Abstract
Purpose
To identify sunitinib alternative schedules (AS) that maintained dose intensity while decreasing adverse events (AEs) in patients with metastatic renal cell cancer (mRCC), and to determine impact of AS on clinical outcomes.
Patients and Methods
A retrospective review of patients ≥ 18 yr of age with clear-cell mRCC who received first-line sunitinib between 1/26/06 and 3/1/11 at a major Comprehensive Cancer Center. Subset of patients switched at first intolerable AE from traditional schedule (28 d on, 14 d off; TS) to 14 d on/7 d off schedule or other AS. Control group underwent standard dose reduction. Progression-free survival (PFS) and overall survival (OS) were estimated by the Kaplan-Meier method. Predictors of PFS and OS were analyzed using Cox regression.
Results
One hundred eighty five patients were included for analysis; 87% were on TS at baseline. During treatment, 53% of patients continued TS and 47% initiated or transitioned to AS. Baseline characteristics were similar. AEs prompting schedule modification included fatigue (64%), hand-foot syndrome (38%) and diarrhea (32%). Median time to AS was 5.6 mo. Median OS was 17.7 mo (95% confidence interval [CI], 10.8-22.2) on TS compared to 33.0 mo (95% CI, 29.3- not estimable) on AS (P < 0.0001). By multivariable analysis; poor ECOG PS, increased LDH, decreased albumin, unfavorable Heng criteria, and TS are associated with decreased OS (P < 0.05).
Conclusion
Sunitinib administered on AS may mitigate AEs and has comparable outcomes as TS for mRCC patients. Prospective investigations of alternate dosing schemas are warranted.
Keywords: renal cell carcinoma, schedule, sunitinib, toxicity
Introduction
Sunitinib is a therapy option for metastatic renal cell carcinoma (mRCC) and was US FDA approved as a fixed, 50 mg per d oral dose given 28 d on, 14 d off (traditional schedule; TS) per a 6 week cycle [1]. In a pivotal phase III clinical trial, sunitinib demonstrated superior efficacy compared to interferon alpha in untreated patients with mRCC. In addition, patients treated with sunitinib also reported superior quality of life despite greater dose interruptions, dose reductions, and adverse events (AEs) [1, 2].
Sunitinib must be maintained for continued disease control and higher exposure has been associated with improved response, time to progression (TTP), and overall survival (OS) [3]. TS was selected for investigation based on bone marrow and adrenal toxicities observed in animal models [4]. However, the optimal algorithm for schedule and dose modifications to manage or prevent AEs is unknown.
Two phase II clinical trials demonstrated antitumor activity with sunitinib 37.5 mg continuous daily dosing with manageable AEs [5, 6]. Motzer and colleagues [7] reported the results of the Renal EFFECT Trial, a phase II trial were patients with mRCC were randomized to sunitinib 50 mg per d on TS or 37.5 mg continuous daily dosing for up to 2 yr. No significant difference was demonstrated between arms for TTP, OS, AEs, or disease related symptoms. However, TS was statistically superior in time to deterioration, a composite end point. The authors concluded that adherence to TS is optimal.
There are limited published series of patients with mRCC who receive sunitinib on an alternative schedule (AS). Herein, we sought to retrospectively evaluate the clinical outcomes and AE rates associated with AS compared to TS.
Patients and Methods
After Institutional Review Board approval, we conducted a retrospective evaluation of mRCC patients at the University of Texas M.D. Anderson Cancer Center (MDACC) between 1/26/2006 to 3/1/2011. Patients 18 yr of age or older with clear cell mRCC, who received first-line anti-angiogenic therapy with sunitinib and available for adequate follow-up (at least one clinic visit at MDACC within 3 mo of sunitinib initiation) were included. Patients were stratified according to schedule at the time of sunitinib discontinuation to TS or AS group (14 days on then7 days off, 7 days on then 3 days off alternating with 7 days on then 4 days off, or other AS). In addition, patients received best supportive care for the management of AEs. Given the retrospective study design, assignment to schemas and response assessment was at physician discretion. Additional subset analyses included; 1) patients on TS compared to AS at sunitinib initiation, and 2) patients maintained on TS compared to AS beyond the median time to schedule change from TS to AS.
Institutional electronic medical records were used to extract patient clinical information. AEs were graded by the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) version 4.0 [8]. An independent, blinded radiology review was conducted to assess the radiographic response to sunitinib using Response Evaluation Criteria in Solid Tumors (RECIST) 1.1 [9].
Statistical analysis
Patient characteristics were summarized using median and range for continuous variables and frequency and percentage for categorical variables. All statistical tests were two-sided and p-values less than 0.05 were statistically significant. To assess the difference between dosing schedules, Wilcoxon rank-sum tests were used for continuous variables and Fisher's exact tests were used for categorical patient variables.
Time on treatment (TOT) was calculated from date of sunitinib initiation to discontinuation or death from any cause. Progression-free survival (PFS) was calculated from date of sunitinib initiation to tumor progression by RECIST or death from any cause. OS was calculated from date of sunitinib initiation to death from any cause. Patients alive and without progression of disease on the last day of sunitinib were censored on that day. The Kaplan-Meier method was used to estimate TOT, PFS, and OS. The Log-rank test was used to assess the difference between the two schedule cohorts. Cox regression models assessed the association between patient characteristics, dosing schedules, and OS or PFS; with goodness-of-fit assessed by the Grambsch-Therneau test and martingale residual plots. Based on the fitted univariate Cox models, variables with P value < 0.10 were included in the full multivariable Cox model. The final multivariable Cox model was obtained by a backward elimination procedure, where variables with P values < 0.05 were statistically significant. All computations were by SAS (version 9.3) and Splus (version 8.2).
Results
Patients
We identified 185 patients who met the study's inclusion criteria (Figure 1). Ninety-eight patients (53%) were maintained on TS throughout sunitinib therapy, whereas 87 patients (47%) were initiated or transitioned to AS before sunitinib discontinuation. Demographic and baseline characteristics were similar between treatment arms (Table 1). A subset of 24 patients who started on AS demonstrated equivalent baseline characteristics to the TS group (supplementary Table S1).
Figure 1. Consort diagram of patients screened for inclusion and cohort groups for comparison. (mRCC = metastatic renal cell carcinoma).
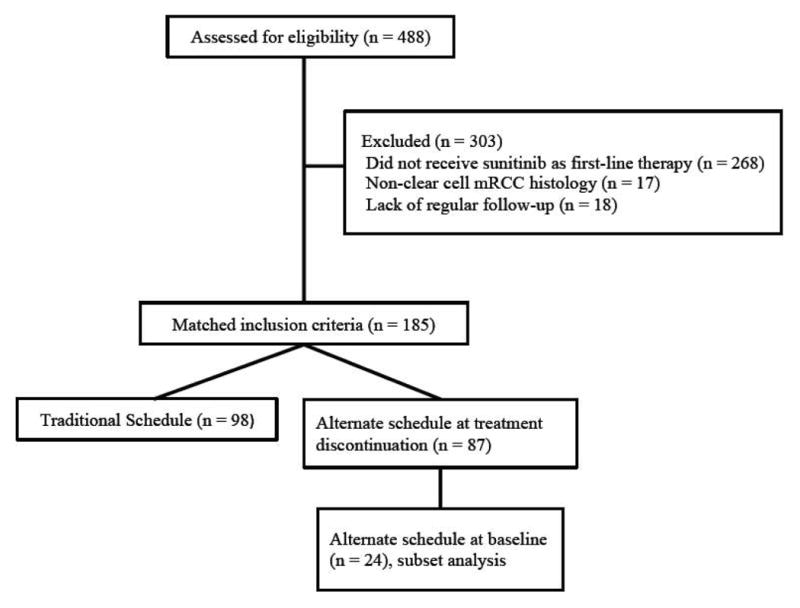
Table 1. Baseline patient characteristics by schedule.
| Characteristic | Traditional schedule | Alternative schedule | Whole population | P value |
|---|---|---|---|---|
| No of patients | 98 | 87 | 185 | |
|
| ||||
| Male | 81 (83%) | 57 (66%) | 138 (75%) | 0.01 |
| Age | 59 (34–82) | 62 (23–80) | 60 (23–82) | 0.13 |
|
| ||||
| Race | ||||
| Caucasian | 72 (74%) | 70 (81%) | 142 (77%) | 0.54 |
| African-American | 6 (6%) | 3 (3%) | 9 (5%) | |
| Other | 20 (20%) | 14 (16%) | 34 (18%) | |
|
| ||||
| ECOG PS | ||||
| 0 | 19 (19%) | 23 (26%) | 42 (23%) | 0.43 |
| 1 | 59 (60%) | 51 (59%) | 110 (60%) | |
| ≥ 2 | 20 (20%) | 13 (15%) | 33 (12%) | |
|
| ||||
| Nephrectomy | 56 (57%) | 52 (60%) | 108 (58%) | 0.77 |
|
| ||||
| MSKCC | ||||
| Good | 4 (4%) | 6 (7%) | 10 (5%) | 0.37 |
| Intermediate | 46 (47%) | 47 (54%) | 93 (50%) | |
| Poor | 48 (49%) | 34 (39%) | 82 (44%) | |
|
| ||||
| Heng Criteria | ||||
| Favorable | 10 (10%) | 13 (15%) | 23 (12%) | 0.16 |
| Intermediate | 60 (61%) | 59 (68%) | 119 (64%) | |
| Poor | 28 (29%) | 15 (17%) | 43 (23%) | |
|
| ||||
| Disease Sites | ||||
| Lungs | 73 (75%) | 61 (70%) | 134 (72%) | 0.51 |
| Bone | 25 (26%) | 37 (43%) | 62 (34%) | 0.01 |
| Liver | 19 (19%) | 18 (21%) | 37 (20%) | 0.85 |
|
| ||||
| Number of metastases | ||||
| 1 | 33 (34%) | 25 (29%) | 58 (31%) | 0.685 |
| 2 | 37 (38%) | 38 (44%) | 75 (41%) | |
| ≥3 | 28 (29%) | 24 (28%) | 52 (28%) | |
|
| ||||
| Laboratory abnormalities | ||||
| Hgb < LLN | 74 (76%) | 65 (75%) | 139 (75%) | 1.00 |
| ANC > 7.3 K/uL | 31 (32%) | 8 (9%) | 39 (21%) | < 0.01 |
| Cor. Ca > 10 mg/dL | 20 (20%) | 20 (23%) | 40 (22%) | 0.72 |
| PLT > 440 K/uL | 16 (16%) | 10 (12%) | 26 (14%) | 0.40 |
ECOG PS = Eastern Cooperative Oncology Group performance status; MSKCC = Memorial Sloan-Kettering Cancer Center; Hgb = hemoglobin; LLN = lower limit of normal; ANC = absolute neutrophil count; Cor. Ca = corrected calcium; PLT = platelets
Treatment
At baseline, 161 patients (87%) were on TS and 24 patients (13%) were on AS. Majority of patients (87%) were initiated on sunitinib 50 mg dose. At a median of 5.6 mo (range 1 - 50.5), 63 patients transitioned from TS to AS. Common sunitinib AS included 14 d on then 7 d off (82%), 7 d on then 3 d off alternating with 7 d on then 4 d off (8%), or other AS (9%). Dose intensity was maintained in the majority of patients upon transition to AS; 79% stayed on 50 mg and 21% were on less than 50 mg. At discontinuation, dose intensity was similar among the treatment cohorts; 67% of patients on TS and 54% on AS were on 50 mg, 22% and 30% were on 37.5 mg, and 10% and 16% were on 25 mg, respectively (P = 0.21). In subset analysis of patients who did not switch schedule schemas from baseline, TS (n= 98) vs AS (n= 24), dose intensity was comparable; 67% of each cohort on 50 mg, 22% of TS and 21% of AS on 37.5 mg, and 10% and 13% on 25 mg (P = 0.94), respectively.
Adverse Events
Of 161 patients initiated on TS, 63 patients had AEs with management that included transition to AS. Common AEs (all grades) associated with schedule modifications are presented in Table 2. Grade 3 or 4 toxicities were rare and included fatigue (10%), hand foot syndrome (8%), and diarrhea (5%). The incidence of the AEs decreased with the transition to AS. The incidence of AEs at next clinic follow-up was less than 30% for all AEs after transition from TS to AS with a median time to follow-up after transition from TS to AS of 1.8 mo.
Table 2. Distribution of adverse events before and after schedule modification.
| Adverse Event | Before schedule modification N = (%) |
At first follow up after schedule modification N = (%) |
|---|---|---|
| Fatigue | 40 (64) | 18 (29) |
|
| ||
| Hand foot syndrome | 24 (38) | 6 (10) |
|
| ||
| Diarrhea | 20 (32) | 4 (6) |
|
| ||
| Mucositis | 14 (22) | 3 (5) |
|
| ||
| Nausea/vomiting | 9 (14) | 7 (11) |
|
| ||
| Dysgeusia | 6 (10) | 0 |
|
| ||
| Rash | 6 (10) | 0 |
|
| ||
| Hypertension | 6 (10) | 3 (5) |
|
| ||
| Anorexia | 5 (8) | 1 (1.5) |
|
| ||
| Mouth sores | 3 (5) | 0 |
|
| ||
| Laboratory Abnormalities | ||
|
| ||
| ANC < 1K/uL | 3 (5) | 1 (1.5) |
| Platelet count < 100 K/uL | 2 (3) | 1 (1.5) |
| TSH level > 4.20 uIU/mL | 2 (3) | 0 |
ANC = absolute neutrophil count; TSH = thyroid stimulating hormone
Efficacy
TOT
The median TOT for patients maintained on TS was 4.1 mo (95% CI, 2.9-4.7) compared to 13.6 mo (95% CI, 9.4-16.1) for patients who were initiated or transitioned to AS (P < 0.0001).
PFS
Among the 185 patients assessed, 158 patients (85%) had progressed or died at last follow-up with a median PFS of 9 mo (95% CI, 6.4-12; Figure 2a). Median PFS for patients treated with TS was 4.3 mo (95% CI, 3.4-6.4) and was 14.5 mo for patients treated with AS (95% CI, 11.3-19.4; P < 0.0001; Figure 2b). Predictive variables of PFS by Univariate Cox proportional hazards models included time to systemic therapy less than one yr, no prior nephrectomy, increased lactate dehydrogenase (LDH), decreased albumin, increased corrected calcium, decreased hemoglobin, poor ECOG PS (Eastern Cooperative Oncology Group performance status), Heng poor prognostic category, increased number of metastases, and TS compared to AS. Based on the final fitted multivariable Cox model for PFS; higher LDH, lower albumin, Heng poor prognostic category, and sunitinib with TS are associated with an increased risk of disease progression (Table 3). The median PFS was 11.6 mo (95% CI, 5.8-18.3; P = 0.11; supplementary Figure S1a) for the 24 patients who were initiated on AS at baseline, and AS was a favorable predictor of PFS (supplementary Table S2). One hundred twenty - two patients received therapy beyond the median time to change in schedule (5.6 mo); 41 patients on TS and 81 patients on an AS. The median PFS of patients treated with TS beyond the median time to change in schedule was 9.5 mo compared to 14.7 mo for patients on an AS (95% CI, 1.03-2.43; P = 0.03; supplementary Figure S2a); again, AS was a favorable predictor of PFS in this subset analysis (supplementary Table S3).
Figure 2.
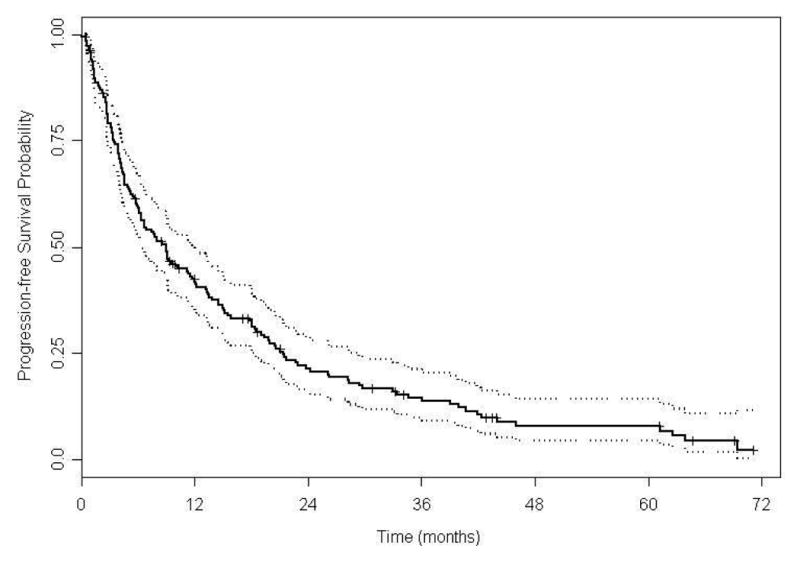
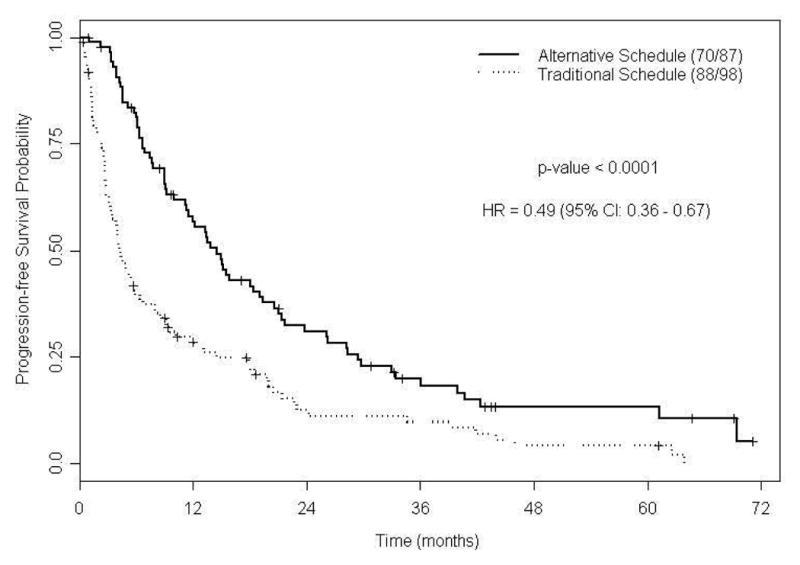
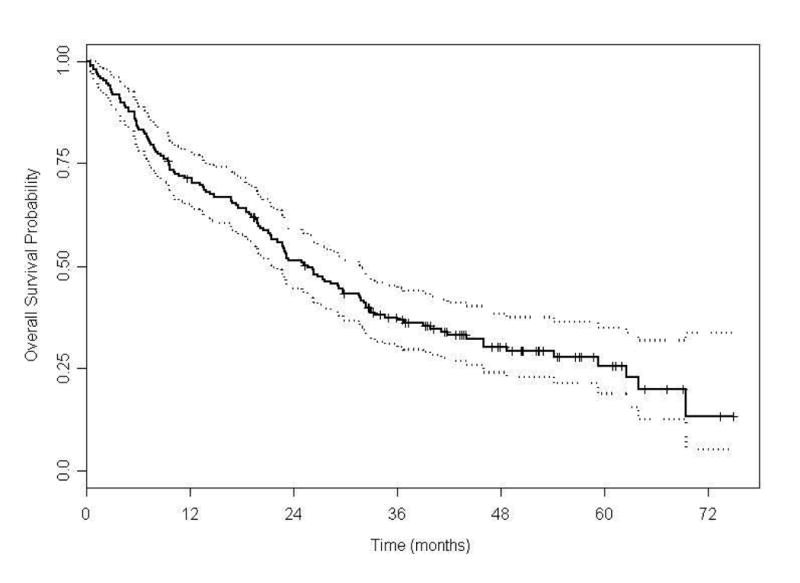
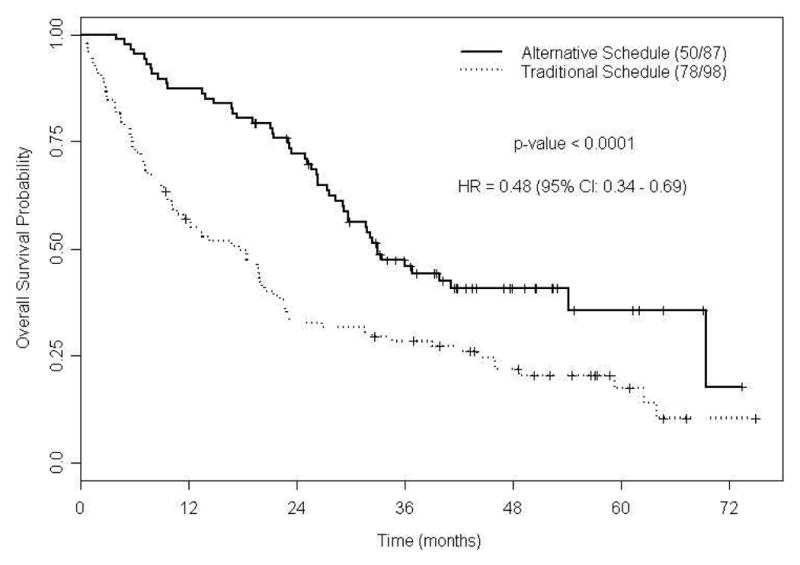
Figure 2A. Kaplan-Meier estimates for progression-free survival (N =185).
Figure 2B. Kaplan-Meier estimates for progression-free survival by sunitinib dosing schedule (N =185).
Figure 2C. Kaplan-Meier estimates for overall survival (N =185).
Figure 2D. Kaplan-Meier estimates for overall survival by sunitinib dosing schedule (N =185).
Table 3. Multivariable Cox proportional hazards model for progression-free survival (Total patients=185, Progression=158).
| Variable | Hazard Ratio | 95% CI | P value |
|---|---|---|---|
| LDH ≥ 927 (vs. <927) | 2.42 | 1.20 – 4.50 | 0.005 |
| Albumin ≤ 4 (vs. >4) | 1.49 | 1.04 – 2.13 | 0.03 |
| Heng Intermediate vs. Favorable | 1.26 | 0.75 – 2.14 | 0.38 |
| Heng Poor vs. Favorable | 2.27 | 1.22 – 4.21 | 0.009 |
| AS at sunitinib discontinuation (vs. TS) | 0.54 | 0.39 – 0.74 | 0.0002 |
LDH = lactate dehydrogenase; AS = alternative schedule; TS = traditional schedule
OS
At last follow-up, 128 patients (69%) had died with median OS of 25.6 mo (95% CI, 21.4-31.6; Figure 2c). The median OS was 17.7 mo (95% CI, 10.8-22.2) in patients treated with TS; while in patients treated with AS the median OS was 33.0 mo (95% CI, 29.3-not estimable; P < 0.0001; Figure 2d). Predictive variables for OS by univariate Cox proportional hazards models are presented in supplementary Table S4. Based on the final fitted multivariable Cox model for OS; poor ECOG PS, higher LDH level, lower albumin level, Heng poor prognostic category, and TS are associated with an increased risk of death (Table 4). In patients started on AS, 14 out of 24 patients died and the median OS was 27.7 mo (95% CI, 21.2-not estimable; P = 0.08; supplementary Figure S1b) and AS was associated with favorable OS (supplementary Table S5). In patients treated beyond the median time to schedule change, the median OS for patients on TS was 35.1 mo, compared to 33.5 mo for patients on an AS (95% CI, 0.69-1.81; P = 0.6; supplementary Figure S2b). In this subset analysis, AS was not predictive of OS (supplementary Table S6).
Table 4. Multivariable Cox proportional hazards model for overall survival (Total patients= 185, Deaths =128).
| Variable | Hazard Ratio | 95% CI | P value |
|---|---|---|---|
| LDH ≥ 927 (vs. <927) | 3.05 | 1.56 – 5.96 | 0.001 |
| Albumin ≤ 4 (vs. >4) | 1.83 | 1.23 – 2.72 | 0.003 |
| ECOG = 1 (vs. 0) | 2.08 | 1.26 – 3.46 | 0.005 |
| ECOG ≥ 2 (vs. 0) | 2.67 | 1.38 – 5.16 | 0.003 |
| Heng Intermediate vs. Favorable | 1.49 | 0.76 – 2.92 | 0.24 |
| Heng Poor vs. Favorable | 2.65 | 1.22 – 5.76 | 0.01 |
| AS at sunitinib discontinuation (vs. TS) | 0.55 | 0.38 – 0.79 | 0.001 |
LDH = lactate dehydrogenase; AS = alternative schedule; TS = traditional schedule
Discussion
Sunitinib is a commonly used agent for the treatment of mRCC with efficacy equivalent or superior to most other agents [2, 11-15]. A challenge with sunitinib is to minimize toxicity and maximize quality of life for patients without compromising efficacy. Data suggest that maximizing dose intensity and area under the curve may increase efficacy [3, 7, 12, 16, 17]. AEs are a substantial barrier to maintaining sunitinib dose intensity. Phase III data demonstrate nearly 50% of patients require dose reductions or interruptions due to AEs [2]. AEs increase during each individual cycle and are worst in the third and fourth week [21]. If the key determinant of benefit from sunitinib is the intensity of exposure, and steady state is achieved quickly, than taking earlier but shorter treatment breaks may permit the same efficacy without exceeding normal tissue tolerance.
Houk et al. [3] demonstrated a relationship between area under the curve of sunitinib and clinical outcome in 639 patients, of whom 443 had pharmacokinetic data. There was a significant association between exposure and the probability of a response in mRCC patients (P < 0.01). A positive relationship was identified between exposure and incidence, but not severity of fatigue.
In addition, a positive relationship was identified between diastolic blood pressure changes and total drug (sunitinib and SU12662) exposure. These data suggest that higher exposure is linked to improved outcome, but does not inform us whether a particular schedule is superior. Rini et al. [18] reported the development of hypertension was linked to superior OS in 544 patients treated with sunitinib in several clinical trials. Furthermore, antecedent hypertension was an independent predictor of improved outcome; suggesting there are inherent, dose-independent, host-specific characteristics that predict for duration of response and OS in these patients. Poprach and colleagues [22] described that skin toxicity was associated with improved PFS and OS in patients treated with sunitinib or sorafenib. The fact that AEs were linked to efficacy leads to the supposition that higher grade of toxicity is necessary for increased efficacy. An alternate hypothesis is the development of AEs identifies patients with inherent pharmacokinetic and pharmacodynamics characteristics that predispose to clinical benefit, but that toxicities can be managed using schedule changes without diminishing clinical benefit. In our current study, we show that altering dosage schedule AS may mitigate toxicity, yet does not appear to decrease efficacy. This raises the possibility that toxicity is a marker of a response phenotype, but maintaining a higher level AEs is not necessary to achieve clinical benefit.
In an effort to identify AS of sunitinib that maintain efficacy and mitigate toxicity, several studies have been published. A phase II trial comparing 37.5 mg of sunitinib given continuous daily dosing to 50 mg of sunitinib on TS was recently published [7]. Median TTP was 7.1 mo for the continuous schedule and 9.9 mo for TS (hazard ratio 0.77; 95% CI = 0.57 to 1.04; P = 0.090), and no difference was seen in OS. The incidence and severity of common AEs were not different. Based on the numerical superiority of PFS and a composite endpoint the authors concluded that TS was superior.
Neri et al. [23] described a single institution experience in which 31 patients with mRCC received sunitinib 50 mg, 14 d on then 7 d off schedule. They reported an ORR of 42% and median 16 mo TOT. Toxicity rates were lower than historical controls and a 9% dose reduction rate was reported in the patients receiving this AS.
Our report expands on these observations and provides insight on patients who either started on an AS or were eventually switched to an AS, in comparison to patients who received TS and underwent conventional dose reductions. The PFS and OS of patients on AS are superior to those on TS. By our analysis of dose and schedule, the superior outcome is not due to higher dose intensity. However, the incidence of AEs decreased as a function of schedule adjustment. We considered the potential for survival bias in the AS group and conducted additional subset analyses. Despite a small sample size, there was a clear numerical difference and a strong trend towards superiority for patients initiated on AS at baseline in comparison to the conventionally treated patients for TOT, PFS, and OS. We also considered the limitation that some patients initiated on sunitinib therapy with TS may not be maintained on treatment for a sufficient time to require a change to AS. We sought to account for this limitation by doing a subset analysis of patients who were maintained on sunitinib therapy beyond the median time to schedule change. In this subset analyses, AS was a predictor of PFS and median PFS was significantly longer in this group. Median OS was comparable and fairly lengthy, which likely can be attributed to effective sequential therapy.
Our results are limited by the retrospective study design; including the inability to assess patient compliance with sunitinib and AEs not noted in the electronic medical records Although not statistically significant, numerically there were more patients with poor prognostic features as noted by Heng criteria and MSKCC risk model in the TS cohort. Despite our best efforts to eliminate bias between treatment groups, such bias may remain.
Conclusions
Our study suggests that a more dynamic approach towards toxicity management in patients receiving sunitinib is warranted and is associated with improved outcomes. Prospective validation in the context of a well-designed and controlled randomized trial is the best path forward.
Supplementary Material
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Escudier B. Sorafenib [corrected] in kidney cancer. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 2007;8(Suppl 9):ix90–3. doi: 10.1093/annonc/mdm301. Epub 2007/09/27. [DOI] [PubMed] [Google Scholar]
- 2.Motzer RJ, Hutson TE, Tomczak P, et al. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. The New England journal of medicine. 2007;356(2):115–24. doi: 10.1056/NEJMoa065044. Epub 2007/01/12. [DOI] [PubMed] [Google Scholar]
- 3.Houk BE, Bello CL, Poland B, et al. Relationship between exposure to sunitinib and efficacy and tolerability endpoints in patients with cancer: results of a pharmacokinetic/pharmacodynamic meta-analysis. Cancer chemotherapy and pharmacology. 2010;66(2):357–71. doi: 10.1007/s00280-009-1170-y. Epub 2009/12/08. [DOI] [PubMed] [Google Scholar]
- 4.Faivre S, Delbaldo C, Vera K, et al. Safety, pharmacokinetic, and antitumor activity of SU11248, a novel oral multitarget tyrosine kinase inhibitor, in patients with cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2006;24(1):25–35. doi: 10.1200/JCO.2005.02.2194. Epub 2005/11/30. [DOI] [PubMed] [Google Scholar]
- 5.Escudier B, Roigas J, Gillessen S, et al. Phase II study of sunitinib administered in a continuous once-daily dosing regimen in patients with cytokine-refractory metastatic renal cell carcinoma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2009;27(25):4068–75. doi: 10.1200/JCO.2008.20.5476. Epub 2009/08/05. [DOI] [PubMed] [Google Scholar]
- 6.Barrios CH, Hernandez-Barajas D, Brown MP, et al. Phase II trial of continuous once-daily dosing of sunitinib as first-line treatment in patients with metastatic renal cell carcinoma. Cancer. 2012;118(5):1252–9. doi: 10.1002/cncr.26440. Epub 2011/09/08. [DOI] [PubMed] [Google Scholar]
- 7.Motzer RJ, Hutson TE, Olsen MR, et al. Randomized phase II trial of sunitinib on an intermittent versus continuous dosing schedule as first-line therapy for advanced renal cell carcinoma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2012;30(12):1371–7. doi: 10.1200/JCO.2011.36.4133. Epub 2012/03/21. [DOI] [PubMed] [Google Scholar]
- 8.National Cancer Institute (U.S) Common terminology criteria for adverse events (CTCAE) Bethesda, Md: U.S Dept of Health and Human Services, National Institutes of Health, National Cancer Institute; 2009. [Google Scholar]
- 9.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) European journal of cancer. 2009;45(2):228–47. doi: 10.1016/j.ejca.2008.10.026. Epub 2008/12/23. [DOI] [PubMed] [Google Scholar]
- 10.Motzer RJ, Bacik J, Murphy BA, et al. Interferon-alfa as a comparative treatment for clinical trials of new therapies against advanced renal cell carcinoma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2002;20(1):289–96. doi: 10.1200/JCO.2002.20.1.289. Epub 2002/01/05. [DOI] [PubMed] [Google Scholar]
- 11.Escudier B, Eisen T, Stadler WM, et al. Sorafenib in advanced clear-cell renal-cell carcinoma. The New England journal of medicine. 2007;356(2):125–34. doi: 10.1056/NEJMoa060655. Epub 2007/01/12. [DOI] [PubMed] [Google Scholar]
- 12.Sternberg CN, Davis ID, Mardiak J, et al. Pazopanib in locally advanced or metastatic renal cell carcinoma: results of a randomized phase III trial. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2010;28(6):1061–8. doi: 10.1200/JCO.2009.23.9764. Epub 2010/01/27. [DOI] [PubMed] [Google Scholar]
- 13.Hudes G, Carducci M, Tomczak P, et al. Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. The New England journal of medicine. 2007;356(22):2271–81. doi: 10.1056/NEJMoa066838. Epub 2007/06/01. [DOI] [PubMed] [Google Scholar]
- 14.Escudier B, Pluzanska A, Koralewski P, et al. Bevacizumab plus interferon alfa-2a for treatment of metastatic renal cell carcinoma: a randomised, double-blind phase III trial. Lancet. 2007;370(9605):2103–11. doi: 10.1016/S0140-6736(07)61904-7. Epub 2007/12/25. [DOI] [PubMed] [Google Scholar]
- 15.Rini BI, Halabi S, Rosenberg JE, et al. Phase III trial of bevacizumab plus interferon alfa versus interferon alfa monotherapy in patients with metastatic renal cell carcinoma: final results of CALGB 90206. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2010;28(13):2137–43. doi: 10.1200/JCO.2009.26.5561. Epub 2010/04/07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Escudier B, Szczylik C, Hutson TE, et al. Randomized phase II trial of first-line treatment with sorafenib versus interferon Alfa-2a in patients with metastatic renal cell carcinoma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2009;27(8):1280–9. doi: 10.1200/JCO.2008.19.3342. Epub 2009/01/28. [DOI] [PubMed] [Google Scholar]
- 17.Rini BI, Escudier B, Tomczak P, et al. Comparative effectiveness of axitinib versus sorafenib in advanced renal cell carcinoma (AXIS): a randomised phase 3 trial. Lancet. 2011;378(9807):1931–9. doi: 10.1016/S0140-6736(11)61613-9. Epub 2011/11/08. [DOI] [PubMed] [Google Scholar]
- 18.Rini BI, Cohen DP, Lu DR, et al. Hypertension as a biomarker of efficacy in patients with metastatic renal cell carcinoma treated with sunitinib. Journal of the National Cancer Institute. 2011;103(9):763–73. doi: 10.1093/jnci/djr128. Epub 2011/04/30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Poprach A, Pavlik T, Melichar B, et al. Skin toxicity and efficacy of sunitinib and sorafenib in metastatic renal cell carcinoma: a national registry-based study. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 2012;23(12):3137–43. doi: 10.1093/annonc/mds145. Epub 2012/06/16. [DOI] [PubMed] [Google Scholar]
- 20.Schmidinger M, Vogl UM, Bojic M, et al. Hypothyroidism in patients with renal cell carcinoma: blessing or curse? Cancer. 2011;117(3):534–44. doi: 10.1002/cncr.25422. Epub 2010/09/17. [DOI] [PubMed] [Google Scholar]
- 21.Gore ME, Szczylik C, Porta C, et al. Safety and efficacy of sunitinib for metastatic renal-cell carcinoma: an expanded-access trial. The lancet oncology. 2009;10(8):757–63. doi: 10.1016/S1470-2045(09)70162-7. Epub 2009/07/21. [DOI] [PubMed] [Google Scholar]
- 22.Poprach A, Pavlik T, Melichar B, et al. Skin toxicity and efficacy of sunitinib and sorafenib in metastatic renal cell carcinoma: a national registry-based study. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 2012;23(12):3137–43. doi: 10.1093/annonc/mds145. Epub 2012/06/16. [DOI] [PubMed] [Google Scholar]
- 23.Neri B, Vannini A, Brugia M, et al. Biweekly sunitinib regimen reduces toxicity and retains efficacy in metastatic renal cell carcinoma: A single-center experience with 31 patients. International journal of urology : official journal of the Japanese Urological Association. 2012 doi: 10.1111/j.1442-2042.2012.03204.x. Epub 2012/11/02. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


