Abstract
Aims/Introduction
The distinct effects of different statins on glycemic control have not been fully evaluated. In this open‐label, prospective, cross‐over clinical trial, we compared the effects of pitavastatin and atorvastatin on glycemic control in type 2 diabetic patients with hypercholesterolemia.
Materials and Methods
A total of 28 Japanese type 2 diabetics with hypercholesterolemia treated with rosuvastatin (2.5 mg/day) for at least 8 weeks were recruited to this quasi‐randomized cross‐over study. At study entry, the patients assigned to sequence 1 received pitavastatin (2 mg/day) for 12 weeks in period 1 and atorvastatin (10 mg/day) for another 12 weeks in period 2, whereas patients assigned to sequence 2 received atorvastatin (10 mg/day) for 12 weeks in period 1 and pitavastatin (2 mg/day) for another 12 weeks in period 2. Blood samples were collected at three visits (baseline, after 12 and 24 weeks).
Results
Lipid control was similar in both statins. The difference in glycated hemoglobin between pitavastatin and atorvastatin treatments was −0.18 (95% confidence interval −0.34 to −0.02; P = 0.03). Compared with atorvastatin, pitavastatin treatment significantly lowered the levels of glycoalbumin, fasting glucose and homeostasis model assessment of insulin resistance.
Conclusions
Our results showed that treatment with pitavastatin had a more favorable outcome on glycemic control in patients with type 2 diabetes compared with atorvastatin. This trial was registered with UMIN (no. 000003554).
Keywords: 3‐Hydroxy‐3‐methylglutaryl coenzyme A reductase inhibitor; Type 2 diabetes mellitus, Hypercholesterolemia
Introduction
Patients with type 2 diabetes are at high risk for the development of cardiovascular diseases, and low‐density lipoprotein (LDL) cholesterol is the most important determinant of the onset of cardiovascular disease in these patients. A previous clinical randomized trial clearly showed that atorvastatin, an inhibitor of 3‐hydroxy‐3‐methylglutaryl coenzyme A reductase, reduces the incidence of cardiovascular disease in patients with type 2 diabetes1.
Despite the beneficial effects of statins on cardiovascular disease, a recent meta‐analysis of 13 randomized studies showed that statin therapy significantly increased the incidence of new cases of diabetes by 9%2. In particular, some reports indicated that atorvastatin adversely affects glycemic control. In contrast, subanalysis of the West of Scotland Coronary Prevention Study showed that pravastatin reduced the rate of new‐onset diabetes by 30%3. Thus, the various types of statins might have differential effects on glucose metabolism4.
Pitavastatin is a new statin marginally metabolized by cytochrome P450 isoenzymes, it has particularly low potential for drug–drug interaction and has a powerful LDL cholesterol‐lowering effect similar to atorvastatin6. With regard to blood glucose control, a recent study reported that pitavastatin was neutral, whereas atorvastatin caused deterioration of glycemic control in patients with type 2 diabetes8. In contrast, some studies did not show such favorable effects for pitavastatin compared with atorvastatin9. Thus, the exact effect of pitavastatin on glycemic control remains controversial. In addition, there have been no studies that have evaluated the effect of pitavastatin on glycemic control as the primary end‐point.
The aim of the present study was to compare the effects of different statins on glycemic control. Because the effect of statins on glucose metabolism is expected to be very mild and can vary among individuals, it is difficult to evaluate the effect of statins on glucose metabolism in groups that have a small number of participants. For this reason, we chose a cross‐over study design, which allows treatment comparisons in one participant rather than between participants, to compare the effects of pitavastatin with that of atorvastatin on glucose metabolism.
Materials and Methods
Study Population and Design
All patients with type 2 diabetes mellitus who visited Juntendo University Hospital (Tokyo, Japan) and Secomedic hospital (Funabashi, Chiba, Japan) between July 2010 and October 2010 were invited to participate in the present study. The inclusion criteria were patients with type 2 diabetes mellitus and hypercholesterolemia treated with 2.5 mg rosuvastatin once daily for at least 8 weeks (rosuvastatin at this dose is regarded to have similar potency in reducing the LDL cholesterol level to 2 mg pitavastatin and 10 mg atorvastatin10). We excluded from the study patients with glycated hemoglobin (HbA1c) higher than 7.4%, glycemic control as defined by the Japan Diabetes Society, unstable glycemic control with HbA1c variation of ≥0.5% during the preceding 6 months and/or patients treated with insulin. In addition, we also excluded patients with severe renal or hepatic disease, overt cardiovascular disease and malignancy. The ethics committees of the participating hospitals approved the study protocol and informed consent was obtained from each participant.
This was a quasi‐randomized, open‐label, two‐sequence, two‐period cross‐over study. The study design is summarized in Figure 1. In the present study, patients taking rosuvastatin (2.5 mg/day) were switched at random to 2 mg pitavastatin or 10 mg atorvastatin once daily in the morning. After 12 weeks of each type of statin treatment, fasting blood samples were collected. Then, the patients on pitavastatin were switched to atorvastatin, whereas the patients on atorvastatin were switched to pitavastatin, and each continued the treatment for another 12 weeks, after which each provided blood samples. As shown Figure 1, the patients assigned to sequence 1 received pitavastatin in period 1 and atorvastatin in period 2. Furthermore, patients assigned to sequence 2 received atorvastatin in period 1 and pitavastatin in period 2. The study patients were quasi‐randomly assigned into the two sequences at a ratio of 1:1. For quasi‐randomization, an independent researcher from our study group determined the allocations based on the enrolment order of the eligible patients in the present study. Specifically, patients with odd numbers were allocated to sequence 1 and patients with even numbers were allocated to sequence 2. At three visits (baseline, after 12 and 24 weeks), blood samples were drawn, and the clinical status and adverse events were recorded. Baseline was defined as the observed value at visit 1 (week 0) for the two sequences in the present study. Apart from statins, the doses of all drugs were unchanged throughout the study period.
Figure 1.
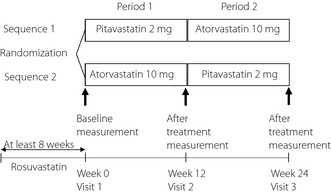
Study protocol. Schematic diagram of the study protocol. Blood sampling and blood pressure measurement were carried out at week 0 for basal data (visit 1). Blood samples obtained at week 12 (visit 2) and week 24 (visit 3) were used for evaluation of the effects of each drug.
Biochemical Tests
Blood samples were obtained between 08:00 hours and 10:00 hours after overnight fast. Serum lipids, glucose, HbA1c and glycoalbumin were measured with standard techniques. The value of HbA1c (%) was estimated as the National Glycohemoglobin Standardization Program (NGSP) equivalent value (%) calculated by the formula HbA1c (%) = {HbA1c [Japan Diabetes Society (JDS)] (%) + 0.4%}11. Homeostasis model assessment of insulin resistance (HOMA‐IR) represented the product of fasting plasma insulin (pmol/L) and fasting plasma glucose levels (mmol/L) divided by 22.5.
Statistical Analysis
A total of 30 quasi‐randomized patients was planned and justified to be necessary to obtain 80% power in detecting a difference of 0.2% in HbA1c between pitavastatin and atorvastatin treatments, assuming a standard deviation (SD) of the difference of 0.35% based on previous studies8, with a two‐sided type I error rate of 0.05 and dropout rate of 10%.
Continuous variables were summarized as mean ± SD or median (range 25 – 75%) and categorical variables were presented as the number and percentage of patients.
The primary end‐point was HbA1c level after statin treatment, whereas the secondary end‐points were the levels of glycoalbumin, fasting glucose, insulin and HOMA‐IR. These end‐points and other measurement variables were analyzed using the mixed effect model, including the sequence terms, sequence period and statin treatment as fixed effects, and patient as a random effect to compare pitavastatin and atorvastatin treatments. Other covariates (e.g. concomitant medications and baseline values) should not be adjusted for comparison of the two statins treatments, because the cross‐over design allows the comparison of the two treatments within the same patient. The significance level was sas software (version 9.2; SAS Institute, Cary, NC, USA).
Results
A total of 30 diabetic patients with hypercholesterolemia were quasi‐randomly assigned to sequence 1 (pitavastatin to atorvastatin, n = 15) and sequence 2 (atorvastatin to pitavastatin, n = 15; Figure 2). Of these, 28 patients completed this trial. Two patients did not come back to the hospital after signing the consent form (n = 1 of each sequence). No serious adverse effects were observed in all study patients including the two dropout cases.
Figure 2.
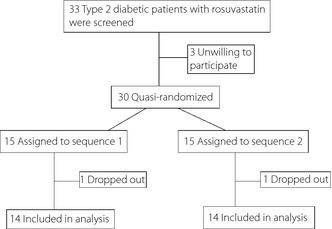
Flow chart of patient recruitment.
The demographic characteristics and mean baseline anthropometric data of patients are shown in Table 1. There were no critical differences in the demographic and baseline data between sequences 1 and 2.
Table 1. Baseline characteristics of study participants at visit 1.
| Variable | Both sequences | Sequence 1: Pitavastatin to atorvastatin | Sequence 2: Atorvastatin to pitavastatin | P‐valuea |
|---|---|---|---|---|
| n = 28 | n = 14 | n = 14 | ||
| Age (years) | 63.3 ± 9.3 | 63.3 ± 10.6 | 63.3 ± 8.2 | 1.00 |
| Sex (male) | 11 (39.3) | 5 (35.7) | 6 (42.9) | 1.00 |
| Smoker | 6 (21.4) | 3 (21.4) | 3 (21.4) | 1.00 |
| Duration of diabetes (years) | 6.3 ± 3.7 | 7.4 ± 4.3 | 5.3 ± 2.8 | 0.15 |
| Concomitant drugs | ||||
| Sulfonylurea (yes) | 9 (32.1) | 4 (28.6) | 5 (35.7) | 1.00 |
| Glinide (yes) | 2 (7.1) | 1 (7.1) | 1 (7.1) | 1.00 |
| Gliptin (yes) | 1 (3.6) | 1 (7.1) | 0 (0.0) | 1.00 |
| α‐Glucosidase inhibitor (yes) | 9 (32.1) | 4 (28.6) | 5 (35.7) | 1.00 |
| TZD (yes) | 8 (28.6) | 3 (21.4) | 5 (35.7) | 0.68 |
| Metoformin (yes) | 7 (25.0) | 3 (21.4) | 4 (28.6) | 1.00 |
| CCB (yes) | 7 (25.0) | 5 (35.7) | 2 (14.3) | 0.38 |
| ARB (yes) | 8 (28.6) | 5 (35.7) | 3 (21.4) | 0.68 |
| Antiplatelet agents (yes) | 3 (10.7) | 2 (14.3) | 1 (7.1) | 1.00 |
| Bodyweight (kg) | 61.4 ± 11.0 | 62.4 ± 11.1 | 60.5 ± 11.3 | 0.65 |
| Body mass index (kg/m2) | 25.1 ± 4.2 | 25.3 ± 3.3 | 24.9 ± 5.0 | 0.84 |
| Systolic BP (mmHg) | 132.1 ± 14.1 | 130.1 ± 12.4 | 134.1 ± 15.7 | 0.46 |
| Diastolic BP (mmHg) | 73.6 ± 11.3 | 70.6 ± 12.5 | 76.7 ± 9.4 | 0.15 |
| HbA1c (%) (NGSP) | 6.79 ± 0.59 | 6.79 ± 0.38 | 6.78 ± 0.76 | 0.95 |
| Glycoalbumin (%) | 17.1 ± 2.5 | 17.2 ± 2.1 | 17.0 ± 2.9 | 0.85 |
| Fasting blood glucose (mmol/L) | 7.28 ± 1.88 | 6.74 ± 1.03 | 7.75 ± 2.32 | 0.16 |
| Insulin (pmol/L) | 59.3 ± 39.5 | 62.9 ± 50.2 | 56.5 ± 30.3 | 0.71 |
| HOMA‐IR (mmol/L) | 2.92 ± 2.31 | 2.79 ± 2.24 | 3.03 ± 2.44 | 0.80 |
| Total cholesterol (mmol/L) | 4.58 ± 0.50 | 4.40 ± 0.46 | 4.76 ± 0.48 | 0.06 |
| HDL‐C (mmol/L) | 1.52 ± 0.28 | 1.49 ± 0.33 | 1.54 ± 0.23 | 0.65 |
| LDL‐C (mmol/L) | 2.57 ± 0.24 | 2.45 ± 0.25 | 2.70 ± 0.15 | 0.005 |
| Triglyceride (mmol/L) | 1.26 ± 0.44 | 1.26 ± 0.43 | 1.26 ± 0.48 | 1.00 |
| AST (U/L) | 22.7 ± 6.9 | 23.4 ± 8.3 | 22.1 ± 5.4 | 0.63 |
| ALT (U/L) | 22.9 ± 10.4 | 24.7 ± 12.1 | 21.1 ± 8.5 | 0.37 |
| Creatine kinase (U/L) | 99.8 ± 69.7 | 105.4 ± 81.1 | 94.2 ± 59.6 | 0.72 |
| Adiponectin (mg/L) | 12.2 ± 8.0 | 14.3 ± 9.5 | 10.1 ± 5.9 | 0.19 |
| hs‐CRP (mg/L) | 0.68 (0.36–0.93) | 0.77 (0.49–0.93) | 0.58 (0.36–1.00) | 0.74 |
Continuous variables are expressed as mean ± standard deviation or median (range 25–75%). Categorical variables are expressed as number of participants (%).
Comparison between the two sequences by two‐sample t‐test for continuous variables and Fisher's exact test for categorical variables. Differences in highly‐sensitive C‐reactive protein (hs‐CRP) were tested by Wilcoxon rank sum test.
ALT, alanine aminotransferase; ARB, angiotensin II type‐1 receptor blockers; AST, aspartate aminotransferase; BP, blood pressure; CCB, calcium channel blocker; HbA1c, glycated hemoglobin; HDL‐C, high‐density lipoprotein cholesterol; HOMA‐IR, homeostasis model assessment of insulin resistance; LDL‐C, low‐density lipoprotein cholesterol; NGSP, National Glycohemoglobin Standardization Program; TZD, thiazolidinedione.
Table 2 summarizes the HbA1c data by each sequence and statin treatment. At the end of the study periods (visits 2 and 3), HbA1c was lower under pitavastatin treatment than under atorvastatin treatment in each sequence. In addition, HbA1c values under pitavastatin and atorvastatin treatments were 6.74% and 6.92%, respectively, and the difference in HbA1c between pitavastatin and atorvastatin treatments was −0.18 (95% confidence interval −0.34 to −0.02; P = 0.03). Furthermore, the sequence effect on HbA1c was not statistically significant (P = 0.26). In addition, changes in HbA1c did not correlate with those in LDL (r = −0.146, P = 0.294).
Table 2. Changes in glycated hemoglobin and during each study part.
| Visit | Sequence 1 (pitavastatin to atorvastatin) | Visit | Sequence 2 (atorvastatin to pitavastatin) | ||
|---|---|---|---|---|---|
| Mean ± SD | Mean change ± SD | Mean ± SD | Mean change ± SD | ||
| n = 14 | n = 14 | n = 14 | n = 14 | ||
| Visit 1 (baseline) | 6.79 ± 0.38 | – | Visit 1 (baseline) | 6.78 ± 0.76 | – |
| Visit 2 (pitavastatin) | 6.66 ± 0.51 | −0.13 ± 0.26 | Visit 2 (atorvastatin) | 7.01 ± 1.23 | 0.23 ± 0.61 |
| Visit 3 (atorvastatin) | 6.84 ± 0.59 | 0.04 ± 0.29 | Visit 3 (pitavastatin) | 6.81 ± 0.98 | 0.04 ± 0.55 |
| HbA1c after treatment | Difference between two statin treatments (95% CI) | P‐value for statin effect | P‐value for sequence effect | ||
| Pitavastatin | Atorvastatin | ||||
| 6.74 | 6.92 | −0.18 (−0.34, −0.02) | 0.03 | 0.26 | |
CI, confidence interval. SD, standard deviation.
Table 3 lists the secondary end‐points and other markers at baseline and at the end of each statin treatment. Compared with the atorvastatin treatment, glycoalbumin, fasting glucose level and HOMA‐IR were significantly lower during pitavastatin treatment. In contrast, the levels of serum lipids, highly‐sensitive C‐reactive protein (hs‐CRP) and adiponectin were comparable between the two treatments.
Table 3. Secondary and other end‐points at baseline (visit 1) and after treatment with pitavastatin and atorvastatin.
| Variable | Baseline (visit 1) | After treatment | P‐value for | ||
|---|---|---|---|---|---|
| Pitavastatin | Atorvastatin | Statin | Sequence | ||
| n = 28 | n = 28 | n = 28 | effecta | effectb | |
| Glycoalbumin (%) | 17.1 ± 2.5 | 17.0 ± 2.8 | 17.6 ± 3.2 | <0.01 | 0.71 |
| Fasting blood glucose (mmol/L) | 7.28 ± 1.88 | 6.70 ± 1.20 | 7.38 ± 1.71 | <0.01 | 0.18 |
| Insulin (pmol/L) | 59.3 ± 39.5 | 44.6 ± 30.8 | 47.2 ± 28.8 | 0.42 | 0.54 |
| HOMA‐IR (mmol/L) | 2.92 ± 2.31 | 1.89 ± 1.24 | 2.29 ± 1.64 | 0.03 | 0.78 |
| Body mass index (kg/m2) | 25.1 ± 4.2 | 25.2 ± 4.0 | 25.3 ± 4.2 | 0.38 | 0.99 |
| Systolic BP (mmHg) | 132.1 ± 14.1 | 127.6 ± 13.4 | 132.0 ± 17.3 | 0.08 | 0.59 |
| Diastolic BP (mmHg) | 73.6 ± 11.3 | 70.9 ± 10.1 | 72.7 ± 9.7 | 0.13 | 0.09 |
| Total cholesterol (mmol/L) | 4.58 ± 0.50 | 4.71 ± 0.69 | 4.75 ± 0.69 | 0.70 | 0.72 |
| HDL‐C (mmol/L) | 1.52 ± 0.28 | 1.54 ± 0.33 | 1.54 ± 0.37 | 0.94 | 0.71 |
| LDL‐C (mmol/L) | 2.57 ± 0.24 | 2.65 ± 0.50 | 2.63 ± 0.39 | 0.82 | 0.55 |
| Triglyceride (mmol/L) | 1.26 ± 0.44 | 1.29 ± 0.52 | 1.26 ± 0.58 | 0.73 | 0.91 |
| AST (U/L) | 22.7 ± 6.9 | 23.6 ± 6.9 | 24.1 ± 7.3 | 0.63 | 0.10 |
| ALT (U/L) | 22.9 ± 10.4 | 24.3 ± 11.1 | 27.0 ± 17.2 | 0.22 | 0.19 |
| Creatine kinase (U/L) | 99.8 ± 69.7 | 120.3 ± 82.7 | 133.2 ± 100.5 | 0.31 | 0.80 |
| Adiponectin (mg/L) | 12.2 ± 8.0 | 12.2 ± 7.0 | 12.2 ± 7.7 | 0.97 | 0.25 |
| hs‐CRP (mg/L) | 0.68 (0.36–0.93) | 0.55 (0.25–1.32) | 0.56 (0.31–0.98) | 0.81 | 0.86 |
Data are mean ± standard deviation. The median highly‐sensitive C‐reactive protein (hs‐CRP) values are shown (range 25–75%). The log transformed hs‐CRP values were applied to the mixed effect model.
Comparison between pitavastatin and atorvastatin treatments by mixed effect model.
Comparison between the two sequences by mixed effect model.
ALT, alanine aminotransferase; AST, aspartate aminotransferase; BP, blood pressure; HDL‐C, high‐density lipoprotein cholesterol; HOMA‐IR, homeostasis model assessment of insulin resistance; LDL‐C, low‐density lipoprotein cholesterol.
Discussion
While several studies have analyzed the beneficial effects of pitavastatin on glucose metabolism compared with atorvastatin, the reported effects were marginal and statistically insignificant8. In contrast, the present study directly showed a more favorable effect for pitavastatin on glucose metabolism compared with atorvastatin, although the two statins are known to have similar LDL cholesterol‐lowering effects 10. These differences might be partly due to the study design. The present study was a prospective and cross‐over study designed to evaluate the distinct effects of statins on HbA1c, which was the primary end‐point, unlike previous studies8. In particular, the strength of the present study was the cross‐over design, which allows treatment comparisons in one participant rather than between participants, as the effect of statins on glucose metabolism is expected to be very mild and can vary among individuals.
Reduction of LDL cholesterol reduces the chance of development of cardiovascular disease in type 2 diabetic patients1. Subanalysis of the Japan Assessment of Pitavastatin and Atorvastatin in Acute Coronary Syndrome (JAPAN‐ACS) study showed that early intensive LDL cholesterol‐lowering therapy with statins resulted in the regression of coronary plaque volume in type 2 diabetic patients with acute coronary syndrome15. In this regard, potent statins are useful in the management of type 2 diabetes. As glycemic control is also important in the prevention of diabetic vasculopathies, the effects of statins on glycemic control seems beneficial in diabetics with hypercholesterolemia. In the present study, we found that pitavastatin had a more favorable effect on glucose metabolism including HbA1c, glycoalbumin, fasting blood glucose and HOMA‐IR compared with atorvastatin, despite a similar LDL cholesterol‐lowering effect. Accordingly, in terms of prevention of cardiovascular events, pitavastatin seems to be the preferred statin for patients with type 2 diabetes.
It was shown recently that hypercholesterolemia can induce islet cholesterol accumulation in mice16, and that exposure of β‐cells to high cholesterol concentrations results in their dysfunction and death17. However, in the present study, the observed changes in HbA1c did not correlate with changes in LDL cholesterol and there were no differences in LDL cholesterol levels between pitavastatin and atorvastatin treatments. Thus, pitavastatin might have a favorable effect on glucose metabolism independent of cholesterol metabolism. In addition, several studies reported that age, body mass index, blood pressure, triglyceride level and sex contribute to the development of diabetes in non‐diabetic subjects treated with statin2. In the present study, these parameters measured at baseline were found to have no effect on the change in HbA1c level (data not shown). Further studies of larger sample sizes are required to address these points.
Recent studies have reported the unfavorable effects of some lipophilic statins on glucose metabolism. Lipophilic statins can be incorporated into various organs, such as the pancreas, adipose tissue and muscle, and thus alter glucose metabolism. In contrast, hydrophilic statins are metabolized only in the liver. For instance, simvastatin, but not pravastatin, inhibits insulin secretion as a result of the blockage of L‐type calcium channels in rat pancreatic β‐cells21. However, the effects of lipophilic statins on glucose metabolism remain controversial22, and the present results showed a differential effect for distinct lipophilic statin on glucose metabolism. On the other hand, it was shown that atorvastatin at both high and low concentrations inhibited the differentiation of 3T3‐L1 preadipocytes and suppressed expression of glucose tranporter type 4, also known as GLUT‐4, leading to impaired glucose uptake in adipocytes24. In contrast, pitavastatin did not impair the differentiation and maturation of 3T3‐L1 preadipocytes, and suppressed GLUT‐4 expression when used at clinical concentrations25. In the present study, HOMA‐IR was significantly lower under pitavastatin than atorvastatin. Although the mechanisms of the differential effects of pitavastatin and atorvastatin on glucose metabolism are still largely unknown, these differences might contribute to their distinct clinical effects.
Pitavastatin is reported to prevent triglyceride accumulation in adipocytes, and elicit an increase in adiponectin messenger ribonucleic acid25. In a recent study, pitavastatin resulted in modest hyperadiponectinemia in patients with and without type 2 diabetes26. However, the two statins tested in the present study did not affect plasma adiponectin levels. Thus, the mechanism of the preferential effect of pitavastatin seems to be independent of such markers, at least when used over a short period of time, as tested in the present study.
A limitation of the present study was the small number of patients studied over a short period of time. This study might also have some selection bias because of the quasi‐randomized design. Thus, further randomized studies using larger samples followed over a longer period are required to confirm the differential effects of statins on glucose metabolism. As we only recruited Japanese patients with good or moderate glycemic control, other studies that include patients with poor glycemic control, subjects at risk of diabetes or patients at high risk for cardiovascular disease are required to further evaluate the effect of pitavastatin on glycemic control. Considering practical issues related to clinical research, we did not set up a washout period based on ethical issues27. The results showed no statistically significant sequence effects for all biochemical parameters listed in Tables 2 and 3. These results suggest no carry‐over effect in the present study, and that the results of analyses are basically valid and can be generalized to other patients. Another shortfall was the lack of evaluation of a daily nutritional diary and activity status. Accordingly, we could not exclude the effects of changes in diet, and nutritional and activity status on glycemic control. However, the roles of these factors on the observed changes seem unlikely, because we excluded patients with unstable glycemic control and the results showed no significant changes in body mass index (BMI) during each treatment.
In conclusion, the present study showed that pitavastatin treatment had more favorable effects on glucose metabolism in patients with type 2 diabetes compared with atorvastatin.
Acknowledgments
We thank all the patients who participated in this study and all the staff at Juntendo University, Department of Medicine, Metabolism & Endocrinology, (Tokyo, Japan), and Secomedic Hospital, Metabolism & Endocrinology (Funabashi, Chiba, Japan). TH received lecture fees from MSD, Novartis and Daiichi Sankyo. RK received lecture fees from MSD, Novartis, Takeda, Astellas, Daiichi Sankyo, AstraZeneca and Shionogi. HW received lecture fees from MSD, Novartis and Daiichi Sankyo. HW also received research funding from MSD, Novartis, Astellas, Daiichi Sankyo, Bayer, Pfizer, Shionogi, AstraZeneca and Kowa, and consultancy fees from Astellas and Daiichi Sankyo.
(J Diabetes Invest, doi: 10.1111/jdi.12032, 2013)
References
- 1.Colhoun HM, Betteridge DJ, Durrington PN, et al Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): multicentre randomised placebo‐controlled trial. Lancet 2004; 364: 685–696 [DOI] [PubMed] [Google Scholar]
- 2.Sattar N, Preiss D, Murray HM, et al Statins and risk of incident diabetes: a collaborative meta‐analysis of randomised statin trials. Lancet 2010; 375: 735–742 [DOI] [PubMed] [Google Scholar]
- 3.Freeman DJ, Norrie J, Sattar N, et al Pravastatin and the development of diabetes mellitus: evidence for a protective treatment effect in the West of Scotland Coronary Prevention Study. Circulation 2001; 103: 357–362 [DOI] [PubMed] [Google Scholar]
- 4.Collins R, Armitage J, Parish S, et al MRC/BHF Heart Protection Study of cholesterol‐lowering with simvastatin in 5963 people with diabetes: a randomised placebo‐controlled trial. Lancet 2003; 361: 2005–2016 [DOI] [PubMed] [Google Scholar]
- 5.Mita T, Watada H, Nakayama S, et al Preferable effect of pravastatin compared to atorvastatin on beta cell function in Japanese early‐state type 2 diabetes with hypercholesterolemia. Endocr J 2007; 54: 441–447 [DOI] [PubMed] [Google Scholar]
- 6.Hiro T, Kimura T, Morimoto T, et al Effect of intensive statin therapy on regression of coronary atherosclerosis in patients with acute coronary syndrome: a multicenter randomized trial evaluated by volumetric intravascular ultrasound using pitavastatin versus atorvastatin (JAPAN‐ACS [Japan assessment of pitavastatin and atorvastatin in acute coronary syndrome] study). J Am Coll Cardiol 2009; 54: 293–302 [DOI] [PubMed] [Google Scholar]
- 7.Yokote K, Bujo H, Hanaoka H, et al Multicenter collaborative randomized parallel group comparative study of pitavastatin and atorvastatin in Japanese hypercholesterolemic patients: collaborative study on hypercholesterolemia drug intervention and their benefits for atherosclerosis prevention (CHIBA study). Atherosclerosis 2008; 201: 345–352 [DOI] [PubMed] [Google Scholar]
- 8.Yokote K, Saito Y. Influence of statins on glucose tolerance in patients with type 2 diabetes mellitus: subanalysis of the collaborative study on hypercholesterolemia drug intervention and their benefits for atherosclerosis prevention (CHIBA study). J Atheroscler Thromb 2009; 16: 297–298 [DOI] [PubMed] [Google Scholar]
- 9.Sasaki J, Ikeda Y, Kuribayashi T, et al A 52‐week, randomized, open‐label, parallel‐group comparison of the tolerability and effects of pitavastatin and atorvastatin on high‐density lipoprotein cholesterol levels and glucose metabolism in Japanese patients with elevated levels of low‐density lipoprotein cholesterol and glucose intolerance. Clin Ther 2008; 30: 1089–1101 [DOI] [PubMed] [Google Scholar]
- 10.Saku K, Zhang B, Noda K. Randomized head‐to‐head comparison of pitavastatin, atorvastatin, and rosuvastatin for safety and efficacy (quantity and quality of LDL): the PATROL trial. Circ J 2011; 75: 1493–1505 [DOI] [PubMed] [Google Scholar]
- 11.Seino Y, Nanjo K, Tajima N, et al Report of the Committee on the classification and Diagnositc Criteria of Diabetes Mellitus. J Diabetes Invest 2010; 1: 212–228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kashiwagi A, Kasuga M, Araki E, et al You have free access to this contentInternational clinical harmonization of glycated hemoglobin in Japan: from Japan Diabetes Society to National Glycohemoglobin Standardization Program values. J Diabetes Invest 2012; 3: 39–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yamakawa T, Takano T, Tanaka S, et al Influence of pitavastatin on glucose tolerance in patients with type 2 diabetes mellitus. J Atheroscler Thromb. 2008; 15: 269–275 [DOI] [PubMed] [Google Scholar]
- 14.Gumprecht J, Gosho M, Budinski D, et al Comparative long‐term efficacy and tolerability of pitavastatin 4 mg and atorvastatin 20‐40 mg in patients with type 2 diabetes mellitus and combined (mixed) dyslipidaemia. Diabetes Obes Metab 2011; 13: 1057–1057 [DOI] [PubMed] [Google Scholar]
- 15.Arai H, Hiro T, Kimura T, et al More intensive lipid lowering is associated with regression of coronary atherosclerosis in diabetic patients with acute coronary syndrome–sub‐analysis of JAPAN‐ACS study. J Atheroscler Thromb 2010; 17: 1096–1107 [DOI] [PubMed] [Google Scholar]
- 16.Brunham LR, Kruit JK, Pape TD, et al Beta‐cell ABCA1 influences insulin secretion, glucose homeostasis and response to thiazolidinedione treatment. Nat Med 2007; 13: 340–347 [DOI] [PubMed] [Google Scholar]
- 17.Kruit JK, Kremer PH, Dai L, et al Cholesterol efflux via ATP‐binding cassette transporter A1 (ABCA1) and cholesterol uptake via the LDL receptor influences cholesterol‐induced impairment of beta cell function in mice. Diabetologia 2010; 53: 1110–1119 [DOI] [PubMed] [Google Scholar]
- 18.Hao M, Head WS, Gunawardana SC, et al Direct effect of cholesterol on insulin secretion: a novel mechanism for pancreatic beta‐cell dysfunction. Diabetes 2007; 56: 2328–2338 [DOI] [PubMed] [Google Scholar]
- 19.Waters DD, Ho JE, DeMicco DA, et al Predictors of new‐onset diabetes in patients treated with atorvastatin: results from 3 large randomized clinical trials. J Am Coll Cardiol 2011; 57: 1535–1545 [DOI] [PubMed] [Google Scholar]
- 20.Culver AL, Ockene IS, Balasubramanian R, et al Statin use and risk of diabetes mellitus in postmenopausal women in the Women's Health Initiative. Arch Intern Med 2012; 172: 144–152 [DOI] [PubMed] [Google Scholar]
- 21.Yada T, Nakata M, Shiraishi T, et al Inhibition by simvastatin, but not pravastatin, of glucose‐induced cytosolic Ca2+ signalling and insulin secretion due to blockade of L‐type Ca2+ channels in rat islet beta‐cells. Br J Pharmacol 1999; 126: 1205–1213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lalli CA, Pauli JR, Prada PO, et al Statin modulates insulin signaling and insulin resistance in liver and muscle of rats fed a high‐fat diet. Metabolism 2008; 57: 57–65 [DOI] [PubMed] [Google Scholar]
- 23.Furuya DT, Poletto AC, Favaro RR, et al Anti‐inflammatory effect of atorvastatin ameliorates insulin resistance in monosodium glutamate‐treated obese mice. Metabolism 2010; 59: 395–399 [DOI] [PubMed] [Google Scholar]
- 24.Nakata M, Nagasaka S, Kusaka I, et al Effects of statins on the adipocyte maturation and expression of glucose transporter 4 (SLC2A4): implications in glycaemic control. Diabetologia 2006; 49: 1881–1892 [DOI] [PubMed] [Google Scholar]
- 25.Ishihara Y, Ohmori K, Mizukawa M, et al Beneficial direct adipotropic actions of pitavastatin in vitro and their manifestations in obese mice. Atherosclerosis 2010; 212: 131–138 [DOI] [PubMed] [Google Scholar]
- 26.Nomura S, Shouzu A, Omoto S, et al Correlation between adiponectin and reduction of cell adhesion molecules after pitavastatin treatment in hyperlipidemic patients with type 2 diabetes mellitus. Thromb Res 2008; 122: 39–45 [DOI] [PubMed] [Google Scholar]
- 27.Nakayama S, Watada H, Mita T, et al Comparison of effects of olmesartan and telmisartan on blood pressure and metabolic parameters in Japanese early‐stage type‐2 diabetics with hypertension. Hypertens Res 2008; 31: 7–13 [DOI] [PubMed] [Google Scholar]
- 28.Yoshii Y, Mita T, Sato J, et al Comparison of effects of azelnidipine and trichlormethiazide in combination with olmesartan on blood pressure and metabolic parameters in hypertensive type 2 diabetic patients. J Diabetes Invest 2011; 2: 490–496 [DOI] [PMC free article] [PubMed] [Google Scholar]


