Abstract
Wnt ligands are secreted morphogens that control multiple developmental processes during embryogenesis and adult homeostasis. A diverse set of receptors and signals have been linked to individual Wnts, but the lack of tools for comparative analysis has limited the ability to determine which of these signals are general for the entire Wnt family, and which define subsets of differently acting ligands. We have created a versatile Gateway library of clones for all 19 human Wnts. An analysis comparing epitope-tagged and untagged versions of each ligand shows that despite their similar expression at the mRNA level, Wnts exhibit considerable variation in stability, processing and secretion. At least 14 out of the 19 Wnts activate β-catenin-dependent signaling, an activity that is cell type-dependent and tracks with the stabilization of β-catenin and LRP6 phosphorylation. We find that the core Wnt modification and secretion proteins Porcupine (PORCN) and Wntless (WLS) are essential for all Wnts to signal through β-catenin-dependent and independent pathways. This comprehensive toolkit provides critical tools and new insights into human Wnt gene expression and function.
Keywords: Wnt signaling, β-catenin, Wnt modification and secretion
1. Introduction
The Wnt signaling pathways regulate key networks during both embryonic development and adult tissue homeostasis. These signaling outcomes play a major role in the regulation of cell fate decisions such as proliferation, survival and differentiation. Because of its well-documented contributions to these processes, misregulation of Wnt signaling contributes to a variety of human diseases, ranging from defects of development and cancers of the colon, breast and skin, to diseases associated with defects of the eye, bone and heart (Klaus and Birchmeier, 2008).
At the center of this complex network are Wnt ligands, a highly conserved family of cysteine-rich secreted morphogens. Wnt proteins are synthesized in the endoplasmic reticulum (ER) where the membrane bound O-acyltransferase porcupine (PORCN) catalyzes their palmitoylation (Takada et al., 2006; Willert et al., 2003). Modified Wnt ligands are carried to the plasma membrane by binding in a palmitoylation dependent manner to the carrier protein Wntless (WLS). At the plasma membrane, Wnts undergo a variety of fates including cell surface binding, local diffusion, binding to lipoprotein carriers, and secretion in vesicles (Banziger et al., 2006; Bartscherer et al., 2006). Once secreted, these Wnts are further regulated by interaction with a variety of extracellular regulators including members of the decoy receptor family SFRP. In total, there are 19 human Wnts that regulate numerous biological processes via diverse receptors and signaling pathways. One well-studied property of many Wnts is their ability to stabilize β-catenin, an activity initiated by binding to LRP5/6 and Frizzled family cell surface receptors (van Amerongen and Nusse, 2009). Importantly, Wnts also signal through Frizzled and additional classes of cell surface receptors including receptor tyrosine kinases, to regulate diverse β-catenin-independent pathways, including the Planar Cell Polarity (PCP) and the Wnt/Calcium pathways (Najdi et al., 2011).
Given the complexity of Wnt biology in humans, many questions regarding the roles and activities of various Wnts remain. The classification of Wnts has been largely based on their signaling properties across different systems rather than direct comparison among Wnts of the same species in a single cell type. For example, WNT1 and 3A are traditionally considered to be potent activators of Wnt/β-catenin signaling while other Wnts such as 5A and 11 are known to activate β-catenin-independent pathways. However, recent reports have demonstrated that WNT5A can activate Wnt/β-catenin signaling depending on the receptors expressed at the plasma membrane. Furthermore, both WNT5A and 11 have been shown to heterodimerize to activate β-catenin-dependent signaling (Cha et al., 2009; Mikels and Nusse, 2006). Similarly, WNT3A has been shown to induce morphogenetic changes by activating Rho-associated kinase or c-Jun N-terminal kinase, depending on cell type (Endo et al., 2008; Kishida et al., 2004). These data confirm that signaling is complex and will be dependent on multiple factors beyond single ligand/receptor interactions. However, the tools to compare the activity of human Wnts have been lacking.
One apparently common pathway for Wnt signaling is that of Wnt production, processing and secretion. However, while a small number of Wnts have been examined for posttranslational modification and secretion, other mammalian Wnt ligands have not been characterized. This information gap becomes more important to address as inhibitors of Wnt secretion enter clinical trials (www.clinicaltrials.gov). These questions and the lack of understanding of Wnt signaling properties have emphasized the requirement for a full standardized set of Wnt expression plasmids. Such a set would allow for a direct side-by-side comparison of Wnt processing, secretion and signaling. For this reason, we started the “Open Source Wnt” plasmid depository and cloned open reading frame sequences for all 19 human Wnts into the same expression plasmid backbone. Using this plasmid set, we have been able to analyze and compare the expression levels and the efficiency of secretion of all human Wnts in a defined set of cell lines, revealing valuable insight on their processing, their signaling potential and their accumulation in conditioned media. We show that, dependent on cell type, up to 14 out of the 19 Wnts are capable of signaling through the β-catenin-dependent pathway to activate the Super8XTOPflash reporter. Wnt/β-catenin signaling is broadly reduced by the secreted Wnt inhibitors Dkk-1 and the SFRP1 and 3 (Secreted Frizzled-Related Proteins). Activation of the Super8XTOPflash reporter, with a few exceptions, is more sensitive than, but tracks with, LRP6 phosphorylation and the stabilization of β-catenin. Finally, we find that PORCN and WLS are essential elements of a common Wnt secretion pathway, as all assessable Wnts require PORCN-dependent palmitoylation to bind to the carrier protein WLS and to signal through β-catenin-dependent and independent pathways.
2. Materials & Methods
2.1. Wnt Cloning
Human WNT cDNAs were PCR-amplified twice: one reaction using WNT-specific sense primers and “STOP” antisense primers, and another reaction using “Non-STOP” antisense primers (Refer to supplementary material Table S1 for cDNA source, primer sequence and PCR conditions). 4 μL of each PCR reaction were TOPO-cloned into the pENTR/D-TOPO Gateway Entry vector following the manufacturer’s protocol (pENTR Directional TOPO Cloning Kits, Invitrogen). Entry clones were then recombined into the pcDNA3.2/V5-DEST Gateway Destination vector at a 1:1 ratio using LR clonase II for 1 hour at 25°C (Gateway LR Clonase II Enzyme Mix, Invitrogen). All clones were sequence-validated at every step of the cloning process.
2.2. qRT-PCR analysis
Isolated RNA was reverse transcribed into cDNA using the High Capacity cDNA kit (Applied Biosystems). cDNA was amplified for 40 cycles with the Maxima SYBR Green/ROX qPCR Master Mix (2X) (Fermentas) using primers specific to the backbone destination vector for all of the Wnts. Primers used were destVect16F1 sense primer (5′-CGCGCCGACCCAGCTTTCTTG -3′) and destVect121R1 antisense primer (5′-CGGTACGCGTAGAATCGAGACCG -3′). Melting and annealing temperatures were 95°C and 60°C.
2.3. Western Analysis
For detection of WNT expression and secretion, HEK293 or NIH3T3 cells were cultured in DMEM media with 10% FBS (Cellgro) and plated at a density of 1,000,000 cells per plate in 10 cm dishes. Cells were transfected using Bio T transfection reagent (Bioland Scientific) 24 hours post plating with plasmids containing V5-tagged Wnts (pcDNA3.2/V5-DEST; 5 μg) or untagged WNT 1 and 3A (pcDNA3.2/V5-DEST; 5 μg). Conditioned media and cell lysates were harvested 48 hours post transfection. The conditioned media was concentrated using StrataClean resin (Agilent Technologies). Media, lysates and a V5 protein standard (Recombinant Yeast Calmodulin Kinase Array Control Protein, Invitrogen) were separated by 10% SDS-PAGE and transferred to a nitrocellulose membrane (Whatman). For non-phospho β-catenin and phospho-LRP6 detection, HEK293 cells were plated at a density of 200,000 cells per well in 6-well plates and transfected using Bio T 24 hours later with plasmids containing untagged Wnts (pcDNA3.2/V5-DEST; 1 μg). Cell lysates were harvested 24 hours post transfection, separated by 10% SDS-PAGE and transferred to a nitrocellulose membrane (Amersham Biosciences). Blots were blocked in 5% milk for 30 minutes and hybridized with primary antibodies against V5 (1:5000 dilution, Invitrogen), Actin (I-19, 1:500, Santa Cruz), Lamin A/C (1:5000, Cell Signaling), WNT1 (N2C3, 1:1300, GeneTex), WNT3A (1:250, R&D Systems), non-phospho β-catenin (Ser33/37/Thr41, 1:500, Cell Signaling), phospho-LRP6 (Ser1490, 1:1000, Cell Signaling), or β-Tubulin (TUBB1, 1:1000, GeneTex) overnight at 4°C. After hybridization, the blots were washed and hybridized with anti-rabbit IgG-HRP (1:5000, Amersham Biosciences), anti-mouse IgG-HRP (1:5000, Amersham Biosciences), anti-rat IgG-HRP (1:50000, Jackson ImmunoResearch) or Bovine anti-goat IgG-HRP (1:15000, Santa Cruz) for 2 hours at RT. The ECL reaction was performed according to the manufacturer’s protocol (Thermo Scientific) and blots were visualized using the LAS-4000 Fujifilm imaging system.
2.4. Luciferase Assays
HEK293 or NIH3T3 cells were cultured in DMEM media with 10% FBS and plated at a density of 200,000 cells per well in 6-well plates. Cells were transfected 24 hours post plating, using BioT, with Super8XTOPflash reporter plasmid (0.1 μg; kind gift from Dr. R.T. Moon), thymidine kinase β-galactosidase plasmid (0.1 μg), Wnt plasmids (pcDNA3.2/V5-DEST; 0.1 μg), Dkk-1 expression plasmid (pcDNA3; 0.4 μg) (Kind gift from Dr. B. Hoang) and expression plasmids containing SFRP1 (pcDNA3.1; 0.4 μg), SFRP2 (pBABE; 0.4 μg) or SFRP3 (pcDNA3.1; 0.5 μg). For the competition experiment, the amount of Wnt plasmid transfected is indicated on the figure (Fig. 4B). For figure 5B, 1 μg of Wnt DNA plasmid was transfected. Cells were harvested 24 hours post transfection and then luciferase activity was measured and normalized using β-galactosidase levels. For figure 8 (knockdown of WLS), three independent experiments were performed using HEK293 cells with an integrated Super8XTOPflash reporter. 100–200 ng of untagged Wnt plasmids were transfected into cells plated in 24-well plates using lipofectamine 2000 (Life Technologies). siRNA targeting WLS (targeting sequences: ACGAATCCCTTCTACAGTA) was transfected into the cells using Dharmafect transfection reagent (Dharmacon). Cell lysates were assayed for luciferase activity 48 hours post transfection. Duplicate samples were assayed for each condition.
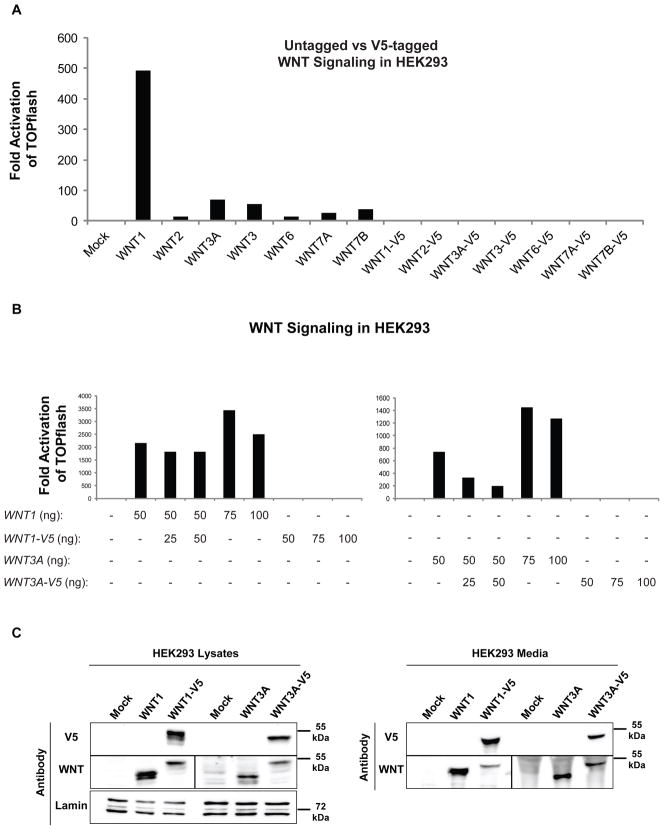
Fig. 4.
Effect of C-terminal tagging on Wnt activity, expression and accumulation. (A) The indicated untagged and V5-tagged versions of the active Wnts were co-transfected with the STF reporter into HEK293 cells. The C-terminal V5 tag significantly reduced Wnt/β-catenin signaling activity. (B) V5-tagged Wnts 1 and 3A were co-transfected with their untagged counterparts into HEK293 cells to determine whether they can compete for cell surface receptors. V5-tagging affects different Wnts in different manners; it interferes with the ability of WNT1-V5 to bind to cell-surface receptors, but does not do so with WNT3A-V5. (C) The expression levels of Wnts 1 and 3A in lysates (left panel) or their accumulation levels in media (right panel) are not significantly affected by C-terminal V5-tagging in HEK293 cells. Cell lysates were also probed for Lamin as a loading control. Both (A) and (B) are representatives of 3 replicate experiments each.
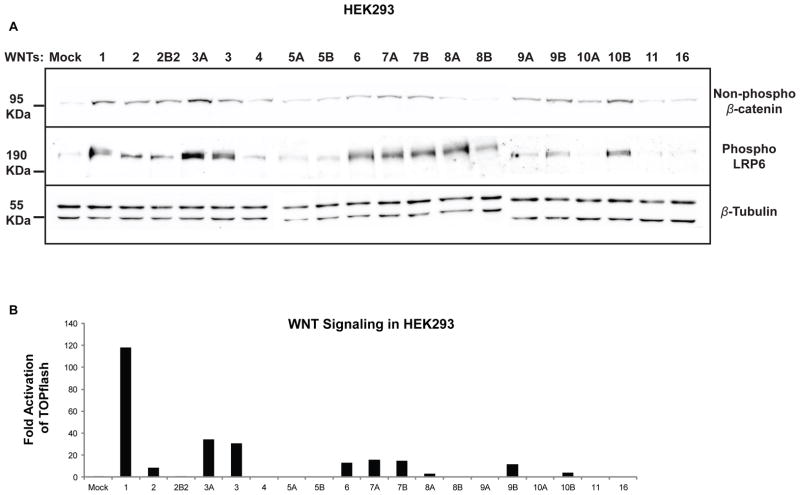
Fig. 5.
Wnt-dependent phosphorylation of LRP6 and stabilization of β-catenin. (A) The 19 untagged Wnts (1 μg each) were tested for their ability to induce non-phosphorylated β-catenin (1st panel) and phosphorylation of LRP6 (2nd panel) in HEK293 cells. All the active Wnts (1, 2, 3A, 3, 6, 7A, 7B, 8A, 9B and 10B) were successful in inducing both, while a subset of the inactive Wnts displayed varying degrees of LRP6 phosphorylation and β-catenin dephosphorylation. β-Tubulin levels were monitored as a loading control (3rd panel). (B) Plasmid encoding untagged Wnts (1000 ng each) was transfected to determine their effect on the STF reporter in HEK293. The activation pattern is similar to that observed with 100ng of plasmid DNA (Fig. 3A). (B) is a representative of 3 replicate experiments.
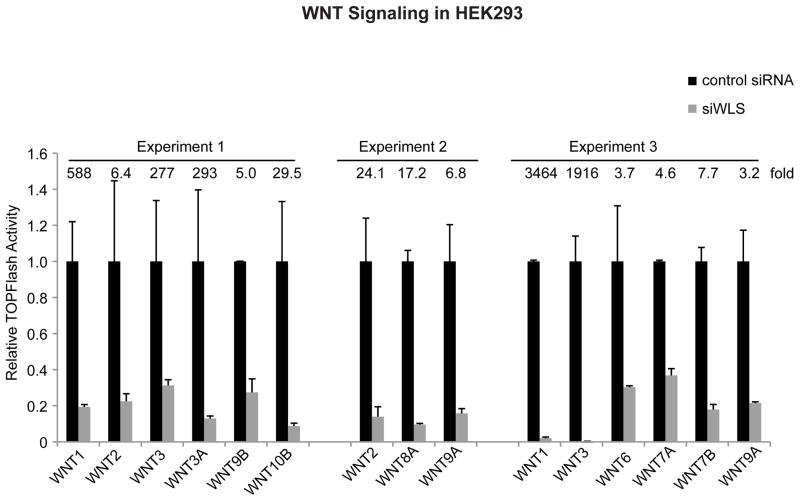
Fig. 8.
Wnt signaling requires WLS. HEK293 cells with an integrated Super8XTopFlash reporter (STF cells (Coombs et al., 2010)) were transfected with the indicated untagged Wnts together with either control or WLS siRNA. Fold activation by the Wnts in the presence of control siRNA is indicated above the columns, and the effect of WLS knockdown is presented as remaining Wnt/β-catenin signaling activity in cells with WLS knockdown.
2.5. Porcupine-Deleted HT1080 Cells
HT1080 cells were acquired from the American Type Culture Collection (VA, USA) and grown in DMEM (Nacali Tesque, Japan) containing 4.5 g/L glucose, pen/strep, 10% FBS, and 1 mM sodium pyruvate in a humidified 37 °C atmosphere. Porcupine was functionally deleted from HT1080 cells (ATCC) using a zinc finger nuclease (Sigma-Aldrich) targeting exon 9 of the human PORCN gene (KP and DMV, manuscript in preparation). For luciferase assays in these lines, cells were seeded at 80,000 per well in 24-well culture dishes one day prior to transfection. 50 ng of untagged Wnt plasmid was transfected along with mCherry transfection control, Super8XTOPflash reporter, and 100 ng of HA-tagged mPORCN construct as indicated, all by lipofectamine 2000 (Life Technologies). Cell lysates were assayed for luciferase activity 24 hours post transfection.
2.6. Dvl-2 Mobility Shift
To test for Wnt-induced Dvl2 mobility shift, HT1080 PORCN null cells, seeded in 24-well dishes as in luciferase assays, were transfected with 400 ng of untagged Wnts indicated, in the presence or absence of 100 ng HA-PORCN expression plasmid. 24 hours following transfection, cell lysates were prepared (100 mM sodium phosphate, pH 7.5, 150 mM NaCl, 1% IGEPAL-CA630, complete protease inhibitor cocktail (Roche)) and separated by SDS-PAGE. After transfer to PVDF membranes and blocking with 3% BSA in TBS-T, blots were incubated overnight with Dvl2 antibodies (1:500, Santa Cruz #13974) followed by HRP-conjugated secondary antibodies and visualization by ECL.
2.7. FLAG Wnt Cloning
Human Wnt cDNAs, without their putative signal sequences, were cloned into p3XFLAG-CMV-8 (Sigma) using the Cold Fusion Cloning Kit (System Biosciences). p3XFLAG-CMV-8 was linearized with HindIII and XbaI. Design of PCR primers for cDNA amplification and cloning into the linearized vector were both carried out according to manufacturer’s recommendations. HindIII and BglII restriction sites were incorporated into the forward and reverse primers respectively to provide unique sites to allow for linearization or excision of cDNA from this library. (Refer to supplementary material Table S2 for primers used for cloning).
2.8. Co-Immunoprecipitation
HeLa cells were transfected (Invitrogen Lipofectamine 2000) with C-terminal V5 tagged Wnts (1000 ng per 10 cm dish) and treated with either DMSO or 2 uM IWP1 (MolPort) overnight. 500 ug of lysate was subject to immunoprecipitation with anti-V5 antibody (Invitrogen), separated by SDS-PAGE and immunoblot carried out with anti-WLS (YJ5, Millipore MABS87 (Coombs et al., 2010)) and anti-V5 antibodies.
3. Results
3.1. Human Wnt Cloning
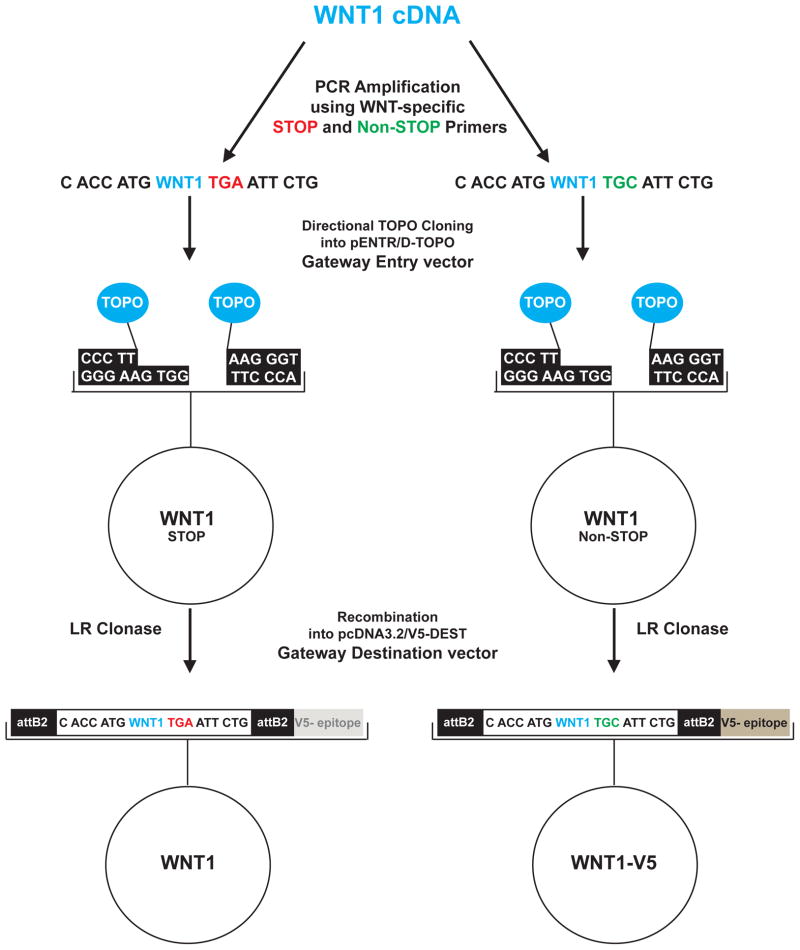
In order to create a synchronized set of Wnt expression plasmids, cDNAs of all 19 human Wnt proteins (1, 2, 2B2, 3A, 3, 4, 5A, 5B, 6, 7A, 7B, 8A, 8B, 9A, 9B, 10A, 10B, 11 and 16) were cloned into the same backbone, thus allowing for direct comparisons to be made among the different Wnts. First, WNT cDNA was amplified using PCR with both WNT-specific “STOP” and “Non-STOP” reverse primers. “STOP” primers contained the TGA STOP codon, while “Non-STOP” primers contained the codon TGC rather than TGA. This one basepair difference in the two reverse primers also introduced an EcoRI restriction site that is unique to clones amplified using the “STOP” primers (TGA ATT CTG), therefore allowing the excision of the WNT coding sequence using restriction digest techniques. For this reason, internal EcoRI sites were destroyed in WNTs 2, 8A and 9A while maintaining the integrity of the amino acid sequence (Table S1). Following PCR amplification, the products were cloned using the TOPO cloning system into a Gateway entry vector, pENTR/D-TOPO. Finally, the WNT coding sequence was transferred, through the use of homologous recombination, into a Gateway destination vector, pcDNA3.2/V5-DEST, which contains a C-terminal V5 epitope tag. Consequently, clones that contain the STOP codon are expressed as untagged Wnt ligands, while those that do not are expressed as V5-tagged at the C-terminus (Fig. 1). In a parallel cloning effort, all Wnts were cloned into a p3XFLAG-CMV-8 vector where the native signal peptide is replaced with the preprotrypsin signal peptide. These N-terminally tagged Wnts were expressed and secreted at 50-fold greater amounts than the Gateway clones, but also had a >1000-fold decrease in signaling activity (Fig. S1). We speculate that the N-terminal tag and use of the vector-supplied signal peptide accounts for both the increased protein production and also for the substantial decrease in activity. The N-tagged Wnts in p3XFLAG were not extensively studied.
Fig. 1.
Wnt cloning scheme using WNT1 as an example. WNT1 cDNA was amplified by PCR using WNT1-specific “STOP” and “Non-STOP” primers. The PCR product was then transferred, using TOPO cloning, into the pENTR/D-TOPO Gateway Entry vector, and subsequently shuttled, using recombination, into the pcDNA3.2/V5-DEST Gateway Destination vector. The outcome of the cloning process is 4 WNT1 plasmids: 2 Entry vectors (WNT1 STOP and WNT1 Non-STOP) and 2 Destination vectors (WNT1 and WNT1-V5).
3.2. Wnt ligand production and secretion profile
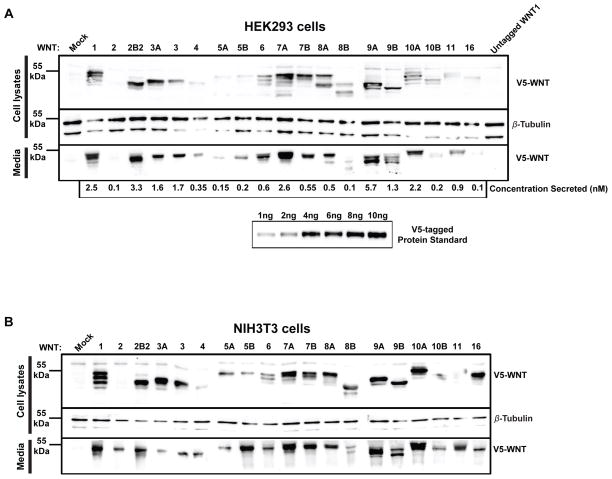
As a first step for validation of the Gateway Wnt clones, the expression and secretion levels of each Wnt were monitored in HEK293 cells (Fig. 2A), a human embryonic kidney cell line, and NIH3T3 cells (Fig. 2B), a mouse fibroblast cell line. Plasmids expressing C-terminal V5-tagged Wnts were transiently transfected and both lysates and media were probed with V5-tag antibody. Transient transfection of the “STOP” expression plasmid for untagged WNT1 was included in the analysis (Fig. 2A, last lane). For this sample, no WNT1-V5 was detected in lysates or media demonstrating that the single nucleotide difference that creates a translation stop codon for the untagged plasmids is effective. While all Wnt ligands were expressed in the lysates and secreted into the media of both cell lines, there was tremendous variation in both protein abundance and secretion into the media. The variation was particularly surprising since all clones share the same backbone plasmid and produce relatively similar amounts of mRNA transcripts in HEK293 cells (Fig. S2). Since mRNA stability was similar, the differences are likely to be due to either differences in rates of translation or differences in protein stability. Less likely, the uniform V5 epitope tag may differentially affect the stability of the different Wnt proteins. One implication of this result is that mRNA abundance may be a relatively poor method to compare the relative abundance of different Wnt ligands.
Fig. 2.
V5-tagged Wnt expression. Cell Lysates and media collected from HEK293 (A) and NIH3T3 (B) cells that had been transfected with V5-tagged Wnts (and untagged WNT1 for HEK293 cells) were separated by SDS-PAGE on a 10% gel and then probed with a V5 antibody to determine expression (1st panels) and secretion levels (3rd panels). Cell lysates were also probed for β-Tubulin as a loading control (2nd panels). A V5-tagged protein standard (2A, 5th panel) was processed on a parallel immunoblot at the same time and used to calculate the amount of V5-tagged Wnts secreted into the media of HEK293 cells (2A, 4th panel). In HEK293 cells, WNT9A was the most secreted Wnt into the media (5.7 nM), while WNT2, 8B and 16 were the least secreted Wnts (0.1 nM).
To quantify the amount of Wnt protein secreted into the media of HEK293 cells, a purified V5-tagged protein of known concentration (Recombinant Yeast Calmodulin Kinase Array Control Protein, see Materials and Methods) was utilized as a V5-tag standard to calculate the different concentrations to which each Wnt is secreted (Fig. 2A; panel 6). WNT1, 2B2, 7A and 9A were among the most abundantly secreted Wnts in HEK293 cells, while WNT2, 5A, 8B and 16 were the least well secreted Wnts (Fig. 2A; panel 4). It is important to point out that there were more protein isoforms in lysates than in media for WNT1, 6, 7A and 10A. The multiple bands detected in lysates might reflect the levels of intracellular processing that Wnt ligands undergo. We observed relative consistency in expression, secretion and processing from one cell line to another, as the patterns observed in HEK293 cells are largely unchanged in NIH3T3 cells. However, WNT2, 5A, 5B and 16 appear to be secreted more efficiently from NIH3T3 cells (Fig. 2B; panel 3).
3.3. Wnt activation of the β-catenin-dependent pathway
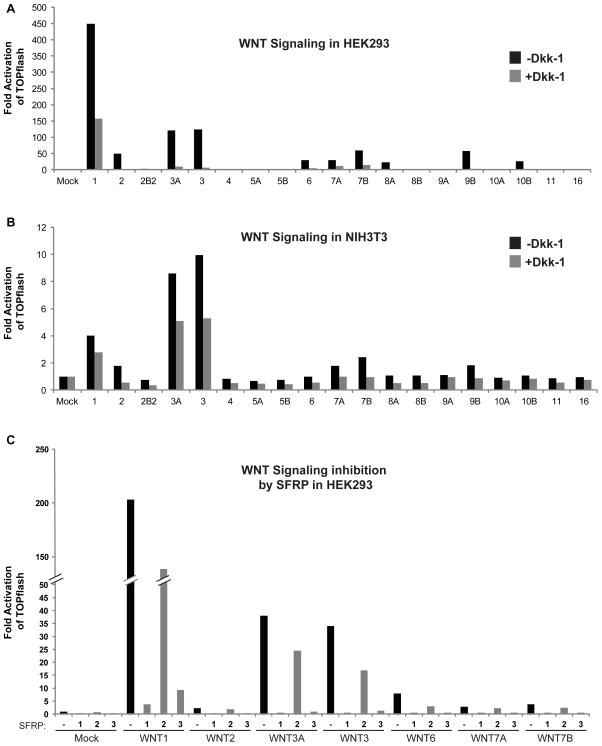
The ability of “non-canonical” Wnts such as 5A and 11 to activate Wnt/β-catenin signaling suggested that in theory, all Wnt ligands might be capable of signaling via the β-catenin-dependent pathway or other β-catenin-independent pathways when provided with the right conditions (Cha et al., 2009; Mikels and Nusse, 2006). To test which Wnts can activate the Wnt/β-catenin signaling in HEK293, NIH3T3 and HT1080 cells, plasmids expressing untagged Wnts were co-transfected with Super8XTOPflash (STF), a luciferase reporter plasmid with 8 multimerized LEF/TCF response elements (Fig. 3A). Out of all 19 Wnts, WNT1 was the most active in HEK293 cells. Additionally, WNT2, 2B2, 3A, 3, 6, 7A, 7B, 8A, 9A, 9B and 10B also exhibited considerable activation of the STF reporter when compared to mock-transfected cells. Meanwhile, only WNT1, 3A and 3 significantly activated the β-catenin-dependent pathway in NIH3T3 cells (Fig. 3B). Since the V5 tagged versions of each Wnt show that they are efficiently produced and secreted from NIH3T3 cells, as with HEK293 cells, the global decrease in reporter activity in mouse NIH3T3 cells may be due to decreased expression of Wnt receptors on the cell surface, species differences in human-mouse ligand interaction, or decreased activity of other downstream Wnt pathway components.
Fig. 3.
Wnt activation of the β-catenin-dependent pathway. All 19 untagged Wnts (100 ng each plasmid) were compared for their ability to activate the Super8XTOPflash (STF) reporter in HEK293 (A) and NIH3T3 (B) cells in 6-well dishes. WNT1 (450 fold), 2 (49 fold), 2B2 (3.4 fold), 3A (120 fold), 3 (123 fold), 6 (30 fold), 7A (29 fold), 7B (59 fold), 8A (23 fold), 9A (2.3 fold), 9B (59 fold) and 10B (26 fold) were significantly active in HEK293 cells, while only WNT1 (4 fold), 3A (8.6 fold), 3 (10 fold) and 7B (2.4 fold) were significantly active in NIH3T3 cells. Co-expression of the Wnt inhibitors Dkk-1 (400 ng plasmid) (A and B) and SFRPs 1 and 3, but not 2 (400 ng plasmid) (C) effectively reduced Wnt/β-catenin signaling activity. In (C), the columns designated 1, 2 and 3 refer to the SFRP used in the experiment. (A) is a representative of 4 replicate experiments. Both (B) and (C) are representatives of 3 replicate experiments each.
All Wnt/β-catenin signaling is thought to require the LRRP5/6 co-receptors. Consistent with this, addition of Dkk-1, an inhibitor of Wnt signaling that binds to the LRP5/6 co-receptor at the plasma membrane, significantly reduced the activating potential of all Wnt proteins in both NIH3T3 and HEK293 cells (Fig. 3A and 3B) (Glinka et al., 1998; Li et al., 2005; Mao et al., 2001). In addition to Dkk-1, we also tested whether the soluble Frizzled-related proteins SFRP1-3 can negatively regulate Wnt-induced activation of Wnt/β-catenin-signaling in HEK293 cells (Fig. 3C). SFRP proteins differ from Dkk-1 in that they directly bind to Wnt ligands to block their actions. Co-transfection of either SFRP1 or SFRP3 effectively reduced the activation of the STF reporter by WNT1, 2, 3A, 3, 6, 7A and 7B. Conversely, SFRP2 was globally a less effective inhibitor of Wnt/β-catenin activation, although this could be due to expression levels rather than a decrease in binding efficiency.
We next asked whether C-terminal tagging of Wnt proteins affects their signaling properties. Plasmids expressing V5-tagged Wnts were co-transfected with the STF reporter into HEK293 cells (Fig. 4A). While the untagged versions of WNT1, 2, 3A, 3, 6, 7A and 7B were highly active (Fig. 3A and 4A), their C-terminal tagged counterparts could not activate the STF reporter, suggesting that C-terminal tagging interferes with their signaling potential. However, C-terminal tagging did not appear to affect expression or secretion of Wnt proteins (Fig. 2A, 2B and 4C). We therefore hypothesized that C-terminal tagging with the Gateway and V5 sequence might interfere with the ability of Wnt ligands to bind to their receptors at the plasma membrane. To test this hypothesis, untagged WNT1 or WNT3A cDNAs were co-transfected with C-terminally tagged WNT1-V5 or WNT3A-V5 cDNAs respectively in HEK293 cells (Fig. 4B). If addition of the V5-tagged Wnt can interfere with the ability of the untagged Wnt to activate Wnt/β-catenin signaling, it would suggest that C-terminally tagged Wnt ligands are still capable of binding to their receptors and competing with untagged Wnts, but that the tag affects signal relay to downstream components of the pathway. Conversely, if the V5-tagged Wnt does not compete away the untagged Wnt-mediated activity, the implication would be that C-terminal tagging hinders a Wnt’s ability to bind to its receptor. In fact we saw both outcomes: while WNT1-induced activation remained unchanged with increasing concentrations of WNT1-V5 cDNA, introduction of WNT3A-V5 cDNA blocked WNT3A-induced activation in a dose-dependent manner. To rule out the possibility that V5-tagging affects WNT1 accumulation in the media, HEK293 lysates and media were probed for the expression and accumulation of untagged Wnts 1 and 3A and their V5-tagged counterparts (Fig. 4C). Both versions of Wnts 1 and 3A were detected in cell lysates and media. Therefore, the inability of WNT1-V5 to interfere with untagged WNT1-mediated signaling is not due to low levels of expression/secretion or its lack of accumulation in the media. These experiments suggested that C-terminal tagging might affect each Wnt ligand in a distinct manner: interfering with its binding to cell-surface receptors in the case of WNT1 or simply affecting its ability to induce activation once bound to its receptor(s) in the case of WNT3A. This is consistent with the recent finding that different Wnts might bind to different propeller domains of LRP6 (Bourhis et al., 2010).
3.4. Wnt-dependent phosphorylation of LRP6 and stabilization of β-catenin
The most readily measured effect of Wnt signaling is the activation of the β-catenin-dependent pathway. For that to occur, the LRP5/6 receptor at the cell surface must be phosphorylated at its PPPSP motif by GSK3 (Glycogen Synthase Kinase 3) and CK1γ (Casein Kinase 1 gamma) in response to the binding of Wnt ligands (Davidson et al., 2005; Tamai et al., 2004; Zeng et al., 2005). Another hallmark of the activation of this pathway is the stabilization of the co-activator β-catenin once released from the destruction complex (Liu et al., 2002; Yost et al., 1996). We therefore monitored LRP6 phosphorylation and unphosphorylated β-catenin abundance by immunoblot analysis in HEK293 cells (Fig. 5A). While transfection of 100 ng of untagged WNT cDNA activated the STF reporter, this amount of Wnt plasmid had little effect on the levels of both phospho-LRP6 and non-phospho β-catenin (data not shown, Fig. 3A). However, transfection of a super-saturating amount, 1000 ng, of untagged WNT cDNA induced readily detectable changes in both phospho-LRP6 and non-phospho β-catenin abundance (Fig. 5A). In order to allow for accurate comparison with the activation of the STF reporter, 1000 ng of untagged WNT cDNA was transfected into HEK293 cells to assay for luciferase levels (Fig. 5B). While this saturating Wnt expression actually elicited less activity than lower expression levels, the patterns of activation remained the same (Fig. 3A and 5B). As expected, all Wnts that activated the STF reporter also induced phosphorylation of LRP6 and β-catenin stabilization to varying degrees (WNT1, 2, 3A, 3, 6, 7A, 7B, 8A, 9B and 10B). However, phospho-LRP6 and non-phospho β-catenin levels did not always track with activation of the STF reporter. For example, WNT2B2, 9A and 10A, which did not activate the β-catenin-dependent pathway in HEK293 cells, caused significant stabilization of β-catenin when compared to mock-transfected cells (Fig. 5A and 5B). This finding suggests that changes in β-catenin abundance and LRP6 phosphorylation do not necessarily correlate with transcriptional activation of the Wnt/β-catenin pathway.
3.5. Analysis of the core Wnt modification and secretion pathway
One goal of the Open Source Wnt project is to test if the core Wnt synthesis and secretion pathway is common to all Wnt proteins. In the current model, all vertebrate and most metazoan Wnts are post-translationally modified by palmitoylation on one or two conserved Cysteine and Serine residues (Takada et al., 2006; Willert et al., 2003). Serine palmitoylation (with a mono-unsaturated palmitate) is required for several Wnts to bind to the integral membrane protein WLS, which then transports the Wnts to the plasma membrane (Coombs et al., 2010). All but one Drosophila wingless protein requires WLS for activity (Ching et al., 2008; Herr and Basler, 2012) suggesting a broad requirement for both PORCN and WLS proteins. Conversely, however, one study suggested that WLS is only required for a small subset of Wnts in Xenopus development (Kim et al., 2009). The standardized Wnt expression library allows us to examine if there is variability in the mechanism by which human Wnts are secreted from cells.
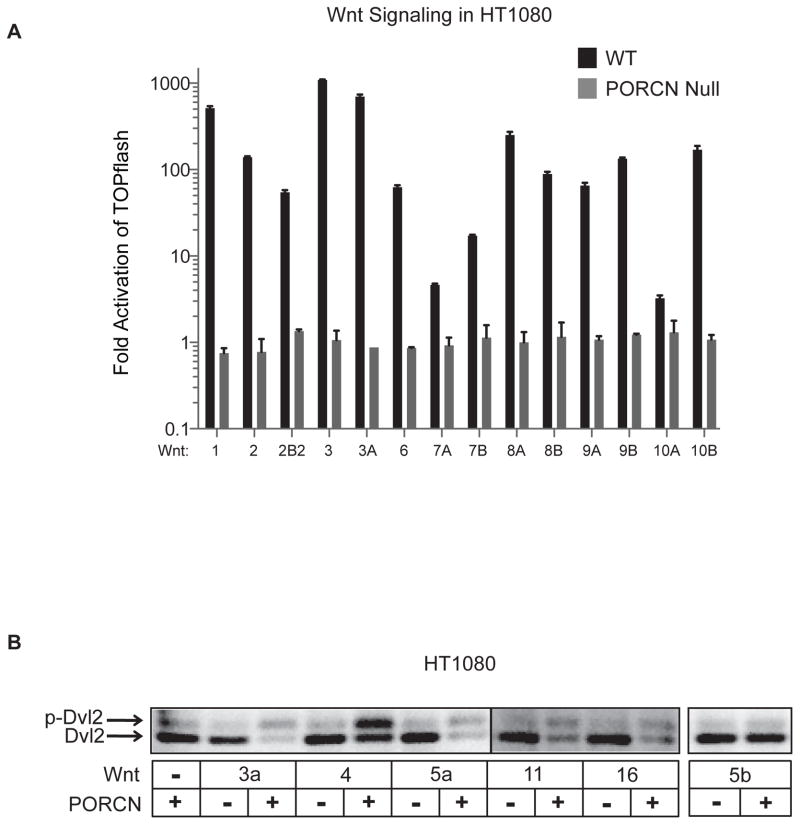
To ask if all human Wnts require PORCN for activity, we created a human fibrosarcoma HT1080 cell line with a zinc-finger nuclease-mediated null mutation in the single copy PORCN gene (PORCN is on the X chromosome, and HT1080 cells are male) (Proffitt and Virshup, manuscript in preparation). The activity of transfected Wnts was tested in paired wildtype and PORCN null cells by STF reporter assays (Fig. 6A) and Dvl2 (Dishevelled 2) mobility shift (Fig. 6B). HT1080 cells were particularly responsive to Wnt/β-catenin signaling, as Wnts 8B and 10A gained activity in HT1080 cells, and many other Wnts gave better than 10-fold activation of the STF reporter (Fig. 6A). In total, fourteen of the nineteen Wnts were able to activate β-catenin-dependent signaling, ranging from 3- to 1000-fold. In all cases, the matched PORCN null cells had no measurable Wnt/β-catenin signaling activity. This was not due to any secondary effect of the PORCN mutation, since the signaling activity of the null cells could be rescued by wildtype but not catalytically inactive PORCN plasmid co-transfection (Fig. 6B and data not shown). A subset of Wnts (Wnts 4, 5A, 5B, 11 and 16) was not able to activate Wnt/β-catenin signaling in HT1080 cells. These Wnts (and WNT3A) were assessed for their ability to activate the CK1-dependent phosphorylation of endogenous Dvl2. WNT3A and four of five additional Wnts were able to stimulate Dvl2 mobility shift in a PORCN-dependent manner (Fig. 6B), indicating that PORCN is also essential for β-catenin-independent Wnt signaling. Of the nineteen Wnts tested, PORCN (and hence Wnt palmitoylation) was essential for the function of eighteen while one Wnt, WNT5B, was unable to be assessed in these assays.
Fig. 6.
Wnts require PORCN for activity. (A) WT and PORCN null HT1080 cells were transfected in parallel with untagged Wnts shown (50 ng per well in a 24-well dish) in combination with the STF reporter. Data is presented as fold activation over background signal with no transfected Wnt. Error bars represent SD. (B) PORCN null HT1080 cells were transfected with Wnts shown, in combination with mPORCN-D expression plasmid as indicated. Western blots were performed to analyze the Wnt and PORCN-dependent Dvl2 electrophoretic mobility shift.
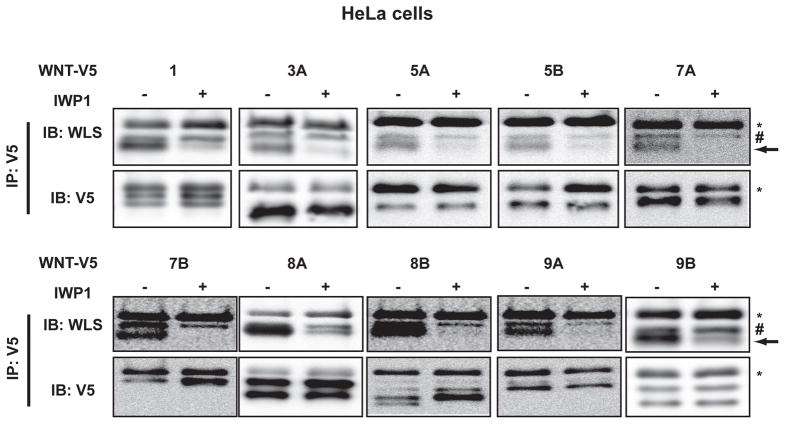
Palmitoylation can affect Wnt export, membrane association, and receptor binding. Palmitoylation is required for WNT3A and WNT5A to bind to WLS for proper transport to the cell membrane. However, whether all of the mammalian Wnts similarly bind to endogenous WLS in a palmitoylation-dependent manner is not known. To test this, we utilized the Wnt-V5 library and the PORCN inhibitor IWP1 (Chen et al., 2009). Wnts that were expressed at sufficiently high levels to be clearly visualized were assessed in HeLa cells (HeLa cells were chosen because they have high endogenous WLS levels). In every evaluable case, Wnts co-immunoprecipitated with WLS, and this interaction was sensitive to the PORCN inhibitor IWP1 (Fig. 7). WNT5B, whose signaling activity we were unable to assess, nonetheless interacted with WLS in a palmitoylation-dependent manner. Thus, the palmitate-dependent interaction of Wnts with WLS is a general feature of human Wnt secretion. This suggested that WLS, like PORCN, would be essential for the signaling activity of all Wnts. To test this directly, WLS was knocked down by siRNA and Wnt/β-catenin signaling activity was assessed. As shown in figure 8, all evaluable Wnts (11 of 19) require WLS for full Wnt/β-catenin signaling activity.
Fig. 7.
All tested Wnts bind WLS in a PORCN dependent manner. HeLa cells were transfected with C-terminal V5 tagged Wnts (1000 ng per 10 cm dish) and treated with either DMSO or the PORCN inhibitor IWP1 (2 μM) overnight. 500 μg of lysate was subject to immunoprecipitation with anti-V5 antibody and immunoblot carried out with anti-WLS and anti-V5 antibodies. Arrow indicates WLS, asterisk (*) indicates IgG heavy chain. Hex (#) indicates non- specific band.
4. Discussion
Here we report the cloning of all 19 human Wnts and the subsequent analysis of their expression in cells, their secretion and accumulation in media, and their signaling properties through the Wnt/β-catenin pathway. All V5-tagged Wnt proteins are produced in HEK293 cells and secreted into the media (Fig. 2). While some Wnts (1, 2B2, 3A, 7A and 9A) are highly expressed, others (2, 4, 5A, 5B and 16) are poorly produced. There are also major differences in the estimated concentrations at which Wnts are secreted into the media, and these largely correlate with the expression levels. Since Wnt mRNA levels are relatively similar, this variation could be due to several factors (Fig. S2). Some Wnts might contain seed sequences for miRNAs to target and therefore downregulate the translation of the message. Another possibility is that different protein structures or folding have varying effects on Wnt protein stability. This is supported by the fact that Wnt proteins are expressed and secreted in relatively similar patterns in two different cell lines, with the exception of WNT16, which is produced at higher levels in NIH3T3 cells. The variation in Wnt protein levels suggests that the use of conditioned media to survey Wnt activity might not be an accurate representation of the signaling potential of each Wnt since they are all expressed and secreted at different levels. Therefore, it is essential to confirm whether Wnt proteins are efficiently secreted and can be detected in the media before reaching conclusions about their signaling properties, a task made easier by these matched untagged and V5-tagged Wnt expression constructs. The greater number of Wnt protein isoforms observed in cell lysates compared with culture media is likely due to the different stages of processing and protein modifications that these particular Wnts undergo inside cells prior to secretion. This could provide a glimpse into the post-translational modifications of Wnt proteins and how different this process is for each ligand. For example, WNT1 has four isoforms as it goes through several steps of processing while WNT3 has only one isoform. Curiously, out of the Wnts that have more than one isoform in HEK293 cells, only Wnts 1 and 8B exhibit a similar processing pattern in NIH3T3 cells, suggesting key differences between the two cell lines in the Wnt biogenesis pathway.
The use of the STF reporter has allowed us to assess and compare the ability of all Wnts to signal through the Wnt/β-catenin pathway (Fig. 3 and 6A). Twelve Wnts are capable of significantly activating the β-catenin-dependent pathway in HEK293 cells, ranging from 2- to 450-fold above background. The inability of the other 7 Wnts to activate the STF reporter may not accurately reflect their potential, since it is possible that the cell-surface receptors required for these Wnts to signal through β-catenin are not expressed in HEK293 cells. Indeed, this has already been shown for WNT5A (Mikels and Nusse, 2006). In our system, WNT8B and Wnt10A were inactive in HEK293 and HeLa cells but activated the STF reporter in HT1080 cells, with WNT8B increasing signal nearly 100 fold above background. This is consistent with the model that the repertoire of cell surface receptors determines the nature of the response to the Wnts. For example, the inhibitory effects of WNT5A in HEK293 cells are typically mediated through the orphan tyrosine kinase receptor Ror2, but when Fz4 and LRP5 are available for binding, WNT5A activates β-catenin signaling (Mikels and Nusse, 2006). A second hypothesis for the inactivity of some Wnts could be inability to interact with other Wnts. The clearest example is that of Wnts 5A and 11, which may heterodimerize together to activate β-catenin signaling (Cha et al., 2009). Since this interaction is dependent on tyrosyl sulfation of both interacting Wnts, defects in Wnt sulfation or the absence of the corresponding Wnt partner could all be reasons for the inability to activate the STF reporter. Overall, these data suggest that all Wnts might be capable of signaling through the β-catenin arm of the pathway as long as the right components are expressed. One might also argue that the expression/secretion profiles affect signaling potential; Wnts that are abundantly secreted might be expected to signal efficiently while those that are poorly produced may not signal at all. However, this is clearly not the case. WNT2, which is barely detectable in western blots (Fig. 2A), is one of the more potent activators of the β-catenin pathway (Fig. 3A). Meanwhile, highly expressed/secreted Wnts such as 2B2, 9A and 10A have little to no activity.
Activation of the β-catenin pathway can be effectively abrogated by the Wnt inhibitors Dkk-1, SFRP1 and SFRP3 (Fig. 3). Inhibition by Dkk-1 is not surprising considering that β-catenin signaling is mediated through the LRP5/6 co-receptor and that Dkk-1 blocks Wnt signaling by binding to LRP5/6. SFRP1 and SFRP3-mediated repression of Wnt activity suggests that those two members of the SFRP family can efficiently bind to all the Wnts that were tested. The ineffective inhibition by SFRP2, on the other hand, might be due to poor expression or to poor binding to Wnt ligands in HEK293 cells (Fig. 3C). However, as others report, SFRP proteins 1 and 2, but not 3 are strong inhibitors of WNT3A-mediated signaling in L cells, a mouse fibroblast cell line (Galli et al., 2006). It was previously reported that C-terminal tagging of Wnts can have negative effects on Wnt ligand activity (data not shown). Here we show that these Gateway-V5-tagged Wnts can no longer activate β-catenin signaling and that inactivity may be due to Wnt-specific variable causes (Fig. 4). This is demonstrated by the finding that the C-terminal tagging of WNT1 likely blocks its ability to bind to cell surface receptors, while our data suggest that WNT3A-V5 can bind to receptors, but does not induce activation of the pathway for other reasons. While the V5-tagged Wnts are inactive and appear to be secreted at a slightly lower concentration than their untagged counterparts, they have proven to be important for monitoring Wnt expression, secretion and accumulation in the media (Fig. 2 and 4C).
In addition to surveying Wnts for activation of the STF reporter, we also examined their ability to induce phosphorylation of LRP6 and stabilization of β-catenin in HEK293 cells (Fig. 5). All Wnts that activate the STF reporter are also capable of inducing LRP6 phosphorylation and β-catenin stabilization, two requirements for the activation of Wnt/β-catenin signaling. Interestingly, some Wnts such as 2B2, 9A and 10A can trigger LRP6 phosphorylation and β-catenin stabilization despite not having any effect on STF activity. The same Wnts are also highly expressed and secreted into the media, suggesting that despite their abundance and stability, these Wnts are not potent enough to significantly affect the transcriptional output of the Wnt pathway. In this case, it is possible that the stabilized β-catenin is bound to E-cadherin complexes at the plasma membrane or not effectively transported into the nucleus (Orsulic et al., 1999). This is certainly true in cancer, where stabilized β-catenin is often predominantly cytosolic rather than nuclear. Alternatively, although stabilized, perhaps there is a threshold of β-catenin accumulation that needs to be reached before it can translocate to the nucleus and activate transcription. Even if β-catenin accumulates and translocates to the nucleus, activation of gene expression is not always guaranteed (Prieve and Waterman, 1999). In other cases, LRP6 phosphorylation and β-catenin stabilization do not go hand in hand. For example, WNT8B upregulates phospho-LRP6, but cannot stabilize β-catenin in HEK293 cells, suggesting that LRP6 phosphorylation may not always be sufficient to inactivate the destruction complex. Whatever is missing in HEK293 cells is present in HT1080 cells, because WNT8B is a strong activator of the STF reporter in that system (Fig. 6). On the other hand, WNT10A stabilizes β-catenin without detectable phospho-LRP6, again suggesting that phospho-LRP6 is an insensitive assay, or that there may be an alternative ligand-receptor combination. While Wnt-mediated transcriptional activation is always accompanied by either LRP6 phosphorylation or the accumulation of β-catenin, the inverse is not true: even when both LRP6 phosphorylation and β-catenin accumulation are observed, STF reporter activation is not guaranteed.
Finally, we investigated whether all Wnts require PORCN-mediated palmitoylation to signal and to bind to the transporter protein WLS. We find that all Wnts that signal through the β-catenin pathway in HT1080 cells are dependent on the presence of PORCN to activate the STF reporter (Fig. 6A). In addition, the Wnts that signal through β-catenin-independent pathways (4, 5A, 11 and 16) also require PORCN to induce the phosphorylation of Dvl2, a marker of both β-catenin-dependent and independent Wnt activity (Fig. 6B). WNT5B is the only Wnt that does not exhibit any signaling activity that we could measure, but it too required PORCN function to bind to WLS. We also find that WLS is required at least for the Wnt/β-catenin signaling activity of all tested Wnts, since WLS knockdown inhibited the STF reporter activity of the 11 Wnts that were assessed in HEK293 cells (Fig 8). Since Wnts 5A and 5B have very similar amino acid sequences, it is possible that the few amino acid differences dictate the differences in their signaling activity. Thus, PORCN and WLS form an essential core Wnt production module. We also confirm that one key function of Wnt palmitoylation is to enable their binding to WLS. Finally, an implication of this work is that inhibition of either PORCN or WLS will block the ability of cells to secrete any active Wnts, which makes them potential drug targets for diseases of excess Wnt activity.
The availability of this complete and standardized set of untagged and V5-tagged Wnt plasmids has made it possible to thoroughly compare and contrast the secretion and signaling profiles of all human Wnt ligands and will be useful to the scientific community to gain new understanding of the Wnt signaling pathway.
Supplementary Material
Table S1. Gateway WNT PCR conditions
Table S2. FLAG WNT PCR conditions
Acknowledgments
We would like to acknowledge Roel Nusse, Janet Heasman, Jeff Rubin and Randall Moon for support, and Karl Willert and Roel Nusse for advice. We also acknowledge members of the Waterman and Virshup laboratory for critique and comments. Special thanks to Jason Yee and Kian Mehrazarin for help with the cloning project and Wen Hwa Lee and Guikai George Wu for advice about GeneTex Wnt antibodies.
Funding
This work was supported by NIH grants CA096878 and CA108697 to MLW. In Singapore, this research was supported by the Duke-NUS Signature Research Program and the Singapore Translational Research (STaR) Investigator Award (to DMV) funded by the Agency for Science, Technology and Research, Singapore, and the Ministry of Health, Singapore.
References
- Banziger C, Soldini D, Schutt C, Zipperlen P, Hausmann G, Basler K. Wntless, a conserved membrane protein dedicated to the secretion of Wnt proteins from signaling cells. Cell. 2006;125:509–522. doi: 10.1016/j.cell.2006.02.049. [DOI] [PubMed] [Google Scholar]
- Bartscherer K, Pelte N, Ingelfinger D, Boutros M. Secretion of Wnt ligands requires Evi, a conserved transmembrane protein. Cell. 2006;125:523–533. doi: 10.1016/j.cell.2006.04.009. [DOI] [PubMed] [Google Scholar]
- Bourhis E, Tam C, Franke Y, Bazan JF, Ernst J, Hwang J, Costa M, Cochran AG, Hannoush RN. Reconstitution of a frizzled8.Wnt3a.LRP6 signaling complex reveals multiple Wnt and Dkk1 binding sites on LRP6. J Biol Chem. 2010;285:9172–9179. doi: 10.1074/jbc.M109.092130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cha SW, Tadjuidje E, White J, Wells J, Mayhew C, Wylie C, Heasman J. Wnt11/5a complex formation caused by tyrosine sulfation increases canonical signaling activity. Curr Biol. 2009;19:1573–1580. doi: 10.1016/j.cub.2009.07.062. [DOI] [PubMed] [Google Scholar]
- Chen B, Dodge ME, Tang W, Lu J, Ma Z, Fan CW, Wei S, Hao W, Kilgore J, Williams NS, Roth MG, Amatruda JF, Chen C, Lum L. Small molecule-mediated disruption of Wnt-dependent signaling in tissue regeneration and cancer. Nat Chem Biol. 2009;5:100–107. doi: 10.1038/nchembio.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ching W, Hang HC, Nusse R. Lipid-independent secretion of a Drosophila Wnt protein. J Biol Chem. 2008;283:17092–17098. doi: 10.1074/jbc.M802059200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coombs GS, Yu J, Canning CA, Veltri CA, Covey TM, Cheong JK, Utomo V, Banerjee N, Zhang ZH, Jadulco RC, Concepcion GP, Bugni TS, Harper MK, Mihalek I, Jones CM, Ireland CM, Virshup DM. WLS-dependent secretion of WNT3A requires Ser209 acylation and vacuolar acidification. J Cell Sci. 2010;123:3357–3367. doi: 10.1242/jcs.072132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson G, Wu W, Shen J, Bilic J, Fenger U, Stannek P, Glinka A, Niehrs C. Casein kinase 1 gamma couples Wnt receptor activation to cytoplasmic signal transduction. Nature. 2005;438:867–872. doi: 10.1038/nature04170. [DOI] [PubMed] [Google Scholar]
- Endo Y, Beauchamp E, Woods D, Taylor WG, Toretsky JA, Uren A, Rubin JS. Wnt-3a and Dickkopf-1 stimulate neurite outgrowth in Ewing tumor cells via a Frizzled3- and c-Jun N-terminal kinase-dependent mechanism. Mol Cell Biol. 2008;28:2368–2379. doi: 10.1128/MCB.01780-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galli LM, Barnes T, Cheng T, Acosta L, Anglade A, Willert K, Nusse R, Burrus LW. Differential inhibition of Wnt-3a by Sfrp-1, Sfrp-2, and Sfrp-3. Dev Dyn. 2006;235:681–690. doi: 10.1002/dvdy.20681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glinka A, Wu W, Delius H, Monaghan AP, Blumenstock C, Niehrs C. Dickkopf-1 is a member of a new family of secreted proteins and functions in head induction. Nature. 1998;391:357–362. doi: 10.1038/34848. [DOI] [PubMed] [Google Scholar]
- Herr P, Basler K. Porcupine-mediated lipidation is required for Wnt recognition by Wls. Dev Biol. 2012;361:392–402. doi: 10.1016/j.ydbio.2011.11.003. [DOI] [PubMed] [Google Scholar]
- Kim H, Cheong SM, Ryu J, Jung HJ, Jho EH, Han JK. Xenopus Wntless and the retromer complex cooperate to regulate XWnt4 secretion. Mol Cell Biol. 2009;29:2118–2128. doi: 10.1128/MCB.01503-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishida S, Yamamoto H, Kikuchi A. Wnt-3a and Dvl induce neurite retraction by activating Rho-associated kinase. Mol Cell Biol. 2004;24:4487–4501. doi: 10.1128/MCB.24.10.4487-4501.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klaus A, Birchmeier W. Wnt signalling and its impact on development and cancer. Nat Rev Cancer. 2008;8:387–398. doi: 10.1038/nrc2389. [DOI] [PubMed] [Google Scholar]
- Li X, Zhang Y, Kang H, Liu W, Liu P, Zhang J, Harris SE, Wu D. Sclerostin binds to LRP5/6 and antagonizes canonical Wnt signaling. J Biol Chem. 2005;280:19883–19887. doi: 10.1074/jbc.M413274200. [DOI] [PubMed] [Google Scholar]
- Liu C, Li Y, Semenov M, Han C, Baeg GH, Tan Y, Zhang Z, Lin X, He X. Control of beta-catenin phosphorylation/degradation by a dual-kinase mechanism. Cell. 2002;108:837–847. doi: 10.1016/s0092-8674(02)00685-2. [DOI] [PubMed] [Google Scholar]
- Mao B, Wu W, Li Y, Hoppe D, Stannek P, Glinka A, Niehrs C. LDL-receptor-related protein 6 is a receptor for Dickkopf proteins. Nature. 2001;411:321–325. doi: 10.1038/35077108. [DOI] [PubMed] [Google Scholar]
- Mikels AJ, Nusse R. Purified Wnt5a protein activates or inhibits beta-catenin-TCF signaling depending on receptor context. PLoS Biol. 2006;4:e115. doi: 10.1371/journal.pbio.0040115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Najdi R, Holcombe RF, Waterman ML. Wnt signaling and colon carcinogenesis: beyond APC. J Carcinog. 2011;10:5. doi: 10.4103/1477-3163.78111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orsulic S, Huber O, Aberle H, Arnold S, Kemler R. E-cadherin binding prevents beta-catenin nuclear localization and beta-catenin/LEF-1-mediated transactivation. J Cell Sci. 1999;112 (Pt 8):1237–1245. doi: 10.1242/jcs.112.8.1237. [DOI] [PubMed] [Google Scholar]
- Prieve MG, Waterman ML. Nuclear localization and formation of beta-catenin-lymphoid enhancer factor 1 complexes are not sufficient for activation of gene expression. Mol Cell Biol. 1999;19:4503–4515. doi: 10.1128/mcb.19.6.4503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takada R, Satomi Y, Kurata T, Ueno N, Norioka S, Kondoh H, Takao T, Takada S. Monounsaturated fatty acid modification of Wnt protein: its role in Wnt secretion. Dev Cell. 2006;11:791–801. doi: 10.1016/j.devcel.2006.10.003. [DOI] [PubMed] [Google Scholar]
- Tamai K, Zeng X, Liu C, Zhang X, Harada Y, Chang Z, He X. A mechanism for Wnt coreceptor activation. Mol Cell. 2004;13:149–156. doi: 10.1016/s1097-2765(03)00484-2. [DOI] [PubMed] [Google Scholar]
- van Amerongen R, Nusse R. Towards an integrated view of Wnt signaling in development. Development. 2009;136:3205–3214. doi: 10.1242/dev.033910. [DOI] [PubMed] [Google Scholar]
- Willert K, Brown JD, Danenberg E, Duncan AW, Weissman IL, Reya T, Yates JR, 3rd, Nusse R. Wnt proteins are lipid-modified and can act as stem cell growth factors. Nature. 2003;423:448–452. doi: 10.1038/nature01611. [DOI] [PubMed] [Google Scholar]
- Yost C, Torres M, Miller JR, Huang E, Kimelman D, Moon RT. The axis-inducing activity, stability, and subcellular distribution of beta-catenin is regulated in Xenopus embryos by glycogen synthase kinase 3. Genes Dev. 1996;10:1443–1454. doi: 10.1101/gad.10.12.1443. [DOI] [PubMed] [Google Scholar]
- Zeng X, Tamai K, Doble B, Li S, Huang H, Habas R, Okamura H, Woodgett J, He X. A dual-kinase mechanism for Wnt co-receptor phosphorylation and activation. Nature. 2005;438:873–8. doi: 10.1038/nature04185. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Gateway WNT PCR conditions
Table S2. FLAG WNT PCR conditions