Abstract
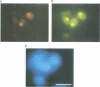
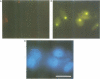
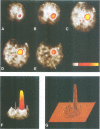
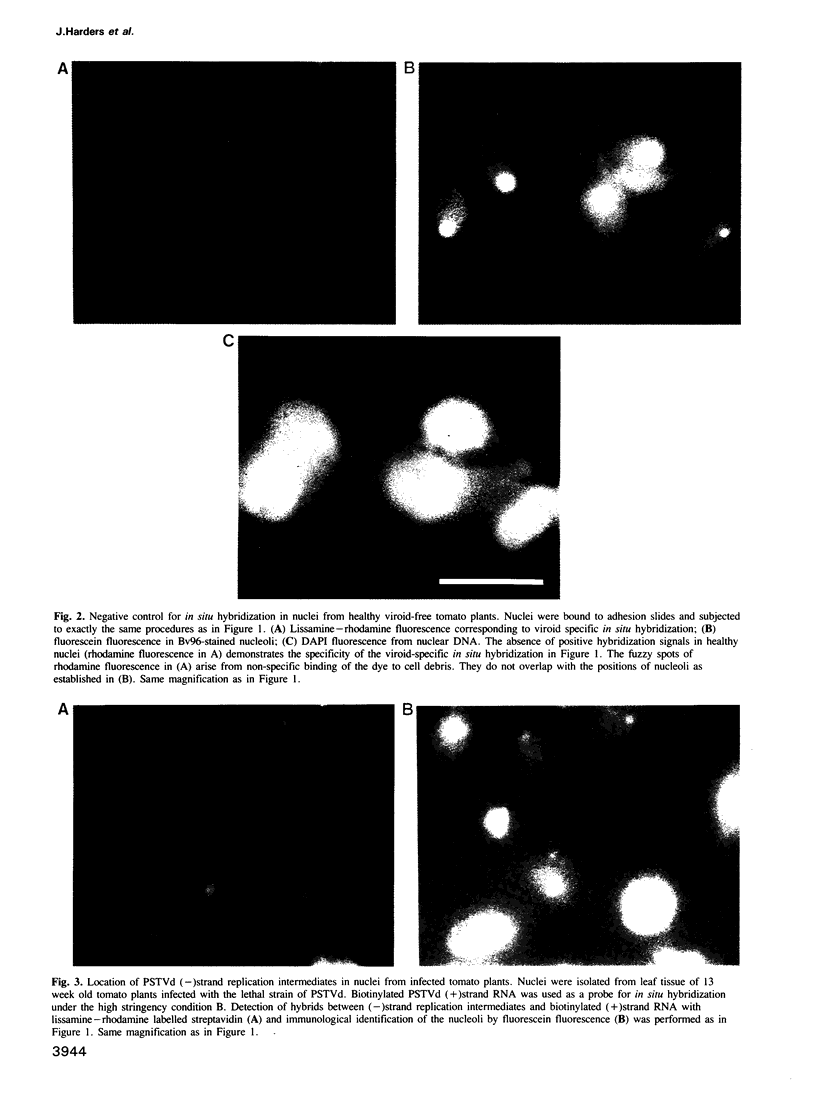
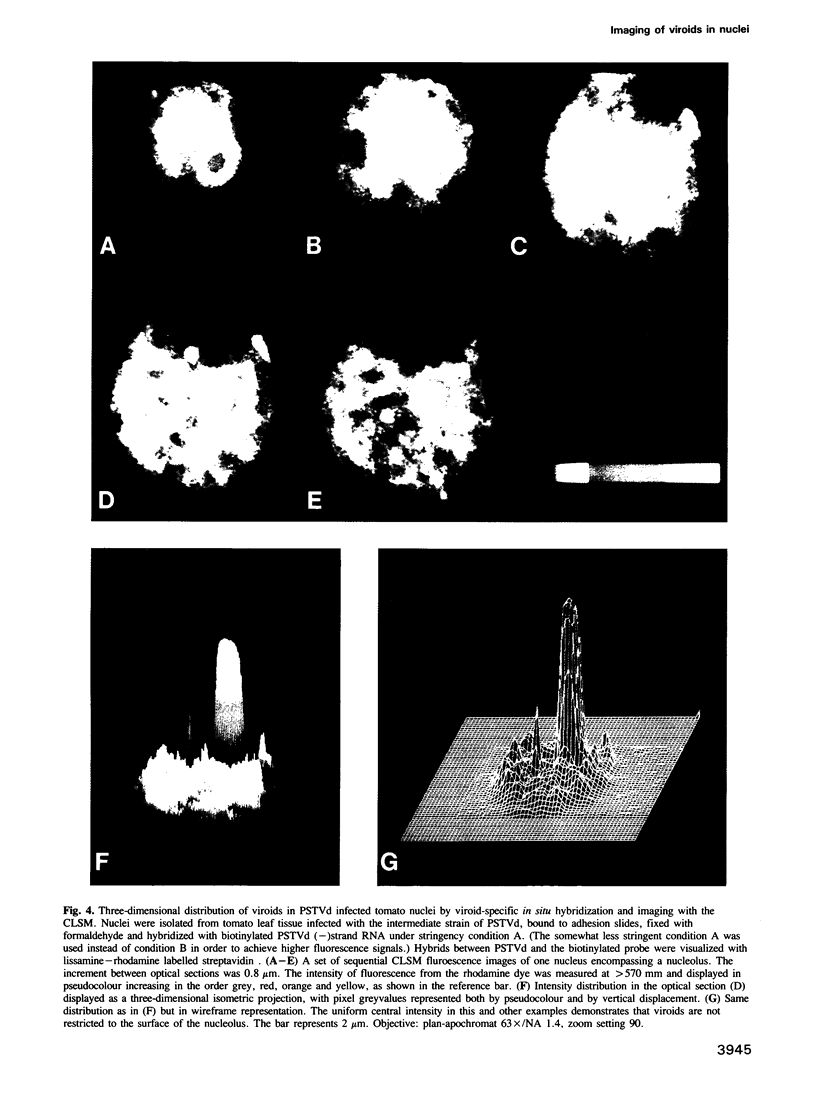
The intracellular localization of viroids has been investigated by viroid-specific in situ hybridization and analysis by digital microscopy of the distribution of the fluorescent hybridization signals. Isolated nuclei from green leaf tissue of tomato plants infected with potato spindle tuber viroid (PSTVd) were bound to microscope slides, fixed with formaldehyde and hybridized with biotinylated transcripts of cloned PSTVd cDNA. The bound probe was detected with lissamine--rhodamine conjugated streptavidin. Nucleoli were identified by immunofluorescence using the monoclonal antibody Bv96 and a secondary FITC-conjugated antibody. In plants infected with either a lethal or an intermediate PSTVd strain, the highest intensity of fluorescence that arose from hybridization with the probe specific for the viroid (+)strand was found in the nucleoli, confirming results of previous fractionation studies. A similar distribution was found for (-)strand replication intermediates of PSTVd using specific (+)strand transcripts as hybridization probes. In order to determine if viroids are located at the surface or in the interior of the nucleoli, the distribution of the fluorescence hybridization signals was studied with a confocal laser scanning microscope (CLSM). It was shown by three-dimensional reconstruction that viroids are neither restricted to the surface of the nucleoli nor to a peripheral zone, but are instead homogeneously distributed throughout the nucleolus. The functional implications of the intranucleolar location of viroids and their replication intermediates are discussed with respect to proposed mechanisms of viroid replication and pathogenesis.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Busch H., Reddy R., Rothblum L., Choi Y. C. SnRNAs, SnRNPs, and RNA processing. Annu Rev Biochem. 1982;51:617–654. doi: 10.1146/annurev.bi.51.070182.003153. [DOI] [PubMed] [Google Scholar]
- Crouch R. J., Kanaya S., Earl P. L. A model for the involvement of the small nucleolar RNA (U3) in processing eukaryotic ribosomal RNA. Mol Biol Rep. 1983 May;9(1-2):75–78. doi: 10.1007/BF00777476. [DOI] [PubMed] [Google Scholar]
- Gröning B. R., Abouzid A., Jeske H. Single-stranded DNA from abutilon mosaic virus is present in the plastids of infected Abutilon sellovianum. Proc Natl Acad Sci U S A. 1987 Dec;84(24):8996–9000. doi: 10.1073/pnas.84.24.8996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas B., Klanner A., Ramm K., Sänger H. L. The 7S RNA from tomato leaf tissue resembles a signal recognition particle RNA and exhibits a remarkable sequence complementarity to viroids. EMBO J. 1988 Dec 20;7(13):4063–4074. doi: 10.1002/j.1460-2075.1988.tb03300.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hecker R., Wang Z. M., Steger G., Riesner D. Analysis of RNA structures by temperature-gradient gel electrophoresis: viroid replication and processing. Gene. 1988 Dec 10;72(1-2):59–74. doi: 10.1016/0378-1119(88)90128-x. [DOI] [PubMed] [Google Scholar]
- Johnson G. D., Nogueira Araujo G. M. A simple method of reducing the fading of immunofluorescence during microscopy. J Immunol Methods. 1981;43(3):349–350. doi: 10.1016/0022-1759(81)90183-6. [DOI] [PubMed] [Google Scholar]
- Jovin T. M., Arndt-Jovin D. J. Luminescence digital imaging microscopy. Annu Rev Biophys Biophys Chem. 1989;18:271–308. doi: 10.1146/annurev.bb.18.060189.001415. [DOI] [PubMed] [Google Scholar]
- Kapuściński J., Yanagi K. Selective staining by 4', 6-diamidine-2-phenylindole of nanogram quantities of DNA in the presence of RNA on gels. Nucleic Acids Res. 1979 Aug 10;6(11):3535–3542. doi: 10.1093/nar/6.11.3535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiss T., Pósfai J., Solymosy F. Sequence homology between potato spindle tuber viroid and U3B snRNA. FEBS Lett. 1983 Nov 14;163(2):217–220. doi: 10.1016/0014-5793(83)80822-9. [DOI] [PubMed] [Google Scholar]
- Kiss T., Tóth M., Solymosy F. Plant small nuclear RNAs. Nucleolar U3 snRNA is present in plants: partial characterization. Eur J Biochem. 1985 Oct 15;152(2):259–266. doi: 10.1111/j.1432-1033.1985.tb09192.x. [DOI] [PubMed] [Google Scholar]
- Parker K. A., Steitz J. A. Structural analysis of the human U3 ribonucleoprotein particle reveal a conserved sequence available for base pairing with pre-rRNA. Mol Cell Biol. 1987 Aug;7(8):2899–2913. doi: 10.1128/mcb.7.8.2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinkel D., Gray J. W., Trask B., van den Engh G., Fuscoe J., van Dekken H. Cytogenetic analysis by in situ hybridization with fluorescently labeled nucleic acid probes. Cold Spring Harb Symp Quant Biol. 1986;51(Pt 1):151–157. doi: 10.1101/sqb.1986.051.01.018. [DOI] [PubMed] [Google Scholar]
- Rackwitz H. R., Rohde W., Sänger H. L. DNA-dependent RNA polymerase II of plant origin transcribes viroid RNA into full-length copies. Nature. 1981 May 28;291(5813):297–301. doi: 10.1038/291297a0. [DOI] [PubMed] [Google Scholar]
- Randles J. W., Rillo E. P., Diener T. O. The viroidlike structure and cellular location of anomalous RNA associated with the cadang-cadang disease. Virology. 1976 Oct 1;74(1):128–139. doi: 10.1016/0042-6822(76)90135-5. [DOI] [PubMed] [Google Scholar]
- Riesner D., Gross H. J. Viroids. Annu Rev Biochem. 1985;54:531–564. doi: 10.1146/annurev.bi.54.070185.002531. [DOI] [PubMed] [Google Scholar]
- Riesner D., Klaff P., Steger G., Hecker R. Viroids. Subcellular location and structure of replicative intermediates. Ann N Y Acad Sci. 1987;503:212–237. doi: 10.1111/j.1749-6632.1987.tb40610.x. [DOI] [PubMed] [Google Scholar]
- Schnölzer M., Haas B., Raam K., Hofmann H., Sänger H. L. Correlation between structure and pathogenicity of potato spindle tuber viroid (PSTV). EMBO J. 1985 Sep;4(9):2181–2190. doi: 10.1002/j.1460-2075.1985.tb03913.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumacher J., Randles J. W., Riesner D. A two-dimensional electrophoretic technique for the detection of circular viroids and virusoids. Anal Biochem. 1983 Dec;135(2):288–295. doi: 10.1016/0003-2697(83)90685-1. [DOI] [PubMed] [Google Scholar]
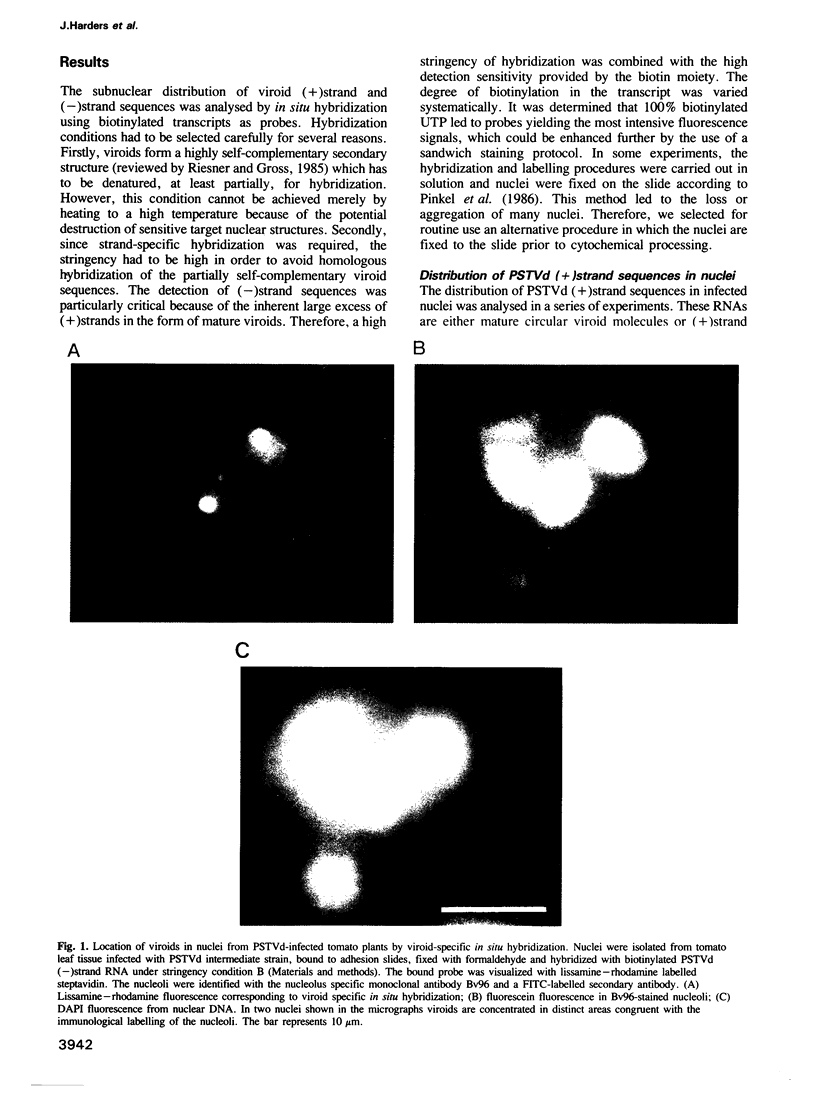
- Schumacher J., Sänger H. L., Riesner D. Subcellular localization of viroids in highly purified nuclei from tomato leaf tissue. EMBO J. 1983;2(9):1549–1555. doi: 10.1002/j.1460-2075.1983.tb01622.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semancik J. S., Tsuruda D., Zaner L., Geelen J. L., Weathers J. G. Exocortis disease: subcellular distribution of pathogenic (viroid) RNA. Virology. 1976 Feb;69(2):669–676. doi: 10.1016/0042-6822(76)90495-5. [DOI] [PubMed] [Google Scholar]
- Spiesmacher E., Mühlbach H. P., Schnölzer M., Haas B., Sänger H. L. Oligomeric forms of potato spindle tuber viroid (PSTV) and of its complementary RNA are present in nuclei isolated from viroid-infected potato cells. Biosci Rep. 1983 Aug;3(8):767–774. doi: 10.1007/BF01120988. [DOI] [PubMed] [Google Scholar]
- Steger G., Müller H., Riesner D. Helix-coil transitions in double-stranded viral RNA. Fine resolution melting and ionic strength dependence. Biochim Biophys Acta. 1980 Feb 29;606(2):274–284. doi: 10.1016/0005-2787(80)90037-4. [DOI] [PubMed] [Google Scholar]
- Steger G., Tabler M., Brüggemann W., Colpan M., Klotz G., Sänger H. L., Riesner D. Structure of viroid replicative intermediates: physico-chemical studies on SP6 transcripts of cloned oligomeric potato spindle tuber viroid. Nucleic Acids Res. 1986 Dec 22;14(24):9613–9630. doi: 10.1093/nar/14.24.9613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi T., Diener T. O. Potato spindle tuber viroid. XIV. Replication in nuclei isolated from infected leaves. Virology. 1975 Mar;64(1):106–114. doi: 10.1016/0042-6822(75)90083-5. [DOI] [PubMed] [Google Scholar]
- Theissen G., Richter A., Lukacs N. Degree of biotinylation in nucleic acids estimated by a gel retardation assay. Anal Biochem. 1989 May 15;179(1):98–105. doi: 10.1016/0003-2697(89)90207-8. [DOI] [PubMed] [Google Scholar]