar and assess the factors that might be associated with anemia.
Methods
Participants.
This cross-sectional survey was conducted in Myanmar from January to May of 2011. Two villages in the rural area of Karchin (K1 and K2) and one village in Shan (S1) were selected with convenience sampling. Cluster sampling was used for screening all 747 women who were in the lactation period. Finally, 733 lactating women qualified and volunteered to participate in this survey (NK1 = 280, NK2 = 284, and NS1 = 169); 14 women did not qualify, mainly because they could not clearly respond to the questions or refused to take the blood test.
Data collection.
Blood testing, physical examinations, and interviews were all performed by local health professionals, who were coordinated with provincial health officers and the Health Poverty Action (HPA), which is a British non-governmental organization founded in 1984 that aims to secure healthcare access for marginalized communities in developing countries. An interviewer-administrated questionnaire was used to collect demographic characteristics, self-reported health-related status (diseases and symptoms that occurred in the last 3 months), and dietary information from the lactating women. The food consumption frequencies of three kinds of food (meat, egg, and dairy products), which are considered to be closely related to iron and protein intakes, were investigated and ranked as (1) never or less than one time per week, (2) one to six times per week, and (3) daily. Preliminary questionnaire testing, training of examiners and interviewers, and a pilot study were completed before data collection.
Height and weight were measured to calculate the body mass index (BMI; kg/m2) and categorized based on the Asian BMI standard (< 18.5 kg/m2 was defined as underweight). Mid-upper arm circumference (MUAC) was measured at the middle of the left arm using single-slotted insertion tapes (LeCheng 10–100 cm; Zhejiang, LeChen Co. Ltd., China). MUAC < 22.5 cm for women indicated a status of malnutrition. Blood samples were obtained from finger pricks of women, and hemoglobin (Hb) levels were measured using the Hemoscan Blood Hemoglobin Photometer (Hemoscan POCT; Chengdu BSD Instrument Co. Ltd., China). The presence and severity of anemia were diagnosed using WHO criteria for women adults,14 which defines moderate anemia as Hb < 110 g/L and severe anemia as < 80 g/L.
Ethics.
This study was conducted in compliance with the Declaration of Helsinki and approved by Peking University. Written consent was obtained from all the participants before data collection.
Statistical analyses.
SPSS Version 15.0 (SPSS Inc., Chicago, IL) was used to carry out all the statistical analysis. All the variables were presented as mean, median, or frequency. χ2 test was performed to determine significance before regression analysis (only the variables with P < 0.05 were used to develop the regression model). Logistic regression (with the method of Backwards Wald) was used to obtain odds ratios (ORs) and 95% confidence intervals (95% CIs) and to analyze the associations between anemia and potential risk factors.
Results
Prevalence of anemia.
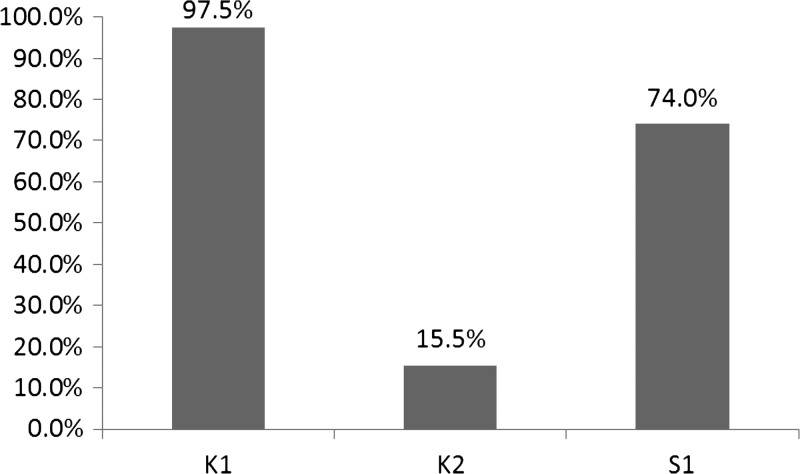
In total, 733 women qualified and participated in this study. The overall mean age was 28.8 ± 6.3 years (range = 16–49 years). The overall anemia prevalence was 60.3%, with 20.3% of women having severe anemia. The prevalence of anemia in three different regions is showed in Figure 1, where K1 village had the highest anemia rate. There were no age differences between anemic and non-anemic women (mean ages of anemic and non-anemic women were 28.9 ± 5.8 and 28.9 ± 6.7 years, respectively; F = 8.024, P = 0.836).
Figure 1.
Prevalence of anemia in lactating women from three villages in Myanmar.
Health indicators of participants.
In this study, 11.9% of women were underweight (BMI < 18.5 kg/m2), and 28.3% of women were considered malnourished per MUAC (MUAC < 22.5 cm). Based on the data from local medical records, malaria occurred in 54 women (7.4%) 3 months before this study, and no women had acquired immunodeficiency syndrome (AIDS) or human immunodeficiency virus (HIV). According to the self-reported health-related symptoms, 18.7% of women reported that they had experienced symptoms of night blindness or poor darkness adaptation in the past year, and 23.6% of women reported recurrent oral ulcer history. Also, 14.9% and 13.2% of women reported fever of unknown origin and unidentified diarrhea, respectively.
Compared with non-anemic lactating women, anemic women had a significantly higher proportion of malnutrition (defined by MUAC < 22.5 cm), self-reported symptoms of night blindness, self-reported recurrent ulcer, and self-reported fever of unknown origin according to single factor analyses (χ2 = 7.706, P = 0.006; χ2 = 74.55, P < 0.001; χ2 = 42.33, P < 0.001; and χ2 = 41.26, P < 0.001, respectively).
Socioeconomic and demographic characteristics, reproductive history, and dietary intake of women with or without anemia.
The median family annual capita income was 833 (interquartile range [IQR] = 375–1,429) Myanmar kyat (MMK; 1 MMK equals $0.15 US). Median incomes in K1, K2, and S1 were 400 (IQR = 207–667), 1,333 (IQR = 1,000–1,962), and 667 (IQR = 289–1,111) MMK, respectively. Lactating women from lower-income families (< 800 MMK), without primary education, who drank spring and river water, and who drank unboiled water were more at risk for anemia in single factor analysis (Table 1). Woman with prolificacy (parity > 2 times) were more susceptive anemia, whereas dystocia history and the time span of breastfeeding were not associated with anemia (Table 1).
Table 1.
The association of sociodemographic characteristics and self-reported health status with anemia for lactating women in single factor analysis
| Variables | Lactating women with anemia N (%) | Lactating women without anemia N (%) | P value |
|---|---|---|---|
| Family annual capita income N (MMK) | 442 | 291 | < 0.001 |
| > 1,000 | 68 (15.4) | 93 (32.0) | |
| 800–1,000 | 102 (23.1) | 107 (36.8) | |
| < 800 | 272 (61.5) | 91 (31.2) | |
| Education of mothers N | 442 | 291 | < 0.001 |
| With primary education | 172 (38.9) | 227 (78.0) | |
| Without primary education | 270 (61.1) | 64 (22.2) | |
| Source of water N | 442 | 291 | < 0.001 |
| Tap water | 124 (28.1) | 118 (40.5) | |
| Well water | 30 (6.8) | 99 (34.0) | |
| Spring and river water | 288 (65.2) | 74 (25.4) | |
| Whether water was boiled N | 439 | 280 | < 0.001 |
| Yes | 193 (44.0) | 195 (69.6) | |
| No | 246 (56.0) | 85 (30.4) | |
| Parity N | 442 | 291 | 0.001 |
| One or two times | 178 (40.3) | 159 (54.6) | |
| More than two times | 264 (59.7) | 132 (45.4) | |
| Dystocia history N | 441 | 289 | 0.679 |
| No | 392 (88.9) | 254 (87.9) | |
| Yes | 49 (11.1) | 35 (12.1) | |
| Time span of breastfeeding N (months) | 434 | 282 | 0.778 |
| 0–6 | 31 (7.1) | 22 (7.8) | |
| 6.1–12 | 193 (44.5) | 131 (46.5) | |
| > 12 | 210 (48.4) | 129 (45.7) |
Anemia is defined as Hb < 110 g/L.
In this survey, none of the women consumed dietary supplements during the lactation period. Additionally, 57.8%, 62.6%, and 66.7% of women never consumed or consumed less than one time per week eggs, meat, and milk, respectively, whereas only 6.7%, 7.4%, and 18.6% of women had eggs, meat, and milk every day, respectively. The frequencies of egg, meat, and milk consumption were not associated with anemia (χ2 = 3.78, P = 0.151; χ2 = 5.28, P = 0.071; and χ2 = 0.774, P = 0.679, respectively).
Multiple logistic regression analysis and anemia-related factors.
Data from 717 subjects were eligible for logistic regression analysis, and the data from the remaining 16 subjects were not used because of missing values. Factors found to be associated with anemia in lactating women included MUAC < 22.5 cm, self-reported symptoms of night blindness or poor dark adaptation, no primary education experience, low family annual capita income (< 800 MMK), drinking spring or river water, and drinking unboiled water (Table 2).
Table 2.
Logistic regression for predictors of anemia in lactating women from Myanmar (N = 717)
| Indicator variable | B | Wald | OR | 95% CI | P value |
|---|---|---|---|---|---|
| Family annual capita income < 800 MMK | 0.43 | 12.24 | 1.53 | 1.21–1.94 | < 0.001 |
| MUAC < 22.5 cm | 0.49 | 4.96 | 1.64 | 1.06–2.53 | 0.026 |
| Symptom of night blindness or poor dark adaptation | 1.14 | 17.02 | 3.12 | 1.82–5.36 | < 0.001 |
| Without primary education experience | 1.89 | 75.87 | 6.59 | 4.31–10.07 | < 0.001 |
| Drinking spring or river water | 0.82 | 46.35 | 2.26 | 1.79–2.86 | < 0.001 |
| Drinking unboiled water | 0.47 | 5.81 | 1.60 | 1.09–2.33 | 0.016 |
Anemia is defined as Hb < 110 g/L.
Discussion
Prevalence of anemia.
Anemia is still widespread in Myanmar, and there is also a high risk of maternal mortality.4 This current survey has supported and extended previous findings by showing that anemia is an important public health problem in lactating women from Myanmar.
In the surveyed populations, anemia during lactation was generally high. The overall prevalence was 60.3%, which is similar with the reported rate (61.1%) among women of reproductive age (15–45 years old); however, this number is much higher than the 42% prevalence in women living elsewhere in southeast Asia.4 It is worth noting that the post-partum period is generally the time with the lowest anemia rate9; however, anemia rates in some vulnerable villages were unacceptably high (exceeding 90% in K1). The consequences of anemia in post-partum women include impaired physical work capacity, low work efficiency, fatigue, depressive symptoms, reduced immune function, and other disease.15,16 In addition, high severe anemia rate is also a major concern in this investigated population (20.8%), and studies report that severe maternal anemia (caused by iron deficiency) can also greatly influence the iron status of their children.17
Health indicators of lactating women.
In this study, we found a high prevalence of underweight, low MUAC, and nutrition deficiency-related symptoms, which suggests that multiple nutrient deficiencies might exist in lactating women. In our results, women had a high rate of self-reported night blindness, which might indicate a vitamin A deficiency.1 Vitamin A deficiency is an increasingly recognized problem among women of reproductive age, especially in low-income countries.18 Previous studies have reported the co-occurrence of vitamin A and iron deficiency, because vitamin A deficiency might affect iron repletion.19–21 Based on the results, symptoms of night blindness and MUAC < 22.5 cm were associated with anemia in lactating women. However, because of the cross-sectional design, the causality between malnutrition and anemia could not been observed.
Previous studies reported that malaria and other infection diseases had high prevalence rates in tropical regions and were considered to contribute to anemia.22 In these three investigated villages, malaria and other infectious diseases, to some extent, were controlled by the previous work of HPA, which included bed net distribution and timely treatment of diseases. However, we also found that 74 women had experienced malaria in last 3 months and that a number of women suffered from fever and/or diarrhea of unknown cause. Although the associations between infection disease and anemia were only found in single factor analysis, the health consequence on women because of infection disease in these regions still needs additional attention.
Risk factors associated with anemia.
The results of this study confirmed that anemia had a high prevalence rate in low-income countries and low-income families. Villages K1 and S1 had significantly lower family incomes and were more disadvantaged compared with village K2. Moreover, lactating women from K2 accepted vitamin supplements (multivitamin or vitamin A) from United Nations International Children's Emergency Fund (UNICEF) in recent years, which might have contributed to the lower prevalence of anemia in that region. According to the UNICEF Humanitarian Action Plan for Myanmar, 60% of pregnant and lactating women were targeted to receive the micronutrients in the hard to reach areas in 2011, and 4,600 pregnant and lactating women were targeted to receive multimicronutrient supplementation in 2013.23,24 However, the data of the vitamin supplement intake rates and duration in the K2 village cannot be obtained for this study and previous reports. Women without primary education had a higher prevalence of anemia. We inferred that women in low-income families and without primary education were more likely to have worse living conditions, limited health knowledge of anemia, and worse nutrition status.
In this study, we also found that drinking spring or river water and drinking unboiled water were associated with anemia. Although we did not collect data on parasite infection, we can infer that drinking unboiled water, especially from a spring or river, easily caused parasite infections, which led to intestinal bleeding and anemia.25,26 Boel and others27 reported that the prevalence of helminth infection in Karen and Burmese pregnant women on the Thai–Burmese border was as high as 70%.
In addition, a large proportion of women did not consume eggs and meat on a daily basis, which might result in an insufficient intake of dietary iron.28 Additionally, according to the anthropometric indicators and self-reported symptoms, malnutrition and nutrient deficiency symptoms were observed in a majority of women, and lack of eggs, meat, and milk, which are rich in protein and other essential nutrients, might be one explanation. However, food intake frequency was not associated with anemia in this study. The lack of association between food and anemia was also found in other populations that had multiple environmental risk factors of anemia.29,30
Limitations.
Because of the case control design, there were inherent limitations. The causality between exposure factors and anemia could not been observed, and recall bias by the studied population might exist. For the subject selection, we could not use a stratified random sampling for subjects, because some villages were at war and not accessible. The anemia prevalence of women in those villages was expected to be higher. Unfortunately, in this study, we did not measure indicators like serum ferritin, transferrin saturation, and erythrocyte protoporphyrin because of the restricted working conditions. Consequently, we could not distinguish the type of anemia. Although the majority of anemia seems to be caused by micronutrient deficiency partly or exclusively, thalassemia was reported to have a high prevalence in Myanmar.8,21 Different types of anemia are managed in different ways. Anemia from genetic causes is attended to differently and intervention is slow compared with iron-deficiency anemia.3 This study focused on exploring the controllable factors associated with anemia. Also, in this study, we did not test for helminths in stool samples or measure nutrient levels in blood, and therefore, helminth infection rate and nutrient deficiency prevalence were unknown. Additional studies still need to explore the underlying cause of anemia.
Another important predicator of anemia is perinatal healthcare.31 The status of perinatal healthcare on an individual level was not investigated. On the regional level, the coverage rates of perinatal healthcare were improved in these three regions (based on the unpublished data obtained by HPA from 2006 to 2010, the antenatal examination rate improved from 59.9% to 80.8%, the rate of hospitalized delivery increased from 20.6% to 28.4%, and the rate of post-partum visit increased from 49.5% to 72.7%). However, the rate of hospitalized delivery is still unacceptably low. Plenty of work will need to be done to improve perinatal healthcare in these regions.
Conclusion.
Lactating women in rural areas of Myanmar had a high prevalence of anemia. According to the results of this study, multiple contributing factors might coexist in an individual or a population. The most significant factors are low family income, no primary education experience, malnutrition, drinking spring or river water, or drinking unboiled water. Anemia usually can be prevented at a low cost, and the benefit/cost ratio of implementing preventive programs is recognized as one of the highest in the field of public health.32 A combination of interventions, including infectious disease control, nutrition promotion, and health education, may be the ideal way to reach comprehensive anemia control in this region.
ACKNOWLEDGMENTS
We thank all the Health Poverty Action staff and local health staffs who were involved in the field work in this tough working condition. Also, we appreciate the time devoted by and invaluable assistance of provincial health officers in all provincial aspects of the survey.
Footnotes
Authors' addresses: Ai Zhao, Peiyu Wang, and Yong Xue, Department of Social Medicine and Health Education, School of Public Health, Peking University Health Science Center, Beijing, People's Republic of China, E-mails: aizhaobjmu@gmail.com, 506238753@qq.com, and xiaochaai@163.com. Yumei Zhang, Department of Nutrition and Food Hygiene, School of Public Health, Peking University Health Science Center, Beijing, People's Republic of China, E-mail: zhangyumei111@gmail.com. Bo Li, Health Poverty Action Eastern Asia Programme Office, Yunnan, People's Republic of China, E-mail: boli_hu@163.com. Jiayin Li, Health Poverty Action Eastern Asia Programme Office, Karchin, Myanmar, E-mail: jiayin_hu@163.com. Hongchong Gao, Capital Medical University, School of Basic Medical Sciences, Beijing, People's Republic of China, E-mail: sisystone@gmail.com.
References
- 1.Black RE, Allen LH, Bhutta ZA, Caulfield LE, de Onis M, Ezzati M, Mathers C, Rivera J. Maternal and child undernutrition: global and regional exposures and health consequences. Lancet. 2008;371:243–260. doi: 10.1016/S0140-6736(07)61690-0. [DOI] [PubMed] [Google Scholar]
- 2.Stoltzfus RJ, Mullany L, Black RE. GWH Organization Iron Deficiency Anaemia. Comparative Quantification of Health Risks: Global and Regional Burden of Disease Attributable to Selected Major Risk Factors. 2005. http://www.who.int/healthinfo/global_burden_disease/cra/en/ Available at. Accessed May 2, 2013.
- 3.Htay TT. Making pregnancy safer in Myanmar: introducing misoprostol to prevent post-partum haemorrhage as part of active management of the third stage of labour. Reprod Health Matters. 2007;15:214–215. doi: 10.1016/S0968-8080(07)30317-0. [DOI] [PubMed] [Google Scholar]
- 4.Mullany LC, Lee CI, Yone L, Paw P, Oo EK, Maung C, Lee TJ, Beyrer C. Access to essential maternal health interventions and human rights violations among vulnerable communities in eastern Burma. PLoS Med. 2008;5:1689–1698. doi: 10.1371/journal.pmed.0050242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhao A, Zhang Y, Peng Y, Li J, Yang T, Liu Z, Lv Y, Wang P. Prevalence of anemia and its risk factors among children 6–36 months old in Burma. Am J Trop Med Hyg. 2012;87:306–311. doi: 10.4269/ajtmh.2012.11-0660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kemmer TM, Bovill ME, Kongsomboon W, Hansch SJ, Geisler KL, Cheney C, Shell-Duncan BK, Drewnowski A. Iron deficiency is unacceptably high in refugee children from Burma. J Nutr. 2003;133:4143–4149. doi: 10.1093/jn/133.12.4143. [DOI] [PubMed] [Google Scholar]
- 7.WHOA Prevention Assessing the Iron Status of Populations: Report of a Joint World 2: WHO. 2005. http://www.who.int/nutrition/publications/micronutrients/anaemia_iron_deficiency/9789241596107/en/ Available at. Accessed May 5, 2013.
- 8.Choi JW, Kim SK. Relationships of lead, copper, zinc, and cadmium levels versus hematopoiesis and iron parameters in healthy adolescents. Ann Clin Lab Sci. 2005;35:428–434. [PubMed] [Google Scholar]
- 9.Food And Nutrition Board IOM . Nutrition During Pregnancy. Washington, DC: National Academy Press; 1990. pp. 37–63. [Google Scholar]
- 10.Bodnar LM, Scanlon KS, Freedman DS, Siega-Riz AM, Cogswell ME. High prevalence of postpartum anemia among low-income women in the United States. Am J Obstet Gynecol. 2001;185:438–443. doi: 10.1067/mob.2001.115996. [DOI] [PubMed] [Google Scholar]
- 11.Pehrsson PR, Moser-Veillon PB, Sims LS, Suitor CW, Russek-Cohen E. Postpartum iron status in nonlactating participants and nonparticipants in the special supplemental nutrition program for women, infants, and children. Am J Clin Nutr. 2001;73:86–92. doi: 10.1093/ajcn/73.1.86. [DOI] [PubMed] [Google Scholar]
- 12.Bodnar LM, Cogswell ME, Scanlon KS. Low income postpartum women are at risk of iron deficiency. J Nutr. 2002;132:2298–2302. doi: 10.1093/jn/132.8.2298. [DOI] [PubMed] [Google Scholar]
- 13.Myint K, Health MF. Myanmar Health Statistic 2010. 2010. http://www.moh.gov.mm/file/Myanmar%20Health%20Statistics%202010.pdf Available at. Accessed May 5, 2013.
- 14.UNCS Fund . Preventing Iron Deficiency in Women and Children: Background and Consensus on Key Technical Issues and Resources for Advocacy, Planning and Implementing National Programmes. New York: UNICEF/UNU/WHO/MI Technical Workshop. International Nutrition Foundation (INF), Micronutrient Initiative (MI). UNICEF; 1998. http://www.inffoundation.org/pdf/prevent_iron_def.pdf Available at. Accessed May 5, 2013. [Google Scholar]
- 15.Bodnar LM, Cogswell ME, McDonald T. Have we forgotten the significance of postpartum iron deficiency? Am J Obstet Gynecol. 2005;193:36–44. doi: 10.1016/j.ajog.2004.12.009. [DOI] [PubMed] [Google Scholar]
- 16.Beard JL, Hendricks MK, Perez EM, Murray-Kolb LE, Berg A, Vernon-Feagans L, Irlam J, Isaacs W, Sive A, Tomlinson M. Maternal iron deficiency anemia affects postpartum emotions and cognition. J Nutr. 2005;135:267–272. doi: 10.1093/jn/135.2.267. [DOI] [PubMed] [Google Scholar]
- 17.Zhao A, Zhang Y, Peng Y, Li J, Yang T, Liu Z, Lv Y, Wang P. Prevalence of anemia and its risk factors among children 6–36 months old in Burma. Am J Trop Med Hyg. 2012;87:306–311. doi: 10.4269/ajtmh.2012.11-0660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.West KP, Jr, Darnton-Hill I. Nutrition and Health in Developing Countries—Nutrition and Health Series. Totowa, NJ: Humana Press; 2008. pp. 377–433. [Google Scholar]
- 19.Roodenburg AJ, West CE, Hovenier R, Beynen AC. Supplemental vitamin A enhances the recovery from iron deficiency in rats with chronic vitamin A deficiency. Br J Nutr. 1996;75:623–636. doi: 10.1079/bjn19960165. [DOI] [PubMed] [Google Scholar]
- 20.Palafox NA, Gamble MV, Dancheck B, Ricks MO, Briand K, Semba RD. Vitamin A deficiency, iron deficiency, and anemia among preschool children in the Republic of the Marshall Islands. Nutrition. 2003;19:405–408. doi: 10.1016/s0899-9007(02)01104-8. [DOI] [PubMed] [Google Scholar]
- 21.Roodenburga AJC, West CE, Beynena AC. Vitamin A status affects the efficacy of iron repletion in rats with mild iron deficiency. J Nutr Biochem. 1996;7:99–105. [Google Scholar]
- 22.Chelala C. Burma: a country's health in crisis. Lancet. 1998;352:556. doi: 10.1016/S0140-6736(05)79276-X. [DOI] [PubMed] [Google Scholar]
- 23.United Nations Children's Fund 2011 UNICEF Humanitarian Action for Children: Building Resilience. 2011. http://www.unicef.org/hac2011/files/HAC2011_EN_PDA_web.pdf Available at. Accessed November 25, 2013.
- 24.United Nations Children's Fund 2013 UNICEF Humanitarian Action for Children. 2013. http://www.unicef.org/appeals/myanmar.html Available at. Accessed November 25, 2013.
- 25.Begue RE, Gonzales JL, Correa-Gracian H, Tang SC. Dietary risk factors associated with the transmission of Helicobacter pylori in Lima, Peru. Am J Trop Med Hyg. 1998;59:637–640. doi: 10.4269/ajtmh.1998.59.637. [DOI] [PubMed] [Google Scholar]
- 26.Latham M, Nations OOTU. Human Nutrition in the Developing World. Rome, Italy: Food and Agricultural Organization; 1997. [Google Scholar]
- 27.Boel M, Carrara VI, Rijken M, Proux S, Nacher M, Pimanpanarak M, Paw MK, Moo O, Gay H, Bailey W, Singhasivanon P, White NJ, Nosten F, McGready R. Complex interactions between soil-transmitted helminths and malaria in pregnant women on the Thai-Burmese border. PLoS Negl Trop Dis. 2010;4:e887. doi: 10.1371/journal.pntd.0000887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bhargava A, Bouis HE, Scrimshaw NS. Dietary intakes and socioeconomic factors are associated with the hemoglobin concentration of Bangladeshi women. J Nutr. 2001;131:758–764. doi: 10.1093/jn/131.3.758. [DOI] [PubMed] [Google Scholar]
- 29.Mikki N, Abdul-Rahim HF, Stigum H, Holmboe-Ottesen G. Anaemia prevalence and associated sociodemographic and dietary factors among Palestinian adolescents in the West Bank. East Mediterr Health J. 2011;17:208–217. [PubMed] [Google Scholar]
- 30.Halileh S, Gordon NH. Determinants of anemia in pre-school children in the occupied Palestinian territory. J Trop Pediatr. 2006;52:12–18. doi: 10.1093/tropej/fmi045. [DOI] [PubMed] [Google Scholar]
- 31.Villar J, Bergsjo P. Scientific basis for the content of routine antenatal care. I. Philosophy, recent studies, and power to eliminate or alleviate adverse maternal outcomes. Acta Obstet Gynecol Scand. 1997;76:1–14. doi: 10.3109/00016349709047778. [DOI] [PubMed] [Google Scholar]
- 32.Kotecha PV. Nutritional anemia in young children with focus on Asia and India. Indian J Community Med. 2011;36:8–16. doi: 10.4103/0970-0218.80786. [DOI] [PMC free article] [PubMed] [Google Scholar]