Abstract
Using a quasi-experimental opportunity offered by greatly restricted air pollution emissions during the Beijing Olympics compared to before and after the Olympics, we conducted the current study to compare ultrafine particles (UFPs) and fine particles (PM2.5) in their associations with biomarkers reflecting multiple pathophysiological pathways linking exposure and cardiorespiratory events. Number concentrations of particles (13.0–764.7 nm) and mass concentrations of PM2.5 were measured at two locations within 9 km from the residence and workplace of 125 participating Beijing residents. Each participant was measured 6 times for biomarkers of autonomic function (heart rate, systolic and diastolic blood pressures), hemostasis (von Willebrand factor, soluble CD40 ligand, and P-selectin), pulmonary inflammation and oxidative stress (exhaled nitric oxide and exhaled breath condensate pH, malondialdehyde, and nitrite), and systemic inflammation and oxidative stress (urinary malondialdehyde and 8-hydroxy-2′-deoxyguanosine, plasma fibrinogen, and white blood cells). Linear mixed models were used to estimate associations of biomarkers with UFPs and PM2.5 measured 1–7 days prior to biomarker measurements (lags). We found that the correlation coefficient for UFPs at two locations (∼9 km apart) was 0.45, and at the same location, the correlation coefficient for PM2.5 vs UFPs was −0.18. Changes in biomarker levels associated with increases in UFPs and PM2.5 were comparable in magnitude. However, associations of certain biomarkers with UFPs had different lag patterns compared to those with PM2.5, suggesting that the ultrafine size fraction (≤100 nm) and the fine size fraction (∼100 nm to 2.5 μm) of PM2.5 are likely to affect PM-induced pathophysiological pathways independently.
Introduction
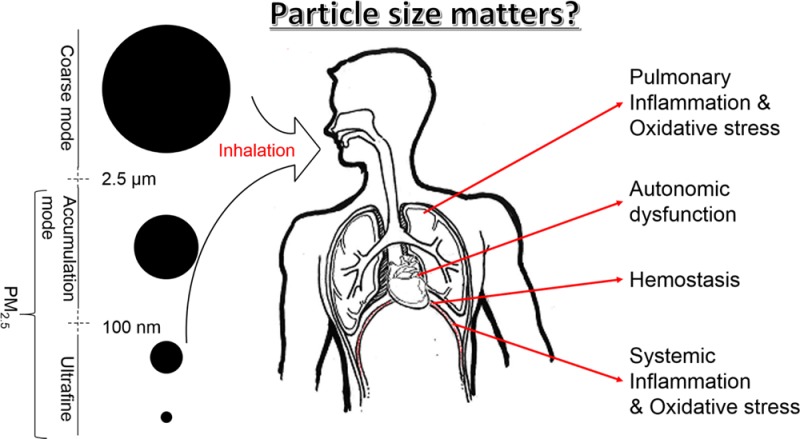
Over the past decades, a large body of literature has provided evidence for associations between exposures to ambient particulate matter (PM) and cardiorespiratory morbidity and mortality.1−3 The vast majority of the epidemiological studies have assessed the relationships between health outcomes and PM2.5 or PM10 mass concentrations.4−6 Unlike a single gaseous pollutant, atmospheric PM is a mixture of heterogeneous components; and particles of different sizes may have different physicochemical and toxicological properties.7 In a simplistic and practical fashion, PM2.5 can be considered the sum of two distinct components, namely ultrafine particles (UFPs, ≤100 nm in aerodynamic diameter) and accumulation-mode particles (AMPs, ∼100 nm to 1.0 μm).8 UFPs make up a large number concentration but contribute little mass to PM2.5.9−12 Furthermore, results from animal studies have suggested that inhaled UFPs deposit more deeply into the lung and may even directly translocate into the circulatory system, thereby exerting adverse health effects via different pathophysiological pathways than larger particles.13
Since the early 1990s, studies using various approaches, including toxicological (in vitro and in vivo), controlled human exposure, and epidemiological methods, have been conducted to examine health effects of UFPs.14−18 To date, the evidence derived from various studies has been inconclusive.11 A few epidemiological studies observed associations of UFPs with acute respiratory symptoms in infants and in adults with asthma,19,20 while other studies did not observe associations between UFPs and emergency department visits.21,22 A major explanation for inconsistent findings across studies is that different studies may have different accuracies in capturing UFP exposure using central-site monitoring data,12 as number concentrations of UFPs generally have a large spatial variation, declining rapidly with distances from sources such as roadways.23,24 At the present time, experimental data are limited to support the notion that the ultrafine fraction of PM2.5 would affect PM-induced pathophysiologic pathways differently than the coarser fraction that dominates PM2.5 mass concentrations.
During the 2008 Beijing Olympics, aggressive air pollution control measures were implemented to temporarily improve Beijing’s air quality,25 leading to substantial reductions in air pollutant levels. By taking advantage of this unique opportunity, we conducted a study to examine relationships between substantial changes in air pollutant concentrations and changes in levels of biomarkers reflecting inflammation, oxidative stress, hemostasis, and autonomic function in a panel of Beijing residents. Findings on the associations between these biomarkers and PM2.5 mass (and gaseous pollutants) have been published.26−28 In the present paper, we aim to associate the same set of biomarkers with UFP number concentrations, and compared UFP and PM2.5 in their associations with the biomarkers in terms of effect size and lag pattern, respectively.
Materials and Methods
Study Population and Study Design
The panel study was conducted before (June 2 to July 7, 2008), during (July 28 to August 29, 2008), and after (September 29 to October 30, 2008) the 2008 Beijing Summer Olympics. The participants of the study have been described previously.26−30 Briefly, 125 nonsmoking individuals, 22–27 years of age, were recruited from the pool of the medical residents at Peking University First Hospital (PKH). All study participants worked on the campus of the hospital and most (92%) resided in dormitories of the nearby (<5 km from the hospital) Peking University Health Sciences Center.27,28
Air Pollutant Measurements
Air pollutants (except particle number concentrations) and meteorological conditions were monitored at the top of a seven-floor building located on the campus of the hospital (PKH) throughout the entire study period.27 The measurement technologies for the air pollutants were reported in previous publications18,26,28,30 and a brief summary was included in the Supporting Information (SI) (Appendix 1).
Particle number concentrations were monitored on the roof of a six-floor building at the main campus of Peking University (PKU), about 9 km from the PKH location, for the same time period (June 2 to October 30, 2008). Particle number concentrations were measured using a twin differential mobility particle sizer system (TDMPS), consisting of two Hauke-type differential mobility analyzers and two condensation particle counters (models 3010 and 3025; TSI Inc., St. Paul, MN). The TDMPS measured particle number concentrations in 26 size bins (ranges) within a range of 13.0 to 764.7 nm at a 10 min interval.
In addition, particle number concentrations were measured at the PKH site for 30 days (October 1–30, 2008), allowing us to compare the particle number concentrations between the PKU site and PKH site. The particle measurement at the PKH site was conducted using a scanning mobility particle sizer system (SMPS, model 3080, TSI Inc., St. Paul, MN), consisting of a long differential mobility analyzer (TSI model 3081) and a Condensational Particle Sizer (TSI model 3025A). The SMPS measured the number concentrations of particles from 14.1 to 736.5 nm at a 5 min interval.
In order to compare the results obtained by the two different systems, the TDMPS and the SMPS were colocated (side-by-side) to measure particles simultaneously for seven days (December 1–7, 2008).
Clinical Visits and Biomarkers Measurements
Participants were invited for clinical visits twice in each of the pre-, during-, and post-Olympic periods, and the two visits in each period were two weeks apart.28 Based on physiological function, the biomarkers were grouped into four categories. In brief, autonomic function indicators included heart rate, systolic blood pressure (SBP) and diastolic blood pressure (DBP). Pulmonary inflammation and oxidative stress were assessed using exhaled breath condensate (EBC) markers, including pH values, nitrite, malondialdehyde (MDA), and fractional exhaled nitric oxide (FeNO). Hemostasis biomarkers included von Willebrand Factor (VWF), soluble CD40 Ligand (sCD40L) and P-selectin (sCD62P). Systemic inflammation and oxidative stress biomarkers included plasma fibrinogen, white blood cells (WBC), urinary MDA, and urinary 8-hydroxy-2′-deoxyguanosine (8-OHDG). The selection basis and measurement methods for these biomarkers have been described in detail previously26−28,30 and in the SI (Appendix 2).
Statistical Analyses
Linear mixed-effect models were applied to examine associations between biomarkers and particle number concentrations, as done previously.27 In each single-pollutant model, temperature, relative humidity (RH), gender, and day of week were adjusted. “Subject” was treated as a random intercept in the models. Best fits for temperature and RH, in the 24 h prior to biomarker measurements and moving averages up to 7 days, were obtained by running the natural splines function with up to 3 degrees of freedom and determined by Akaike information criterion (AIC). Measurements of heart rate, EBC nitrite, FeNO, sCD62P, sCD40L, urinary MDA, and 8-OHdG were log-transformed in the mixed-effect models, because the values of these biomarkers were right-skewed.
Pollutant concentrations measured 1–7 days prior to biomarker measurement were used to assess the lag pattern of the effects as follows: lag 0 (0–23 h), lag 1 (24–47 h), lag 2 (48–71 h), and so on, up to lag 6 (144–167 h). For all pollutant-biomarker combinations, we created “lag plots” representing the percent changes in biomarker levels associated with one interquartile range (IQR) increase in pollutant for lags 0 through lag 6.
We used two-pollutant models to examine whether the biomarker-pollutant association, obtained in the single-pollutant model as described above, can be retained after controlling for a second copollutant. To maximize the amount of variation in the biomarkers that would be accounted for by the added second pollutant, we chose the lag demonstrating the strongest statistical significance (smallest p value) for both the pollutant of primary consideration and the copollutant in the two-pollutant models analysis. Subject, temperature, RH, gender, and day of week were adjusted in the two-pollutant models in the same way as in the single-pollutant models. We then compared the estimated biomarker changes associated with PM2.5 and UFPs from the single pollutant models to those from the two-pollutant models. The detailed description on the statistical method was published previously.28
Results
Comparison of Particle Number Concentrations between Two Instruments
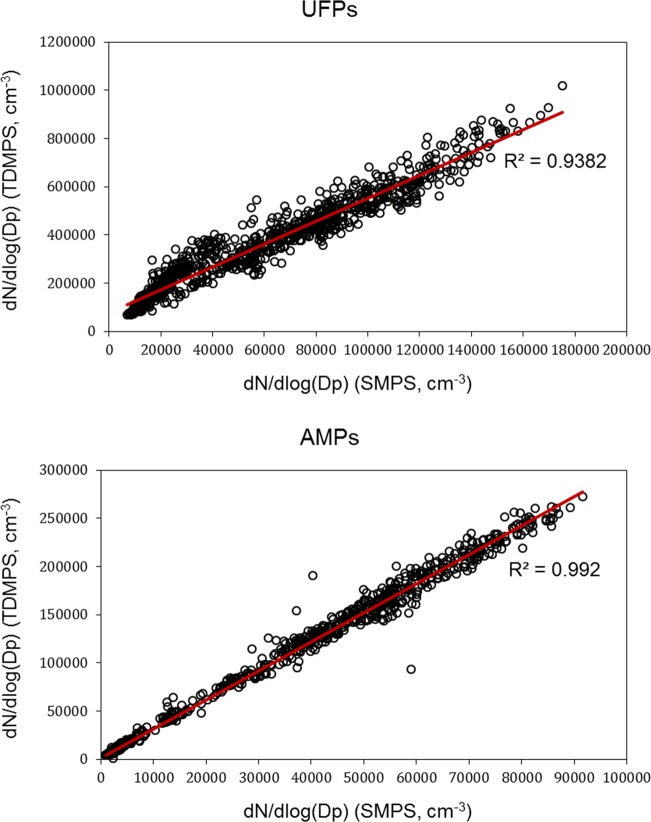
Particle number concentrations were measured by two different systems, that is, TDMPS and SMPS, at the same location for seven days, and the results were compared in Figure 1. Number concentrations were derived separately for UFPs (13.0–108.2 nm) and accumulation-mode particles (AMPs: 108.3–764.7 nm). Measurements made by the two systems had strong correlations (R2 > 0.94) for both UFPs and AMPs. However, particle number concentrations measured by the TDMPS were about 3–5 times higher than those measured by the SMPS (Figure 1). One explanation for TDMPS providing higher UFP concentrations was that the particle size range TDMPS measured was from 13.0 to 764.7 nm which was slightly wider than that (14.1 to 736.5 nm) SMPS measured. Another possible reason might be due to the intrinsic properties of the two different systems in counting particles.
Figure 1.
UFP and accumulation-mode particle number concentrations measured by two systems colocated for seven days.
Comparison of Particle Number Concentrations between Two Locations
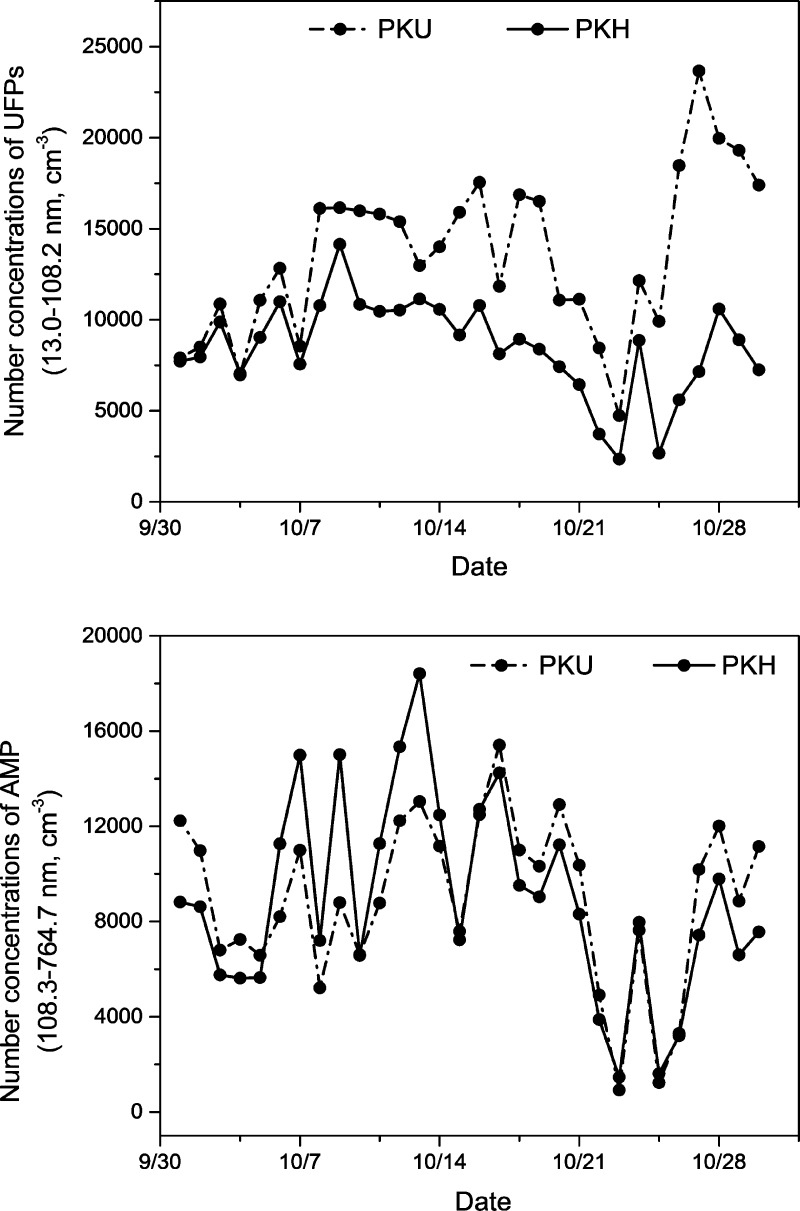
Figure 2 shows the daily average concentration of UFPs and AMPs for the 30-day period when particle number concentrations were simultaneously measured at the PKU and PKH locations. As shown, a similar day-to-day changing pattern was observed for both UFPs and AMPs between the two locations. Concentrations of AMPs between the two locations tracked each other better than those of UFPs. Concentrations of UFPs measured at the PKH site appeared to be systematically lower (by 34% on average) than those measured at PKU site. The difference may be explained by the fact that the TDMPS provided a higher particle number concentration than the SMPS as shown in Figure 1. The difference may reflect the actual spatial variation.
Figure 2.
Daily average concentrations of UFPs and AMPs at two locations, that is, the PKU and PKH sites.
Linear regression analyses were conducted to compare the number concentrations of UFPs and AMPs between the two locations. We found a higher Spearman correlation (r = 0.80) for AMPs than for UFPs (r = 0.45). More data are shown in the SI (Figure S1).
Particle Number Concentrations and Correlations with Other Pollutants
At the PKU site, number concentrations were measured for particles with a size ranging from 13.0 to 764.7 nm for 94 days. The size distribution of particles was shown in SI (Figure S2). The means ± standard deviations for 24 h averaged concentrations were 10623 ± 4313 cm–3 for UFPs and 5156 ± 2076 cm–3 for AMPs. A 16% reduction in UFP concentrations and a 32% reduction in AMP concentrations were observed from the pre- to during-Olympic period. Larger increases (66% in UFPs and 46% in AMPs) were observed from the during- to post-Olympic period.
Spearmen correlation coefficients between pollutant pairs and the meteorological parameters were summarized in Table 1. UFP was positively correlated with NO2 (r = 0.65, p < 0.001), elemental carbon (EC) (r = 0.43, p < 0.001), SO2 (r = 0.31, p = 0.026), AMP (r = 0.20, p = 0.128), and CO (r = 0.18, p = 0.166), or but negatively (and weakly) correlated with PM2.5 (r=-0.18, p = 0.168). In contrast, PM2.5 was generally more strongly correlated with AMP (r = 0.79, p < 0.001), SO2 (r = 0.73, p < 0.001), CO (r = 0.62, p < 0.001), and EC (r = 0.59, p < 0.001).
Table 1. Spearman Correlations Coefficients among Measured Air Pollutantsa.
| PM2.5 | EC | CO | SO2 | NO2 | temp | RH | UFPs | |
|---|---|---|---|---|---|---|---|---|
| PM2.5 | 1 | |||||||
| EC | 0.59** | 1 | ||||||
| CO | 0.62** | 0.54** | 1 | |||||
| SO2 | 0.73** | 0.71** | 0.52** | 1 | ||||
| NO2 | 0.32 | 0.81** | 0.50** | 0.59** | 1 | |||
| temp | 0.40* | –0.16 | 0.19 | 0.12 | –0.54** | 1 | ||
| RH | 0.25 | –0.36* | 0.19 | –0.24 | –0.31 | 0.24 | 1 | |
| UFPs | –0.18 | 0.43** | 0.18 | 0.31 | 0.65** | –0.47** | –0.56** | 1 |
| AMP | 0.79** | 0.84** | 0.55** | 0.84** | 0.61** | 0.036 | –0.12 | 0.20 |
Temp, temperature; RH, relative humidity; AMP, accumulation-mode particles (108.3–764.7 nm); UFP, ultrafine particles (13.0–108.2 nm). *Denotes statistical significance (p < 0.01). ** Denotes statistical significance (p < 0.001).
Single-Pollutant Models
Figure 3A-D and Table S1–S4 in the SI showed the estimated change in each individual biomarker associated with an IQR increase in PM2.5 or UFP concentrations from one to seven lag days. The associations between PM2.5 and the biomarkers have been reported in previous publications.26,27,30 These associations were presented again here so that we can compare the size of the estimated biomarker changes associated with IQR increases in UFP and PM2.5 concentrations.
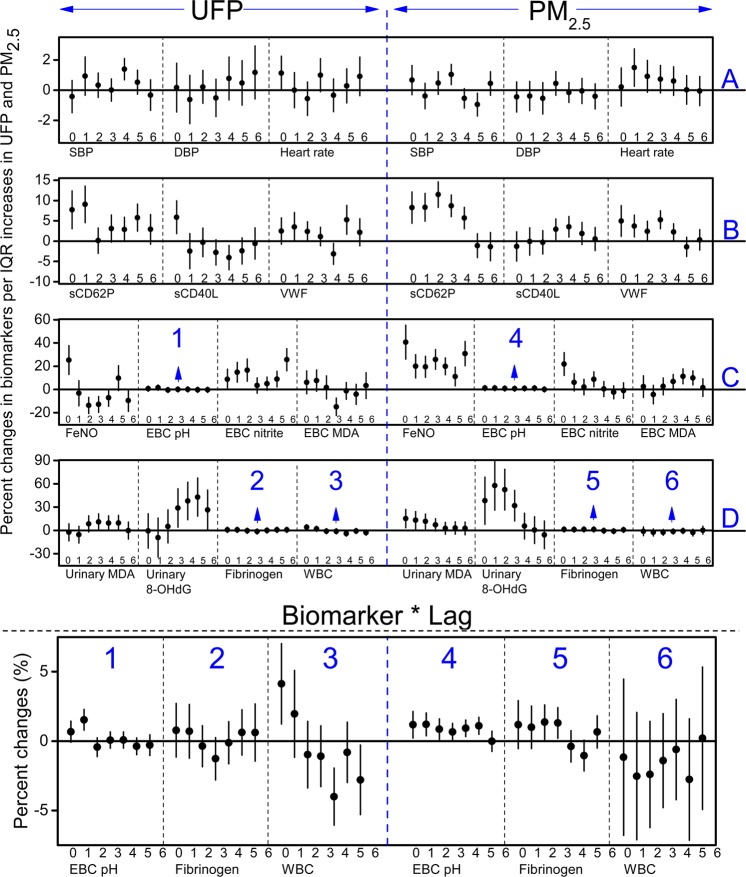
Figure 3.
Estimated means and 95% confident intervals for the percent changes in the biomarkers reflecting (A) autonomic function, (B) hemostasis, (C) pulmonary inflammation and oxidative stress, and (D) systemic inflammation and oxidative stress associated with interquartile range changes in UFPs and PM2.5, controlling for temperature, RH, sex, and day of the week. Blocks numbered with 1–6 were presented in an enlarged scale under the main plot.
For the three parameters related to autonomic function (Figure 3A and SI Table S1), systolic blood pressure (SBP) showed significant associations with PM2.5 and UFPs at lag 3 and lag 4, respectively; diastolic blood pressure (DBP) was associated with neither PM2.5 nor UFPs; and heart rate showed a significant association with PM2.5 at lag 1, while its association with UFPs appeared marginally significant at lag 0 then decreased in the following lag days. As described in the Materials and Methods section, the percent changes in biomarkers were standardized to the scale of an IQR increase in UFPs or PM2.5. The largest percent changes in SBP associated with UFPs and PM2.5 were 1.38 (95% CI: 0.67, 2.09) at lag 4 and 1.03 (95% CI: 0.36, 1.70) at lag 3, respectively. The largest percent changes in heart rate associated with UFPs and PM2.5 were 1.12 (95% CI: −0.01, 2.26) at lag 0 and 1.49 (95% CI: 0.24, 2.75) at lag 1, respectively.
For the biomarkers pertinent to hemostasis (Figure 3B and SI Table S2), sCD62P showed significant associations with both UFPs and PM2.5 at lag 0 and 1, and the association between sCD62P and PM2.5 continued to be significant until lag 4. In contrast, sCD40L was significantly associated with UFPs at lag 0, while its association with PM2.5 was significant at lag 3 and 4. VWF began to have a significant association with PM2.5 from lag 0 and remained significant until lag 4, while it was only significantly positively associated with UFPs at lag 5. The largest percent changes in sCD62P associated with IQR increases in UFPs at lag 1 and PM2.5 at lag 2 were 9.05 (95% CI: 4.55, 13.74) and 11.44 (95% CI: 8.28, 14.70), respectively. The largest percent changes in sCD40L per IQR increases were 5.87 (95% CI: 1.83, 10.08) with UFPs at lag 0 and 3.53 (95% CI: 0.99, 6.14) with PM2.5 at lag 4. The largest percent changes in VWF were 5.25 (95% CI: 1.70, 8.80) with UFPs at lag 5 and 5.26 (95% CI: 3.04, 7.47) with PM2.5 at lag 3.
For biomarkers related to pulmonary inflammation and oxidative stress (Figure 3C and SI Table S3), FeNO was significantly associated with UFPs at lag 0, while its association with PM2.5 was significant at all seven lag days. EBC pH value showed a significant association with UFPs at lag 1, and it was significantly associated with PM2.5 from lag 0 to lag 5. EBC nitrite appeared to have opposite patterns of the associations with UFPs versus those with PM2.5. EBC MDA was significantly associated with PM2.5 at lag 3, 4, and 5, while no significant and positive associations were observed between EBC MDA and UFPs at any lags. After the standardization to the IQR scale, the largest percent change in FeNO associated with UFPs was 25.34 (95% CI: 12.96, 39.09) at lag 0, and with PM2.5 it was 40.71 (95% CI: 26.10, 57.02) at lag 0. The largest percent changes in EBC pH value were 1.54 (95% CI: 0.79, 2.28) associated with UFPs and 1.21 (95% CI: 0.39, 2.03) associated with PM2.5 both at lag 1. The largest percent change in EBC nitrite associated with UFPs at lag 6 was 25.64 (95% CI: 16.12, 35.94), and was 21.90 (95% CI: 12.04, 32.63) in association with PM2.5 at lag 0.
For biomarkers related to systemic inflammation and oxidative stress (Figure 3D and SI Table S4), the lag patterns of urinary MDA and 8-OHdG were different for UFPs than those for PM2.5. The associations of these two biomarkers with PM2.5 began as significant at the first two lag days and then decreased to null, while their associations with UFPs were nonsignificant at the first three lags and then became significant starting from lag 3. Plasma fibrinogen showed no significant association with UFPs, while it was significantly associated with PM2.5 at lag 3. WBC showed a significant association with UFPs at lag 0, but no association was observed between WBC and PM2.5. The largest percent changes in urinary MDA per IQR increase were 10.89 (95% CI: 0.56, 22.28) for UFPs at lag 3 and 15.27 (95% CI: 3.44, 28.44) for PM2.5 at lag 0. The largest percent changes in 8-OHdG were 28.56 (95% CI: 4.08, 59.53) for UFPs at lag 3 and 57.58 (95% CI: 26.06, 96.99) for PM2.5 at lag 1.
Two-Pollutant Models
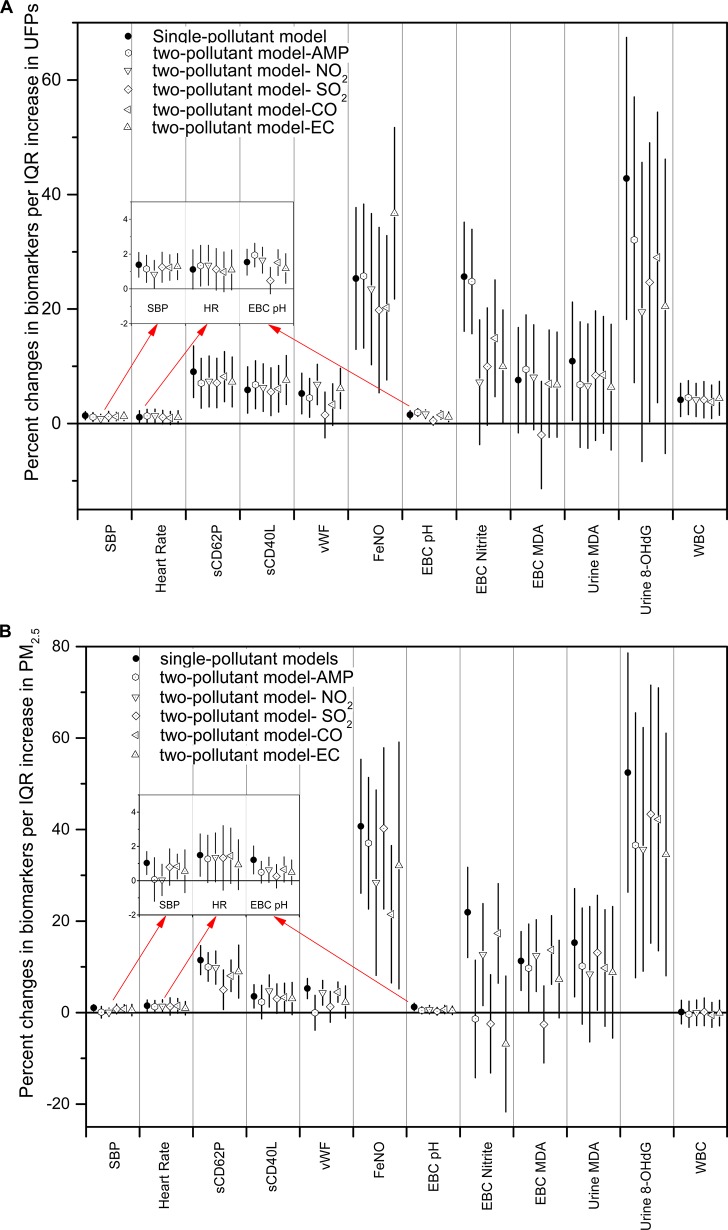
Figure 4A and 4B presented the largest percent changes in the 12 biomarkers per IQR increases in UFPs and PM2.5 estimated by single- and two-pollutant models. DBP and fibrinogen were not included in the two-pollutant models since they did not show significant associations with either UFPs or PM2.5 in single pollutant models
Figure 4.
Estimated means and 95% confident intervals for the percent change in the biomarkers with one IQR increase in (A) UFPs and (B) PM2.5, controlling for temperature, RH, sex, day of the week, and a second pollutant, including AMP, sulfur dioxide (SO2), nitrogen dioxide (NO2), carbon monoxide (CO), and elemental carbon (EC).
Figure 4A showed that the largest percent changes in the biomarkers of autonomic function and hemostasis, including SBP, heart rate, sCD62P, sCD40L, and VWF, associated with increases in UFP were not notably changed after controlling for any of the five copollutants regarding to the effect size and the significance, except for the changes in VWF after controlling for SO2 as the copollutant. The changes in WBC associated with UFPs were not remarkably changed in the two-pollutant models as well. The largest changes in VWF, EBC pH, and EBC MDA associated with UFPs were significantly reduced in both the effect size and the significance after controlling for SO2 in the two-pollutant models. The largest percent change in FeNO associated with the increase in UFPs increased after controlling for EC as the copollutant. For EBC nitrite, urinary MDA and 8-OHdG, their largest changes associated with increases in UFP were reduced in the effect size in copollutant models, with some of them became nonsignificant.
Figure 4B showed that the largest percent changes in all the biomarkers associated with increases in PM2.5 were reduced both in the effect size and the statistical significance by controlling for copollutants. For example, the changes in SBP were reduced in two-pollutant models after controlling for AMP, NO2, or EC, so were the changes in sCD62P and VWF after controlling for SO2 as the copollutant. The largest changes in EBC pH, VWF, EBC nitrite, and EBC MDA associated with increases in PM2.5 were remarkably reduced in both the effect size and significance after controlling for AMP, SO2, or EC. For FeNO and 8-OHdG, their changes associated with increases in PM2.5 were reduced but remained significant in the two-pollutant models.
Discussion
In the current study, we utilized central-site concentrations as a surrogate of UFP exposure for study subjects residing and working within a 9 km radius. Similar to the associations between PM2.5 and the biomarkers, significant associations were consistently observed between UFPs and the biomarkers related to multiple physiological pathways. This suggests the usability of central-site UFP data to estimate exposures of pathophysiological relevance in our study participants.
Central-site monitoring data have been commonly used as surrogates for population exposures to PM2.5 and PM10 mass in epidemiologic studies.5,16,21,27,31 However, due to large spatial variability in UFP number concentrations, there is a concern on the usage of the UFP monitoring data from a limited number of central sites even in relatively small areas.12,32,33 In a Health Effects Institute report, the investigators pointed out that UFP number concentrations monitored at different locations within cities were reasonably correlated in time, with similar patterns of rising and falling over the course of a day.11 Moore and colleagues (2009) measured number concentrations of total particles and found hourly median correlation coefficients varied from 0.3 to 0.56 across 14 cities in the Los Angeles area.34 Consistently found in the current study, daily average concentrations of UFPs at two locations in Beijing changed in a similar pattern across the 30 monitoring days with a moderate correlation coefficient of 0.45. Even though the correlations of UFPs between two locations within Beijing city were not as strong as those observed for the accumulation-mode particles (r = 0.80), they might be usable to support epidemiologic studies on the short-term effects of UFPs on human health.11
Another concern on the studies of UFP health effects is the correlation between UFPs and other traffic-related pollutants.35 The effects of UFPs observed in the current study were likely to be independent from PM2.5 since we observed a weak correlation between UFPs and PM2.5 (r = −0.18). In Rochester, New York with relatively low ambient PM2.5 and UFP concentrations (mean ± SD concentration of PM2.5 and UFPs were 8.67 ± 6.06 μg/m3 and 4049 ± 2168 particles/cm3), Rich et al. (2012) observed that UFPs and PM2.5 were also poorly correlated (r = 0.11).18 Chung et al. (2001) and Herner et al. (2006) suggested inverse correlations between UFPs and PM2.5 as the processes they experience in the atmosphere, that is, coagulation and condensation, can transfer materials from UFPs size to the accumulation mode size.36,37
Given that some traffic-related pollutants were correlated with UFPs and/or PM2.5 (Table 1), we examined whether the associations observed through single-pollutant models were robust by adding a copollutant. Associations of biomarkers with UFPs obtained from the single-pollutant models seems more robust than with PM2.5 after controlling for the copollutants, in terms of either the size of association estimates or statistical significance.
On one hand, the percent changes of the biomarkers associated with increases in UFPs were reduced in a smaller extent of the magnitude than those associated with increases in PM2.5 by controlling for copollutants (a 26% average reduction in the 60 biomarker-UFP association estimates versus a reduction of 60% in the 60 biomarker-PM2.5 association estimates). On the other hand, the statistical significance was less affected for the biomarker-UFP associations because only 13 out of the 60 biomarker-UFP associations in contrast to 30 out of the 60 biomarker-PM2.5 associations (Figure 4A and 4B) lost statistical significance by controlling for the copollutants. It is also notable that, by controlling for AMP, only two biomarkers’ associations (urinary MDA and 8-OHdG) with UFPs showed considerable reductions, while seven biomarkers’ associations with PM2.5 were significantly reduced by controlling for AMP (Figure 4B). This is not surprising because PM2.5 showed higher correlations with the copollutants (except NO2) than UFPs (Table 1). The reductions in the effect size and the loss of statistical significance of the associations between biomarkers and the two measures of particles were substantial after controlling for copollutants, especially for PM2.5. Therefore, the covariation between the two particle measures and other traffic-related pollutants should be considered to interpret the association results from single-pollutant models.
The current study was consistent with Rich et al. (2012) in findings on the associations of some biomarkers with UFPs and PM2.5 in short-term exposure, even though the subjects and exposure levels of particles of the two studies were not comparable.18 In both of the studies, increases in SBP and fibrinogen were associated with increases in UFPs and PM2.5 at lag 3–4, but no clear pattern of associations between DBP and UFPs or PM2.5 were observed. While no significant association was observed between WBC and UFPs in the study of Rich et al. (2012), a significant increase in WBC was associated with increases in UFPs in the current study. Based on a limited number of human studies conducted to date, the evidence is not sufficiently strong to support the notion that there are substantial differences in the effects of short-term exposure to UFPs from those of PM2.5.11 In the current study by comparing UFPs to PM2.5 in their associations with different biomarkers, we found that the percent changes in biomarkers associated with IQR increases in the two measures of particles were comparable in magnitude for most of the biomarkers (Figure 3).
However, we observed differences between UPFs and PM2.5 in the changing patterns of their associations with biomarkers reflecting hemostasis, pulmonary inflammation, and oxidative stress, and systemic inflammation and oxidative stress. For example, the three biomarkers in hemostasis showed a “V-shaped” association pattern with UFPs from lag 0 to 6, while those with PM2.5 were quite the reverse for sCD62P and sCD40L (Figure 3B); EBC nitrite showed a declining association with PM2.5 from lag 0 to lag 6, while its largest association with UFPs occurred at lag 6 (Figure 3C); and urinary MDA and urinary 8-OHdG were significantly associated with UFPs starting from lag 3, while their associations with PM2.5 were significant from lag 0 (Figure 3D). Furthermore, WBC showed significant association with UFPs at lag 0 but not with PM2.5 throughout the 7 lag days (Figure 3D), while EBC MDA showed a significant association with PM2.5 at lag 3–5 but not with UFPs (Figure 3C). The comparisons suggest potential differences in timing of “actions” between UFPs and larger particles (which dominate PM2.5 mass concentration) on the respiratory and the circulatory systems.
The differences between UFPs and PM2.5 in the associations with the biomarkers are likely to reflect their differences in deposition, clearance, and translocation after inhalation. The deposited particles can be cleared by alveolar macrophages through particle phagocytosis.11 Compared with larger particles, UFPs appear to be cleared more slowly and retained longer within the lung after deposition.38,39 Biological effects mediated by UFPs may be cumulative due to their longer retention in the lung than those mediated by larger particles.11 This assumption may partly explain the difference between PM2.5 and UFPs in the variation of their associations with some of the biomarkers across lag days. For instance, the association of EBC nitrite with UFPs stayed significant for most of the lag days and showed an increasing pattern from lag 0 to 6 (except lag 3 and 4), whereas its association with PM2.5 became nonsignificant after lag 1 and decreased from lag 1 to 6 (Figure 3C). The findings on EBC nitrite may suggest that UFPs were retained longer in the lung than larger particles after the deposition.
It has also been hypothesized that some of the systemic effects of PM are due to a spillover of the pulmonary effects.6 If this is the case, we should expect to observe a more prolonged effect of UFPs than PM2.5. Our data on systemic oxidative stress (urinary MDA and 8-OHdG) and inflammation (VWF) indeed appear to support this hypothesis. The associations of the two urinary biomarkers with UFPs became significant from lag 3 to lag 5, in contrast, their associations with PM2.5 were “immediately” significant from lag 0 and then decreased (Figure 3D). VWF showed a significant association with PM2.5 at lag 0 but with UFPs at lag 1 (Figure 3B). However, some of the biomarkers seemed to provide results against this assumption; for example, sCD40L showed a significant association with UFPs at lag 0, and the association decreased to nonsignificant afterward (Figure 3B). This is perhaps due to another mechanism that “competes” with the spillover hypothesis, as discussed below.
Translocation of particles in the body might be another reason for the differences between UFPs and PM2.5 in their associations with the biomarkers. Animal studies have found translocations of UFPs, but not fine or coarse particles (i.e., particles >100 nm in size), into the circulatory system,40,41 even though the mechanism for UFP translocation is still unclear. We assume that the blood markers will have quicker and/or stronger associations with UFPs than with PM2.5 due to the translocation of UFPs into bloodstream, although the assumption was not fully supported by all the blood biomarkers. We observed that WBC was significantly associated with UFPs at lag 0 but not with PM2.5 throughout the seven lag days (Figure 3D). sCD40L was significantly associated with UFPs at lag 0, but its association with PM2.5 started to be significant from lag 3 to 4 (Figure 3B). The difference in biomarker responses to UFPs verses to PM2.5 may reflect the inherent differences in underlying biology, measurement error, sampling variation, or the confounding effects of the other correlated pollutants. For example, the associations of sCD62P and sCD40L with UFPs were not remarkably changed in term of the significance when controlling for any of the copollutants; whereas the associations of VWF with UFPs became nonsignificant in the copollutant models (Figure 4B). Speculation on the reasons for these inconsistent findings may provide insights to reveal the underlying mechanisms through which UFPs and PM2.5 adversely affect human health.
The major limitation of the current study is the characterization of UFP exposure. Just like in any study using central-site monitoring data, there exists a possible nondifferential exposure misclassification due to utilization of central-site air pollution levels rather than personal exposure assessment. Second, due to practical constraints, both UFPs and PM2.5 were measured at a height around 20 m above ground. Compared to the vertical profile of PM2.5, UFPs showed a larger vertical variation.42,43 Even so, we do not tend to attribute the observed differences in biomarker-pollutant associations between UFPs and PM2.5 to potential vertical differences in pollutant concentrations, as our study design was a within-person comparison and no within-person changes in elevations would be expected across the six visits. Other limitations for this type of observational study have been discussed in our previous publications.26−28
Our findings suggest that number concentrations of UFPs monitored in a central site may be useful in a panel study design that mainly relies on within-person comparisons and when subjects work and resides within a relatively small area (<9 km radius). Because UFPs and PM2.5 were poorly correlated, the lag-pattern differences between UPFs and PM2.5 suggest that the ultrafine size fraction (≤100 nm) and the fine size fraction (∼100–2.5 μm) of PM2.5 are likely to affect PM-induced pathophysiological pathways independently. This finding suggests that controlling policies need to consider both the ultrafine and the fine size fraction of PM2.5 in order to protect human health.
Acknowledgments
This research was jointly funded by NIEHS (1R01 ES0158640, P30 ES05022, and 5P30ES007048), the Health Effects Institute (4760-RPFA05-3), and partly funded by Beijing Environmental Protection Agency (OITC-G08026056). The views expressed in this manuscript are solely of the authors and do not necessarily reflect those of the funding agencies.
Supporting Information Available
Additional information as noted in the text. This material is available free of charge via the Internet at http://pubs.acs.org.
The authors declare no competing financial interest.
Funding Statement
National Institutes of Health, United States
Supplementary Material
References
- Martinelli N.; Olivieri O.; Girelli D. Air particulate matter and cardiovascular disease: a narrative review. Eur. J. Intern. Med. 2013, 244295–302. [DOI] [PubMed] [Google Scholar]
- Pope C. A. 3rd; Burnett R. T.; Thun M. J.; Calle E. E.; Krewski D.; Ito K.; Thurston G. D. Lung cancer, cardiopulmonary mortality, and long-term exposure to fine particulate air pollution. J. Am. Med. Assoc. 2002, 28791132–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pope C. A. 3rd; Dockery D. W. Health effects of fine particulate air pollution: lines that connect. J. Air Waste Manage. Assoc. 2006, 566709–42. [DOI] [PubMed] [Google Scholar]
- Dockery D. W.; Pope C. A. 3rd; Xu X.; Spengler J. D.; Ware J. H.; Fay M. E.; Ferris B. G. Jr.; Speizer F. E. An association between air pollution and mortality in six U.S. cities. N. Engl. J. Med. 1993, 329241753–9. [DOI] [PubMed] [Google Scholar]
- Pope C. A. 3rd; Thun M. J.; Namboodiri M. M.; Dockery D. W.; Evans J. S.; Speizer F. E.; Heath C. W. Jr. Particulate air pollution as a predictor of mortality in a prospective study of U.S. adults. Am. J. Respir. Crit. Care Med. 1995, 1513 Pt 1669–74. [DOI] [PubMed] [Google Scholar]
- Brook R. D.; Rajagopalan S.; Pope C. A. 3rd; Brook J. R.; Bhatnagar A.; Diez-Roux A. V.; Holguin F.; Hong Y.; Luepker R. V.; Mittleman M. A.; Peters A.; Siscovick D.; Smith S. C. Jr.; Whitsel L.; Kaufman J. D. Particulate matter air pollution and cardiovascular disease: An update to the scientific statement from the American Heart Association. Circulation 2010, 121212331–78. [DOI] [PubMed] [Google Scholar]
- WHO’s global air-quality guidelines. Lancet 2006, 368, (9544), 1302. [DOI] [PubMed] [Google Scholar]
- US EPA. Air Quality Criteria for Particulate Matter; U.S. Environmental Protection Agency: Research Triangle Park, NC, 2004. [Google Scholar]
- Nel A.; Xia T.; Madler L.; Li N. Toxic potential of materials at the nanolevel. Science 2006, 3115761622–627. [DOI] [PubMed] [Google Scholar]
- Oberdorster G.; Oberdorster E.; Oberdorster J. Nanotoxicology: An emerging discipline evolving from studies of ultrafine particles. Environ. Health Perspect. 2005, 1137823–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HEI. Review panel on ultrafine particles. In Understanding the Health Effects of Ambient Ultrafine Particles, HEI Perspectives 3; Health Effects Institute: Boston, MA, 2013. [Google Scholar]
- Sioutas C.; Delfino R. J.; Singh M. Exposure assessment for atmospheric ultrafine particles (UFPs) and implications in epidemiologic research. Environ. Health Perspect. 2005, 1138947–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberdorster G.; Sharp Z.; Atudorei V.; Elder A.; Gelein R.; Kreyling W.; Cox C. Translocation of inhaled ultrafine particles to the brain. Inhalation Toxicol. 2004, 166–7437–45. [DOI] [PubMed] [Google Scholar]
- Elder A. C.; Gelein R.; Finkelstein J. N.; Cox C.; Oberdorster G. Pulmonary inflammatory response to inhaled ultrafine particles is modified by age, ozone exposure, and bacterial toxin. Inhalation Toxicol. 2000, 12Suppl 4227–46. [DOI] [PubMed] [Google Scholar]
- Oberdörster G.; Finkelstein J.; Johnston C.; Gelein R.; Cox C.; Baggs R.; Elder A.. Acute Pulmonary Effects of Ultrafine Particles in Rats and Mice; Health Effects Institute: Boston, MA, 2000. [PubMed] [Google Scholar]
- McCreanor J.; Cullinan P.; Nieuwenhuijsen M. J.; Stewart-Evans J.; Malliarou E.; Jarup L.; Harrington R.; Svartengren M.; Han I. K.; Ohman-Strickland P.; Chung K. F.; Zhang J. Respiratory effects of exposure to diesel traffic in persons with asthma. N. Engl. J. Med. 2007, 357232348–58. [DOI] [PubMed] [Google Scholar]
- Samet J. M.; Rappold A.; Graff D.; Cascio W. E.; Berntsen J. H.; Huang Y. C.; Herbst M.; Bassett M.; Montilla T.; Hazucha M. J.; Bromberg P. A.; Devlin R. B. Concentrated ambient ultrafine particle exposure induces cardiac changes in young healthy volunteers. Am. J. Respir. Crit. Care Med. 2009, 179111034–42. [DOI] [PubMed] [Google Scholar]
- Rich D. Q.; Zareba W.; Beckett W.; Hopke P. K.; Oakes D.; Frampton M. W.; Bisognano J.; Chalupa D.; Bausch J.; O’Shea K.; Wang Y.; Utell M. J. Are ambient ultrafine, accumulation mode, and fine particles associated with adverse cardiac responses in patients undergoing cardiac rehabilitation?. Environ. Health Perspect. 2012, 12081162–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen Z. J.; Loft S.; Ketzel M.; Stage M.; Scheike T.; Hermansen M. N.; Bisgaard H. Ambient air pollution triggers wheezing symptoms in infants. Thorax 2008, 638710–6. [DOI] [PubMed] [Google Scholar]
- von Klot S.; Wolke G.; Tuch T.; Heinrich J.; Dockery D. W.; Schwartz J.; Kreyling W. G.; Wichmann H. E.; Peters A. Increased asthma medication use in association with ambient fine and ultrafine particles. Eur. Respir. J. 2002, 203691–702. [DOI] [PubMed] [Google Scholar]
- Peel J. L.; Tolbert P. E.; Klein M.; Metzger K. B.; Flanders W. D.; Todd K.; Mulholland J. A.; Ryan P. B.; Frumkin H. Ambient air pollution and respiratory emergency department visits. Epidemiology 2005, 162164–74. [DOI] [PubMed] [Google Scholar]
- Leitte A. M.; Schlink U.; Herbarth O.; Wiedensohler A.; Pan X. C.; Hu M.; Richter M.; Wehner B.; Tuch T.; Wu Z.; Yang M.; Liu L.; Breitner S.; Cyrys J.; Peters A.; Wichmann H. E.; Franck U. Size-segregated particle number concentrations and respiratory emergency room visits in Beijing, China. Environ. Health Perspect. 2011, 1194508–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y.; Hinds W.; Kim S.; Shen S.; Sioutas C. Study of ultrafine particles near a major highway with heavy-duty diesel traffic. Atmos. Environ. 2002, 36274323–4335. [Google Scholar]
- Karner A.; Eisinger D.; Niemeier D. Near-roadway air quality: synthesizing the findings from real-world data. Environ. Sci. Technol. 2010, 44145334–5344. [DOI] [PubMed] [Google Scholar]
- Wang M.; Zhu T.; Zheng J.; Zhang R. Y.; Zhang S. Q.; Xie X. X.; Han Y. Q.; Li Y. Use of a mobile laboratory to evaluate changes in on-road air pollutants during the Beijing 2008 Summer Olympics. Atmos. Chem. Phys. 2009, 9218247–8263. [Google Scholar]
- Huang W.; Wang G.; Lu S. E.; Kipen H.; Wang Y.; Hu M.; Lin W.; Rich D.; Ohman-Strickland P.; Diehl S.; Zhu P.; Gong J.; Tong J.; Zhu T.; Zhang J. Inflammatory and oxidative stress responses of healthy young adults to changes in air pollution levels during the Beijing Olympics. Am. J. Respir. Crit. Care Med. 2012, 186111150–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich D. Q.; Kipen H. M.; Huang W.; Wang G.; Wang Y.; Zhu P.; Ohman-Strickland P.; Hu M.; Philipp C.; Diehl S.; Lu S. E.; Tong J.; Gong J.; Thomas D.; Zhu T.; Zhang J. Association between changes in air pollution levels during the Beijing Olympics and biomarkers of inflammation and thrombosis in healthy young adults. J. Am. Med. Assoc. 2012, 307192068–2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J.; Zhu T.; Kipen H.; Wang G.; Huang W.; Rich D.; Zhu P.; Wang Y.; Lu S.; Ohman-stricklan P.; Diehl S. R.; Hu M.; Tong J.; Gong J.; Thomas D.. Cardiorespiratory Biomarker Responses in Healthy Young Adults to Drastic Air Quality Changes Surrounding the 2008 Beijing Olympics, Report Number: 174; Health Effects Institute: Boston, MA, 2013. [PMC free article] [PubMed] [Google Scholar]
- Kipen H.; Rich D.; Huang W.; Zhu T.; Wang G.; Hu M.; Lu S. E.; Ohman-Strickland P.; Zhu P.; Wang Y.; Zhang J. J. Measurement of inflammation and oxidative stress following drastic changes in air pollution during the Beijing Olympics: A panel study approach. Ann. N. Y. Acad. Sci. 2010, 1203, 160–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong J.; Zhu T.; Kipen H.; Wang G.; Hu M.; Ohman-Strickland P.; Lu S. E.; Zhang L.; Wang Y.; Zhu P.; Rich D. Q.; Diehl S. R.; Huang W.; Zhang J. J. Malondialdehyde in exhaled breath condensate and urine as a biomarker of air pollution induced oxidative stress. J. Expos. Sci. Environ. Epidemiol. 2013, 233322–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoek G.; Brunekreef B.; Goldbohm S.; Fischer P.; van den Brandt P. A. Association between mortality and indicators of traffic-related air pollution in the Netherlands: a cohort study. Lancet 2002, 36093411203–9. [DOI] [PubMed] [Google Scholar]
- Pekkanen J.; Kulmala M. Exposure assessment of ultrafine particles in epidemiologic time-series studies. Scand. J. Work, Environ. Health 2004, 30Suppl 29–18. [PubMed] [Google Scholar]
- Hoek G.; Boogaard H.; Knol A.; de Hartog J.; Slottje P.; Ayres J. G.; Borm P.; Brunekreef B.; Donaldson K.; Forastiere F.; Holgate S.; Kreyling W. G.; Nemery B.; Pekkanen J.; Stone V.; Wichmann H. E.; van der Sluijs J. Concentration response functions for ultrafine particles and all-cause mortality and hospital admissions: results of a European expert panel elicitation. Environ. Sci. Technol. 2010, 441476–82. [DOI] [PubMed] [Google Scholar]
- Moore K.; Krudysz M.; Pakbin P.; Hudda N.; Sioutas C. Intra-community variability in total particle number concentrations in the San Pedro Harbor Area (Los Angeles, California). Aerosol Sci. Technol. 2009, 43, 587–603. [Google Scholar]
- Knol A. B.; de Hartog J. J.; Boogaard H.; Slottje P.; van der Sluijs J. P.; Lebret E.; Cassee F. R.; Wardekker J. A.; Ayres J. G.; Borm P. J.; Brunekreef B.; Donaldson K.; Forastiere F.; Holgate S. T.; Kreyling W. G.; Nemery B.; Pekkanen J.; Stone V.; Wichmann H. E.; Hoek G. Expert elicitation on ultrafine particles: likelihood of health effects and causal pathways. Part. Fibre Toxicol. 2009, 6, 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung A.; Herner J. D.; Kleeman M. J. Detection of alkaline ultrafine atmospheric particles at Bakersfield, California. Environ. Sci. Technol. 2001, 35112184–90. [DOI] [PubMed] [Google Scholar]
- Herner J.; Ying Q.; Aw J.; Gao O.; Chang D.; Kleeman M. Dominant mechanisms that shape the airborne particle size and composition distribution in central California. Aerosol Sci. Technol. 2006, 4015827–844. [Google Scholar]
- Möller W.; Felten K.; Sommerer K.; Scheuch G.; Meyer G.; Meyer P.; Haussinger K.; Kreyling W. G. Deposition, retention, and translocation of ultrafine particles from the central airways and lung periphery. Am. J. Respir. Crit. Care Med. 2008, 1774426–32. [DOI] [PubMed] [Google Scholar]
- Kreyling W. G.; Dirscherl P.; Ferron G. A.; Heilmann P.; Josten M.; Miaskowski U.; Neuner M.; Reitmeir P.; Ruprecht L.; Schumann G.; Takenaka S.; Ziesenis A.; Heyder J. Health effects of sulfur-related environmental air pollution. III. Nonspecific respiratory defense capacities. Inhalation Toxicol. 1999, 115391–422. [DOI] [PubMed] [Google Scholar]
- Geiser M.; Rothen-Rutishauser B.; Kapp N.; Schurch S.; Kreyling W.; Schulz H.; Semmler M.; Im Hof V.; Heyder J.; Gehr P. Ultrafine particles cross cellular membranes by nonphagocytic mechanisms in lungs and in cultured cells. Environ. Health Perspect. 2005, 113111555–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapp N.; Kreyling W.; Schulz H.; Im Hof V.; Gehr P.; Semmler M.; Geiser M. Electron energy loss spectroscopy for analysis of inhaled ultrafine particles in rat lungs. Microsc. Res. Tech. 2004, 635298–305. [DOI] [PubMed] [Google Scholar]
- He M. L.; Dhaniyala S. Vertical and horizontal concentration distributions of ultrafine particles near a highway. Atmos. Environ. 2012, 46, 225–236. [Google Scholar]
- Chan C. Y.; Xu X. D.; Li Y. S.; Wong K. H.; Ding G. A.; Chan L. Y.; Cheng X. H. Characteristics of vertical profiles and sources of PM2.5, PM10 and carbonaceous species in Beijing. Atmos. Environ. 2005, 39285113–5124. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.