Abstract
Aims/Introduction
Many patients with diabetes now use 5‐, 6‐ or 8‐mm needles for insulin injection. However, it is unclear whether needle length, particularly for shorter needles, affects the pharmacokinetic properties of insulin.
Materials and Methods
This was a three‐way, randomized, cross‐over, single‐center study involving 12 healthy Japanese adult males (age 27.4 ± 4.14 years; weight 64.2 ± 5.2 kg; body fat percentage 18.2 ± 1.5%). Participants received a subcutaneous (abdomen) dose of insulin lispro (1.5 U for participants weighing 55 to <65.0 kg; 2.0 U for participants weighing 65.0 to <80.0 kg) delivered using a 32‐G × 4 mm (32G × 4), 31‐G × 8 mm (31G × 8) or 32‐G × 6 mm (32G × 6) needle with a 3–7‐day washout between doses. Pharmacokinetic parameters of exogenous insulin were identified using non‐linear least squares, where the total insulin concentration was fit to the measured plasma insulin concentration using an overall combined model that accounted for C‐peptide/insulin secretion in addition to the injected dose.
Results
Maximum concentration and area under the curve for 0 to infinity min for insulin were bioequivalent for the 32G × 4 needle relative to the 32G × 6 and the 31G × 8 needles. The time to the maximum insulin concentration was bioequivalent for the 32G × 4 needle relative to the 32G × 6 needle, but not the 31G × 8 needle.
Conclusions
The use of 4‐mm needles is unlikely to change the pharmacokinetic properties of insulin when injected subcutaneously in adults. This trial was registered with UMIN‐CTR (no. UMIN000004469).
Keywords: Insulin, Needle length, Pharmacokinetics
Introduction
The goal of injections of insulin is to reliably deliver insulin into the subcutaneous space without leakage or discomfort1. Recent developments in insulin pen technology have overcome many of the perceived barriers associated with insulin injection. However, discomfort associated with needle insertion still has an adverse effect on quality of life.
The discomfort often associated with needle insertion is at least partly related to the diameter (gauge) and length of the needle, the wall thickness and the shape of the needle tip. Because of this, needle manufacturers have endeavored to overcome these limitations through redesigning and refining needle properties. Such improvements have had substantial benefits on quality of life and patients often prefer the newer needles2. However, needle length is subject to some limitations, as it governs the depth of injection and must be long enough to reach the subcutaneous tissue, without penetrating the underlying muscle.
Many of the currently available needles are 5, 6 or 8 mm long. In Japan, 5‐ or 6‐mm needles are more frequently used than 8‐mm needles. Some patients use longer needles (mainly 8 mm) for no specific reason other than their long‐held habit or their physicians' preference.
Although subcutaneous tissue thickness varies between injection sites, it is possible that 8‐mm needles inserted perpendicular to the skin could be inserted into muscle tissue8. Intramuscular injection is associated with increased pain relative to subcutaneous injection and can increase the rate of uptake of insulin, because of differences in tissue vascularity9.
The use of shorter needles might overcome these problems. Shorter needles have reduced the likelihood of insertion into muscle and are often better tolerated. However, issues such as what is the minimal length of an insulin pen needle or whether shorter needles could be used in patients from demographically different backgrounds, have not been fully evaluated. A recent study showed that injection‐site skin thickness does not markedly differ among demographically diverse adults with diabetes8. Conversely, subcutaneous thickness shows a much wider range. The results of that study suggested that a 4–5‐mm pen needle could enter the subcutaneous tissue with minimal risk of intramuscular injection, and can be safely and effectively used by most diabetic adults.
A 4‐mm‐long 32‐G needle was recently introduced. A clinical trial carried out in USA patients with type 1 or 2 diabetes showed equivalent glycemic control, but reduced pain and greater preference for the 32‐G × 4 mm (32G × 4) needle relative to the two other needles12.
Although glucose control is an essential outcome of such studies, it is also important to evaluate possible implications on insulin pharmacokinetic properties and confirm that needle length does not adversely influence the properties of the injected insulin. Studies in other patient populations are also necessary, considering possible differences in subcutaneous fat depth. Therefore, the aim of the present study was to compare the new 32‐G × 4 mm (32G × 4) needle with two other available needles with lengths of 6 mm (32G) and 8 mm (31G) in terms of insulin pharmacokinetics in healthy Japanese adult males.
Materials and Methods
Objective
The primary objective of the present three‐way, randomized, cross‐over, single‐center study was to compare the blood insulin pharmacokinetic properties of three different insulin pen needles.
Participants
Healthy, non‐diabetic adult males aged 20–40 years were eligible for the present study. Further inclusion criteria included weight 55–80 kg, body mass index 18.5–25.0 kg/m2, body fat percentage 15–20%, systolic blood pressure 90–140 mmHg, diastolic blood pressure <90 mmHg, pulse rate 40–100 b.p.m., glycated hemoglobin <5.2% (Japan Diabetes Society value; National Glycohemoglobin Standardization Program value <5.6%)13, fasting blood glucose <110 mg/dL and blood glucose <140 mg/dL at 2 h after a 75‐g glucose load. The protocol was described to the participants and written informed consent was obtained. The present study was approved by the institutional review board at Kyushu Clinical Pharmacology Research Clinic (Fukuoka, Japan).
Study Design
The present study was carried out at the LTA Clinical Pharmacology Center, Sumida Hospital (Tokyo, Japan). The following needles that are commonly used in Japan were used in the present study: Becton Dickinson Company 32G × 4 and 31‐G × 8 mm (31G × 8), and Novo Nordisk 32G × 6. HumaPen Luxura HD (Eli Lilly Japan K.K., Kobe, Japan) pens were used to administer insulin lispro (Humalog; Eli Lilly Japan K.K.). A single pen was used for each patient, and all pens used were from the same manufacturing batch. This pen meets JIS T 3226‐1:2005 and ISO 11608‐1:2000 for accuracy of injection devices.
Each participant attended the institute four times, with one screening visit and three test visits. At the screening visit, biochemical blood and urine tests were carried out, informed consent was obtained, and the participants were randomized to one of six groups using a computer‐generated randomization list. The order of needles to be used differed between each group to minimize possible bias (Figure 1).
Figure 1.
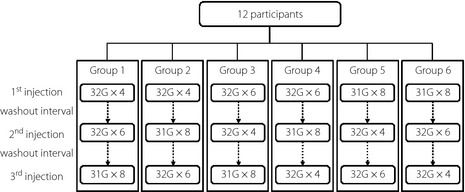
A total of 12 participants were randomized to six groups. The order of needle use differed between each group to minimize possible bias.
The three test visits were carried out at 3–7‐day intervals to allow washout, and to reduce the physical and mental burden on the participants. After a 12‐h fast, the participants arrived at the clinic, and height, weight, body temperature, blood pressure and heart rate were measured, and a physical examination was carried out. At approximately 09.00 hours, the participants received a dose of insulin (1.5 U for participants weighing 55 to <65.0 kg; 2.0 U for participants weighing 65.0 to <80.0 kg) with the insulin pen fitted with the allocated needle. The dose was decided based on the doses used in previous studies 15. Needles were to be replaced if they were blocked, bent or damaged on inspection.
All injections were administered into the abdomen, approximately 5 cm left of the navel. After cleaning the participant's abdomen using a disposable alcohol gauze swab, the insulin was injected without lifting a skin fold for the 32G × 4 needle, and with a lifted skin fold for the 31G × 8 and the 32G × 6 needles to reduce the risk of intramuscular injection and standardize injection depth. All needles were inserted perpendicularly to the skin surface.
Blood samples (through an indwelling venous catheter placed at the antebrachial region) were taken at 15 min and just before injection, and at 10, 20, 30, 40, 50, 60, 90, 120 and 180 min after injection. Blood pressure and heart rate were measured during this time and another physical examination was carried out after obtaining the last blood sample. The duration of blood sampling was selected based on the Guidelines for Bioequivalence Studies of Generic Products (2006), which recommends that sampling should be continued until the area under the curve (AUC) for time T (AUCT) exceeds 80% of the AUC to infinity17. Considering that the reported time16 to the maximum insulin concentration (Tmax) for insulin lispro is approximately 37 min, and the half‐life is 53 min for a 0.025‐U/kg dose, the AUCT should be determined for 196 min; thus, we considered that the AUC for 0 to infinity min (AUC0–∞) would be appropriate.
All tests were carried out in a single‐blind manner, with the participants blinded to which needle was being used at each test. The investigator was aware of which needle was being used. All procedures were carried out by physicians, nurses and medical technicians who were well familiarized with the procedures. All injections were carried out by a single physician with extensive experience of using insulin pens and needles.
Biochemical Assays
Serum immunoreactive insulin (IRI) and C‐peptide concentrations were measured in all blood samples using validated chemiluminescence assays. Biochemical tests were carried out by the Mitsubishi Chemical Medience Corporation (Tokyo, Japan).
Pharmacokinetic Modeling and Parameter Identification
To determine the pharmacokinetics of the injected insulin, the contribution of endogenous insulin must be considered, because the participants were not diabetic. We used a combined model that involves kinetic analysis of both endogenous plasma insulin and C‐peptide, as well as of exogenous insulin (Figure 2). System modeling and parameter identification were carried out using Matlab version 7.13 (Mathworks, Natick, MA, USA). Endogenous insulin and C‐peptide components of the model were taken from previous studies that have accurately described the insulin and C‐peptide secretion, and their appearance in plasma under changing conditions17.
Figure 2.
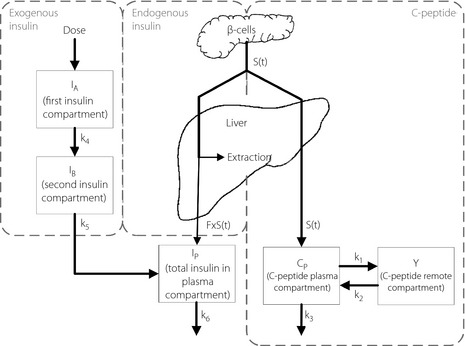
Schematic diagram of the extended combined model that accounts for the kinetics of endogenous insulin and C‐peptide, in addition to exogenous insulin. Insulin and C‐peptide are assumed to be secreted by β‐cells in an equimolar fashion (i.e. S[t]). A fraction, F, of the secreted insulin survives hepatic transit, appears in the systemic circulation and is distributed within the single plasma compartment. All of the C‐peptide survives hepatic portal transport and is assumed to follow two‐compartment distribution kinetics. The injected insulin goes through two compartments before being distributed into the plasma compartment along with the endogenous insulin.
S(t) denotes equimolar insulin–C‐peptide release from β‐cells. All of the C‐peptide survives hepatic portal transport and follows two‐compartment distribution kinetics (Equations (1) and (2)). C(t) and Y(t) are C‐peptide concentrations in the plasma and the remote compartments, respectively. A fraction of the secreted insulin, FS(t), survives hepatic portal transport and enters the plasma compartment (Equation (5)), whereas the remainder is extracted by the liver. The injected insulin passes through two compartments before entering the plasma compartment, along with endogenous insulin, to yield the total plasma insulin concentration that was measured experimentally.
Mass transfer equations:
| (1) |
| (2) |
| (3) |
| (4) |
| (5) |
Plasma insulin concentration:
| (6) |
The units on the left hand side of Equations (1) are in nmol/min. After dividing plasma insulin in nmol by the plasma insulin distribution volume in L in Equation (6), a conversion factor of 166.667 yielded the insulin concentration in units of μU/mL.
The rates k1, k2 and k3, which describe the secretion of C‐peptide to its appearance in plasma and the C‐peptide distribution volume in the plasma, VCP, were previously determined in a large population, and were mathematically related to the participants' sex, age and body surface area20 with a high degree of correlation17. The same mathematical relationships were used to predetermine k1, k2, k3 and VCP for all of the participants.
The plasma C‐peptide concentrations were interpolated minute to minute between the measured samples. A 31‐min symmetric (–15, +15) min Savitzky–Golay finite impulse response derivative filter21, equivalent to a moving linear fit (first‐order polynomial), was used to determine the rate of change of plasma C‐peptide, , from 0 to 165 min. An ordinary differential equation (ODE) solver was used to integrate Equation (2) to deconvolute plasma C‐peptide concentrations in nmol/L and estimate insulin–C‐peptide secretion (S[t]) via Equation (1) per needle injection experiment in all of the participants. To determine C‐peptide secretion in units of nmol/min, the C‐peptide concentration was multiplied by VCP to yield C‐peptide in nmol before deconvolution. The mean secretion rates and the mean measured C‐peptide concentrations, from which they were derived, are shown in Figure 3.
Figure 3.
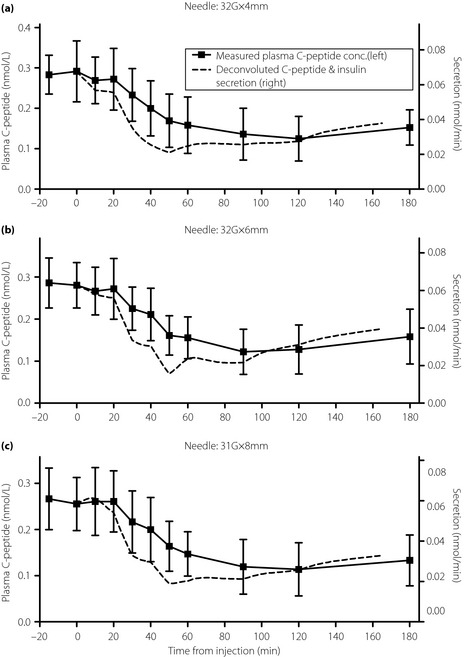
Plots of the measured plasma C‐peptide concentrations (squares; mean and standard deviation) and interpolated values (solid line, left axis) along with C‐peptide and insulin secretion rates (dashed line, right axis; mean values). (a) 32‐G × 4 mm (32G × 4) needle. (b) 32‐G × 6 mm (32G × 6) needle. (c) 31‐G × 8 mm (31G × 8) needle. The left and right axis scales are normalized so that the C‐peptide concentration and secretion overlap at 0 min.
After determining insulin secretion, S(t), for each of the 36 experiments, the parameters k4, k5 and k6 that define the pharmacokinetics of the injected insulin as well as F, the fraction of secreted insulin that survived hepatic transport and VIP, the distribution volume of insulin in plasma, were determined by non‐linear least squares on the set of ODEs defined by Equations (3), (4), (5) and (6). The system was assumed to be at steady state immediately before insulin injection. The parameters were determined by minimizing the sum‐squared error between the measured total insulin concentration and the modeled plasma insulin concentration, in which the parameters were constrained to be positive.
After parameter determination in each experiment, the model was simulated with fit parameters in which insulin secretion was set to 0 to yield the estimated exogenous plasma insulin curve attributable to the injected insulin. Conversely, the plasma insulin curve attributable to endogenous secretion was obtained by setting the dose to 0. The sum of both profiles yields the total fit plasma insulin curve.
Calculation of Tmax, Maximum Concentration and AUC0–∞
The primary end‐point was to test for bioequivalence of insulin injected with the 32G × 4 needle relative to the 32G × 6 and the 31G × 8 needles by three pharmacokinetic metrics; Tmax (the time after the injection corresponding to the maximal exogenous plasma insulin concentration), maximum concentration (Cmax; the corresponding insulin concentration) and AUC0–∞ (the area under the curve from time 0 to infinity). The solution to Equations (3), (4), (5) and (6), with no endogenous insulin secretion, is given by Equation (7), which calculates the exogenous plasma insulin concentration attributable to the injected dose:
| (7) |
where:
| (8) |
| (9) |
| (10) |
Equation (7) was simulated with the parameters VIP k4, k5 and k6 for each experiment. Tmax and Cmax were derived from the peak of the simulated plasma insulin curve in response to the dose. AUC0–∞ was calculated by integrating Equation (7) from 0 to infinity, yielding:
| (11) |
Normalized Cmax and normalized AUC0–∞ were calculated by dividing Cmax and AUC0–∞ by the dose.
Statistical Analyses
Based on previous reports16 and considering the possible risks/ethical concerns of injecting insulin in healthy individuals, we enrolled 12 participants, with two participants allocated to each needle order group. Bioequivalence analyses were carried out as previously described22. For Cmax and AUC0–∞, bioequivalence of the 32G × 4 needle relative to either the 32G × 6 needle or the 31G × 8 needle was accepted if the mean difference (ratio) of the log‐converted Cmax and AUC0–∞ was within the range log(0.80)–log(1.25). For Tmax, bioequivalence of the 32G × 4 needle relative to either the 32G × 6 needle or the 31G × 8 needle was accepted if the mean difference (ratio) of Tmax between pairs of needles was within ±20%.
To identify the pharmacokinetic factors influencing Cmax, Tmax and AUC0–∞ for exogenous insulin resulting from the dose, analysis of variance was carried out with adjustment for needle type and order of needles as dependent variables. Values of P < 0.05 were considered statistically significant. Statistical analyses were carried out using Prism version 5.04 (GraphPad Software, San Diego, CA, USA).
Results
All 12 participants completed all three tests, and all of their data were used in the analyses. No participants withdrew from the study and no needle replacements were required for blocked or damaged needles. The characteristics of the 12 participants are presented in Table 1. The pre‐estimated C‐peptide rate constants and C‐peptide distribution volume in the plasma of the C‐peptide secretion submodel used to derive insulin secretion are also shown in Table 1.
Table 1. Participant characteristics and calculated C‐peptide secretion parameters.
| Mean ± SD | Median (range) | |
|---|---|---|
| Age (years) | 27.4 ± 4.14 | 26 (23–36) |
| Height (cm) | 172.4 ± 5.1 | 171.4 (165.3–179.6) |
| Weight (kg) | 64.2 ± 5.2 | 66 (55.8–70.7) |
| BMI (kg/m2) | 21.6 ± 1.3 | 21.2 (19.9–23.7) |
| Body fat percentage (%) | 18.2 ± 1.5 | 18.6 (15.7–19.9) |
| C‐peptide secretion parameters | ||
| VCP (L) | 3.99 ± 0.1 | 4.03 (3.82‐4.1) |
| k1 (per min) | 0.0522 ± 0.0006 | 0.052 (0.0515–0.0534) |
| k2 (per min) | 0.0496 ± 0.0003 | 0.0496 (0.0490–0.0499) |
| k3 (per min) | 0.0593 ± 0.0007 | 0.0595 (0.0579–0.06) |
BMI, body mass index; k1, k2 and k3, rate constants used in the C‐peptide secretion submodel; SD, standard deviation; VCP, distribution volume of C‐peptide in plasma.
The fit parameters from the insulin submodel for each needle were averaged across the 12 participants and are shown in Table 2. In this model, parameters k4 and k5 are interchangeable; although the two rates are identifiable, their order is not. In Table 2, the smaller of the two parameters is reported as k4, and the larger value is reported as k5. Tmax, Cmax and AUC0–∞ values are also reported in Table 2.
Table 2. Insulin pharmacokinetic parameters and metrics.
| 32G × 4 | 32G × 6 | 31G × 8 | |
|---|---|---|---|
| Identified parameters | |||
| k4 (per min) | 0.0275 ± 0.0105 | 0.0252 ± 0.007 | 0.0236 ± 0.0069 |
| k5 (per min) | 0.0378 ± 0.0164 | 0.037 ± 0.0135 | 0.0365 ± 0.0221 |
| k6 (per min) | 0.2737 ± 0.0334 | 0.2567 ± 0.0587 | 0.2559 ± 0.0456 |
| VIP (L) | 4.35 ± 0.37 | 4.46 ± 0.43 | 4.39 ± 0.33 |
| F | 0.39 ± 0.11 | 0.34 ± 0.11 | 0.37 ± 0.10 |
| PK metrics | |||
| Tmax (min) | 39.39 ± 9.26 | 39.76 ± 6.09 | 43.01 ± 8.03 |
| Cmax (μU/mL) | 16.99 ± 4.17 | 16.95 ± 3.22 | 17.19 ± 4.08 |
| AUC0‐∞ (min μU/mL) | 1420 ± 255 | 1506 ± 259 | 1539 ± 279 |
| Cmax/dose (μU/mL) | 9.65 ± 2.79 | 9.69 ± 2.41 | 9.70 ± 2.45 |
| AUC0‐∞/dose (min μU/mL) | 795 ± 104 | 845 ± 115 | 860 ± 101 |
Values are means ± standard deviation. The identified exogenous parameters were determined by fitting the overall model to the total measured insulin by the non‐linear least squares method. The parameters for C‐peptide and insulin secretion compartments were calculated using mathematical relationships as a function of participant characteristics. Although the two‐rate constants k4 and k5 are identifiable, they are interchangeable. In this table, k4 is reported as the smaller of the two and k5 is reported as the larger of the two. The maximum insulin concentration (Tmax), maximum concentration (Cmax) and area under the curve for 0 to infinity min (AUC0–∞) are derived from the simulated exogenous plasma insulin curve resulting from the identified parameters when insulin secretion was set to 0. PK, pharmacokinetic.
For each of the three needles, the fit (total) plasma insulin curves, the estimated plasma insulin curve resulting from the injection and the estimated plasma insulin attributable to endogenous secretion were averaged across the 12 participants, and are plotted in Figure 4. The means and standard deviations of the insulin concentrations measured at each time are also plotted.
Figure 4.
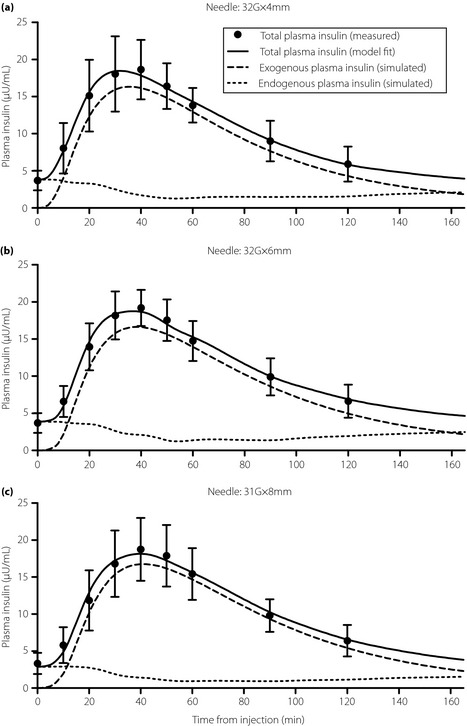
Measured plasma insulin concentrations (circles; mean and standard deviation) and the model fits of total insulin (solid line). The simulated exogenous plasma insulin curves (dashed line; mean values) and of simulated endogenous plasma insulin curves (dotted line; mean values). The simulated exogenous and endogenous plasma insulin curves were generated by simulating the model using the identified parameters with insulin secretion set to 0 and the injection dose set to 0, respectively. The sum of the exogenous and endogenous curves yield the model fit of total insulin. (a) 32‐G × 4 mm (32G × 4) needle. (b) 32‐G × 6 mm (32G × 6) needle. (c) 31‐G × 8 mm (31G × 8) needle.
The insulin pharmacokinetic metrics (i.e. Tmax, Cmax and AUC0–∞) are summarized for each needle in Table 2. The comparisons of Tmax between the 32G × 4 needle (39.39 ±9.26 min) and either the 32G × 6 needle (39.76 ± 6.09 min) or the 31G × 8 needle (43.01 ± 8.03 min) were not statistically significant (P = 0.9098 and P = 0.3177, respectively). Similarly, the comparisons of Cmax between the 32G × 4 needle (16.99 ± 4.17 μU/mL) and either the 32G × 6 needle (16.95± 3.22 μU/mL) or the 31G × 8 needle (17.19 ± 4.08 μU/mL) were not significant (P = 0.9823 and P = 0.9059, respectively). Last, the comparisons of AUC0–∞ between the 32G × 4 needle (1420 ± 255 min μU/mL) and either the 32G × 6 needle (1506 ± 259 min μU/mL) or the 31G × 8 needle (1539 ±279 min μU/mL) were not statistically significant (P = 0.4212 and P = 0.2843, respectively). As shown in Table 3, Cmax and AUC0–∞ for the 32G × 4 needle were bioequivalent to both the 32G × 6 needle and the 31G × 8 needle, as the 90% confidence intervals for both comparisons were within the prespecified limits. Interestingly, Tmax tended to be longer for the 31G × 8 needle relative to the 32G × 4 and 32G × 6 needles. Consequently, the 32G × 4 needle was bioequivalent to the 32G × 6 needle, but not the 31G × 8 needle. The type of needle and the needle order did not affect Tmax, Cmax or AUC0–∞ (P = 0.1309, P = 0.8025 and P = 0.8318, respectively; two‐way analysis of variance).
Table 3. Bioequivalence analysis of 32‐G × 4 mm vs 32‐G × 6 mm and 31‐G × 8 mm needles for insulin pharmacokinetics.
| Ratioa | 90% CI | Lower/upper limitsb | |
|---|---|---|---|
| Tmax (min) | |||
| 32G × 4 vs 32G × 6 | −0.3667 | −5.8597, 5.1264 | −7.9517, 7.9517 |
| 32G × 4 vs 31G × 8 | −3.6167 | −9.6907, 2.4574 | −8.6017, 8.6017 |
| Cmax (μU/mL) | |||
| 32G × 4 vs 32G × 6 | −0.0109 | −0.1713, 0.1496 | −0.2230, 0.2231 |
| 32G × 4 vs 31G × 8 | −0.0164 | −0.1893, 0.1565 | −0.2230, 0.2231 |
| AUC0–∞ (min μU/mL) | |||
| 32G × 4 vs 32G × 6 | −0.0608 | −0.1827, 0.0611 | −0.2230, 0.2231 |
| 32G × 4 vs 31G × 8 | −0.0810 | −0.2076, 0.0457 | −0.2230, 0.2231 |
CI, confidence interval; Tmax, time to the maximum concentration; Cmax, maximum concentration; AUC0–∞, area under the curve from 0 to infinity min.
Ratio of the mean values.
±20% for time to the maximum concentration (Tmax), log (0.80), log (1.25) for maximum concentration (Cmax) and area under the curve from 0 to infinity min (AUC0–∞).
Because we expected the possibility of mild hypoglycemia in the present study, we were determined to treat only severe hypoglycemia (<50 mg/dL) or mild hypoglycemia (<70 mg/dL) with any symptoms consistent with hypoglycemia, such as sickness or general fatigue. Therefore, mild hypoglycemia without symptoms was not included as an adverse event in the present study. None of the participants developed severe hypoglycemia, and none of the participants who showed temporary mild hypoglycemia reported any symptoms.
Discussion
In the present study of healthy Japanese adult males, we found that Cmax and AUC0–∞ for insulin lispro injected using a 32G × 4 needle were bioequivalent to those for the 32G × 6 needle and 31G × 8 needle, whereas Tmax was bioequivalent between the 32G × 4 needle and the 32G × 6 needle.
Our findings expand on those of a cross‐over study that compared the pharmacodynamic characteristics of the 32G × 4 needle with two other needles (31G × 5 mm and 31G × 8 mm) in two 3‐week treatment periods12. The percent absolute change in serum fructosamine, a marker of glycemic control over 2–3 weeks, was bioequivalent for the 32G × 4 needle relative to both comparators. In a study in obese subjects, a 31G × 5‐mm needle was associated with comparable metabolic control to a 31G × 8‐mm needle24. However, it was reported that approximately 5% of injections with a 5‐mm needle in children were intramuscular25.
In the present study, Tmax tended to be longer for the 31G × 8 needle than the shorter needles, which meant that bioequivalence for the 32G × 4 and the 31G × 8 needles was not shown. However, Tmax was not significantly different between the 32G × 4 and 31G × 8 needles (P = 0.3177, t‐test). Considering that Cmax and AUC0–∞ were comparable for all three needles with bioequivalence for both parameters relative to the 32G × 4 needle, we suspect this finding is related to the timing of blood samples (every 10 min), which might mask the true timing of the peak insulin concentration, and the small sample size, which was too small given the large standard deviation of Tmax. More frequent blood sampling and more subjects might be necessary in future studies to confirm whether the differences in Tmax are due to the method used, or whether the depth of injection in the abdomen affects the duration of insulin exposure.
Regarding the impact of injection depth on insulin uptake, one study compared radioactivity levels at the injection sites where radiolabeled regular insulin was injected subcutaneously to a depth of 3 mm beneath the skin surface or to 2 mm above the muscle fascia in the thigh and abdomen, under ultrasound guidance26. The authors found no significant differences in insulin uptake between the deep and superficial injections at either site26.
The results of the present study should be considered in light of some limitations. First, the insulin assay was non‐specific for insulin lispro, measuring both insulin lispro and endogenous insulin. To overcome this, our combined model accounted for C‐peptide and insulin secretion dynamics, which we obtained from previous publications. We added exogenous insulin submodel compartments to the endogenous insulin and C‐peptide combined model. This allowed us to estimate exogenous insulin and endogenous insulin plasma concentrations by fitting the total measured insulin to the overall combined model. Studies in patients with type 1 diabetes would help to confirm these findings. Second, bioequivalence of the 32G × 6 and 32G × 8 needles was not evaluated. However, this was not a prespecified analysis. Based on the descriptive data, it is likely that these needles would provide the same pharmacokinetics in clinical settings. Finally, the present study was carried out in Japanese adult males, with injections into the abdomen. Nevertheless, the results can be generalizable to other ethnicities, age‐groups, or even obese people, as even the shortest needle penetrates through the skin. The results of a recent study on skin/subcutaneous tissue thickness8 concluded that skin thickness varies minimally between patient groups of differing demographics (e.g. age, body mass index, sex and race), and rarely exceeds 3 mm. However, differences in subcutaneous tissue thickness, subcutaneous vascularity and adiposity between common injection sites (e.g. thigh, buttocks and abdomen) might influence the resulting insulin pharmacokinetic profiles, and the risk of intramuscular injection, highlighting the importance of good injection technique. Studies in children and other populations are required to confirm and expand on the present results.
Pharmacokinetic studies in obese, particularly abdominally obese, patients will be needed to confirm these results. Future studies could also include intramuscular injection as an additional control.
In conclusion, the new 32G × 4 needle was bioequivalent to two widely used needles, namely the 32G × 6 and 31G × 8 needles in terms of the peak insulin concentration (Cmax) and total insulin exposure (AUC0–∞). The 32G × 4 needle was also bioequivalent to the 32G × 6 needle, but not the 31G × 8 needle, for the time to reach peak insulin concentration (Tmax), which was longer in the latter needle than in the 32G × 4 and 32G × 6 needles. Taken together, we believe the use of a 4‐mm needle does not adversely affect the pharmacokinetic properties of insulin compared with longer needles, when injected subcutaneously in adults.
Acknowledgements
This study was supported by Nippon Becton Dickinson Company, Ltd. The authors would like to thank Dr Ippei Ikushima and participating staff at LTA Clinical Pharmacology Center, Sumida Hospital (Tokyo, Japan) for carrying out the study. We thank Nicholas D Smith PhD for his editorial support. Finally, we thank Garry Steil PhD at Harvard University, Junko Kuri MD at Nippon Becton Dickinson Company, Ltd. and Kerstin Rebrin MD PhD at Becton Dickinson for their guidance on identifying the pharmacokinetic parameters of the injected insulin accounting for endogenous C‐peptide/insulin secretion.
ST is an employee of Nippon Becton Dickinson Company, Ltd., and SK is an employee of Becton Dickinson and Company, the manufacturers of two of the needles compared in this study. TH was an advisor of Nippon Becton Dickinson Company, Ltd., from November 2009 to December 2011. TO and HW have no relevant conflicts of interest to declare.
(J Diabetes Invest, doi: 10.1111/jdi.12035, 2013)
The material presented in this manuscript was included on a poster entitled 'Comparison of insulin pharmacokinetics with three different insulin needles' (original title is in Japanese) at The 54th Annual Meeting of the Japan Diabetes Society (Pres #I‐P‐50, May 19–21, 2011, Sapporo, Japan).
References
- 1.Frid A, Hirsch L, Gaspar R, et al New injection recommendations for patients with diabetes. Diabetes Metab 2010; 36(Suppl 2): 3–18 [DOI] [PubMed] [Google Scholar]
- 2.Asakura T, Seino H, Nunoi K, et al Usability of a microtapered needle (TN3305) for insulin treatment in Japanese patients with diabetes mellitus: a comparative clinical study with a standard thin wall needle. Diabetes Technol Ther 2006; 8: 489–494 [DOI] [PubMed] [Google Scholar]
- 3.Hanas R, Lytzen L, Ludvigsson J. Thinner needles do not influence injection pain, insulin leakage or bleeding in children and adolescents with type 1 diabetes. Pediatr Diabetes 2000; 1: 142–149 [DOI] [PubMed] [Google Scholar]
- 4.Iwanaga M, Kamoi K. Patient perceptions of injection pain and anxiety: a comparison of NovoFine 32‐gauge tip 6 mm and Micro Fine Plus 31‐gauge 5 mm needles. Diabetes Technol Ther 2009; 11: 81–86 [DOI] [PubMed] [Google Scholar]
- 5.McKay M, Compion G, Lytzen L. A comparison of insulin injection needles on patients' perceptions of pain, handling, and acceptability: a randomized, open‐label, crossover study in subjects with diabetes. Diabetes Technol Ther 2009; 11: 195–201 [DOI] [PubMed] [Google Scholar]
- 6.Miyakoshi M, Kamoi K, Iwanaga M, et al Comparison of patient's preference, pain perception, and usability between Micro Fine Plus 31‐gauge needle and Microtapered NanoPass 33‐gauge needle for insulin therapy. J Diabetes Sci Technol 2007; 1: 718–724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Siegmund T, Blankenfeld H, Schumm‐Draeger PM. Comparison of usability and patient preference for insulin pen needles produced with different production techniques: “thin‐wall” needles compared to “regular‐wall” needles: an open‐label study. Diabetes Technol Ther 2009; 11: 523–528 [DOI] [PubMed] [Google Scholar]
- 8.Gibney MA, Arce CH, Byron KJ, et al Skin and subcutaneous adipose layer thickness in adults with diabetes at sites used for insulin injections: implications for needle length recommendations. Curr Med Res Opin 2010; 26: 1519–1530 [DOI] [PubMed] [Google Scholar]
- 9.Henriksen JE, Djurhuus MS, Vaag A, et al Impact of injection sites for soluble insulin on glycaemic control in type 1 (insulin‐dependent) diabetic patients treated with a multiple insulin injection regimen. Diabetologia 1993; 36: 752–758 [DOI] [PubMed] [Google Scholar]
- 10.Vaag A, Handberg A, Lauritzen M, et al Variation in absorption of NPH insulin due to intramuscular injection. Diabetes Care 1990; 13: 74–76 [DOI] [PubMed] [Google Scholar]
- 11.Vaag A, Pedersen KD, Lauritzen M, et al Intramuscular versus subcutaneous injection of unmodified insulin: consequences for blood glucose control in patients with type 1 diabetes mellitus. Diabet Med 1990; 7: 335–342 [DOI] [PubMed] [Google Scholar]
- 12.Hirsch LJ, Gibney MA, Albanese J, et al Comparative glycemic control, safety and patient ratings for a new 4 mm x 32G insulin pen needle in adults with diabetes. Curr Med Res Opin 2010; 26: 1531–1541 [DOI] [PubMed] [Google Scholar]
- 13.Tominaga M, Makino H, Yoshino G, et al Japanese standard reference material for JDS Lot 2 haemoglobin A1c. I: comparison of Japan Diabetes Society‐assigned values to those obtained by the Japanese and USA domestic standardization programmes and by the International Federation of Clinical Chemistry reference laboratories. Ann Clin Biochem 2005; 42: 41–46 [DOI] [PubMed] [Google Scholar]
- 14.Kashiwagi A, Kasuga M, Araki E, et al International clinical harmonization of glycated hemoglobin in Japan: from Japan Diabetes Society to National Glycohemoglobin Standardization Program values. J Diabetes Invest 2012; 3: 39–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Owens DR. Human Insulin: Clinical Pharmacological Studies in Normal Man. MTP Press, Boston, 1986 [Google Scholar]
- 16.Urae A, Maeda A, Bowsher RR, et al Safety, pharmacokinetics and pharmacodynamics of fast‐acting Insulin, LY275585, a Phase I study: comparison with regular human insulin (Humulin R). J Clin Ther Med 2000; 16: 1601–1611 [Google Scholar]
- 17.Van Cauter E, Mestrez F, Sturis J, et al Estimation of insulin secretion rates from C‐peptide levels. Comparison of individual and standard kinetic parameters for C‐peptide clearance. Diabetes 1992; 41: 368–377 [DOI] [PubMed] [Google Scholar]
- 18.Watanabe RM, Steil GM, Bergman RN. Critical evaluation of the combined model approach for estimation of prehepatic insulin secretion. Am J Physiol 1998; 274: E172–E183 [DOI] [PubMed] [Google Scholar]
- 19.Watanabe RM, Bergman RN. Accurate measurement of endogenous insulin secretion does not require separate assessment of C‐peptide kinetics. Diabetes 2000; 49: 373–382 [DOI] [PubMed] [Google Scholar]
- 20.Mosteller RD. Simplified calculation of body‐surface area. N Engl J Med 1987; 317: 1098. [DOI] [PubMed] [Google Scholar]
- 21.Savitzky A, Golay MJE. Smoothing and differentiation of data by simplified least squares procedures. Anal Chem 1964; 36: 1627–1639 [Google Scholar]
- 22.Pharmaceutical and Food Safety Bureau . Guideline for Bioequivalence Studies of Generic Products. December, 2006
- 23.World Health Organization Expert Committee on Specifications for Pharmaceutical Preparations . Fortieth Report: Technical Report No. 937. 2006 [PubMed]
- 24.Kreugel G, Keers JC, Kerstens MN, et al Randomized trial on the influence of the length of two insulin pen needles on glycemic control and patient preference in obese patients with diabetes. Diabetes Technol Ther 2011; 13: 737–741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hofman PL, Derraik JG, Pinto TE, et al Defining the ideal injection techniques when using 5‐mm needles in children and adults. Diabetes Care 2010; 33: 1940–1944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Frid A, Linde B. Intraregional differences in the absorption of unmodified insulin from the abdominal wall. Diabet Med 1992; 9: 236–239 [DOI] [PubMed] [Google Scholar]


