Abstract
Research on the “emotional brain” remains centered around the idea that emotions like fear, happiness, and sadness result from specialized and distinct neural circuitry. Accumulating behavioral and physiological evidence suggests, instead, that emotions are grounded in core affect – a person's fluctuating level of pleasant or unpleasant arousal. A neuroimaging study revealed that participants' subjective ratings of valence (i.e., pleasure/displeasure) and of arousal evoked by various fear, happiness, and sadness experiences correlated with neural activity in specific brain regions (orbitofrontal cortex and amygdala, respectively). We observed these correlations across diverse instances within each emotion category, as well as across instances from all three categories. Consistent with a psychological construction approach to emotion, the results suggest that neural circuitry realizes more basic processes across discrete emotions. The implicated brain regions regulate the body to deal with the world, producing the affective changes at the core of emotions and many other psychological phenomena.
Emotion research focuses predominantly on the idea that a limited number of emotions (e.g., fear, sadness, happiness, anger, disgust) are psychologically and biologically basic (e.g., Ekman, 1999). This view is widespread in psychology, providing inspiration for everything from psychopathology interventions to popular television shows. Yet recent reviews of accumulating behavioral, psychophysiological, and neural evidence question this theoretical perspective (e.g., Barrett, 2006; Lindquist, Wager, Kober, Bliss-Moreau, & Barrett, 2012). An emerging alternative view is that diverse human emotions result from the interplay of more basic “ingredients,” namely, domain-general processes that contribute to many psychological phenomena (including discrete emotions) (e.g., Barrett, 2009a). One such ingredient in this “psychological construction” approach is core affect (Barrett & Bliss-Moreau, 2009), characterized as simple feelings of valence and arousal (Russell, 2003; Wundt, 1987). Here, we present neural evidence that sadness, fear, and happiness experiences share core affective properties.
The hypothesis that emotions are grounded in continuous and fluctuating affective states described as pleasant or unpleasant with some level of arousal is as old as psychological science itself (c.f. Wundt, 1897). Recent formulations of this hypothesis refer to these states as “core affect” (Russell, 2003; Russell & Barrett, 1999) because they arise in the core of the body (or representations of body state change). Core affect is detectable in the face (Cacioppo, Berntson, Larsen, Poehlmann, & Ito, 2000), in the voice (Russell, Bachorowski, & Fernandez-Dols, 2003), in peripheral nervous system activation (Cacioppo et al., 2000), and in reports of subjective experience (Barrett, 2004). The capacity to experience core affect is psychologically universal (Mesquita, 2003; Russell, 1991) and present in infants (Lewis, 2000), although many of the sensory patterns that predict pleasure and pain are learned through experience. Physiologists, neuroscientists, and economists alike consider core affect a common mental currency that underlies decision-making, choice, and action (Cabanac, 2002; Damasio, 1999; Grabenhorst & Rolls, 2011).
Amidst this progress in understanding the nature and functions of core affect, understanding its exact relation to the experience of emotion remains limited by a key assumption. Studies often confound core affect and emotion by assuming that each emotion category is associated with a specific core affective state: fear is unpleasant and highly arousing, sadness is unpleasant and less arousing, happiness is pleasant and less arousing. Yet, the core affective feelings evoked during an emotion depend on the situation (and how the situation is conceptualized): fear can be pleasant and highly arousing when rocketing downwards in a rollercoaster car, unpleasant and less arousing when detecting the first bodily signs of the flu, and so on (Barrett, 2009b; Wilson-Mendenhall, Barrett, Simmons, & Barsalou, 2011). In this study, we assessed the relationship between each individual's core affective ratings and their brain activity within, as well as across, the emotion categories fear, happiness, and sadness. Capitalizing on the normal variability in everyday life emotion experiences, we developed familiar scenarios for each emotion category that systematically varied in valence and arousal. Although fear, happiness, and sadness are typically studied as either unpleasant or pleasant (and sometimes as either high or low arousal), we created both unpleasant and pleasant scenarios that varied in arousal within each emotion category. This novel stimulus set included scenarios describing the pleasant fear of risk-taking, the pleasant sadness of nostalgia, and the unpleasant happiness of relief. Thus, we investigated core affect as a dynamic ingredient of emotional experience that varies within an emotion category (i.e., fear can be pleasant or unpleasant; more or less arousing) instead of a one-to-one description of the category (i.e., fear is unpleasant, highly arousing).
Manipulating core affect within each emotion category, we examined whether the affective feelings evoked by diverse instances of fear, happiness, and sadness are grounded in a common neural system. We first predicted that the experience of valence (i.e., individuals' ratings of felt pleasure/displeasure) varying across the fear, happiness, and sadness scenarios would correlate with activity in orbitofrontal cortex (OFC), a region implicated in many studies of reward and value (for reviews see Grabenhorst & Rolls, 2011; Kringelbach & Rolls, 2004). Critically, we further predicted that this correlation between valence ratings and OFC activity would be observed within each emotion category, which we could examine because we designed the scenarios in each category to evoke emotional experiences varying in hedonic valence. Our second prediction was that the experience of arousal varying across the fear, happiness, and sadness scenarios would correlate with activity in the amygdala, a region implicated in detecting and coordinating responses to motivationally salient positive and negative events (for reviews see Costafreda, Brammer, David, & Fu, 2008; Lindquist et al., 2012). We further predicted that this correlation between arousal ratings and amygdala activity would be observed within each emotion category, which we could examine because we designed the scenarios in each category to evoke emotional experiences varying in arousal. As part of a network that represents and regulates the body, orbitofrontal cortex and the amygdala, specifically, are uniquely positioned to coordinate bodily responses dynamically as interpretations of the external world unfold (Barrett & Bliss-Moreau, 2009).
Method
Participants
Sixteen right-handed, native English speakers ranging in age from 19-30 (8 female) received $100 in compensation. Participants had no history of psychiatric illness and were not taking psychotropic medication.
Neuroimaging Design
The fMRI paradigm was designed to evoke affective feelings through immersion in scenarios depicting real-world fear, happiness, and sadness experiences (Wilson-Mendenhall et al., 2011). Emotion induction techniques that draw on the imagination are powerful, often producing changes in cognition, experience, behavior, and physiology that rival real life manipulations (for a review see Lench, Flores, & Bench, 2011). Furthermore, the neural overlap observed during imagery and perception suggests the brain easily emulates how it feels to experience events in the real world (e.g., Kosslyn, Ganis, & Thompson, 2001).
We included two critical trial types in our design to separate neural activity associated with the emotion induction process from neural activity associated with the affect evoked during the emotion (Fig. S1A). In 144 complete trials, participants first immersed in a scenario designed to induce fear, happiness, or sadness (i.e., scenario immersion), and then focused on and rated the valence or arousal quality of the evoked feeling (i.e., valence/arousal focus). We instructed participants to focus on their internal feeling state before rating it because empirical evidence shows that attention enhances sensory detection and discrimination (Chun, Golomb, & Turk-Browne, 2011). In 36 partial trials (the second type of trial), participants immersed in a scenario, but did not focus on or rate their affective experience. The unpredictable partial trials were critical for mathematically separating neural activity during scenario immersion events (that occurred on both complete and partial trials) from neural activity during the subsequent valence/arousal focus events (that only occurred on partial trials) (Ollinger, Corbetta, & Shulman, 2001; Ollinger, Shulman, & Corbetta, 2001). Because our hypotheses concerned the core affective feelings evoked during the emotion, all brain activations reported here reflect brain activity during the focus events that occurred once the emotion was induced.
In each of the six runs in the neuroimaging experiment, complete and partial trials were presented from six critical conditions created by crossing affective dimension (valence, arousal) with emotion category (fear, happiness, sadness). To encourage swift immersion and to facilitate focusing on a specific affective dimension of the emotional experience, trials were blocked by valence and by arousal (i.e., during valence blocks, participants focused on and rated valence, and during arousal blocks, they focused on and rated arousal). One arousal block and one valence block was presented in each run, with block order counterbalanced across runs (illustrated in Fig. S1B and described further in the Supplemental Online Material (SOM)). Within each block, four complete trials per category and one partial trial per category were presented in an optimized pseudo-random order amidst jittered baseline periods (ranging from 3-15 s in increments of 3 with average ISI = 6.3 s; optimized using optseq2 software).
Materials
During training sessions and during the scan session, participants listened to scenarios designed to induce fear, sadness, and happiness (see Table 1). A full paragraph-long form of each scenario provided a richly detailed and affectively compelling description of an event inducing fear, sadness, or happiness to guide vivid immersion during training sessions. A corresponding core form of each scenario served to minimize presentation time in the scanner so the number of trials necessary for a powerful design could be implemented (Table 1 displays the shorter, core form in italics). In both forms, scenarios were explicitly categorized as fear, sadness, or happiness to avoid ambiguity.
Table 1.
Example fear, happiness, and sadness scenarios in each valence × arousal quadrant. Italics signify the core form of the scenario presented during the fMRI session.
| High Arousal | Low Arousal | |
|---|---|---|
| Pleasant | You are sitting stiffly in a rollercoaster car, creeping up one click at a time. You reach the peak of the hill and are suddenly whizzing downwards. Your heart is pounding and your stomach drops as crisp air blasts your face. You delight in the uncontrollable rush dipping and swirling high above the ground. You feel an invigorating fear. | You are sipping punch at a school reunion, scanning the growing crowd. You notice your high school crush from across the room returning your gaze. Your crush looks away and you smile to yourself in the private moment. A soft amusement begins to arise as your mind becomes lost in a familiar fantasy. You feel a lovely fear. |
| You are performing a challenging piano solo, your fingers working the keys. You finish the piece and receive thunderous applause as you rise. You bend at the waist into a deep bow and sense your heart thumping rapidly. Glowing with satisfaction, you continue to feed off the crowd's energy. You feel a proud happiness. | You are lounging on a cushy floor pillow, opening a new magazine. You glance up as your puppy trots over and wiggles into your lap. As her small body relaxes, you sense both your hearts beating evenly. Tenderly petting her soft fur cultivates a lovely sense of ease. You feel an affectionate happiness. | |
| You are running in a charity race, your first time covering this long a distance. You see the finish line and remember your aunt's lost battle with cancer. Covered in sweat and heart pumping, you pick up your pace. The cheerful chanting ahead instills an overwhelming sense of courage. You feel a beneficial sadness. | You are inching under the sheets, slowly getting settled at the late hour. You long for a good night's sleep after spending all your waking hours working. You sense your stiff neck relax as you rest your head on a pillow. You curl up and let go of the day, finally a moment of lovely calm. You feel a peaceful sadness. | |
| Unpleasant | You are walking to your car alone, the city parking deck dimly lit. You hear an explosive bang and see a man running with a pointed gun. You quickly drop behind a car and attempt to control your shallow breathing. You try to dismiss the horrendous vision of what will happen if he finds you. You feel a perilous fear. | You are sitting down after lunch out, your desktop reappearing at your touch. You notice a pressing e-mail from your boss that you forgot to address. Taking a deep breath, you lengthen your spine in an attempt to reenergize. You slowly re-read the message with the burden of responding quickly. You feel an inconvenient fear. |
| You are walking down the hall, trying to get to a meeting on time. You run into a difficult colleague and end a tense exchange with a biting remark. Your stomach tightens the moment the last sarcastic jab escapes your lips. The cutting retort echoes poisonously in your head as your colleague sulks away. You feel a disturbing happiness. | You are rocking in your favorite chair, gently flipping your cell phone open and closed. You want to share a recent promotion with your brother who is unavailable overseas. Wishing you could call him, you close your eyes and release a held breath. You continue fiddling with your phone, a tender solitude clouding your mind. You feel a lonely happiness. | |
| You are walking into a friend's house, dropping by to return a movie. You witness your significant other in an intimate embrace with your friend. Your stomach is nauseated, the shocking infidelity settling into your body. Your mind is spinning trying to understand the terrible betrayal of trust. You feel a devastating sadness. | You are sitting at the table, spooning a heap of food on your plate. You taste the casserole made from a new recipe and are disappointed. Setting down your fork momentarily, you hear your stomach quietly rumbling. You look at your plate and avoid taking another bite of disagreeable blandness. You feel a dissatisfied sadness. | |
To vary the core affective properties as much as possible within each emotion category, scenarios were developed to evoke typical valence (i.e., unpleasant fear, pleasant happiness, and unpleasant sadness) and atypical valence (i.e., pleasant fear, unpleasant happiness, and pleasant sadness) (see Table 1). The atypical scenarios described familiar experiences such as the pleasant fear involved in zooming downwards on a rollercoaster or encountering a secret crush, the pleasant sadness involved in inspiring others through one's own loss or unwinding after sacrificing the evening to work, and the unpleasant happiness involved in confronting a surly colleague or being unable to share good news. Ratings collected during the training sessions described in the procedure validated that the emotions induced by the typical and atypical scenarios were familiar and relatively easy to imagine from a first-person perspective (Fig. S2). Variation in arousal was similarly introduced through the event itself and through vivid descriptions of actions and physiological reactions. Please see the SOM for details on the construction and selection of scenarios and for the complete stimulus set.
Procedure
The experiment consisted of two training sessions and an fMRI scan session. The first training session occurred 24-48 hours before the second training session, which occurred just prior to the scan session (Fig. S1C). The training sessions were designed to give participants practice a) vividly imagining the full versions of the scenarios they would hear later in the scanner or during practice trials b) reinstating the rich imagery of each full scenario upon hearing the core version, and c) focusing on and rating the valence or arousal quality of the feeling state induced by a scenario. During the first training session, participants listened to the full versions of the scenarios, immersing themselves with eyes closed, and rated their personal familiarity with each induced emotion. After a short break, they listened to the core versions of the same scenarios, reinstating imagined details from the full versions, and then rated the internal, external, and thought imagery experienced (further encouraging immersion in the imagined scenarios).
When participants returned to the lab 24-48 hours later, they began the second session by listening to and vividly imagining each full scenario again. Participants provided one rating of how much they experienced “being there” immersed in the feeling of fear, happiness, or sadness described in the scenario. Imagining the full versions in the second session ensured that participants were reacquainted with scenario details just prior to hearing the core versions during the scan session.
Participants then practiced the task that they would perform in the scanner, using scenarios that were not included in the critical scans. Participants were informed that one block of valence trials and one block of arousal trials would occur in each imaging run (with the cue word 'valence' or `arousal' indicating the block start). They then practiced each trial type, beginning with 15 s complete trials. During complete trials, participants were instructed to immerse themselves fully as they listened to a core version of a scenario lasting no longer than 8 s. A 1 s `beep, beep, beep' that followed indicated that immersion in the emotional experience should continue as the participant centered in on the valence or arousal quality of the feeling (depending on the block), maintaining focus for 3 s. Finally, a 1 s cowbell cued participants to rate their introspective sense of valence or arousal within the next 2 s using the appropriate scale. At this point, participants had received much practice using the 5-point valence and arousal scales with their eyes closed. During 9 s partial trials, participants heard a 1 s `whoosh' sound when the 8 s scenario concluded, which signified the end of the trial. During baseline rest trials, participants cleared their mind during the 3-15 s period of no sound as they waited to hear the next scenario begin. Participants then practiced several short arousal and valence blocks with all trial types intermixed, similar to the critical scans. Detailed descriptions of all training procedures are provided in the SOM.
Imaging and Analysis
Images were collected at the Emory Biomedical Imaging Technology Center on a 3T Siemens Trio scanner and preprocessed using standard methods in AFNI (Cox, 1996) (see the SOM for details). Two critical regression analyses were performed on each participant's preprocessed data that used canonical Gamma functions convolved with boxcars reflecting event duration to model the hemodynamic response. In the first analysis, the onset times were specified for five conditions: cues beginning each block, scenario events during valence blocks, scenario events during arousal blocks, focus events during valence blocks, and focus events during arousal blocks. Scenario events included the 9 s during which participants immersed in the scenario and heard the brief auditory cue that followed in complete and partial trials. Modeling the scenarios in complete and partial trials as a single condition allowed for the mathematical separation of the scenario events from the focus events in complete trials. The focus events included the 6 s during which participants focused on and rated the valence or arousal quality of the evoked feeling.
Each participant's valence ratings were specified trial-by-trial in the valence focus condition, and his or her arousal ratings specified trial-by-trial in the arousal focus condition. The following numerical codes were used for valence (1-very unpleasant 2-somewhat unpleasant 3-neutral 4-somewhat pleasant 5-very pleasant) and arousal (1-low 2-medium-low 3-medium 4-medium-high 5-high). Any missing rating was replaced with the mean rating (1% of trials on average). For the focus conditions, both the onset times and ratings were entered into the regression using the amplitude modulation option in AFNI. This option specified two regressors for each focus condition, which were used to detect: 1) voxels in which activity was correlated with the ratings (also known as a parametric regressor); 2) voxels in which activity was constant for the condition and was not correlated with the ratings.
Each participant's betas produced from the first parametric regressor for the focus conditions (i.e., indicating the strength of the correlation with valence or arousal ratings) were next entered into a random effects group analysis. In this analysis, the critical statistic for each condition was a t test indicating if the mean across subjects differed significantly from zero (zero indicating no correlation between brain activity and the ratings). To test our regional hypotheses, the group analysis was computed within anatomical masks of orbitofrontal cortex (OFC) and of the amygdala (Eickhoff et al., 2005). A voxel-wise threshold of p < .005 was used in conjunction with an extent threshold that produced a p < .05 corrected threshold within each mask (12 voxels for medial OFC, 9 voxels for lateral OFC, 3 voxels for amygdala).
Any significant cluster identified in the first analysis was used to mask a second analysis, which analyzed the emotion categories separately. The critical difference from the first analysis was that each scenario and focus condition was split into three conditions for the emotion categories fear, happiness, and sadness. Otherwise the analysis was exactly the same. Table S1 provides descriptive statistics for the valence and arousal ratings by emotion category. Participant betas produced from the parametric regressors for the six focus conditions (i.e., fear-valence, happiness-valence, sadness-valence, fear-arousal, happiness-arousal, sadness-arousal) were then entered into a random effects group analysis in an identical manner to the first analysis. At the group level, voxel-wise t statistics representing significant correlations with either valence or arousal for each category (p < .05) were entered into a conjunction analysis. The conjunction was only computed within clusters identified in the first analysis to determine if these voxels were significantly correlated with valence or arousal in each emotion category. This key analysis allowed us to examine whether each voxel that correlated with valence or arousal in the first analysis, which was conducted across categories, was correlated with valence or arousal in one or more emotion categories when each category was modeled separately.
Results
Valence
We predicted, and found, that neural activity in orbitofrontal cortex (OFC) correlated with ratings of subjective valence both across and within the three emotion categories. Activity in medial OFC significantly correlated with valence ratings when we collapsed across all fear, happiness, and sadness scenarios (p < .005, peak -2 38 -13, 24 voxels).1 Illustrated in Figure 1A, activity in this medial OFC cluster increased as the unpleasantness that participants experienced decreased and pleasantness increased (i.e., activity was positively correlated with the bipolar valence scale in which 1 = very unpleasant, 3 = neutral, 5 = very pleasant).
Figure 1.
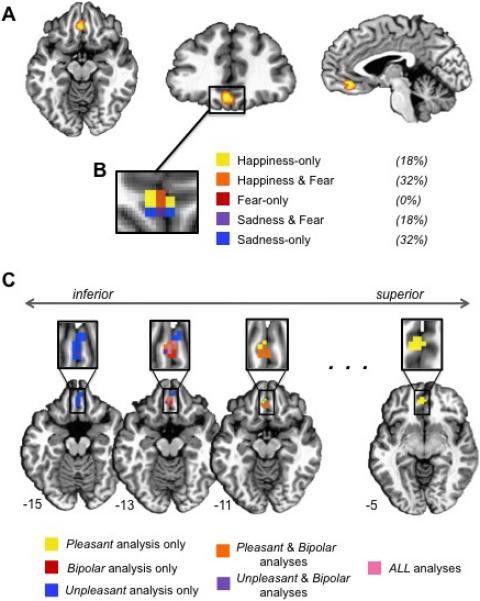
A) The medial OFC cluster that correlated positively with bipolar valence ratings across categories is shown in three views: axial (z = -11), coronal (y = 34), and sagittal (x = -2). B) The magnified box shows the within-category results (i.e., correlations between valence ratings and OFC activity within each emotion category). The percentages indicate the proportion of voxels that correlated with valence ratings in one or more categories across the entire 3D cluster (not just the 2D slice displayed). C) A conjunction analysis of the three medial OFC clusters observed in the unipolar-focused and bipolar valence analyses (across categories) is displayed on axial slices, from inferior to superior. The ellipsis signifies that axial slices between -11 and -5 look very similar to the -11 slice shown.
Remarkably, this correlation held within each emotion category when the three emotion categories were modeled independently (p < .05 for each category). Within the medial OFC cluster identified from the correlation across categories, 92% of the voxels showed a significant correlation with the valence ratings of at least one emotion category, with 50% of the voxels correlating with valence ratings in more than one emotion category (Fig. 1B). Taken together, these results show that as activity changes in medial OFC, so does the subjective experience of valence (i.e., pleasure/displeasure) during all three emotions. Because this result was independently observed within three emotion categories, our findings suggest that valence is a basic property of human emotional experience.
Although qualitatively different systems supporting positive and negative evaluation are often postulated (e.g., Cacioppo, Gardner, & Berntson, 1997), other theorists have emphasized that multiple sources of value information must be compared and integrated for action selection (e.g., Barrett & Bliss-Moreau, 2009; Cabanac, 2002). To determine if medial OFC activity was driven by positive affect, negative affect, or both, we recoded the ratings to reflect unipolar scales, one weighted for pleasant intensity (2-very pleasant 1-somewhat pleasant 0-neutral 0-somewhat unpleasant 0-very unpleasant) and the other weighted for unpleasant intensity (2-very unpleasant 1-somewhat unpleasant 0-neutral 0-somewhat pleasant 0-very pleasant). Correlations in medial OFC were observed for both unipolar codings in the same direction as the original bipolar coding, with activity increasing as participants experienced more pleasantness (i.e., positive correlation with the pleasant intensity scale; p < .005, peak -2 44 -4, 36 voxels) and less unpleasantness (i.e., negative correlation with the unpleasant intensity scale; p < .005, peak -2 32 -16, 19 voxels). Figure 1C illustrates differences that emerged, however, in the spatial location of the correlations within medial OFC: ratings reflecting the pleasant weighting correlated with activity in the superior aspect of medial OFC, whereas ratings reflecting the unpleasant weighting correlated with activity in the inferior aspect of medial OFC. Figure 1C also illustrates that the original bipolar ratings correlate with activity that overlaps the unipolar clusters centrally. Animal work has revealed somewhat similar valence gradients in subcortical structures tightly coupled with action (e.g., bivalent rostrocaudal gradients in the nucleus accumbens shell) (Reynolds & Berridge, 2002). To our knowledge, this is the first time such an inferior-superior cortical gradient for affective valence has been identified in humans.
Arousal
We predicted, and found, that neural activity in the amygdala correlated with subjective arousal ratings both across and within the three emotion categories. Activity in left amygdala significantly correlated with arousal ratings when we collapsed across all fear, sadness, and happiness scenarios (p < .005, peak -23 -2 -10, 6 voxels).2 Illustrated in Figure 2A, activity in this amygdala cluster increased as subjective arousal experiences became more intense. This correlation held within the sadness and happiness categories (but not within fear) when each category was modeled independently (p < .05 for each category; Fig. 2B). Although the arousal ratings varied substantially within each category (see Table S1), arousal ratings for scenarios inducing fear varied less than the arousal ratings for scenarios inducing happiness or sadness (Levene's test p < .05), with fear scenarios rated more arousing on average (M = 4.13) than happiness scenarios (M = 3.40) or sadness scenarios (M = 3.38). We addressed this restriction of range within the fear category by conducting a follow-up analysis that split each category into a (relatively) high and low arousal condition (see the SOM for details). As Figure 2C illustrates, significantly greater left amygdala activity was observed in the high versus low arousal condition for fear, and for the other emotion categories (p < .05). Taken together, these analyses show that as activity changes in left amygdala, so does the subjective experience of arousal during all three emotions. Because this result was independently observed within three emotion categories, our findings suggest that arousal is a basic property of human emotional experience.
Figure 2.
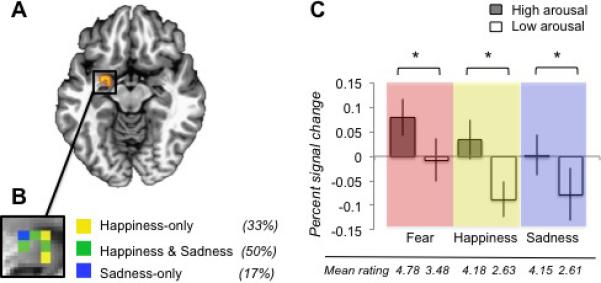
A) The left amygdala cluster that correlated positively with arousal ratings across categories is shown (z = -10). B) The magnified box shows the within-category results, with percentages indicating the proportion of voxels that correlated with arousal ratings in one or more categories across the entire 3D cluster (not just the 2D slice displayed). C) The mean participant betas (in percent signal change) are shown for the high and low arousal conditions of each category, with a star (*) indicating a significant difference (p < .05). The mean behavioral rating for the high and low conditions of each category is provided below the graph.
Discussion
Our results support the century-old scientific hypothesis that core affect is a common building block of emotion experience, showing that subjective ratings of core affect correlate with brain activity both within and across emotion categories. The valence (pleasure or displeasure) and arousal that participants experienced during varied instances of fear, sadness, and happiness correlated with neural activity in medial orbitofrontal cortex and left amygdala, respectively. These brain regions are highly connected structures that have continual access to information about the state of the body and the state of the world, thereby able to influence the body in relation to what is necessary to deal with the world (Barrett & Bliss-Moreau, 2009). Integrating external sensory information with internal homeostatic and interoceptive information, in the context of prior experience, is vital not only for safely navigating the physical and social environment, but also for creating richly textured subjective experiences.
Consistent with the idea that core affect is a basic “ingredient” of many psychological phenomena, the affect experienced during discrete emotions in our study shares neural correlates with the affect experienced during simple sensations. Investigations of the affect-inducing properties of taste, smell, touch, and temperature have revealed activity in orbitofrontal cortex and amygdala, among other connected regions, that varies with the valence and intensity of sensory stimuli (for reviews see Kringelbach & Rolls, 2004; Rolls, 2010). To date, the findings among studies examining the valence and arousal properties of more complex stimuli such as faces (Gerber et al., 2008), scenes (Anders, Eippert, Weiskopf, & Veit, 2008; Anders, Lotze, Erb, Grodd, & Birbaumer, 2004), sounds (Anders et al., 2008), and words or phrases (Colibazzi et al., 2010; Lewis, Critchley, Rotshtein, & Dolan, 2007; Posner et al., 2009) have been less consistent. Because our experiment addressed several methodological challenges (by using rich scenarios to induce familiar emotion experiences, collecting online ratings to avoid memory confounds, and measuring brain activity once the emotion was induced), it is significant that this study produced results consistent with studies of sensory affect. It will be important for future work to examine if these effects replicate for other emotion categories and in larger samples.
In contrast to studying the discreteness of five or so emotions, our results support another theoretical approach – studying the fundamental neural processes that underlie a wide variety of emotions (Barrett, 2009a). We propose that this psychological construction view, which is consistent with a number of emerging scientific models of emotion (e.g., Clore & Ortony, 2008; Coan, 2010; Cunningham & Zelazo, 2007), has much to contribute to psychological science.
Supplementary Material
Acknowledgments
Preparation of this manuscript was supported by an NIH Director's Pioneer Award DPI OD003312 to Lisa Feldman Barrett at Northeastern University with a sub-contract to Lawrence Barsalou at Emory University. We thank Lynne Nygaard for her assistance and guidance in recording the scenarios. We thank Yesenia Mares, Sarah Brown, Robert Smith, and the Emory Biomedical Imaging Technology Center for assistance with data collection. Finally, thanks to L. Nygaard, S. Hamann, and K. Wallen for their comments on an earlier version of this manuscript.
Footnotes
Whole-brain analyses revealed that no other cluster showed a significant correlation with the valence ratings (p < .05 corrected at a voxel-wise threshold of p < .005 and cluster threshold of 36 voxels).
Whole-brain analyses revealed two additional clusters in visual cortex that exhibited positive correlations with participant arousal ratings (p < .05 corrected at a voxel-wise threshold of p < .005 and cluster threshold of 36 voxels) (see Table S2). The amygdala is strongly connected with visual cortex (Amaral, Behniea, & Kelly, 2003), which may explain the heightened activity there.
References
- Amaral DG, Behniea H, Kelly JL. Topographic organization of projections from the amygdala to the visual cortex in the macaque monkey. Neuroscience. 2003;118(4):1099–1120. doi: 10.1016/s0306-4522(02)01001-1. [DOI] [PubMed] [Google Scholar]
- Anders S, Eippert F, Weiskopf N, Veit R. The human amygdala is sensitive to the valence of pictures and sounds irrespective of arousal: an fMRI study. Soc Cogn Affect Neurosci. 2008;3(3):233–243. doi: 10.1093/scan/nsn017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anders S, Lotze M, Erb M, Grodd W, Birbaumer N. Brain activity underlying emotional valence and arousal: a response-related fMRI study. Hum Brain Mapp. 2004;23(4):200–209. doi: 10.1002/hbm.20048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett LF. Feelings or words? Understanding the content in self-report ratings of experienced emotion. J Pers Soc Psychol. 2004;87(2):266–281. doi: 10.1037/0022-3514.87.2.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett LF. The Future of Psychology: Connecting Mind to Brain. Perspect Psychol Sci. 2009a;4(4):326–339. doi: 10.1111/j.1745-6924.2009.01134.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett LF. Variety is the spice of life: A psychological construction approach to understanding variability in emotion. Cogn Emot. 2009b;23(7):1284–1306. doi: 10.1080/02699930902985894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett LF, Bliss-Moreau E. Affect as a Psychological Primitive. Adv Exp Soc Psychol. 2009;41:167–218. doi: 10.1016/S0065-2601(08)00404-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabanac M. What is emotion? Behav Processes. 2002;60(2):69–83. doi: 10.1016/s0376-6357(02)00078-5. [DOI] [PubMed] [Google Scholar]
- Cacioppo JT, Berntson GG, Larsen JH, Poehlmann KM, Ito TA. The psychophysiology of emotion. In: Lewis R, Haviland-Jones JM, editors. The handbook of emotion. 2 ed. Guilford Press; New York: 2000. pp. 173–191. [Google Scholar]
- Cacioppo JT, Gardner WL, Berntson GG. Beyond bipolar conceptualizations and measures: the case of attitudes and evaluative space. Pers Soc Psychol Rev. 1997;1(1):3–25. doi: 10.1207/s15327957pspr0101_2. [DOI] [PubMed] [Google Scholar]
- Chun MM, Golomb JD, Turk-Browne NB. A taxonomy of external and internal attention. Annu Rev Psychol. 2011;62:73–101. doi: 10.1146/annurev.psych.093008.100427. [DOI] [PubMed] [Google Scholar]
- Clore GL, Ortony A. Appraisal theories: How cognition shapes affect into emotion. In: Lewis M, Haviland-Jones JM, Barrett LF, editors. Handbook of emotions. 3rd ed. Guilford Press; New York: 2008. pp. 628–642. [Google Scholar]
- Coan JA. Emergent ghosts of the emotion machine. Emot Rev. 2010;2(3):274–285. [Google Scholar]
- Colibazzi T, Posner J, Wang Z, Gorman D, Gerber A, Yu S, et al. Neural systems subserving valence and arousal during the experience of induced emotions. Emotion. 2010;10(3):377–389. doi: 10.1037/a0018484. [DOI] [PubMed] [Google Scholar]
- Costafreda SG, Brammer MJ, David AS, Fu CH. Predictors of amygdala activation during the processing of emotional stimuli: a meta-analysis of 385 PET and fMRI studies. Brain Res Rev. 2008;58(1):57–70. doi: 10.1016/j.brainresrev.2007.10.012. [DOI] [PubMed] [Google Scholar]
- Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29(3):162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Cunningham WA, Zelazo PD. Attitudes and evaluations: a social cognitive neuroscience perspective. Trends Cogn Sci. 2007;11(3):97–104. doi: 10.1016/j.tics.2006.12.005. [DOI] [PubMed] [Google Scholar]
- Damasio AR. How the brain creates the mind. Scientific American. 1999;281(6):112–117. doi: 10.1038/scientificamerican1299-112. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Stephan KE, Mohlberg H, Grefkes C, Fink GR, Amunts K, et al. A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. Neuroimage. 2005;25(4):1325–1335. doi: 10.1016/j.neuroimage.2004.12.034. [DOI] [PubMed] [Google Scholar]
- Ekman P. Basic Emotions. In: Dalgleish T, Power T, editors. Handbook of cognition and emotion. Wiley; New York: 1999. pp. 45–60. [Google Scholar]
- Gerber AJ, Posner J, Gorman D, Colibazzi T, Yu S, Wang Z, et al. An affective circumplex model of neural systems subserving valence, arousal, and cognitive overlay during the appraisal of emotional faces. Neuropsychologia. 2008;46(8):2129–2139. doi: 10.1016/j.neuropsychologia.2008.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabenhorst F, Rolls ET. Value, pleasure and choice in the ventral prefrontal cortex. Trends Cogn Sci. 2011;15(2):56–67. doi: 10.1016/j.tics.2010.12.004. [DOI] [PubMed] [Google Scholar]
- Kosslyn SM, Ganis G, Thompson WL. Neural foundations of imagery. Nat Rev Neurosci. 2001;2(9):635–42. doi: 10.1038/35090055. [DOI] [PubMed] [Google Scholar]
- Kringelbach ML, Rolls ET. The functional neuroanatomy of the human orbitofrontal cortex: evidence from neuroimaging and neuropsychology. Prog Neurobiol. 2004;72(5):341–372. doi: 10.1016/j.pneurobio.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Lench HC, Flores SA, Bench SW. Discrete emotions predict changes in cognition, judgment, experience, behavior, and physiology: A meta-analysis of experimental emotion elicitations. Psychol Bull. 2011;137(5):834–855. doi: 10.1037/a0024244. [DOI] [PubMed] [Google Scholar]
- Lewis M. The emergence of human emotions. In: Lewis M, Haviland-Jones JM, editors. Handbook of Emotions. 2 ed. Guilford Press; New York: 2000. pp. 265–280. [Google Scholar]
- Lewis PA, Critchley HD, Rotshtein P, Dolan RJ. Neural correlates of processing valence and arousal in affective words. Cereb Cortex. 2007;17(3):742–748. doi: 10.1093/cercor/bhk024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindquist K, Wager TD, Kober H, Bliss-Moreau E, Barrett LF. The brain basis of emotion: A meta-analytic review. Behavioral Brain Sciences. 2012;35:121–143. doi: 10.1017/S0140525X11000446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesquita B. Emotions as dynamic cultural phenomena. In: Davidson R, Goldsmith H, Scherer KR, editors. The handbook of the affective sciences. Oxford University Press; New York: 2003. pp. 871–890. [Google Scholar]
- Ollinger JM, Corbetta M, Shulman GL. Separating processes within a trial in event-related functional MRI. NeuroImage. 2001;13(1):218–229. doi: 10.1006/nimg.2000.0711. [DOI] [PubMed] [Google Scholar]
- Ollinger JM, Shulman GL, Corbetta M. Separating processes within a trial in event-related functional MRI. Neuroimage. 2001;13(1):210–217. doi: 10.1006/nimg.2000.0710. [DOI] [PubMed] [Google Scholar]
- Posner J, Russell JA, Gerber A, Gorman D, Colibazzi T, Yu S, et al. The neurophysiological bases of emotion: An fMRI study of the affective circumplex using emotion-denoting words. Hum Brain Mapp. 2009;30(3):883–895. doi: 10.1002/hbm.20553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds SM, Berridge KC. Positive and negative motivation in nucleus accumbens shell: bivalent rostrocaudal gradients for GABA-elicited eating, taste “liking”/“disliking” reactions, place preference/avoidance, and fear. J Neurosci. 2002;22(16):7308–7320. doi: 10.1523/JNEUROSCI.22-16-07308.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolls ET. The affective and cognitive processing of touch, oral texture, and temperature in the brain. Neurosci Biobehav Rev. 2010;34(2):237–245. doi: 10.1016/j.neubiorev.2008.03.010. [DOI] [PubMed] [Google Scholar]
- Russell JA. Culture and the Categorization of Emotions. Psychological Bulletin. 1991;110(3):426–450. doi: 10.1037/0033-2909.110.3.426. [DOI] [PubMed] [Google Scholar]
- Russell JA. Core affect and the psychological construction of emotion. Psychol Rev. 2003;110(1):145–172. doi: 10.1037/0033-295x.110.1.145. [DOI] [PubMed] [Google Scholar]
- Russell JA, Bachorowski JA, Fernandez-Dols JM. Facial and vocal expressions of emotion. Annual Review of Psychology. 2003;54:329–349. doi: 10.1146/annurev.psych.54.101601.145102. [DOI] [PubMed] [Google Scholar]
- Russell JA, Barrett LF. Core affect, prototypical emotional episodes, and other things called emotion: dissecting the elephant. J Pers Soc Psychol. 1999;76(5):805–819. doi: 10.1037//0022-3514.76.5.805. [DOI] [PubMed] [Google Scholar]
- Wilson-Mendenhall CD, Barrett LF, Simmons WK, Barsalou LW. Grounding emotion in situated conceptualization. Neuropsychologia. 2011;49(5):1105–1127. doi: 10.1016/j.neuropsychologia.2010.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wundt W. Outlines of psychology. In: Judd CH, editor. Trans. Wilhelm Engelmann; Leipzig: 1897. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


