Abstract
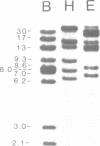
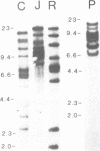
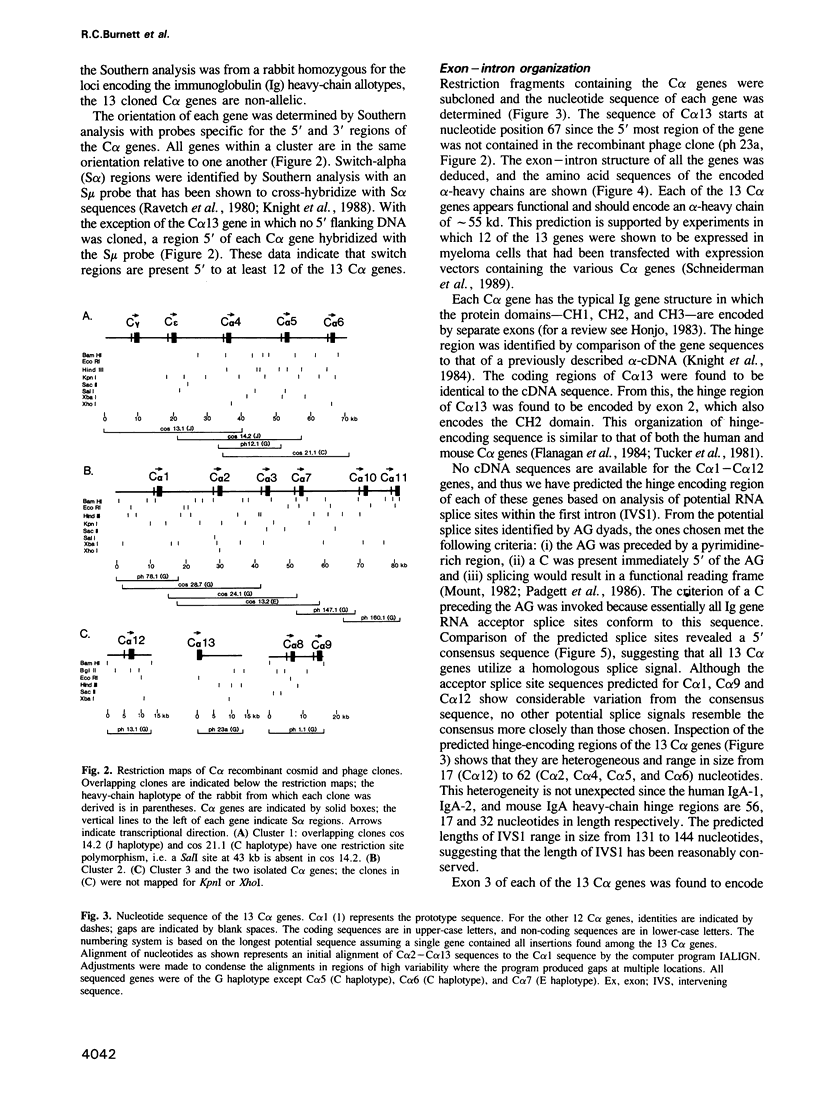
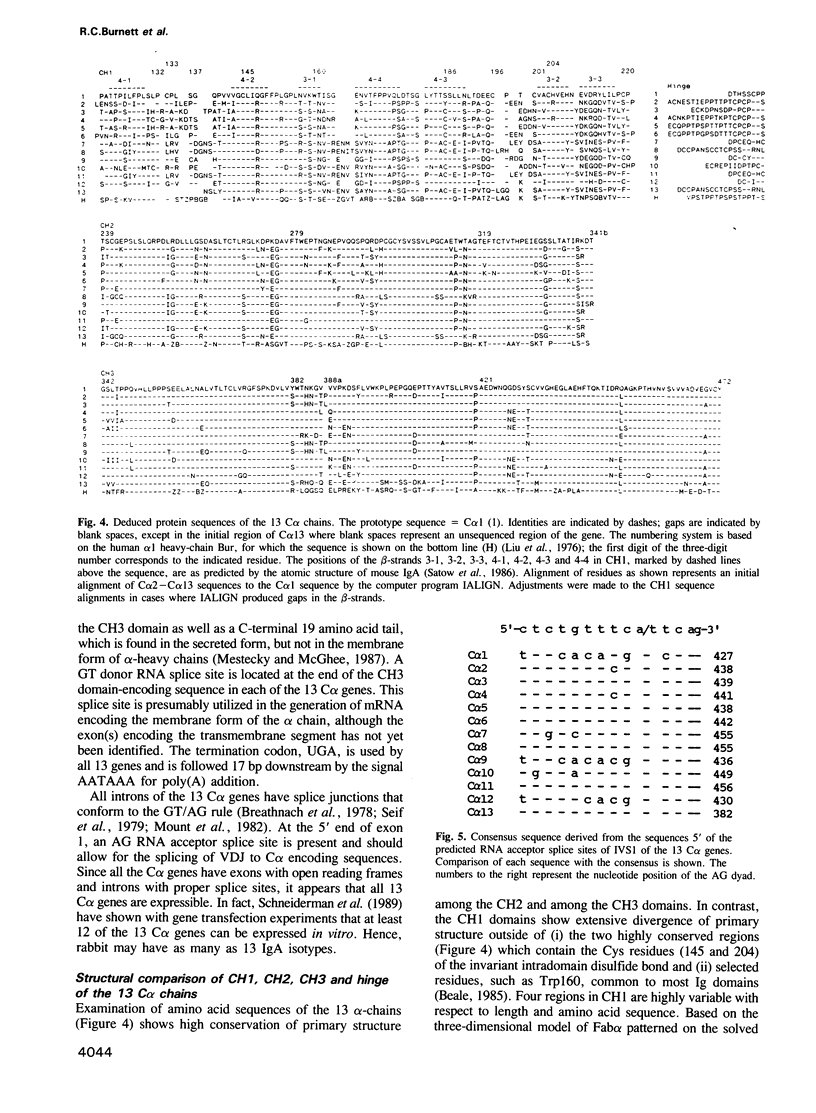
Southern analysis has previously shown that the rabbit genome contains multiple genes coding for the constant regions of IgA heavy chains. In the present study, clones containing these C alpha genes have been isolated from cosmid and phage libraries. Restriction mapping and Southern analysis of the clones identified 13 non-allelic C alpha genes; 11 of the genes were clustered in individual or overlapping clones. The clustered genes are separated by 8-18 kb, and in total, the C alpha genes span a minimum of 160 kb of DNA. Southern analysis has shown that all genes within a cluster have the same transcriptional orientation, and that switch sequences are present 5' of at least 12 of the 13 genes. The nucleotide sequence of each C alpha gene was determined, and it appears that all genes are functional; thus, rabbit may have as many as 13 IgA isotypes. Comparisons of the protein sequences encoded by the 13 C alpha genes showed that the CH2 and CH3 domains of the alpha-chains are highly conserved, whereas the CH1 and hinge regions are highly diverse. Southern analysis of genomic DNA samples from other species within the order Lagomorpha showed that all samples had multiple C alpha hybridizing fragments. Thus, it is likely that all lagomorphs have multiple IgA isotypes and hence complex secretory immune systems.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beale D. A comparison of the amino acid sequences of the extracellular domains of the immunoglobulin superfamily. Possible correlations between conservancy and conformation. Comp Biochem Physiol B. 1985;80(2):181–194. doi: 10.1016/0305-0491(85)90194-4. [DOI] [PubMed] [Google Scholar]
- Breathnach R., Benoist C., O'Hare K., Gannon F., Chambon P. Ovalbumin gene: evidence for a leader sequence in mRNA and DNA sequences at the exon-intron boundaries. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4853–4857. doi: 10.1073/pnas.75.10.4853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway T. P., Dray S., Lichter E. A. Identification and genetic control of three rabbit gamma-A immunoglobulin allotypes. J Immunol. 1969 Mar;102(3):544–554. [PubMed] [Google Scholar]
- Dayhoff M. O., Barker W. C., Hunt L. T. Establishing homologies in protein sequences. Methods Enzymol. 1983;91:524–545. doi: 10.1016/s0076-6879(83)91049-2. [DOI] [PubMed] [Google Scholar]
- Deisenhofer J. Crystallographic refinement and atomic models of a human Fc fragment and its complex with fragment B of protein A from Staphylococcus aureus at 2.9- and 2.8-A resolution. Biochemistry. 1981 Apr 28;20(9):2361–2370. [PubMed] [Google Scholar]
- Elliott B. W., Jr, Friedenson B., Knight K. L. Free sulfhydryl groups of rabbit secretory IgA. J Immunol. 1980 Oct;125(4):1611–1617. [PubMed] [Google Scholar]
- Flanagan J. G., Lefranc M. P., Rabbitts T. H. Mechanisms of divergence and convergence of the human immunoglobulin alpha 1 and alpha 2 constant region gene sequences. Cell. 1984 Mar;36(3):681–688. doi: 10.1016/0092-8674(84)90348-9. [DOI] [PubMed] [Google Scholar]
- Honjo T. Immunoglobulin genes. Annu Rev Immunol. 1983;1:499–528. doi: 10.1146/annurev.iy.01.040183.002435. [DOI] [PubMed] [Google Scholar]
- Knight K. L., Burnett R. C., McNicholas J. M. Organization and polymorphism of rabbit immunoglobulin heavy chain genes. J Immunol. 1985 Feb;134(2):1245–1250. [PubMed] [Google Scholar]
- Knight K. L., Martens C. L., Stoklosa C. M., Schneiderman R. D. Genes encoding alpha-heavy chains of rabbit IgA: characterization of cDNA encoding IgA-g subclass alpha-chains. Nucleic Acids Res. 1984 Feb 10;12(3):1657–1670. doi: 10.1093/nar/12.3.1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight K. L., Suter M., Becker R. S. Genetic engineering of bovine Ig. Construction and characterization of hapten-binding bovine/murine chimeric IgE, IgA, IgG1, IgG2, and IgG3 molecules. J Immunol. 1988 May 15;140(10):3654–3659. [PubMed] [Google Scholar]
- Liu Y. S., Low T. L., Infante A., Putnam F. W. Complete covalent structure of a human IgA1 immunoglobulin. Science. 1976 Sep 10;193(4257):1017–1020. doi: 10.1126/science.821146. [DOI] [PubMed] [Google Scholar]
- Mendez E., Prelli F., Frangione B., Franklin E. C. Characterization of a disulfide bridge linking the J chain to the alpha chain of polymeric immunoglobulin A. Biochem Biophys Res Commun. 1973 Dec 19;55(4):1291–1297. doi: 10.1016/s0006-291x(73)80034-8. [DOI] [PubMed] [Google Scholar]
- Messing J., Vieira J. A new pair of M13 vectors for selecting either DNA strand of double-digest restriction fragments. Gene. 1982 Oct;19(3):269–276. doi: 10.1016/0378-1119(82)90016-6. [DOI] [PubMed] [Google Scholar]
- Mestecky J., McGhee J. R. Immunoglobulin A (IgA): molecular and cellular interactions involved in IgA biosynthesis and immune response. Adv Immunol. 1987;40:153–245. doi: 10.1016/s0065-2776(08)60240-0. [DOI] [PubMed] [Google Scholar]
- Mestecky J., Schrohenloher R. E., Kulhavy R., Wright G. P., Tomana M. Site of J chain attachment to human polymeric IgA. Proc Natl Acad Sci U S A. 1974 Feb;71(2):544–548. doi: 10.1073/pnas.71.2.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mount S. M. A catalogue of splice junction sequences. Nucleic Acids Res. 1982 Jan 22;10(2):459–472. doi: 10.1093/nar/10.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muth K. L., Hanly W. C., Knight K. L. Serologic cross-reactions among rabbit secretory IgA molecules: evidence for multiple subclasses of secretory IgA-f molecules. Mol Immunol. 1983 Sep;20(9):989–999. doi: 10.1016/0161-5890(83)90040-8. [DOI] [PubMed] [Google Scholar]
- Padgett R. A., Grabowski P. J., Konarska M. M., Seiler S., Sharp P. A. Splicing of messenger RNA precursors. Annu Rev Biochem. 1986;55:1119–1150. doi: 10.1146/annurev.bi.55.070186.005351. [DOI] [PubMed] [Google Scholar]
- Ravetch J. V., Kirsch I. R., Leder P. Evolutionary approach to the question of immunoglobulin heavy chain switching: evidence from cloned human and mouse genes. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6734–6738. doi: 10.1073/pnas.77.11.6734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satow Y., Cohen G. H., Padlan E. A., Davies D. R. Phosphocholine binding immunoglobulin Fab McPC603. An X-ray diffraction study at 2.7 A. J Mol Biol. 1986 Aug 20;190(4):593–604. doi: 10.1016/0022-2836(86)90245-7. [DOI] [PubMed] [Google Scholar]
- Schneiderman R. D., Hanly W. C., Knight K. L. Expression of 12 rabbit IgA C alpha genes as chimeric rabbit-mouse IgA antibodies. Proc Natl Acad Sci U S A. 1989 Oct;86(19):7561–7565. doi: 10.1073/pnas.86.19.7561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seif I., Khoury G., Dhar R. BKV splice sequences based on analysis of preferred donor and acceptor sites. Nucleic Acids Res. 1979 Jul 25;6(10):3387–3398. doi: 10.1093/nar/6.10.3387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmetz M., Winoto A., Minard K., Hood L. Clusters of genes encoding mouse transplantation antigens. Cell. 1982 Mar;28(3):489–498. doi: 10.1016/0092-8674(82)90203-3. [DOI] [PubMed] [Google Scholar]
- Tucker P. W., Slightom J. L., Blattner F. R. Mouse IgA heavy chain gene sequence: implications for evolution of immunoglobulin hinge axons. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7684–7688. doi: 10.1073/pnas.78.12.7684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamawaki-Kataoka Y., Miyata T., Honjo T. The complete nucleotide sequence of mouse immunoglobin gamma 2a gene and evolution of heavy chain genes: further evidence for intervening sequence-mediated domain transfer. Nucleic Acids Res. 1981 Mar 25;9(6):1365–1381. doi: 10.1093/nar/9.6.1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C., Kratzin H., Götz H., Hilschmann N. Zur Strukturregel der Antikörper. Die Primärstruktur eines monoklonalen IgA 1-Immunglobulins (Myelomprotein Tro). VII. Darstellung, Reinigung und Charakterisierung der Disulfidbrücken. Hoppe Seylers Z Physiol Chem. 1979 Dec;360(12):1919–1940. [PubMed] [Google Scholar]