Abstract
Background
Many natural compounds were tested for the ability to suppress viral replication. The present manuscript details an analysis of high dose vitamin C therapy on patients with EBV infection.
Material/Methods
The data were obtained from the patient history database at the Riordan Clinic. Among people in our database who were treated with intravenous vitamin C (7.5 g to 50 g infusions) between 1997 and 2006, 178 patients showed elevated levels of EBV EA IgG (range 25 to 211 AU) and 40 showed elevated levels of EBV VCA IgM (range 25 to 140 AU). Most of these patients had a diagnosis of chronic fatigue syndrome, with the rest being diagnosed as having mononucleosis, fatigue, or EBV infection.
Results
Our data provide evidence that high dose intravenous vitamin C therapy has a positive effect on disease duration and reduction of viral antibody levels.
Plasma levels of ascorbic acid and vitamin D were correlated with levels of antibodies to EBV. We found an inverse correlation between EBV VCA IgM and vitamin C in plasma in patients with mononucleosis and CFS meaning that patients with high levels of vitamin C tended to have lower levels of antigens in the acute state of disease.
In addition, a relation was found between vitamin D levels and EBV EA IgG with lower levels of EBV early antigen IgG for higher levels of vitamin D.
Conclusions
The clinical study of ascorbic acid and EBV infection showed the reduction in EBV EA IgG and EBV VCA IgM antibody levels over time during IVC therapy that is consistent with observations from the literature that millimolar levels of ascorbate hinder viral infection and replication in vitro.
MeSH Keywords: Administration, Intravenous - methods, Ascorbic Acid Deficiency - therapy, Epstein-Barr Virus Infections - history, Vitamin D - blood
Background
The Epstein-Barr virus (EBV) is a member of the herpes family that targets lymphocytes and epithelial cells [1–6]. It binds to B-lymphocytes via the CD21 cell surface protein, and establishes life-long persistence in memory B-cells [7–10]. While the infection is usually benign, it can in some cases lead to acute infectious mononucleosis and can impair the immune system [11,12]. EBV is linked to several malignancies, including Burkett’s lymphoma, post-transplant lymph-proliferative disease, Hodgkin’s disease, and several autoimmune diseases [13–15]. EBV inhibits the ability of lymphocytes to respond properly to antigens such as mitogenic lectins, concanavalin A, phytohemagglutinin, and pokeweed mitogen, among others [16,17].
EBV infections can be detected by immunoglobulin assays. Most subjects show IgM antibodies to EBV viral capsid antigen (VCA) at the onset of infection, which decline after two to six months. IgG antibodies to the EBV VCA may be detected a few weeks or months after the onset of infection, and can persist for life [9]. In addition, IgG antibodies to the EBV early diffuse antigen (EA) can also be detected during acute infections [18,19]. Antibodies to the Epstein-Barr nuclear antigen (EBNA) indicate the presence of a past infection. The profile of antibodies that used to distinguish between the various stages of EBV infection is summarized in www.cdc.gov. High lymphocytes counts, particularly atypical high numbers of activated CD8 T-lymphocytes, and the presence of Downey cells characterized by enlarged cytoplasm and condensed nuclei are also present in primary EBV infection [20].
There is currently no treatment for removing EBV infections. Our clinic has been long interested in the use of vitamin C (ascorbic acid, ascorbate) to combat viral infections. Ascorbic acid is an essential nutrient that functions as a key water soluble antioxidant and is involved in synthesis of collagen, carnitine, and neurotransmitters [21–23]. It affects wound healing, energy metabolism, nervous system function, and immune cell health [24–27]. Oral supplementation with vitamin C typically gives rise to plasma ascorbate concentrations less than 0.2 mM, while high dose intravenous infusion of the vitamin can raise plasma concentrations higher than 14 mM [28–30]. These “pharmacologic” plasma ascorbate concentrations achieved by intravenous infusion have been linked with benefits to endothelial function, cellular immune function, antioxidative capacity, pain relief, and treatment of cancer and other illnesses [31–37].
The motivation for using intravenous infusions of vitamin C (IVC) to treat viral illnesses comes, in part, from observations that virally infected patients exhibit vitamin C deficiency [38–40]. This in turn suggests that clinical management of viral infections may benefit from supplementation. Improved recovery of subjects with viral infection upon supplementation with pharmacologic doses of vitamin C has been observed clinically [40–43]. In a multicenter cohort study, sixty-seven symptomatic Herpes-Zoster patients were given intravenous vitamin C in addition to standard treatment for shingles [43]. Pain assessments were made and dermatologic symptoms such as hemorrhagic lesions were followed during twelve weeks of treatment. Pain scores, number of dermatomes and number of efflorescences all showed statistically significant decreases during the treatment.
Several mechanisms of action have been proposed for this potential benefit. Since viral infections are often associated with oxidative stress, the ability of ascorbate replenishment to promote a reducing environment could be important in detoxification and neutralization of reactive oxygen species associated with infection [44]. Vitamin C is also necessary for neutrophil function, as they typically accumulate ascorbic acid at eighty times the plasma concentration [45]. Also considered as potential mechanisms are the ability of ascorbic acid to stimulate the production of interferon and other anti-viral cytokines, its ability to down regulate inflammation, and its direct antiviral properties [46–54].
The direct anti-viral activity of ascorbate has been studied extensively in vitro (animal studies are complicated by the fact that most laboratory animals synthesize ascorbic acid). In one study, for example, millimolar concentrations of ascorbate or dehydroascorbate dramatically reduced the ability of three different viral types (herpes simplex virus type 1, influenza virus type A, and picornaviridea virus 1) to infect cell monolayers [51]. Note that millimolar concentrations of ascorbate are not physiologically achievable through oral vitamin supplementation, but can be attained as a result of intravenous vitamin C infusion. Suspensions of herpes simplex viruses (types 1 and 2), cytomegalovirus, and parainfluenza virus type 2 were inactivated upon exposure to sodium ascorbate (at pharmacologic concentrations in the millimolar range) plus copper, and ascorbic acid concentrations in the millimolar range were effective against human influenza viruses [42,52]. In chick embryo fibroblast, infection with Rous sarcoma viruses was inhibited by ascorbic acid [54].
The present manuscript details an analysis of EBV progression, via antibody assays, in patients undergoing intravenous vitamin C therapy. Our results, detailed below, add further evidence to the idea that ascorbic acid may be useful in treating viral infections.
Material and Methods
The Epstein-Barr antibodies were detected in human serum by enzyme linked immunosorbent assay (ELISA) according to manufacturer’s instructions (INCSTAR Corporation, Minnesota). The absorbance of solution was measured at 450 nm. The normal range of antibodies to EBV viral capsid antigens IgG, IgM and EBV early antigen IgG were in range 0–20 AU. PCR-based method of EBV DNA detection in serum in conjunction with serological tests is a useful additional test to the panel of tests offered at our clinical laboratory. However, test was not avalable and we did not have data for analysis.
The levels of vitamin C and vitamin D in blood were attained by standard clinical procedures.
The study was conducted under Institutional Review Board Approval of Riordan Clinic. Demographics were limited to ensure confidentiality, and informed consent was obtained from all patients. From the database of patients with EBV infection treated with IVC, we selected subjects for whom plasma antibody’ levels before and after treatment were available. The details of the Riordan IVC protocol have been described elsewhere [29,30]. Briefly, patients are given IVC infusions at the 7.5, 15, 25, and 50 gram dosages. Dosages are adjusted by the physician based on the patients’ tolerance and plasma ascorbic acid levels attained post infusion.
As hemolysis has been reported in patients with glucose-6-phosphate dehydrogenase (G6PD) deficiency when given high-dose IVC, the G6PD level was assessed for all patients before beginning IVC.
The protocol also suggests adding magnesium to reduce the incidence of vein irritation and spasm.
Statistical methods. The data were analysed by Systat software (Systat, Inc) and Kaleidagraph software. Variables were presented as mean values ±SD, or as medians with corresponding 25th percentiles. Association between different factors was assessed using linear models. Statistical significance was accepted if the null hypothesis could be rejected at p≤0.05.
Results
The data were obtained from the patient history database at the Riordan Clinic, a nutritional medicine treatment and research clinic. Among people in our database who were treated at the clinic with intravenous vitamin C (7.5 g to 50 g infusions) for various illness, we found 178 patients who showed elevated levels of EBV IgG (range 25 to 211 AU) and forty who showed elevated levels of EBV VCA IgM (range 25 to 140 AU). These subjects, all being treated between 1997 and 2006, formed the basis of our study. Most of these patients (110 subjects) had a diagnosis of chronic fatigue syndrome, with the rest being diagnosed as having mononucleosis, fatigue, or EBV infection.
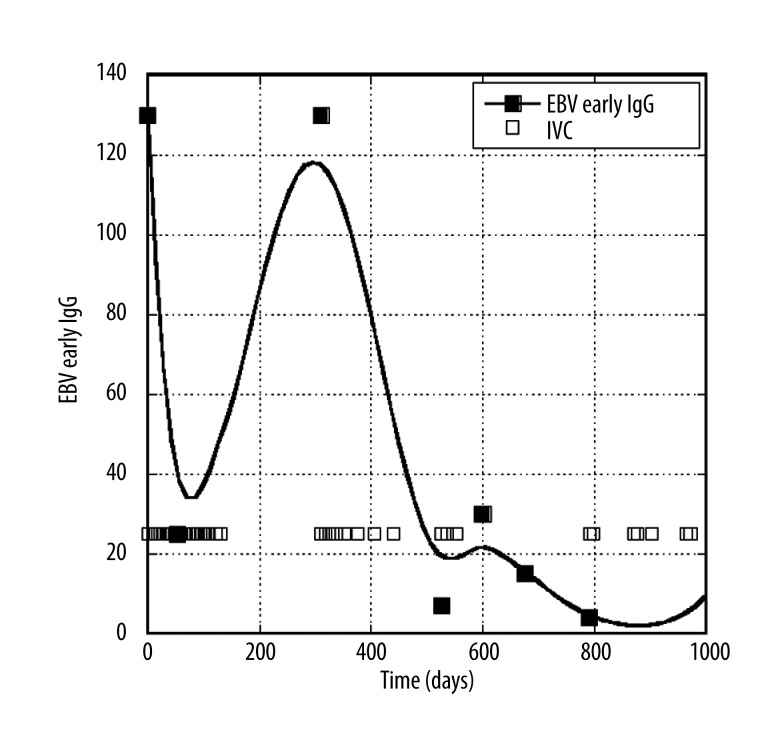
Figure 1 shows how EBV EA IgG antibody levels changed over time in a typical patient. This patient not only had high EBV antibody levels (130 AU) at the start of treatment, but showed a high percentage of lymphocytes (50%, with 10% being atypical morphology). After 13 treatments of IVC, at dose of 25 grams each the EA IgG level decreased to 25 AU. The patient at this point stopped treatments, and saw a rebound in antigen load. Resumption of therapy brought the antibody levels back down to within normal ranges.
Figure 1.
Changes in EBV EA antibodies over time in a patient. Boxes represent times of IVC administration.
We had detailed information about treatments, along with several follow-up measurements, for thirty-five subjects. Their EBV EA IgG levels before and after treatments are shown in Table 1.
Table 1.
EBV EA IgG values before and after IVC therapy in 35 subjects. Percentage of decrease in early antibody IgG levels (Δ) is given.
| Subjects | EBV Early Ag, IgG | % of change | Days | Treatments (IVC) numbers and doses | Total IVCs | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Before | After | Δ (%) | 7.5 g | 15 g | 25 g | 30 g | 50 g | |||
| 1 | 195 | 151 | 22.56 | 90 | 2 | 9 | 11 | |||
| 2 | 195 | 50 | 74.36 | 200 | 7 | 16 | 23 | |||
| 3 | 257 | 211 | 17.90 | 138 | 6 | 6 | ||||
| 4 | 58 | 30 | 48.28 | 50 | 13 | 13 | ||||
| 5 | 38 | 30 | 21.05 | 75 | 1 | 1 | ||||
| 6 | 130 | 25 | 80.77 | 50 | 13 | 13 | ||||
| 7 | 130 | 7 | 94.62 | 200 | 10 | 10 | ||||
| 8 | 123 | 36 | 70.73 | 119 | 8 | 8 | ||||
| 9 | 67 | 30 | 55.22 | 178 | 5 | 5 | ||||
| 10 | 26 | 21 | 19.23 | 60 | 5 | 5 | ||||
| 11 | 37 | 67 | −81.08 | 132 | 8 | 8 | ||||
| 12 | 108 | 80 | 25.93 | 100 | 8 | 8 | ||||
| 13 | 130 | 46 | 64.62 | 118 | 8 | 8 | ||||
| 14 | 48 | 38 | 20.83 | 125 | 2 | 2 | ||||
| 15 | 22 | 15 | 31.82 | 100 | 8 | 8 | ||||
| 16 | 44 | 8 | 81.82 | 108 | 1 | 6 | 7 | |||
| 17 | 44 | 25 | 43.18 | 43 | 1 | 6 | 7 | |||
| 18 | 138 | 98 | 28.99 | 118 | 1 | 10 | 11 | |||
| 19 | 83 | 68 | 18.07 | 91 | 1 | 6 | 7 | |||
| 20 | 80 | 64 | 20.00 | 134 | 2 | 2 | 4 | |||
| 21 | 91 | 3 | 96.70 | 177 | 1 | 17 | 18 | |||
| 22 | 47 | 5 | 89.36 | 243 | 7 | 11 | 18 | |||
| 23 | 47 | 25 | 46.81 | 100 | 7 | 11 | 18 | |||
| 24 | 57 | 64 | −12.28 | 87 | 1 | 8 | 9 | |||
| 25 | 29 | 6 | 79.31 | 67 | 10 | 10 | ||||
| 26 | 39 | 19 | 51.28 | 209 | 1 | 1 | ||||
| 27 | 48 | 38 | 20.83 | 125 | 2 | 2 | ||||
| 28 | 108 | 80 | 25.93 | 50 | 8 | 8 | ||||
| 29 | 42 | 19 | 54.76 | 110 | 20 | 20 | ||||
| 30 | 29 | 6 | 79.31 | 60 | 10 | 10 | ||||
| 31 | 64 | 17 | 73.44 | 107 | 7 | 7 | ||||
| 32 | 60 | 45 | 25.00 | 55 | 1 | 1 | ||||
| 33 | 43 | 50 | −16.28 | 102 | 1 | 4 | 5 | |||
| 34 | 101 | 86 | 14.85 | 70 | 3 | 2 | 7 | 12 | ||
| 35 | 39 | 28 | 28.21 | 24 | 1 | 6 | 7 | |||
The average EBV EA IgG level before treatment was 80±55 (SD) AU, while the average after treatment was 46±43 (SD) AU. This was an average improvement of roughly forty percent, and the difference was highly statistically significant (p=0.001). Out of thirty-five subjects, thirty-two showed improvement (positive Δ values in Table 1) and three showed increased antibody levels (negative Δ in Table 1).
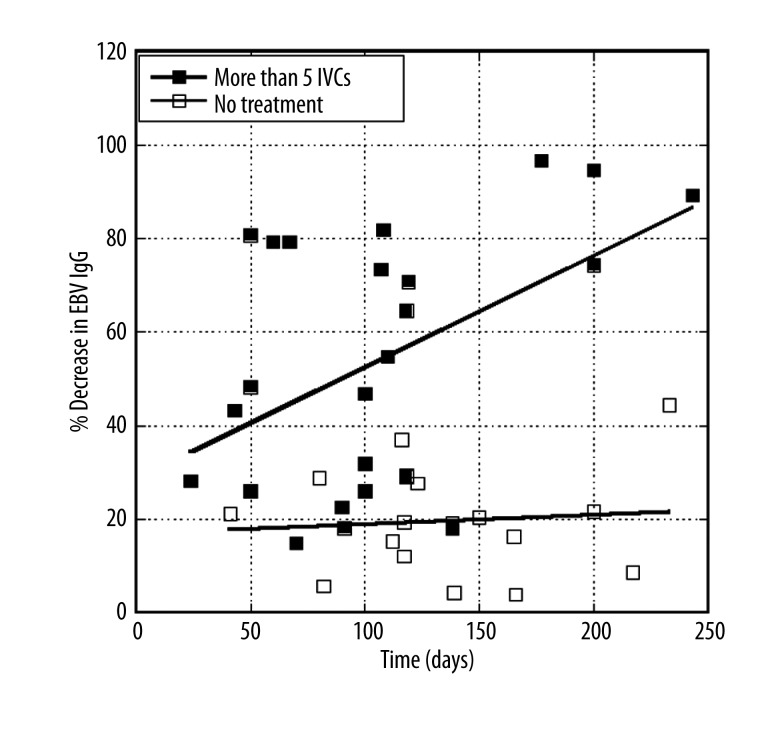
Analysing these data further, we broke down patients into two groups: patients who did not receive IVC treatment and patients who received five or more treatments. Figure 2 shows how EBV EA IgG levels changed with time of treatment for patients in these two groups.
Figure 2.
Percent decrease in EBV EA IgG levels over time for patients receiving no IVC, or ≥5 IVC. Lines represent linear least squares fits to data.
According to the data, the percent decrease in antibodies (percent improvement, as far as reducing infection is concerned) is much higher in the ≥5 IVC group, than in the untreated group. The average values (±SD) for percentage of improvement are 17±13% for untreated subjects and 46±39% for subjects treated more than five times. Compared to untreated controls, the rate of decrease in the EBV antibodies for treated subjects are significantly different (p<0.002) from those of the untreated subjects.
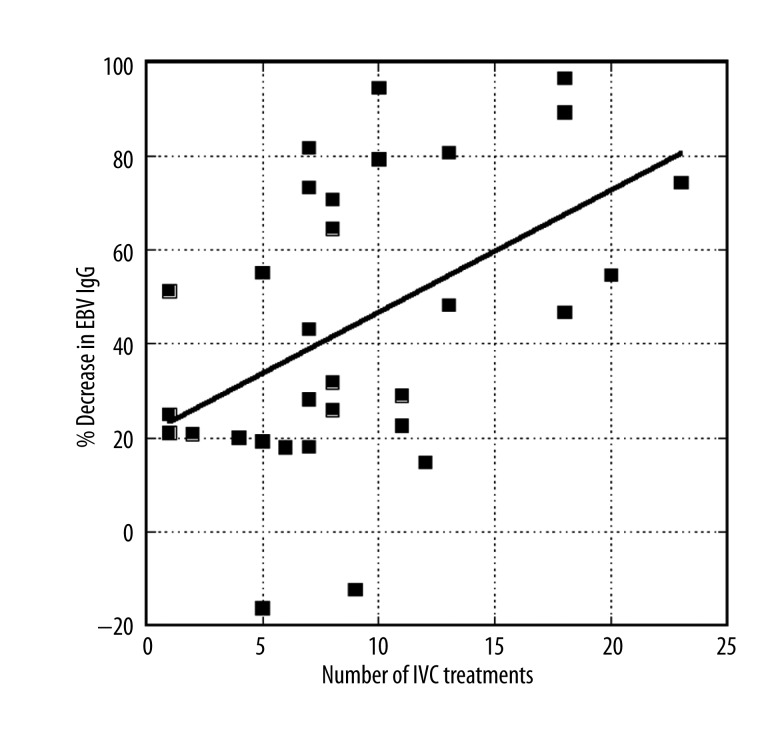
The effect of number of treatments is confirmed in Figure 3, which shows how the percent improvement (decrease in EBV EA IgG) increased as the number of treatments increased. The change in the percentage of improvement with number of treatments (slope of the line in Figure 3) is 2.7±0.7% per treatment (p<0.001).
Figure 3.
Percentage of decrease in EBV EA IgG levels over time for patients receiving IVC as a function of number of IVC treatments.
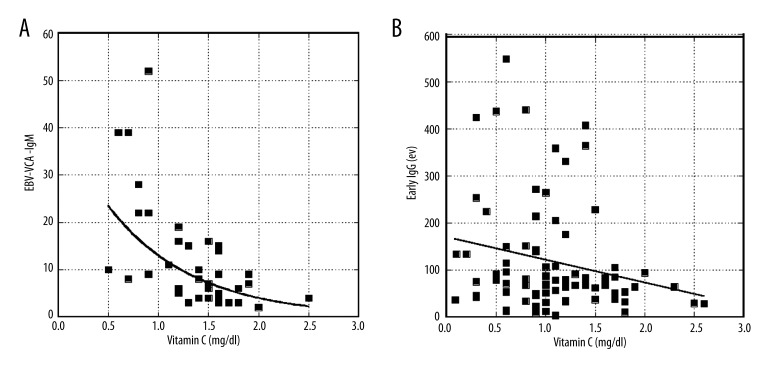
We also found evidence that EBV antibody expression correlates with plasma ascorbic acid concentrations. Figure 4 shows the inverse relationships between plasma ascorbic acid levels (pre-treatment) and EBV EA IgG and VCA IgM. The trend is for subjects with high plasma ascorbic acid levels to have lower EBV antigen loads; this is particularly true for VCA IgM.
Figure 4.
Antibody levels are plotted against plasma ascorbate concentrations for EBV VCA IgM (A) and EBV Early Antigen IgG (B). Curve fits are exponential: y=42 exp (−1.18x) (r=0.62) for A and y=114 exp(−0.37x) (r=0.21) for B. Estimated p-values are p<0.001 for A and p=0.08 for B.
In addition, we found that the peak ascorbic acid plasma levels that can be achieved after IVC infusions varied slightly dependent upon the severity of the infection. For example, in one patient a decrease in EBV EA IgG from 95 AU to 30 AU was accompanied by an increase in peak plasma ascorbate concentration (after 25 g IVC) from 5.6 mM to 8.8 mM. In another patient, a decrease in EBV EA IgG from 30 AU to 10 AU was accompanied by an increase in peak plasma ascorbate concentration (after 15 g IVC) from 5.0 mM to 7.1 mM.
We attempted to examine this further by looking at peak ascorbic acid levels in patients after the same dosage of IVC (15 g) above or below certain antibody load cut-offs. The average peak plasma ascorbic acid concentration was 7.0±2.1 mM for patients with EA IgG values below 70 AU and 5.9±1.4 mM for patients with EA IgG values above 70 AU. For VCA IgM, the average peak plasma ascorbic acid concentration was 7.1±1.1 mM for patients with IgM values below 30 AU and 4.4±3.1 mM for patients with IgM values above 30 AU. This indicates that subjects with higher EBV infection burdens (as indicated by antibody levels) are highly depleted of vitamin C, meaning that they require more treatments to replenish tissue ascorbic acid stores.
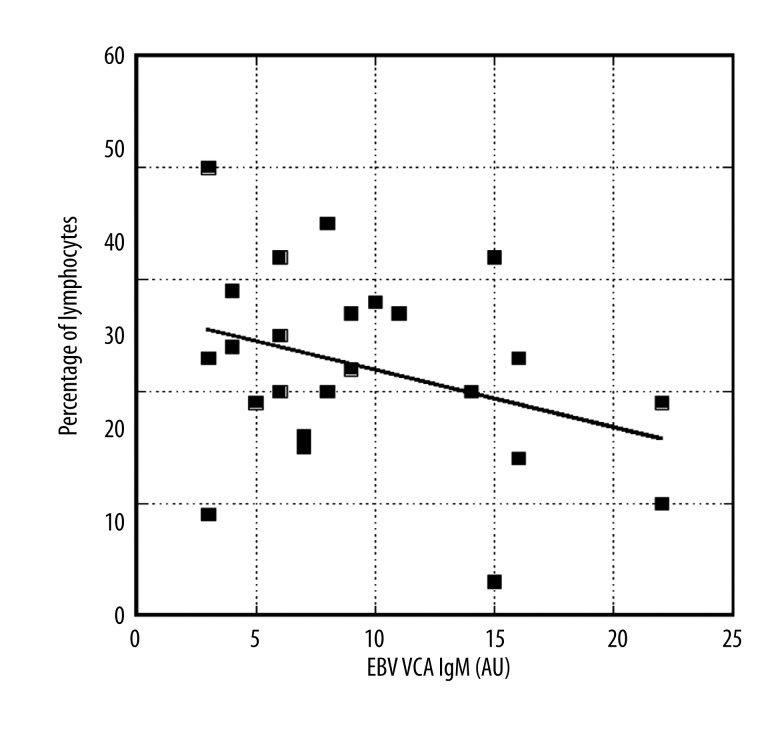
In addition, our data demonstrated that during acute phase viral infection, reduced lymphocyte counts correlated inversely with EBV VCA IgM (Figure 5). Moreover, the percentages of atypical lymphocytes increased from ten percent at VCA IgM levels below 20 AU to values between sixteen and twenty percent for IgM levels above 40 AU.
Figure 5.
Lymphocyte percentages as functions of EBV VCA IgM levels. Line represents a linear regression (y=37–0.51x, r=0.34).
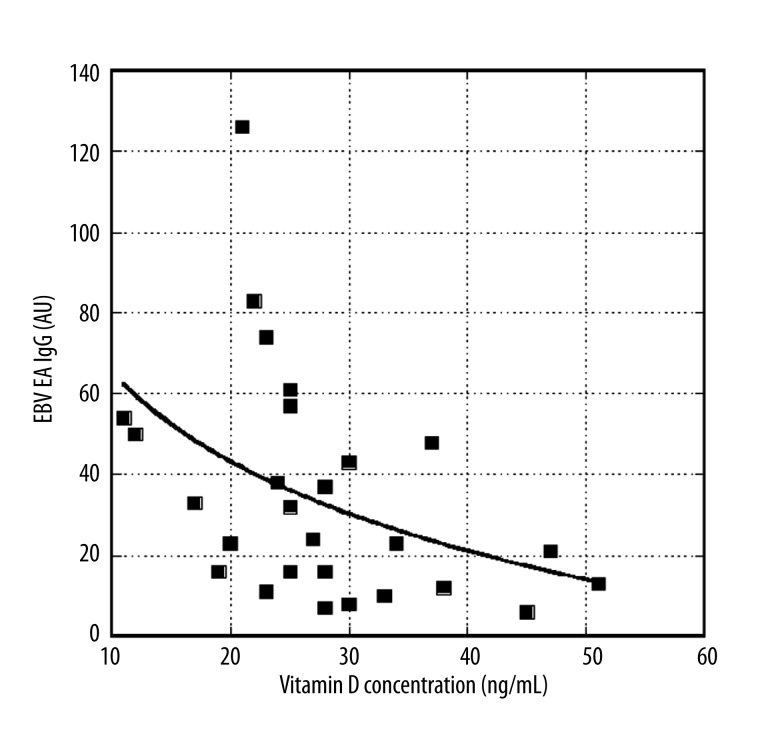
Finally, we analysed the other values of clinical tests for these patients (vitamins and minerals) to find other variables that correlate with EBV antibodies. Thus far, we have found that vitamin D concentration correlates with EBV EA IgG antibody levels (Figure 6).
Figure 6.
Effect of vitamin D levels on EBV EA IgG levels. Curve fit is exponential: y=79 exp(−0.041x) (r=0.44)
Discussion
There has been very little success treating acute EBV infection and mononucleosis with drugs. Corticosteroids may be helpful in treating complications of infectious mononucleosis including central nervous system involvement, myocarditis, tonsillar enlargement causing airway obstruction, and hemolytic anemia [55]. However, a double-blind study showed that acyclovir had no significant effect on symptoms of EBV-related infectious mononucleosis [56]. The combination of acyclovir and prednisolone did not affect the symptom duration or development of specific cellular immunity against EBV [57].
Our data provide evidence that high dose (7.5 to 50 grams) intravenous vitamin C therapy may have a positive effect on disease duration and may reduce viral antibody levels. This is, to our knowledge, the first clinical study of ascorbic acid and EBV infection. The reduction in EBV EA IgG and EBV VCA IgM antibody levels over time during IVC therapy is consistent with observations from the literature that millimolar levels of ascorbate hinder viral infection and replication in vitro [42,51,52]. The benefit seems to be dependent upon the number of IVC treatments given, as patients given five or more IVCs had significantly greater reduction in EBV EA IgG when compared to untreated controls. We also can confirm observations that relate vitamin C depletion to EBV infection. The possible mechanisms for this involve the effect of viral infection on cellular glucose uptake rates and increased oxidative stress [58]. Viruses are thought to increase cellular expression of the glucose transporter: this in turn would increase the rate of ascorbic acid uptake into the cell, since ascorbic acid enters cells as dehydroascorbate via these same glucose transporters [59–61].
Another important finding is the potential role of vitamin D in reducing viral antibody levels. Vitamin D has an important “non-classic” influence on the body’s immune system by modulating the innate and adaptive immune system, influencing the production of important endogenous antimicrobial peptides such as cathelicidin, and regulating the inflammatory cascade [62]. Vitamin D may modulate the production of cytokines, suppressing inflammation, and reduce the severity of viral infection. Multiple epidemiological studies in adults and children have demonstrated that vitamin D deficiency is associated with increased risk and greater severity of infection, particularly of the respiratory tract [63]. Cell culture experiments support the thesis that vitamin D has direct anti-viral effects particularly against enveloped viruses [64]. It may be worth exploring the combination of vitamin D with vitamin C and other antioxidants in treating EBV infected subjects.
Conclusions
The clinical study of ascorbic acid and EBV infection showed the reduction in EBV EA IgG and EBV VCA IgM antibody levels over time during IVC therapy that is consistent with observations from the literature that millimolar levels of ascorbate hinder viral infection and replication in vitro.
Acknowledgements
This research was supported by Allan P. Markin. We would like to thank V. Guilliams and P. Taylor for help in data collection. We thank Dr. J. Casciari for help in manuscript’s preparation.
Footnotes
Competing interests
The author have no direct financial interest in the subject matter discussed in the submitted manuscript.
Source of support: Departmental sources
References
- 1.Bornkamm GW, Hammerschmidt W. Molecular virology of Epstein-Barr virus. Philos Trans R Soc Lond B Biol Sci. 2001;356:437–59. doi: 10.1098/rstb.2000.0781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Farrell PJ. Epstein-Barr virus genome. In: Robertson ES, editor. Epstein-Barr virus Norfolk. Caister: Academic Press; 2005. pp. 263–87. [Google Scholar]
- 3.Okano M, Thiele GM, Davis JR, et al. Epstein-Barr virus and human diseases: recent advances in diagnosis. Clin Microbiol Rev. 1988;1:300–12. doi: 10.1128/cmr.1.3.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Swaminathan S. Molecular biology of Epstein-Barr virus and Kaposi’s sarcoma-associated herpesvirus. Semin Hematol. 2003;40(2):107–15. doi: 10.1053/shem.2003.50011. [DOI] [PubMed] [Google Scholar]
- 5.Tsurumi T, Fujita M, Kudoh A. Latent and lytic Epstein-Barr virus replication strategies. Rev Med Virol. 2005;15(1):3–15. doi: 10.1002/rmv.441. [DOI] [PubMed] [Google Scholar]
- 6.Kieff E, Rickinson AB. Epstein-Barr virus and its replication. In: Knipe DM, Howley PM, Griffin DE, et al., editors. Fields virology. 5 ed. II. Philadelphia: Lippincott Williams & Milkins; 2007. pp. 2603–54. [Google Scholar]
- 7.Speck P, Haan KM, Longnecker R. Epstein-Barr virus entry into cells. Virology. 2000;277:1–5. doi: 10.1006/viro.2000.0624. [DOI] [PubMed] [Google Scholar]
- 8.Hadinoto V, Shapiro M, Greenough TC, et al. On the dynamics of acute EBV infection and the pathogenesis of infectious mononucleosis. Blood. 2008;111:1420–27. doi: 10.1182/blood-2007-06-093278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Odumade OA, Hogquist KA, Balfour HH., Jr Progress and Problems in Understanding and Managing Primary Epstein-Barr Virus Infections. Clin Microbiol Rev. 2011;24:193–209. doi: 10.1128/CMR.00044-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eligio P, Delia R, Valeria G. EBV Chronic Infections. Medit J Hemat Infect Dis. 2010;2(1):1–19. doi: 10.4084/MJHID.2010.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vetsika EK, Callan M. Infectious mononucleosis and Epstein-Barr virus. Expert Rev Mol Med. 2004;6:1–16. doi: 10.1017/S1462399404008440. [DOI] [PubMed] [Google Scholar]
- 12.Sauce D, Larsen M, Curnow SJ, et al. EBV-associated mononucleosis leads to long-term global deficit in T-cell responsiveness to IL-15. Blood. 2006;108:11–18. doi: 10.1182/blood-2006-01-0144. [DOI] [PubMed] [Google Scholar]
- 13.Thacker EL, Mirzaei F, Ascherio A. Infectious mononucleosis and risk for multiple sclerosis: a meta-analysis. Ann Neurol. 2006;59:499–503. doi: 10.1002/ana.20820. [DOI] [PubMed] [Google Scholar]
- 14.James JA, Harley JB, Scofield RH. Epstein-Barr virus and systemic lupus erythematosus. Curr Opin Rheumatol. 2006;18:462–67. doi: 10.1097/01.bor.0000240355.37927.94. [DOI] [PubMed] [Google Scholar]
- 15.Harley JB, Harley IT, Guthridge JM, James JA. The curiously suspicious: a role for Epstein-Barr virus in lupus. Lupus. 2006;15:768–77. doi: 10.1177/0961203306070009. [DOI] [PubMed] [Google Scholar]
- 16.Rouse BT, Horohov DW. Immunosuppression in viral infections. Rev Infect Dis. 1986;8:850–73. doi: 10.1093/clinids/8.6.850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Groux HG, Torpier D, Monte Y, et al. Activation-induced death by apoptosis in CD4+ T cells from human immunodeficiency virus-infected asymptomatic individuals. J Exp Med. 1992;175:331–40. doi: 10.1084/jem.175.2.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nystad TW, Myrmel H. Prevalence of primary versus reactivated Epstein-Barr virus infection in patients with VCA IgG-, VCA IgM and EBNA-1-antibodies and suspected infectious mononucleosis. J Clin Virol. 2007;38:292–97. doi: 10.1016/j.jcv.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 19.Klutts JS, Ford BA, Perez NR, Gronowski AM. Evidence based approach for interpretation of Epstein-Barr virus serological patterns. J Clin Microbiol. 2009;47:3204–10. doi: 10.1128/JCM.00164-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Taga K, Taga H, Tosato G. Diagnosis of atypical cases of infectious mononucleosis. Clin Infect Dis. 2001;33:83–88. doi: 10.1086/320889. [DOI] [PubMed] [Google Scholar]
- 21.Polidori MC, Mecocci P, Levine M, Frei B. Short-term and long-term vitamin C supplementation in humans dose-dependently increases the resistance of plasma to ex vivo lipid peroxidation. Arch Biochem Biophys. 2004;423:109–15. doi: 10.1016/j.abb.2003.12.019. [DOI] [PubMed] [Google Scholar]
- 22.Karanth S, Yu WH, Walczewska A, et al. Ascorbic acid acts as an inhibitory transmitter in the hypothalamus to inhibit stimulated luteinizing hormone-releasing hormone release by scavenging nitric oxide. PNAS. 2000;97(4):1891–96. doi: 10.1073/pnas.97.4.1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rice ME, Russo-Menna I. Differential compartmentalization of brain ascorbate and glutathione between neurons and glia. Neuroscience. 1998;82(4):1213–23. doi: 10.1016/s0306-4522(97)00347-3. [DOI] [PubMed] [Google Scholar]
- 24.Hickey S, Saul AW. In: Vitamin C: The Real Story, the Remarkable and Controversial Healing Factor. Anderson J, editor. Laguna Beach (CA): Beasic Health Publications; 2008. [Google Scholar]
- 25.Klenner FR. The significance of high daily intake of ascorbic acid in preventive medicine. In: Williams R, Kalita DK, editors. Physician’s Handbook on Orthomolecular Medicine. 3rd ed. New York: Pergamon Press; 1979. pp. 51–59. [Google Scholar]
- 26.Webb AL, Villamor E. Update: Effects of antioxidant and non-antioxidant vitamin supplementation on immune function. Nutrition Reviews. 2007;65:181–217. doi: 10.1111/j.1753-4887.2007.tb00298.x. [DOI] [PubMed] [Google Scholar]
- 27.Siegel BV, Morton JI. Vitamin C and immunity: influence of ascorbate on prostaglandin E2 synthesis and implications for natural killer cell activity. Int J Vitam Nutr Res. 1984;54:339–42. [PubMed] [Google Scholar]
- 28.Padayatty SJ, Sun H, Wang Y, et al. Vitamin C pharmacokinetics: implications for oral and intravenous use. Ann Intern Med. 2004;140(7):533–37. doi: 10.7326/0003-4819-140-7-200404060-00010. [DOI] [PubMed] [Google Scholar]
- 29.Mikirova N, Casciari J, Riordan N, Hunninghake R. Clinical experience with intravenous administration of ascorbic acid: achievable levels in blood for different states of inflammation and disease in cancer patients. J Transl Med. 2013;11:191. doi: 10.1186/1479-5876-11-191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Riordan HD, Hunninghake RB, Riordan NH, et al. Intravenous ascorbic acid: protocol for its application and use. P R Health Sci J. 2003;22(3):287–90. [PubMed] [Google Scholar]
- 31.Du WD, Yuan ZR, Sun J, et al. Therapeutic efficacy of high-dose vitamin C on acute pancreatitis and its potential mechanisms. World J Gastroenterol. 2003;9(11):2565–69. doi: 10.3748/wjg.v9.i11.2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yeom CH, Jung GC, Song KJ. Changes of terminal cancer patients’ health-related quality of life after high dose vitamin C administration. J Korean Med Sci. 2007;22(1):7–11. doi: 10.3346/jkms.2007.22.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jackson J, Riordan HD, Hunninghauke R, Riordan N. High dose intravenous vitamin C and long time survival of a patient with cancer of the head and pancreas. J Ortho Med. 1995;10:87–88. [Google Scholar]
- 34.Riordan HD, Jackson J, Riordan N, Shultz M. High-dose intravenous vitamin C in the treatment of a patient with renal cell carcinoma of the kidney. J Ortho Med. 1998;13:72–73. [Google Scholar]
- 35.Sherman DL, Keaney JF, Jr, Biegelsen ES, et al. Pharmacological conncentrations of ascorbic acid are required for the beneficial effect on endothelial vasomotor function in hypertension. Hypertension. 2000;35(4):936–41. doi: 10.1161/01.hyp.35.4.936. [DOI] [PubMed] [Google Scholar]
- 36.Kennes B, Dumont I, Brohee D, et al. Effect of vitamin C supplements on cell-mediated immunity in old people. Gerontology. 1983;29:305–10. doi: 10.1159/000213131. [DOI] [PubMed] [Google Scholar]
- 37.Anderson R, Dittrich OC. Effects of ascorbate on leucocytes: Part IV. Increased neutrophil function and clinical improvement after oral ascorbate in 2 patients with chronic granulomatous disease. S Afr Med J. 1979;1:56476–80. [PubMed] [Google Scholar]
- 38.Jain SK, Pemberton PW, Smith A, et al. Oxidative stress in chronic hepatitis C: not just a feature of late stage disease. J Hepatol. 2002;36(6):805–11. doi: 10.1016/s0168-8278(02)00060-0. [DOI] [PubMed] [Google Scholar]
- 39.Chen JY, Chang CY, Feng PH, et al. Plasma vitamin C is lower in postherpetic neuralgia patients and administration of vitamin C reduces spontaneous pain but not brush-evoked pain. Clin J Pain. 2009;25(7):562–69. doi: 10.1097/AJP.0b013e318193cf32. [DOI] [PubMed] [Google Scholar]
- 40.Levy TE, editor. Curing the Incurable: Vitamin C, Infectious Diseases, and Toxins. Bloomington: Xlibris corporation; 2002. [Google Scholar]
- 41.Harakeh S, Jariwalla RJ, Pauling L. Suppression of human immunodeficiency virus replication by ascorbate in chronically and acutely infected cells. Proc Natl Acad Sci USA. 1990;87:7245–49. doi: 10.1073/pnas.87.18.7245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cheng LL, Liu YY, Li B, et al. An in vitro study on the pharmacological ascorbate treatment of influenza virus (abstract) Zhonghua Jie He He Hu Xi Za Zhi. 2012;35(7):520–23. [PubMed] [Google Scholar]
- 43.Schencking M, Vollbracht C, Weiss G, et al. Intravenous vitamin C in the treatment of shingles: results of a multicenter prospective cohort study. Med Sci Monit. 2012;18(4):CR215–24. doi: 10.12659/MSM.882621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wintergerst ES, Maggini S, Hornig DH. Immune-enhancing role of vitamin C and zinc and effect on clinical conditions. Ann Nutr Metab. 2006;50:85–94. doi: 10.1159/000090495. [DOI] [PubMed] [Google Scholar]
- 45.Wintergerst ES, Maggini S, Hornig DH. Immune-enhancing role of vitamin C and zinc and effect on clinical conditions. Ann Nutr Metab. 2006;50:85–94. doi: 10.1159/000090495. [DOI] [PubMed] [Google Scholar]
- 46.Gerber WF. Effect of ascorbic acid, sodium salicylate and caffeine on the serum interferon level in response to viral infection. Pharmacology. 1975;13:228–33. doi: 10.1159/000136908. [DOI] [PubMed] [Google Scholar]
- 47.Karpinska T, Kawecki Z, Kandefer-Szerszen M. The influence of ultraviolet irradiation, L-ascorbic acid and calcium chloride on the induction of interferon in human embryo fibroblasts. Arch Immunol Ther Exp (Warsz) 1982;30:33–37. [PubMed] [Google Scholar]
- 48.Mandl J, Szarka A, Banhegyi G. Vitamin C: update on physiology and pharmacology. Br J Pharmacol. 2009;157(7):1097–110. doi: 10.1111/j.1476-5381.2009.00282.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mikirova NA, Casciari JJ, Taylor PR, Taylor P. Effect of high-dose intravenous vitamin C on inflammation in cancer patients. J Transl Med. 2012;10:189. doi: 10.1186/1479-5876-10-189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mikirova NA, Rogers AM, Casciari JJ, Taylor P. Effect of high dose intravenous ascorbic acid on the level of inflammation in patients with rheumatoid arthritis. Modern Res Infl. 2012;1(2):26–32. [Google Scholar]
- 51.Furuya A, Uozaki M, Yamasaki H, et al. Antiviral effects of ascorbic acid and dehydroascorbic acids in vitro. Int J Mol Med. 2008;22:541–45. [PubMed] [Google Scholar]
- 52.White LA, Freeman CY, Forrester BD, Chappell WA. In vitro effect of ascorbic acid on infectivity of herpeviruses and paramyxoviruses. J Clin Microbiol. 1986;24(4):527–31. doi: 10.1128/jcm.24.4.527-531.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Murata A, Oyadomari R, Ohashi T, Kitagawa K. Mechanism of inactivation of bacteriophage deltaA containing single-stranded DNA by ascorbic acid. J Nutr Sci Vitaminol (Tokyo) 1975;21:261–69. doi: 10.3177/jnsv.21.261. [DOI] [PubMed] [Google Scholar]
- 54.Bissell MJ, Hatie C, Farson DA, et al. Ascorbic acid inhibits replication and infectivity of avian RNA tumor virus. Cell Biol. 1980;77(5):2711–15. doi: 10.1073/pnas.77.5.2711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Johannsen EC, Schooley RT, Kaye KM. Epstein-Barr virus (infectious mononucleosis) In: Mandell GL, Bennett JE, Dolin R, editors. Principles and Practive of Infectious Diseases. 6th ed. Vol. 2. Philadelphia (PA): Churchill Livingstone; 2005. pp. 1801–21. [Google Scholar]
- 56.Torre D, Tambini R. Acyclovir for treatment of infectious mononucleosis: a meta-analysis. Scand J Infect Dis. 1999;31:543–47. doi: 10.1080/00365549950164409. [DOI] [PubMed] [Google Scholar]
- 57.Tynell E, Aurelius E, Brandell A, et al. Acyclovir and prednisolone treatment of acute infectious mononucleosis: a multicenter, double-blind, placebo-controlled study. J Infect Dis. 1996;174(2):324–31. doi: 10.1093/infdis/174.2.324. [DOI] [PubMed] [Google Scholar]
- 58.Yongjun Y, Maguire TG, Alwine JC. Human cytomegalovirus activates glucose transporter 4 expression to increase glucose update during infection. J Virol. 2011;85(4):1573–80. doi: 10.1128/JVI.01967-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rose RC. Transport of ascorbic acid and other water-soluble vitamins. Biochim Biophys Acta. 1988;947(2):335–66. doi: 10.1016/0304-4157(88)90014-7. [DOI] [PubMed] [Google Scholar]
- 60.Vera JC, Rivas CI, Fischbarg J, Golde DW. Mammalian facilitative hexose transporters mediate the transport of dehydroascorbic acid. Nature. 1993;364:79–82. doi: 10.1038/364079a0. [DOI] [PubMed] [Google Scholar]
- 61.Vera JC, Rivas CI, Velasquez FV, et al. Resolution of the facilitated transport of dehydroascorbic acid from its intracellular accumulation as ascorbic acid. J Biol Chem. 1995;270(40):23706–12. doi: 10.1074/jbc.270.40.23706. [DOI] [PubMed] [Google Scholar]
- 62.Gunville CF, Mourani PM, Ginde AA. The Role of Vitamin D in Prevention and Treatment of Infection. Inflamm Allergy Drug Targets. 2013;12(4):239–45. doi: 10.2174/18715281113129990046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cantorna MT, Yu S, Bruce D. The paradoxical effects of vitamin D on type 1 mediated immunity. Mol Aspects Med. 2008;29:369–75. doi: 10.1016/j.mam.2008.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Beardb JA, Beardena A, Strikera R. Vitamin D and the anti-viral state. J Clin Virol. 2011;50(3):194–200. doi: 10.1016/j.jcv.2010.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]