Abstract
Purpose
To investigate the angiogenic changes in primary tumor tissue of renal cell carcinoma (RCC) patients treated with vascular endothelial growth factor (VEGF)-targeted therapy.
Experimental design
Phase II trials of VEGF pathway targeted therapy given prior to cytoreductive surgery were performed with metastatic RCC patients with the primary tumor in situ, to investigate the necessity of nephrectomy. Primary tumor tissues were obtained and assessed for angiogenesis parameters. Results were compared to similar analyses on untreated tumors.
Results
Sunitinib or bevacizumab pretreatment resulted in a significant reduction of microvessel density in the primary tumor. Also, an increase in vascular pericyte coverage was found in sunitinib-pretreated tumors, consistent with efficient angiogenesis inhibition. Expression of several key regulators of angiogenesis was suppressed in pretreated tissues, among which VEGFR-1 and -2, angiopoietin-1 and -2 and PDGF-B. In addition, apoptosis in tumor and endothelial cells was induced. Interestingly, in sunitinib-pretreated tissues a dramatic increase of the number of proliferating endothelial cells was observed, which was not the case in bevacizumab-pretreated tumors. A positive correlation with the interval between halting the therapy and surgery was found, suggesting a compensatory angiogenic response caused by the discontinuation of sunitinib treatment.
Conclusion
This study describes for the first time the angiostatic response in human primary renal cancers at the tissue level upon treatment with VEGF targeted therapy. Discontinuation of treatment with tyrosine kinase inhibitors leads to accelerated angiogenesis. The results of the current study contribute important data to the ongoing discussion on the discontinuation of treatment with kinase inhibitors.
Keywords: Angiogenesis, angiostasis, preoperative treatment, renal cell cancer, sunitinib, Sutent
Introduction
New blood vessel formation is a critical aspect of tumor progression (1, 2). Angiogenesis inhibitors have now established their role in the clinical practice of cancer therapy. Most applications of anti-angiogenic compounds are in combination with other treatment strategies (3-7). Metastatic renal cell carcinoma (mRCC) is treated with the anti-angiogenic tyrosine kinase inhibitor sunitinib as a monotherapy strategy. This cancer type is therefore an attractive disease to study the effect of exposure of tumor tissue to treatment with angiogenesis inhibitors. However, as surgery is often the first treatment pillar, the effect of angiogenesis inhibitors on the primary tumor tissue and blood vessels has never been extensively studied in patients.
In mRCC sunitinib treatment leads to progression free survival (PFS) of 11.5 months and an overall survival (OS) of 26 months (8), which may extend up to 4 years with adequate sequential therapy (9). Increased effectivity has renewed the controversy surrounding cytoreductive nephrectomy (CN) for patients with primary mRCC. Downsizing of the primary tumor occurs frequently and additional CN may not change clinical outcome. However, 91% of patients included in the pivotal phase III trial of sunitinib versus interferon had a nephrectomy (10). It is therefore unknown whether the reported outcome applies to mRCC patients with the primary tumor in situ. Retrospective data suggest that CN as an adjunct to targeted therapy is beneficial in selected patients with good performance and intermediate Memorial Sloan Kettering Cancer Centre (MSKCC) risk (11). Presurgical targeted therapy may help to further identify those who suffer from early progression and may not benefit from surgery (12). In addition, it provides primary tumor tissue for translational research into prognostic and predictive biomarkers. Two large phase III trials (CARMENA, EORTC SURTIME) now investigate the role and sequence of CN in patients receiving sunitinib (13). Previously, we conducted a phase II trial to investigate downsizing of the primary tumor following 2 courses of sunitinib. Secondary endpoints focused on safety of presurgical sunitinib and on evaluating this strategy to identify patients who progress rapidly and may not benefit from CN. The clinical outcome has recently been reported including data from a UK trial in a meta analysis (14, 15).
Part of the study was to investigate angiogenesis parameters in primary tumor tissues of RCC patients preoperatively treated with sunitinib. We compared the data retrospectively with a similar study where pretreatment was performed with bevacizumab (16). We found that preoperative treatment with either sunitinib or bevacizumab resulted in measurable inhibition of angiogenesis as assessed by suppressed microvessel density, increased apoptosis and pericyte coverage of microvessels and repression of several molecular angiogenic key regulators. Interestingly, while the number of proliferating endothelial cells (EC), a more acute feature of ongoing angiogenesis, was suppressed by bevacizumab, we found an increased number of growing EC after sunitinib, depending on the length of the time interval between halting the therapy and nephrectomy. This study describes for the first time the angiogenic changes in human primary renal cancers at a tissue level and contributes important data to the ongoing discussion on the discontinuation of treatment with tyrosine kinase inhibitors.
Material and Methods
Patient characteristics and tissue used for evaluation
Primary tumors from patients with metastatic clear cell renal cell carcinoma (mRCC) treated with presurgical sunitinib (n=21) from a retrospective study and 2 phase II clinical studies were used for evaluation and compared to tissue from a phase II trial with presurgical bevacizumab (n=29). Clear cell RCC tissues from non treated patients were used as controls (n=70). The 3 trials were performed independently and contained protocols for investigating angiogenesis in primary tumor tissue. Subsequently, a retrospective collective analysis of available tumor samples was performed. Patient characteristics of the trials are presented in Table 1. One of the phase II trials was designed to investigate the effects of preoperative sunitinib treatment in patients with primary metastatic clear-cell RCC. The tissues of 12 primary tumors from this trial were evaluated. The main objective of the study (registered under EudraCT 2006-006491-38, https://eudract.ema.europa.eu/) was to investigate the response rate of the primary tumor following pretreatment with sunitinib. Details of the trial and patient characteristics have been published previously (14, 15). Briefly, patients with histologically confirmed mRCC of clear-cell subtype with a resectable asymptomatic primary in situ were included and received sunitinib at 50 mg/day for 2 cycles of each 4 weeks on treatment and 2 weeks off treatment. At completion of the 2nd cycle patients underwent cytoreductive nephrectomy as per protocol 1 day after discontinuation of sunitinib. Three patients stopped earlier due to adverse events. Another 9 primary tumors were provided from a second phase II study of presurgical sunitinib in patients with primary clear cell mRCC. These patients were restaged after one cycle of systemic therapy, began a second cycle of systemic therapy with sunitinib, and discontinued therapy 1 day before nephrectomy (clinicaltrials.gov identifier: NCT00715442).
Table 1.
Patient characteristics from three phase II trials of presurgical VEGF targeted therapy from which the primary tissues were used for analysis in this study
| Presurgical drug | Sunitinib (EudraCT 2006-006491-38) a |
Sunitinib (NCT00715442) a |
Bevacizumab (NCT00113217) a |
|---|---|---|---|
| Number of patients | 12 | 9 | 29 |
| age | 56 (range:39-75) |
61 (range: 49-76) |
61 (range: 41-74) |
| Gender -male -female |
10 2 |
6 3 |
22 7 |
| MSKCC risk score -intermediate -poor |
11 1 |
6 3 |
25 4 |
| Number of metastatic sites 1 2 3 4 |
2 5 5 |
5 3 1 |
2 17 6 4 |
| Metastatic sites Lung Bone Lymph node Liver other |
10 5 4 3 5 |
9 1 4 |
25 7 17 2 19 |
| Median reduction primary tumor (percent longest diameter) |
11% (range: +2.2 - −36%) |
5% (range 0- −10%) |
1% (range +11- −33%) |
| Progression free survival (median and range) |
8.5 months (range 4-48 months) |
14 months (range 3-40 months) |
5.5 months (range 1-24 months) |
| Overall survival (median and range) |
20.5 months (range 5-48 months) |
35 months (range 6-40 months) |
18.6 (range 3-40 months) |
clinical trial identifier (clinicaltrials.gov)
Previous data from a retrospective study (17) suggested that a one day interval was safe. In this study 17 patients were evaluated who had received sunitinib at various lengths (3 to 11 courses) with discontinuation 2 to 21 days before surgery. From 3 of these patients who underwent surgery we used tissues to evaluate if there was an association with blood vessel changes and the time interval of presurgical discontinuation of sunitinib. To these were added the 3 patients from the phase II trial who interrupted sunitinib earlier than 1 day before surgery (total number of patients who discontinued therapy between 4-21 days prior to surgery n=6). To compare the findings in tumor tissue following presurgical sunitinib, 29 primary tumors were provided from a phase II trial of presurgical bevacizumab (clinicaltrials.gov identifier: NCT00113217). The trial was similar in design and included patients with primary metastatic clear cell renal cell carcinoma. Patients received 4 doses of bevacizumab administered i.v. every 14 days, and discontinued bevacizumab 28 days before surgery. Characteristics and details of the trial have been published (16).
Immunohistochemistry
To investigate microvessel density and the quantity of proliferating endothelial cells CD31/34 and Ki-67 double staining (18) was performed (sunitinib pretreatment, n=21, and bevacizumab pretreatment, n=29). RCC clear-cell tissues without pretreatment (n=70) were used as controls. For the CD31/34 and Ki-67 double staining paraffin sections (6 μm thickness) were deparaffinized in xylene and rehydrated in alcohol series. To block endogenous peroxidase activity, sections were treated with 3% H2O2 in methanol for 20 min., after which antigen retrieval was carried out by heating the sections in a Tris-EDTA buffer (10 mM Tris-1mM EDTA, pH 8) for 15 min. in a microwave. Subsequently, the slides were incubated for 30 minutes in 0.5% BSA in PBS, blocking non-specific antigen binding. Sections were incubated for 1 hour with a rabbit-polyclonal Ki-67 (Neomarker, dilution 1:50), followed by a polyclonal biotin-labeled swine anti-rabbit IgG (Dako; 1/200) for 30 minutes and avidin-biotin complex HRP (DAKO; 1/500) for 30 minutes. Diaminobenzidine (DAB, Sigma) with 0.03% NiCl2 was used as a black chromogen to be able to distinguish the black stained proliferating nuclei from the brown melanin. After the second primary antibody incubation of a mixture of CD31 (DAKO; 1/50) and CD34 (Monosan; 1/200) of 1 hour, followed by a biotin-labeled goat anti-mouse IgG (DAKO; 1/200) for 30 minutes and another 30 minutes incubation with an avidin-biotin complex AP (DAKO; 1/200), the slides were developed with alkaline phosphatase substrate kit III (Vector Laboratories, Burlingame, CA USA). The slides were treated with insulmount (Klinipath, Duiven, The Netherlands) to prevent alkaline phosphatase bleaching, and after 12 hours mounted with enthalan (Merck, Darmstadt, Germany).
To visualize apoptosis in tissue sections, staining was performed with anti-poly(ADP-ribose) polymerase (PARP) p85 Fragment polyclonal antibody directed against the 85kDa caspase-cleaved fragment (p85) of human PARP (Promega, Wisconsin, USA). Sections were subjected to antigen retrieval using citrate for 10 min at 95°C. To block non-specific binding sites, sections were incubated with normal goat serum (5% in PBS) for 10 min followed by incubation o/n with anti-PARP p85 antibody. After incubation in Powervision-goat anti rabbit HRP (Klinipath), peroxidase activity was demonstrated by incubation in 3,3-diaminobenzidine tetrachloride (Sigma; St Louis, MO, USA). Finally, sections were counterstained with hematoxylin, dehydrated, and mounted in Pertex (Sigma-Aldrich, Steinheim, Germany). Between incubation steps, the sections were extensively rinsed in PBS. Within each test, isotype matched control antibodies were included and found to be negative. All sections were examined by two investigators in a double-blind manner.
Immunohistochemical analyses
Microvessel density was determined by 2-4 independent observers in 10 randomly selected fields (200x), and presented as the amount of blood vessels/mm2. A second parameter for angiogenesis was evaluated through detection of active neovascularisation. To that end, a similar approach was performed to enumerate the amount of proliferating endothelial cells. For quantification of pericyte coverage, the number of vessels per high-power field lacking association of SMA positive cells was quantified. PARP expression was quantified as negative (no staining cells in the tissue, given value: 0), <2% of positive cells (given value: 1), or >2% of positive cells (given value: 2). Scores between the groups were compared and used for statistical analyses.
Immunofluorescence multi-color staining
Triple staining was performed as described previously with minor modifications (19, 20). In brief, aceton fixed cryosections were pre-incubated for 30 min. in 5% (v/v) normal goat serum (Sanquin, Central Laboratory of The Netherlands Red Cross Blood Transfusion Service, Amsterdam, The Netherlands) and incubated for 30 min. in a mixture of mouse-anti CD31 (1:100 final dilution, DAKO A/S, Glostrup, Denmark), mouse-anti CD34 (1:50, Monoson, Sanbio, Uden, The Netherlands) and rabbit anti-Ki67 (1:200, DAKO); this was followed by subsequent incubation steps in biotin-labeled goat-anti mouse (1:400, DAKO), normal mouse serum (1:100, Sanquin), FITC-conjugated mouse anti-CD3 (1:20, DAKO) and a mixture of Alexa 568 labeled streptavidin (1:80, Molecular Probes Europe BV) and Alexa 633 labeled goat anti-rabbit antibody (1:800, Molecular Probes) before mounting in Vectashield (Vector Laboratories). Between incubation steps, the sections were rinsed extensively in PBS. Within each test, isotype matched control antibodies were included and found to be negative.
Confocal Laser-scanning microscopy (CLSM) analysis
FITC, Alexa 568 and Alexa 633 signals were collected separately on a LEICA TCS SP confocal system (Leica Microsystems, Heidelberg, Germany) equipped with an argon-krypton-helium/neon laser. Images were taken using a ×40 numerical aperture × 1.4 objective. Color photomicrographs were taken from electronic overlays.
RNA isolation and cDNA synthesis
Total RNA was isolated from ten 20 μm thick sections using the RNeasy mini kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. Samples, a selection of tissues of which frozen tissue was available, included sunitinib pretreated (different time intervals of discontinuation, n=12) and clear-cell RCC without pretreatment as control (n=5). For RNA isolation the acidic phenol extraction method was used according to laboratory protocol. Briefly, frozen tissue was lysed with TRIzol (Invitrogen, Lucern, Switzerland) and RNA was extracted with NaAC 4.5 pH (3M), acidic phenol 4.5 pH (AM9720, Ambion) and chloroform (0.1:1:0.2). RNA concentrations were measured using the NanoDrop-2000 Spectrophotometer (Thermo Scientific, Wilmington, USA). One μg total RNA was incubated for 5 min. at 70°C, and cDNA synthesis was performed for 1.5 hours at 42°C with 400 U of M-MLV reverse transcriptase RNase H (Promega, Leiden, the Netherlands) in 20 μl of 1x first strand buffer (Promega), and 1mM dNTPs in the presence of 10 U RNase inhibitor rRNasin (Promega) and 0.5 μg random primers (Promega). The reverse transcriptase activity was inactivated by incubation at 95°C for 5 minutes and following addition of 1xTE up to a final volume of 50 μL the cDNAs were stored at −20°C.
Real-time quantitative RT-PCR
The primers used for real-time quantitative PCR (qRT-PCR) were targeted against reference genes β-actin, cyclophilin A, β-2 microglobulin and the following angiogenesis related genes: vascular endothelial cell growth factor receptor (VEGFR)-1 and -2, (VEGF)-A, placental growth factor ( PLGF), angiopoietin (ANG)-1 and -2, epithelial growth factor (EGF) and platelet derived growth factor (PDGF)-B. Also primers for VE-cadherin and CD31 were used where indicated. All primers were designed to meet several requirements concerning GC-content, annealing temperature and amplicon length and synthesized by Sigma-Genosys (Cambridgeshire, UK). Real-time qRT-PCR was performed on a CFX96 (Bio-Rad) using the iQ SYBR® Green PCR mix (Bio-Rad). The PCR reaction was performed in a 25 μl volume containing 1.5 μl cDNA, 1x iQ SYBR® Green PCR mix (Bio-Rad), and 400 nM of each primer. The PCR profile was as follows; 10 minutes at 95°C, followed by 40 cycles of 15 seconds at 95°C and 30 seconds at 60°C. The Ct values and specific melting point of the amplicons were analyzed using CFX Manager (Bio-Rad). The parameter Ct (threshold cycle) was defined as the cycle number at which the fluorescent signal passed a fixed value (threshold) above baseline. Expression relative to the reference genes was calculated using the 2^-dCt method (21).
Statistical analysis
Statistical calculations were performed with SPSS software. We used the student’s t-test for statistical analyses of Figures 1-4. Regression analyses were used for the correlation analyses in Figure 5. Statistical significance was set in all analyses at p<0.05.
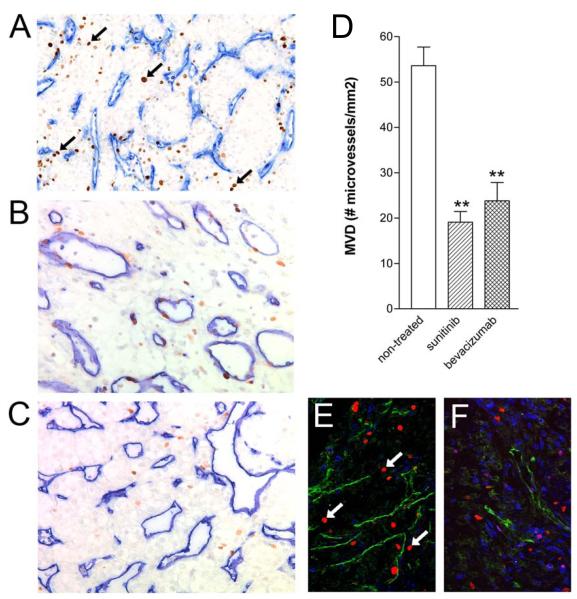
Figure 1.
Suppressed microvessel density in the primary tumor after 2 cycles of sunitinib treatment.
Primary tumor tissue sections of patients with RCC (n=21) were stained with a mixture of CD31/CD34 antibodies to visualize blood vessels (blue), and with anti-Ki-67 (brown, arrows) to monitor proliferation status of both the endothelial- and tumor cell compartments. Representative tissues of untreated (n=70, A), sunitinib treated (n=24, B) and bevacizumab treated (n=29, C) tissues are shown (n values are the number patient tissues with interpretable stainings). Panel D shows the quantification (means ± SEM) of all three patient groups (**p=0.001). Panels E (untreated) and F (sunitinib treated) represent immunofluorescence images, CD31/34 showing in green, Ki-67 in red, and CD3 in blue. Arrows indicate Ki-67-positive nuclei of proliferating EC.
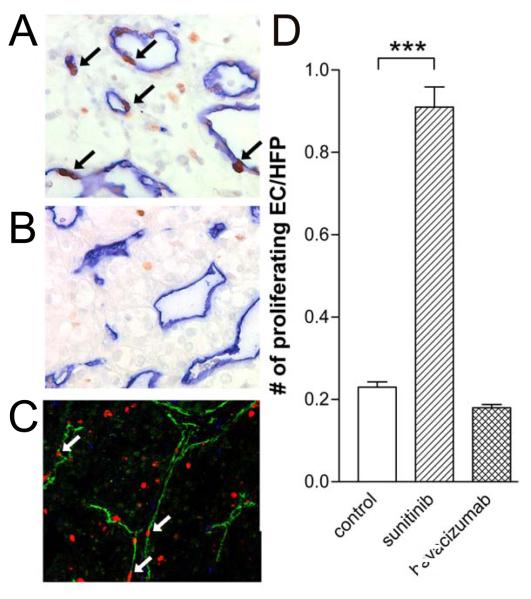
Figure 4.
Enhanced numbers of proliferating endothelial cells in tissues of sunitinib treated patients.
CD31/34 and Ki-67 staining by standard light microscopy (blue and brown, respectively, arrows identify proliferating nuclei of endothelial cells) and immunofluorescence microscopy (green and red, respectively, arrows identify proliferating endothelial cells) in tumor tissues of sunitinib treated patients. Number of nuclei of double stained cells is quantified in C (mean ± SEM, ***p=0.0031).
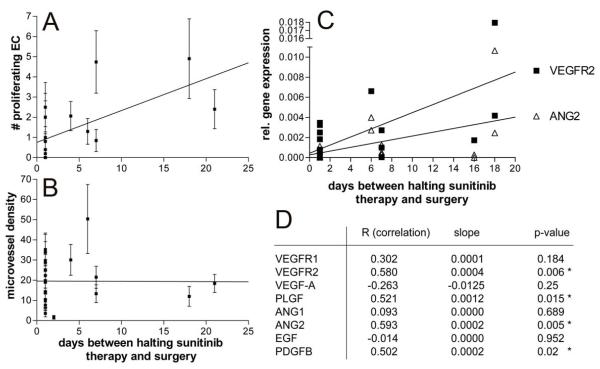
Figure 5.
Onset of angiogenesis after discontinuation of sunitinib treatment.
Positive correlation (p<0.001) between the number of proliferating endothelial cells with the time-interval between treatment stop and cytoreductive surgery (A), and lack of this correlation with microvessel density (B). Panel C represents the linear regression analyses of the gene expression with the time-period between halting the sunitinib therapy and surgery. The correlation plots in C shows the most significantly correlating genes VEGFR2 and ANG2. Correlation and p-values for these and the other tested genes are shown in Table 2.
Results
Clinical studies
The outcome of patients from the retrospective study following pretreatment with sunitinib and the two phase II trials of presurgical sunitinib and bevacizumab have been published previously (14, 16, 17). Briefly, in the retrospective analysis of 17 patients, 4 had a partial response (PR), 12 had stable disease (SD), and 1 had progressive disease (PR). No further outcome data were reported (17).
In the phase II study of presurgical sunitinib only 1 primary tumor responded partially by RECIST (4.5 %). Median reduction of longest diameter was – 9.5% (range 2.2 to – 36%). A >10% reduction of diameter was significantly associated with a high probability to survive 2 years (p=0.01). At metastatic sites, 7 patients developed a PR (31.8%), 7 SD (31.8%) and 8 PD (36.4%). By Memorial Sloan-Kettering Cancer Center criteria (MSKCC) all patients had intermediate risk. Median progression free survival (PFS) was 7 months (range 0-41 months). Median follow-up was 23 months (range 2-41 months). Median OS has not been reached (14). In the presurgical phase II bevacizumab trial 52 patients were enrolled on study, and 50 were included in the analysis. By MSKCC criteria, 82% of patients had intermediate-risk, and 18% had poor-risk, features. Forty-two patients underwent nephrectomy. Median progression-free survival was 11.0 months (95% CI, 5.5 to 15.6 months). Median overall survival was 25.4 months (95% CI, 11.4 months to not estimable) (16).
Sunitinib treatment inhibits primary tumor microvessel density
Primary tumor tissue sections of patients with RCC were stained with a mixture of CD31 and CD34 antibodies to visualize blood vessels, and with anti-Ki-67 to monitor proliferation status of both the endothelial- and tumor cell compartments. Untreated RCC tissues were observed to have a high microvessel density and large numbers of proliferating tumor cells (Figure 1A and 1E). Preoperative sunitinib treatment resulted in a markedly suppressed number of blood vessels in the primary tumor (Figure 1B, D and F, p<0.001 for both sunitinib and bevacizumab) as well as an inhibition of the number of proliferating tumor cells (Figure 1B and 1F). A histological observation indicated that the fewer blood vessels in the treated tumors were larger and more often appeared to have a lumen, as compared to vessels in untreated tumors (Figure 1B). The less compressed aspect of the treated vessels is expected to be a sign of normalized interstitial pressure in the tumor tissue. A similar study on RCC tumor tissues of patients treated with preoperative bevacizumab, showed similar results (Figure 1C and 1D).
Pericyte coverage of microvessels and apoptosis after angiostatic pretreatment of renal cell carcinoma
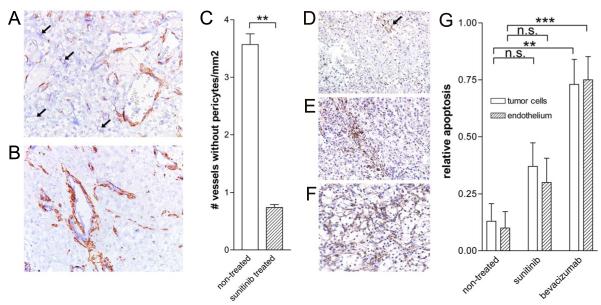
Recently formed angiogenic tumor endothelium is often characterized by a low level of pericyte coverage (22, 23). Treatment of tumors with angiogenesis inhibitors in animal models is reported to normalize this phenotype resulting in more pericyte supported vessels (22). We investigated whether this phenomenon is also observable in human primary tumors treated with sunitinib and bevacizumab, by double staining for CD31/34 and smooth muscle actin. In the tumors of non-treated control RCC patients many microvessels were found lacking support of pericytes (Figure 2A). Interestingly, in the sunitinib treated tumors this number was markedly lower, showing a 4.8-fold decrease, and suggesting a significant anti-angiogenic and vascular maturization effect (Figure 2A-C).
Figure 2.
Pericyte coverage and apoptosis is enhanced after sunitinib treatment.
Panels A and B. CD31/34 (blue) and smooth muscle actin (SMA, brown) stained RCC tissues. Arrows in A indicate small blood vessels that are not associated with pericytes. C. Quantification (means ± SEM) of the number of vessels lacking pericyte coverage (identified by arrows, ** represents statistical significance, p<0.0001). D-F. PARP staining (arrow in D) of non-treated, sunitinib treated and bevacizumab treated tumors, respectively. G. Quantification of the PARP staining in tumor and endothelial cell compartments (mean ± SEM, **p<0.002, ***p<0.001).
Staining for poly ADP-ribose polymerase (PARP) was used to monitor for apoptosis induction by the treatment. A small percentage of PARP staining (1-2%) cells was visible in the non-treated control tumors (Figure 2D). Sunitinib treatment led to an enhanced number (approximately 3-fold increase) of PARP+ apoptotic cells, both in tumor cells as well as in endothelial cells, although for neither compartment this reached significance (Figure 2E and 2G). This parameter increased by >7-fold in the tumors of patients treated with bevacizumab and did reach significance for both the tumor (p<0.01) and endothelial cell compartments (p<0.001, Figure 2F and 2G).
In addition, we analyzed a potential correlation with pre- and post-treatment volume of tumor necrosis for some of the sunitinib pretreated tumors. We were not able to find an association (data not shown).
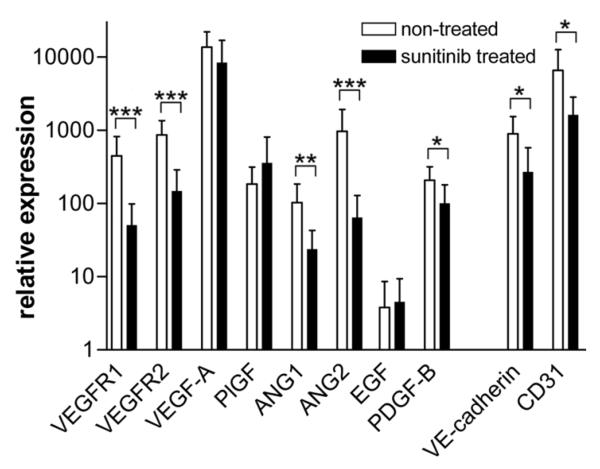
Sunitinib treatment suppresses several key angiogenesis regulators in the primary tumor
To get more insight into the molecular regulation of the effect of sunitinib, real-time quantitative RT-PCR was performed on RNA of frozen tumor tissue of sunitinib treated RCC patients, and results were compared to non-treated cryo-tissue. It was found that gene expression of VEGFR1 and -2, angiopoietin-1 and -2, and PDGF-B was significantly suppressed in the sunitinib treated tumors. In contrast, VEGF itself and two other cytokines placental growth factor (PLGF) and epithelial growth factor (EGF) remained unchanged. Expectedly, the message for the endothelial specific markers VE-cadherin and CD31 were suppressed, independently confirming the inhibition of vessel density after sunitinib treatment (Figure 3).
Figure 3.
Regulation of gene expression in the primary tumor tissue after treatment with sunitinib.
Real-time quantitative PCR was performed for 10 angiogenesis related genes, i.e. VEGF receptor-1 and -2, VEGF-A, placental growth factor (PLGF), angiopoietin (ANG)-1 and -2, epithelial growth factor (EGF), platelet derive growth factor (PDGF)-B, VE-cadherin and CD31. Measurements were performed in RNA preparations of cryo-tumor tissue of sunitinib treated (n=12) and untreated (n=5) patients. Relative expression is shown (mean ± SEM) compensated for the expression of 2 house hold genes (β-actin, cyclophilin A). Statistical significance is indicated by *p<0.05, **p<0.01 and ***p<0.001.
Increased number of proliferating endothelial cells after sunitinib, but not after bevacizumab treatment
Since ongoing angiogenesis can also be quantified by the number of proliferating endothelial cells, we quantified the amount of CD31/34+/Ki-67+ cells. Considering all the suppressed parameters in pretreated tumors, as described above, we expected a decrease in the number of proliferating endothelial cells after the sunitinib treatment. Interestingly, however, a markedly increased number of proliferating endothelial cells was observed by standard immunohistochemistry, as well as fluorescence microscopy (Figure 4A and 4C, respectively) in the tumors of patients that received sunitinib pretreatment. A larger than 4-fold significant (p<0.003) increase in Ki-67 positive endothelial nuclei was noted (Figure 4D) in these patients as compared to non-treated control patients. This increase of proliferating EC was not observed in bevacizumab pretreated patients (Figure 4B and 4D).
Relationship to discontinuation of therapy
An explanation for the enhanced number of growing endothelial cells after sunitinib treatment is that all patients stopped therapy before surgery, and that halting the treatment with sunitinib may induce and unleash a compensatory boost of endothelial cell proliferation. To investigate this possibility, correlation between the number of proliferating endothelial cells and the number of days from halting therapy to the moment of cytoreductive surgery was investigated. Indeed, a strong and significant positive correlation (p<0.001) was found (Figure 5A). This correlation was not observed for microvessel density, a parameter that would take more time to adapt (Figure 5B). Clinical observations of rapid regrowth of tumors after discontinuation of sunitinib treatment were reported. We believe that the boost of endothelial growth may explain this observation.
To understand the molecular regulation of this response, the real-time qPCR data as described above were also correlated to the interval to surgery. Interestingly, for VEGFR-2, angiopoietin-2 (both plotted in Figure 5C), PLGF, and PDGF-B a significant positive association between the number of proliferating endothelial cells and the number of days from halting therapy to the moment of cytoreductive surgery was observed (Figure 5D), further supporting the concept of a rapid onset of angiogenesis after discontinuation of sunitinib therapy.
Discussion
Historically, the treatment of mRCC was unsatisfying, with a lack of meaningful response to most chemotherapeutic agents and radiotherapy. With the advent of agents targeting VEGF signaling, a substantial improvement in response rate and progression free survival was observed. As an example, sunitinib demonstrated an impressive response rate of approximately 40% (10). Sunitinib is a tyrosine kinase inhibitor that blocks the signaling pathways of a series of growth factor receptors, among which are VEGFR-1 and -2, PDGFR-b, c-KIT and FLT-3. Half-life of this agent is 40 to 60 hours. The “off target” effects of sunitinib conspire to produce considerable side effects in patients receiving these agents (24). These side effects make it necessary to administer therapy with an interrupted cycle of 4 weeks on treatment and 2 weeks off treatment. There is clinical evidence that metastases occasionally regrow considerably during the 2-week treatment break (25, 26). Elegant preclinical work has shown that treatment of the RIP-Tag2 mouse tumors or implanted Lewis lung carcinomas with sunitinib resulted in depletion of the tumor endothelial cells, but that rapid regrowth occurred when treatment was discontinued (27). These findings parallel what may be occurring in the human tumors assessed in the current study. Bevacizumab, which is an anti-VEGF-A antibody approved for the treatment of advanced RCC, has a half-life of 21 days. This significant difference in pharmacokinetics can have a significant effect on tumor endothelial growth kinetics.
We found that angiogenesis is clearly inhibited in the primary tumor in response to sunitinib. Several lines of evidence support this observation: (i) A lower tumor microvessel density was present in sunitinib treated patients, as compared to non-treated controls. This further supports the observations by Mancuso et al (27) that the plasticity of vessels can be very high. Turnover of tumor cells is also high, illustrated by the number of proliferating tumor cells. The high activity in the tumor tissue underlies the rapid responses in vessel density. (ii) The number of highly angiogenic blood vessels, visible by their lack of pericyte coverage, was significantly reduced in sunitinib treated tumors. This is a somewhat paradoxical finding, as PDGF inhibition would be expected to decrease pericyte coverage, but this effect may be counterbalanced by the normalizing effect of VEGF blockade in RCC. In addition, our study enumerated the number of vessels without pericytes, representing the population of newly formed vessels, rather than the total number of pericytes. We conclude that vessel neoformation is inhibited. As we did not enumerate the total number of pericytes in the tissue, we cannot draw any conclusion on the direct effect of sunitinib on pericytes. Treatment of primary tumors with combination VEGF and PDGF receptor therapy in an animal model of breast cancer led to decreased pericyte coverage and increased number of metastases (28). Whether the association between pericyte coverage and clinical outcome holds true for RCC needs to be further studied. (iii) Sunitinib induced an increased number of PARP-positive cells, not only in the tumor cell compartment, but also in the endothelial cell fraction, suggesting death of newly formed blood vessels. (iv) The expression of several key angiogenesis regulators is found to be high in non-treated tumor tissues and markedly repressed as a result of sunitinib treatment.
The most important finding of this study is the enhanced number of proliferating endothelial cells in the tumors of sunitinib treated patients, which was not observed in bevacizumab treated patients. This parameter has been described as a better parameter of ongoing angiogenesis than microvessel density (29, 30). One explanation is that an apparent induction of angiogenesis is illustrated by the sunitinib-induced synchronization of endothelial cells in a certain stage of cell cycle, e.g. G2/M, in which cells are positive for Ki-67. Such a situation has been suggested for treatment of endothelial cells with the angiogenesis inhibitor platelet factor-4 (31). This would then mean that the cells are not growing and undergoing perfect angiostasis. Staining of cell cultures for DNA with propidium iodide, before and after sunitinib exposure, however, did not reveal any synchronization of cell cycle (data not shown). The only other explanation is therefore the presence of a compensatory boost of angiogenesis after discontinuation of the treatment, a conclusion that is supported by the finding of the molecular upregulation of angiogenesis mediators (Figures 3 and 5). The observed rapid onset of neovascularization after discontinuation of presurgical treatment of patients in the tumor tissue may partly play a role in the clinical observation of rapid progression in 36% of patients pretreated with sunitinib within a period of a median of 29 days following nephrectomy in primary metastatic disease (15, 32). This relatively high frequency within a short period of time with most patients (73%) stabilizing again under reinitiation of treatment suggests that discontinuation rather than resistance to sunitinib is the cause. However, in preclinical models and clinical practice intermittent higher dose therapy with short-term discontinuation has been beneficial. In an animal xenograft model, higher dose intermittent sorafenib was superior to continuous sorafenib in controlling growth of 786-0 cells implanted in the flank of nu/nu mice (33). A randomized study comparing sunitinib 50 mg on a 4 week on, 2 week off schedule versus 37.5 mg continuous dosing showed a trend towards improved outcome for the intermittent dosage group (34). Therefore, the relative benefit of higher dose therapy with breaks versus continuous lower dose treatment may be in favour of intermittent therapy, except for the subset of patients with highly aggressive disease who experience a significant rebound effect. Another explanation may be that the commonly practised discontinuation of 2 weeks is too short a period in the majority of patients to observe clinical progression. Case reports have been published of rapid disease progression days after discontinuation of sunitinib (25, 26), but this does not reflect clinical reality in the majority of patients. We observed in the period of presurgical interruption, which was shorter than 2 weeks with the exception of 2 cases, that though EC proliferation increases in primary tumor tissue, microvessel density did not. This suggests that a longer time off drug would be needed before an increase in microvessel density would ensue and subsequently translate into clinical progression. As most intermittent treatment protocols reinitiate therapy after 2 weeks it is likely that EC proliferation is abrogated before onset of new blood vessels. Currently, presurgical treatment protocols require a period off treatment in the recovery period following cytoreductive surgery. Together with presurgical discontinuation this may add up to 6 weeks treatment interruption and may be longer in those with surgical complications. These patients may therefore be at risk of disease progression. The ongoing EORTC SURTIME trial compares immediate cytoreductive nephrectomy followed by an uninterrupted treatment with sunitinib 4 weeks on and 2 weeks off, until progression, to delayed cytoreductive nephrectomy after 3 cycles of sunitinib (4 weeks on, 2 weeks off) with a period off treatment of 4 weeks before continuation of treatment. The results of this trial will provide further data on the potential significance of prolonged treatment interruption in metastatic renal cell carcinoma. Some insight into the effect of treatment discontinuation can also be elucidated from patients who have achieved CR after treatment with anti-angiogenic therapy, with or without metastasectomy (35, 36), though it has to be acknowledged that these are highly selected patients with a favourable course of the disease. In each study, a number of individuals demonstrated disease recurrence, underscoring the fact that these agents are not cytocidal against tumor epithelial cells, and may eventually need to be restarted in the majority of patients. In another series of patients who had stable disease or better with anti-angiogenic therapy, treatment discontinuation because of toxicity resulted in 63% of the patients progressing after a median of 10 months (37). Of these, 32% developed new metastatic sites.
This study has a number of limitations. The number of patients in the pretreated groups is very small and may not allow evaluation of statistically meaningful correlations between the expression of certain angiogenesis genes and outcome parameters or the time off treatment prior to surgery. Although in individual patients with a very short survival and rapid disease progression upregulation of genes was comparable to the pattern observed in the untreated control group, these findings have to be interpreted with caution. The observed upregulation may reflect a primary or very early resistance to the anti-angiogenic effect of sunitinib or rapid proliferation after withdrawal of sunitinib but other confounding factors cannot be ruled out. Tumor heterogeneity may play an important role in the variation of expression with different patterns in the same primary tumor at the time of sampling. Ideally, pretreatment tissue would be compared to post-treatment tissue in the same patients. Unfortunately, in most cases, acquiring a sufficiently large pretreatment sample for robust analysis is quite difficult. In addition, a very recent publication described significant intratumor heterogeneity in RCC and suggests that pretreatment tumor samples may not represent the areas from which metastases originated (38). Consequently, they may not be representative when assessing pre- and postreatment tumor changes, which may have implications for the interpretation of results from genomic profiling. In the present study, we therefore performed inter-individual comparisons using full tumor sections of treated and un-treated patients. Although intra-individual comparisons using pretreatment biopsy materials would have been possible, it was felt that having larger tumor areas to compare, would be more accurate than comparing the small cores of Tru cut biopsies, which may indeed not be representative.
Acquiring tissue for analysis is obviously a challenge, and better techniques are needed. Ideally, noninvasive measures of early success with anti-angiogenic therapy should be developed. These could include imaging modalities and measure of circulating factors and cells. Dynamic contrast ultrasound can be used to assess response to anti-angiogenic therapy by evaluating area under the curve of blood flow (39). Imaging with 111In bevacizumab is also being developed, and may be a useful tool for measuring tumor response (40). Multiplex bead-based analysis platforms may allow noninvasive measurement of the changes in tumor microenvironment induced by therapy (41).
Although these speculations may be preliminary, this work demonstrates that different angiogenesis expression patterns can be detected between pretreated and untreated tumors, and between different anti-angiogenic agents. It is clear that larger numbers of patients are needed to rule out inter-tumoral variations and define association with clinical benefit.
Statement on translational relevance.
Current cancer treatment with tyrosine kinase inhibitors often involves treatment discontinuations due to potentially significant side effects. It is now demonstrated that this results in a compensatory boost of angiogenesis. These data support the clinical observation of rapid regrowth during the 2-week break from sunitinib therapy in some patients. As these results are not observed in patients pretreated with bevacizumab, possibly due to the long half-life of this antibody-based drug, clear differences in endothelial growth kinetics exist as a function of agent choice. The therapeutic and mechanistic relevance of this regrowth are not clear, as both agents demonstrate a salutary effect in clinical practice. The current results raise the possibility that tumor growth during therapy, as well as delayed regrowth after therapy, are important parameters for improving patient outcome. Linking these tissue-based findings to clinically relevant readouts will be important.
Table 2.
Correlations between qPCR-based gene expression assessment and the number of days from halting sunitinib therapy.
| R (correlation)a | slopea | p-value | |
|---|---|---|---|
| VEGFR1 | 0.302 | 0.0001 | 0.184 |
| VEGFR2 | 0.580 | 0.0004 | 0.006 * |
| VEGF-A | −0.263 | −0.0125 | 0.25 |
| PLGF | 0.521 | 0.0012 | 0.015 * |
| ANG1 | 0.093 | 0.0000 | 0.689 |
| ANG2 | 0.593 | 0.0002 | 0.005 * |
| EGF | −0.014 | 0.0000 | 0.952 |
| PDGFB | 0.502 | 0.0002 | 0.02 * |
correlation assessments performed for VEGF receptor-1 and -2, VEGF-A, placental growth factor, angiopoietin-1 and -2, epidermal growth factor, platelet derived growth factor, respectively.
Acknowledgement
The authors would like to thank Steven Bosch, Koen Marijt and Maaike van Berkel for expert technical assistance, and our colleagues from the Pathology Department of the Netherlands Cancer Institute for providing paraffin sections.
References
- 1.Griffioen AW, Molema G. Angiogenesis: potentials for pharmacologic intervention in the treatment of cancer, cardiovascular diseases, and chronic inflammation. Pharmacol Rev. 2000;52:237–68. [PubMed] [Google Scholar]
- 2.Carmeliet P, Jain RK. Molecular mechanisms and clinical applications of angiogenesis. Nature. 2011;473:298–307. doi: 10.1038/nature10144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dings RP, Loren M, Heun H, McNiel E, Griffioen AW, Mayo KH, et al. Scheduling of radiation with angiogenesis inhibitors anginex and Avastin improves therapeutic outcome via vessel normalization. Clin Cancer Res. 2007;13:3395–402. doi: 10.1158/1078-0432.CCR-06-2441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dings RP, Vang KB, Castermans K, Popescu F, Zhang Y, Oude Egbrink MG, et al. Enhancement of T-cell-mediated antitumor response: angiostatic adjuvant to immunotherapy against cancer. Clin Cancer Res. 2011;17:3134–45. doi: 10.1158/1078-0432.CCR-10-2443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Teicher BA, Holden SA, Ara G, Korbut T, Menon K. Comparison of several antiangiogenic regimens alone and with cytotoxic therapies in the Lewis lung carcinoma. Cancer Chemother Pharmacol. 1996;38:169–77. doi: 10.1007/s002800050466. [DOI] [PubMed] [Google Scholar]
- 6.Nowak-Sliwinska P, Weis A, Van Beijnum JR, Wong TJ, van den Bergh H, Griffioen AW. Angiostatic kinase inhibitors to sustain photodynamic angio-occlusion. J Cell Mol Med. 2011 doi: 10.1111/j.1582-4934.2011.01440.x. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Griffioen AW, Vyth-Dreese FA. Angiostasis as a way to improve immunotherapy. Thromb Haemost. 2009;101:1025–31. doi: 10.1160/th08-08-0552. [DOI] [PubMed] [Google Scholar]
- 8.Motzer RJ, Hutson TE, Tomczak P, Michaelson MD, Bukowski RM, Oudard S, et al. Overall survival and updated results for sunitinib compared with interferon alfa in patients with metastatic renal cell carcinoma. J Clin Oncol. 2009;27:3584–90. doi: 10.1200/JCO.2008.20.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Escudier B, Goupil MG, Massard C, Fizazi K. Sequential therapy in renal cell carcinoma. Cancer. 2009;115:2321–6. doi: 10.1002/cncr.24241. [DOI] [PubMed] [Google Scholar]
- 10.Motzer RJ, Hutson TE, Tomczak P, Michaelson MD, Bukowski RM, Rixe O, et al. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med. 2007;356:115–24. doi: 10.1056/NEJMoa065044. [DOI] [PubMed] [Google Scholar]
- 11.Choueiri TK, Xie W, Kollmannsberger C, North S, Knox JJ, Lampard JG, et al. The impact of cytoreductive nephrectomy on survival of patients with metastatic renal cell carcinoma receiving vascular endothelial growth factor targeted therapy. J Urol. 2011;185:60–6. doi: 10.1016/j.juro.2010.09.012. [DOI] [PubMed] [Google Scholar]
- 12.Wood CG, Margulis V. Neoadjuvant (presurgical) therapy for renal cell carcinoma: a new treatment paradigm for locally advanced and metastatic disease. Cancer. 2009;115:2355–60. doi: 10.1002/cncr.24240. [DOI] [PubMed] [Google Scholar]
- 13.Bex A, Jonasch E, Kirkali Z, Mejean A, Mulders P, Oudard S, et al. Integrating surgery with targeted therapies for renal cell carcinoma: current evidence and ongoing trials. European urology. 2010;58:819–28. doi: 10.1016/j.eururo.2010.08.029. [DOI] [PubMed] [Google Scholar]
- 14.Bex A, Blank C, Meinhardt W, van Tinteren H, Horenblas S, Haanen J. A Phase II Study of Presurgical Sunitinib in Patients with Metastatic Clear-cell Renal Carcinoma and the Primary Tumor In Situ. Urology. 2011 doi: 10.1016/j.urology.2011.05.034. [DOI] [PubMed] [Google Scholar]
- 15.Powles T, Blank C, Chowdhury S, Horenblas S, Peters J, Shamash J, et al. The outcome of patients treated with sunitinib prior to planned nephrectomy in metastatic clear cell renal cancer. European urology. 2011;60:448–54. doi: 10.1016/j.eururo.2011.05.028. [DOI] [PubMed] [Google Scholar]
- 16.Jonasch E, Wood CG, Matin SF, Tu SM, Pagliaro LC, Corn PG, et al. Phase II presurgical feasibility study of bevacizumab in untreated patients with metastatic renal cell carcinoma. J Clin Oncol. 2009;27:4076–81. doi: 10.1200/JCO.2008.21.3660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van der Veldt AA, Meijerink MR, van den Eertwegh AJ, Bex A, de Gast G, Haanen JB, et al. Sunitinib for treatment of advanced renal cell cancer: primary tumor response. Clin Cancer Res. 2008;14:2431–6. doi: 10.1158/1078-0432.CCR-07-4089. [DOI] [PubMed] [Google Scholar]
- 18.van der Schaft DW, Pauwels P, Hulsmans S, Zimmermann M, van de Poll-Franse LV, Griffioen AW. Absence of lymphangiogenesis in ductal breast cancer at the primary tumor site. Cancer Lett. 2007;254:128–36. doi: 10.1016/j.canlet.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 19.Res PC, Couwenberg F, Vyth-Dreese FA, Spits H. Expression of pTalpha mRNA in a committed dendritic cell precursor in the human thymus. Blood. 1999;94:2647–57. [PubMed] [Google Scholar]
- 20.Vyth-Dreese FA, Kim YH, Dellemijn TA, Schrama E, Haanen JB, Spierings E, et al. In situ visualization of antigen-specific T cells in cryopreserved human tissues. J Immunol Methods. 2006;310:78–85. doi: 10.1016/j.jim.2005.12.011. [DOI] [PubMed] [Google Scholar]
- 21.Thijssen VL, Brandwijk RJ, Dings RP, Griffioen AW. Angiogenesis gene expression profiling in xenograft models to study cellular interactions. Exp Cell Res. 2004;299:286–93. doi: 10.1016/j.yexcr.2004.06.014. [DOI] [PubMed] [Google Scholar]
- 22.Goel S, Duda DG, Xu L, Munn LL, Boucher Y, Fukumura D, et al. Normalization of the vasculature for treatment of cancer and other diseases. Physiol Rev. 2011;91:1071–121. doi: 10.1152/physrev.00038.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yonenaga Y, Mori A, Onodera H, Yasuda S, Oe H, Fujimoto A, et al. Absence of smooth muscle actin-positive pericyte coverage of tumor vessels correlates with hematogenous metastasis and prognosis of colorectal cancer patients. Oncology. 2005;69:159–66. doi: 10.1159/000087840. [DOI] [PubMed] [Google Scholar]
- 24.Walraven M, Witteveen PO, Lolkema MP, van Hillegersberg R, Voest EE, Verheul HM. Antiangiogenic tyrosine kinase inhibition related gastrointestinal perforations: a case report and literature review. Angiogenesis. 2011;14:135–41. doi: 10.1007/s10456-010-9197-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wolter P, Beuselinck B, Pans S, Schoffski P. Flare-up: an often unreported phenomenon nevertheless familiar to oncologists prescribing tyrosine kinase inhibitors. Acta Oncol. 2009;48:621–4. doi: 10.1080/02841860802609574. [DOI] [PubMed] [Google Scholar]
- 26.Desar IM, Mulder SF, Stillebroer AB, van Spronsen DJ, van der Graaf WT, Mulders PF, et al. The reverse side of the victory: flare up of symptoms after discontinuation of sunitinib or sorafenib in renal cell cancer patients. A report of three cases. Acta Oncol. 2009;48:927–31. doi: 10.1080/02841860902974167. [DOI] [PubMed] [Google Scholar]
- 27.Mancuso MR, Davis R, Norberg SM, O’Brien S, Sennino B, Nakahara T, et al. Rapid vascular regrowth in tumors after reversal of VEGF inhibition. J Clin Invest. 2006;116:2610–21. doi: 10.1172/JCI24612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cooke VG, LeBleu VS, Keskin D, Khan Z, O’Connell JT, Teng Y, et al. Pericyte depletion results in hypoxia-associated epithelial-to-mesenchymal transition and metastasis mediated by met signaling pathway. Cancer Cell. 2012;21:66–81. doi: 10.1016/j.ccr.2011.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hillen F, van de Winkel A, Creytens D, Vermeulen AH, Griffioen AW. Proliferating endothelial cells, but not microvessel density, are a prognostic parameter in human cutaneous melanoma. Melanoma Res. 2006;16:453–7. doi: 10.1097/01.cmr.0000232291.68666.4c. [DOI] [PubMed] [Google Scholar]
- 30.Baldewijns MM, Thijssen VL, Van den Eynden GG, Van Laere SJ, Bluekens AM, Roskams T, et al. High-grade clear cell renal cell carcinoma has a higher angiogenic activity than low-grade renal cell carcinoma based on histomorphological quantification and qRT-PCR mRNA expression profile. Br J Cancer. 2007;96:1888–95. doi: 10.1038/sj.bjc.6603796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gupta SK, Singh JP. Inhibition of endothelial cell proliferation by platelet factor-4 involves a unique action on S phase progression. J Cell Biol. 1994;127:1121–7. doi: 10.1083/jcb.127.4.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Powles T, Blank C, Chowdhury S, Horenblas S, Peters J, Shamash J, et al. The outcome of patients treated with sunitinib prior to planned nephrectomy in metastatic clear cell renal cancer. European urology. 60:448–54. doi: 10.1016/j.eururo.2011.05.028. [DOI] [PubMed] [Google Scholar]
- 33.Wang X, Zhang L, Goldberg SN, Bhasin M, Brown V, Alsop DC, et al. High dose intermittent sorafenib shows improved efficacy over conventional continuous dose in renal cell carcinoma. J Transl Med. 2011;9:220. doi: 10.1186/1479-5876-9-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Motzer RJ, Hutson TE, Olsen MR, Hudes GR, Burke JM, Edenfield WE, et al. Randomized Phase II Trial of Sunitinib on an Intermittent Versus Continuous Dosing Schedule As First-Line Therapy for Advanced Renal Cell Carcinoma J Clin Oncol. 2012 doi: 10.1200/JCO.2011.36.4133. In press. [DOI] [PubMed] [Google Scholar]
- 35.Johannsen M, Florcken A, Bex A, Roigas J, Cosentino M, Ficarra V, et al. Can tyrosine kinase inhibitors be discontinued in patients with metastatic renal cell carcinoma and a complete response to treatment? A multicentre, retrospective analysis. European urology. 2009;55:1430–8. doi: 10.1016/j.eururo.2008.10.021. [DOI] [PubMed] [Google Scholar]
- 36.Albiges L, Oudard S, Negrier S, Caty A, Gravis G, Joly F, et al. Complete remission with tyrosine kinase inhibitors in renal cell carcinoma. J Clin Oncol. 2012;30:482–7. doi: 10.1200/JCO.2011.37.2516. [DOI] [PubMed] [Google Scholar]
- 37.Sadeghi S, Albiges L, Wood LS, Black SL, Gilligan TD, Dreicer R, et al. Cessation of vascular endothelial growth factor-targeted therapy in patients with metastatic renal cell carcinoma: Feasibility and Clinical Outcome. Cancer. 2011 doi: 10.1002/cncr.26666. [DOI] [PubMed] [Google Scholar]
- 38.Gerlinger M, Rowan AJ, Horswell S, Larkin J, Endesfelder D, Gronroos E, et al. Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. N Engl J Med. 2012;366:883–92. doi: 10.1056/NEJMoa1113205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lassau N, Chebil M, Chami L, Bidault S, Girard E, Roche A. Dynamic contrast-enhanced ultrasonography (DCE-US): a new tool for the early evaluation of antiangiogenic treatment. Target Oncol. 2010;5:53–8. doi: 10.1007/s11523-010-0136-7. [DOI] [PubMed] [Google Scholar]
- 40.Desar IM, Stillebroer AB, Oosterwijk E, Leenders WP, van Herpen CM, van der Graaf WT, et al. 111In-bevacizumab imaging of renal cell cancer and evaluation of neoadjuvant treatment with the vascular endothelial growth factor receptor inhibitor sorafenib. J Nucl Med. 2010;51:1707–15. doi: 10.2967/jnumed.110.078030. [DOI] [PubMed] [Google Scholar]
- 41.Zurita AJ, Jonasch E, Wang X, Khajavi M, Yan S, Du DZ, et al. A cytokine and angiogenic factor (CAF) analysis in plasma for selection of sorafenib therapy in patients with metastatic renal cell carcinoma. Ann Oncol. 2011;23:46–52. doi: 10.1093/annonc/mdr047. [DOI] [PMC free article] [PubMed] [Google Scholar]