Abstract
Inflammatory responses are increasingly implicated in the pathogenesis of neurodegenerative diseases such as in Alzheimer’s disease (AD). Interleukin-33 (IL-33), a member of IL-1 family, is constitutively expressed in the central nervous system and thought to be an important mediator of glial cell response to neuropathological lesions. Proinflammatory molecules are highly expressed at the vicinity of amyloid plaques (APs) and neurofibrillary tangles (NFTs), the hallmarks of AD pathology. We have investigated the expression of IL-33 and ST2 in relation to APs and NFTs in human AD and non-AD control brains by immunohistochemistry. Sections from the entorhinal cortex, where APs and NFTs appear in early stages of AD, were used for immunohistochemistry. Mouse primary astrocytes were cultured and incubated with amyloid-β1–42 (Aβ1–42), component of plaque for 72 h and analyzed for the expression of IL-33 by flow cytometry. We found strong expression of IL-33 and ST2 in the vicinity of Aβ and AT8 labelled APs and NFTs respectively, and in the glial cells in AD brains when compared to non-AD control brains. IL-33 and ST2 positive cells were also significantly increased in AD brains when compared to non-AD brains. Flow cytometric analysis revealed incubation of mouse astrocytes with Aβ1–42 increased astrocytic IL-33 expression in vitro. These results suggest that IL-33, an alamin cytokine, may induce inflammatory molecule release from the glial cells and may play important role in the pathogenesis of AD.
Keywords: Alzheimer’s disease, amyloid plaques, glia maturation factor, IL-33, neurofibrillary tangles, ST2
INTRODUCTION
Alzheimer’s disease (AD) is the most common form of dementia among the elderly, affecting 5 million Americans and more than 35 million individuals worldwide [1, 2]. This disorder causes rapid neuron loss and deterioration. The etiology of AD remains unknown and there is no definite treatment yet available [3, 4]. Neuropathologically, AD is characterized by the presence of abundant amyloid plaque (AP) deposition and neurofibrillary tangles (NFTs) in brain. These lesions contain hyperphosphorylated tau associated with loss of neurons and an abundance of reactive glia in AD brain [5]. The plaques are generally associated with activated microglia and surrounded by reactive astrocytes [6]. The number of NFTs in the medial temporal lobe, including the entorhinal cortex (Brodmann’s area 28) and hippocampal formation, is associated with the cognitive memory impairment in AD patients [7]. Research is increasingly focused on the role of inflammatory responses in the progression of AD, and how it might accelerate the course of the disease [7, 8]. Inflammation occurs relatively early in the course of AD pathology [9].
Our recent studies have shown that glia maturation factor, a proinflammatory molecule, is highly expressed in the vicinity of APs and NFTs in temporal cortex of human AD brains [5, 10, 11]. IL-33, a member of the interleukin-1 (IL-1β) family, may also have an important function in AD [8]. IL-33 acts as a proinflammatory cytokine as well as an intra-cellular nuclear factor with transcriptional regulatory properties. IL-33 may be specifically proinflammatory in the central nervous system (CNS) [8]. Microglia and astrocytes express IL-33 receptors containing a trans-membrane portion called ST2. The glial cells respond by proliferating and releasing tumor necrosis factor-alpha (TNF-α), IL-1β, and nitric oxide [12]. IL-33 is reported to release IL-1β and TNF-α from inflammatory cells [13]. TNF-α and IL-1β are potent inducers of IL-33 from other cell types such as sub-epithelial myofibroblasts [14]. It is known that both IL-1β and TNF-α were increased in the brains of AD patients when compared to age-matched control subjects [15].
IL-33 release in excess above the physiological level may induce proinflammatory reactions and may act as a prominent inflammatory mediator of neuroinflammation in the CNS. Further, IL-33 is known to activate inflammatory cells, including glial cells [16, 17]. IL-33 has been shown to be increased in the tissues of various inflammatory and autoimmune diseases [18, 19]. Therefore, in the present study we have analyzed the expression of IL-33 and its receptor, ST2 in relation to APs and NFTs distribution in the brains of AD patients. Using immunohistochemistry, we found that both IL-33 and ST2 are localized at the vicinity of APs in human AD brains.
MATERIALS AND METHODS
Human brain tissues
Ten postmortem human AD and six non-AD brains were obtained from the University of Iowa Deeded Body Program. AD and non-AD case details were shown in Table 1. Temporal lobe blocks were removed and fixed in 4% paraformaldehyde in phosphate buffered saline (PBS). Blocks were preserved in 30% sucrose solution until they sunk, and sections were cut at 40–50 μm thickness on a freezing microtome. Sections from the entorhinal cortex, where APs and NFTs appear in early stages of AD, were used. These sections from AD and non-AD brains were immunostained for tau (NFTs, brown color) with AT8 antibody for AD pathology (Fig. 1A, B). The sections were also stained with 6E10 antibody to detect APs in the entorhinal cortex of AD and non-AD brains. The number of APs in the entorhinal cortex (boxed areas) was counted under the microscope using high power objectives and averaged for each case. AD brain entorhinal cortex showed significantly increased number of APs when compared to non-AD brain entorhinal cortex (Fig. 1C). We have used section from these AD and non-AD brains for this investigation with IL-33 or ST2 expression. The University of Iowa Institutional approved guidelines were followed in carrying out all the experimental procedures [5].
Table 1.
Case details
| Cases | AD
|
Non-AD
|
|||||
|---|---|---|---|---|---|---|---|
| Age (y) Gender | Brain Wt. (g) | Dementia (y) | PMI (h) | Age (y) Gender | Brain Wt. (g) | PMI (h) | |
| 1 | 74F | 875 | 12 | 3.0 | 69F | 1200 | 5.0 |
| 2 | 77F | 978 | 9 | 5.0 | 80F | 1075 | 3.5 |
| 3 | 85F | 966 | 8 | 6.5 | 80F | 1028 | 4.0 |
| 4 | 88F | 1022 | 9 | 3.0 | 66M | 1338 | 6.5 |
| 5 | 89F | 1039 | 11 | 3.5 | 77M | 1160 | 4.0 |
| 6 | 79M | 1158 | 7 | 5.0 | 80M | 1080 | 11 |
| 7 | 80M | 1165 | 11 | 4.2 | |||
| 8 | 77M | 1267 | 4 | 3.0 | |||
| 9 | 72M | 1320 | 10 | 6.0 | |||
| 10 | 80M | 1350 | 5 | 7.5 | |||
| Mean ± SD | 80 ± 5.6 | 1114 ± 162 | 8.6 ± 2.6 | 4.7 ± 1.6 | 75 ± 6.3 | 1146 ± 112 | 5.6 ± 2.8 |
PMI, postmortem interval.
Fig. 1.

Representative photomicrograph showing the entorhinal cortex from non-AD (A) and AD (B) brains immunostained for tau (NFTs, brown color) with AT8 antibody to detect AD pathology. C) Brain sections were also immunostained with 6E10 antibody to detect APs in the entorhinal cortex (boxed areas) of AD and non-AD brains. The number of APs was counted in 5–10 non-overlapping visual fields under the microscope using high power objectives and averaged for each case. AD (n = 10) brains entorhinal cortex showed significantly increased (p = <0.05, t test) number of APs when compared to non-AD (n = 6) brain entorhinal cortex. We have used brain sections from these AD and non-AD cases to immunostain for IL-33 or ST2.
Immunohistochemistry and thioflavin-S histochemistry
Free-floating sections from the entorhinal cortex were processed for immunohistochemistry. Briefly, the sections were heated in citrate buffer (10 mM citric acid in dH2O) for 1 min for antigen retrieval and then incubated for 30 min in 0.3% H2O2 in PBS as we have reported previously [20]. Then the sections were blocked for 1 h with 5% normal goat serum containing 3% bovine serum albumin (BSA) with 0.3% Triton-X 100, diluted in PBS. IL-33 rabbit polyclonal antibody (5 μg/ml), Abcam, Cambridge, MA) and ST2 rabbit polyclonal antibody (2 μg/ml; ProSci, Poway, CA) were used to incubate overnight at 4°C. Then the sections were incubated with biotinylated goat anti-rabbit IgG (Vector Laboratories, Burlingame, CA) for 1 h at room temperature (RT) and then developed for 1 h in avidin-biotin-complex (1 : 2000 dilutions) standard solution (Vector Laboratories). Following this, the sections were incubated in DAB peroxidase substrate solution (Vector Laboratories). Sections were washed 3 times in PBS at RT in between the incubation steps. The sections were counterstained with 1% aqueous Thioflavin-S (Sigma, St. Louis, MO) fluorescent marker to detect APs and NFTs in the AD brain sections and then mounted with Fluorogel containing Tris buffer (Electron Microscopy Sciences, Hatfield, PA). For the staining specificity of IL-33 and ST2, equal protein concentrations of isotype matched IgG antibodies instead of the primary antibodies were used.
Single immunofluorescence detection of IL-33 or ST2 with thioflavin-S histochemistry for APs
Tissue sections were incubated overnight at 4°C with mouse monoclonal IL-33 antibody (Pierce, Thermo Scientific, Rockford, IL) or rabbit polyclonal ST2 antibody diluted to 20 μg/ml and 2 μg/ml, respectively, as we have reported previously [5]. The next day, sections were incubated with secondary antibodies for 1 h at RT. Goat anti-mouse IgG and goat anti-rabbit IgG were used for the detection of IL-33 and ST2, respectively. The secondary antibodies were conjugated with Alexa Fluor 568 (Invitrogen-Molecular Probes, Eugene, OR). After the incubation, sections were counterstained using Thioflavin-S fluorescent marker for APs detection, washed with dH2O, and mounted with Fluorogel. Sections were washed three times in PBS at RT in between the incubation steps.
Quantitation of IL-33 and ST2 immunohistochemistry and statistical analysis
To quantitate the IL-33 and ST2 immunostaining, we have counted IL-33-positive and ST2-positive cells in the entorhinal cortex of AD (and non-AD brains as we have reported previously [20]. The counting was performed under 400× in five different fields and then averaged. Difference between AD and non-AD brains was analyzed using unpaired t-test. A p value less than 0.05 was considered statistically significant. The data were presented as the number of IL-33 or ST2-positive cells/95 mm2.
Double immunofluorescence detection of IL-33 and ST2 with APs and NFTs
Double immunofluorescence was carried out to detect IL-33 (rabbit IL-33 polyclonal antibody; Abcam) or ST2 (rabbit ST2 polyclonal antibody; ProSci) with Aβ (1 μg/ml; Beta Amyloid 1–16 (6E10) monoclonal antibody; Covance, Dedham, MA) for APs or AT8 (phospho-PHF-tau pSer202/Thr205 antibody (AT8); 100 ng/ml; Thermo Scientific; Rockford, IL) for NFTs. We have performed double immunofluorescence for IL-33 (mouse monoclonal IL-33 antibody) with astrocytes or microglia as we have reported previously. In this, we have used antibodies to the glial fibrillary acidic protein (GFAP; rabbit polyclonal GFAP antibody, Millipore, Temecula, CA) or ionized calcium binding adaptor molecule 1 (Iba-1; rabbit polyclonal antibody, 1 μg/ml; Wako, Richmond, VA) to label activated astrocytes and activated microglia, respectively. After overnight incubation with the primary antibodies at 4°C, sections were incubated for 1 h at RT with the secondary antibodies. Mouse mono-clonal antibodies were visualized with goat anti-mouse IgG conjugated with fluorescent dye, and rabbit poly-clonal antibodies were visualized with goat anti-rabbit IgG conjugated with fluorescent dye. Alexa Fluor 488 (green color) or Alexa Fluor 568 (red color; Life Technologies, Grand Island, NY, City) was used to visualize. The immunohistochemistry sections were observed and the images were obtained on a Leica DMI6000 B inverted microscope (Maryland Heights, MO) and Nikon DIAPHOT (Garden City, NY).
Fluorescence-activated cell sorting (FACS) of IL-33 expression in mouse astrocytes
Mouse (C57BL/6) primary astrocytes were cultured as described previously with Dulbecco’s Modified Eagle Medium Nutrient Mixture F-12 (DMEM, Life Technologies, Grand Island, NY) [21, 22]. Animals were cared as per the guidelines of the University of Iowa Institutional Animal Care and Use committee (IACUC). Mouse astrocytes were incubated with Aβ1–42 (1 μM; EZBiolab, Carmel, IN) for 72 h at 37°C in 75 cm2 tissue culture flasks (n = 3). After the incubation period was over, the astrocytes were detached by trypsinization and processed for FACS analysis as per the procedure described by R&D Systems (Minneapolis, MN). The expression of intracellular IL-33 was analyzed using monoclonal anti-mouse IL-33 phycoerythrin-conjugated antibody (R&D System) by flow cytometry (BD LSR II with violet, BD Biosciences, San Jose, CA) using 561 nm wavelength excitation and monitoring emitted fluorescence with a detector optimized to collect peak emissions at 585 nm.
RESULTS
IL-33 and ST2 are co-localized with plaques in AD brain by immunohistochemistry
We have analyzed the expression of IL-33 and its receptor ST2 in the affected entorhinal cortex of AD brains in relation to the distribution of APs. IL-33 detection was carried out by immunohistochemistry (Fig. 2A, C, brown color), and Thioflavin-S staining (green color) was performed to detect the APs in AD brains. In the representative AD case, NFTs and APs (white arrow heads) were found in the entorhinal cortex. Results show high expression of IL-33 in entorhinal cortex where APs were abundant in AD brains (Fig. 2A, C) when compared to non-AD brains (Fig. 2E). IL-33 was found to be co-localized with two types of plaques: those with dense, highly fluorescent cores and those that were diffuse. IL-33 was highly expressed in a pattern surrounding the APs by glial cells (Fig. 2A, C, black arrows). Next, we have performed immunohistochemistry with DAB substrate staining for the detection of ST2 (Fig. 2B, D, F) and Thioflavin-S staining for APs. We demonstrate that ST2 (arrows) was diffusely expressed within APs and also more concentrated around the lesions in the entorhinal cortex of AD patients (Fig. 2B). Figure 2C, D shows the photomicrographs of low magnification and Isotype matched IgG for staining control from AD brain.
Fig. 2.
Immunohistochemical analysis of IL-33 and its receptor, ST2, expression and their co-localization with APs of entorhinal cortex in human AD (n = 10) and non-AD brains (n = 6). We performed immunohistochemistry using DAB substrate (brown color) for IL-33 (A, C) and ST2 expression (B, D) in AD brains, and non-AD brains (E, F). Thioflavin-S fluorescence (green color) staining was used to detect APs in the brain (A-F). A) IL-33 expression was co-localized with the APs in the entorhinal cortex. IL-33 and ST2 expression was also seen in glial cells (black arrows) surrounding the plaques (white arrows heads). ST2 (black arrows) receptor expression was also co-localized and seen within lesion (white arrow heads) but more concentrated around the APs in the affected entorhinal cortex of AD brain. C and D show lower magnification along with Isotype matched IgG as staining control from AD brains, and E and F are from non-AD brains. Merged images show co-localization of IL-33 or ST2 with APs in AD brains. Original magnification A, B = 400×; C, D, E, F = 200×. ThioS, Thioflavin S.
IL-33 and ST2 expression is increased in the entorhinal cortex of AD brains
To quantitate the IL-33 and ST2, the number of IL-33-positive and ST2 positive cells were counted in the entorhinal cortex of AD and non-AD brains. Both IL-33 and ST2-positive cells were significantly increased (p = <0.05, t test) in the AD brains when compared to non-AD brains (Fig. 3). The data were presented as the number of IL-33 or ST2-positive cells/95 mm2.
Fig. 3.

IL-33 and ST2 expression is increased in the entorhinal cortex of AD brain. We have counted IL-33-positive and ST2 positive cells in the entorhinal cortex of AD (n = 10) and non-AD (n = 6) brains using the immunohistochemistry slides. The counting was performed under the microscope using high magnification objectives at five different fields in the section and then averaged. The data were presented as the number of IL-33 or ST2-positive cells/95 = mm2. The data were presented as mean ± SEM, *p<0.05, t test.
IL-33 and ST2 are co-localized with plaques and tangles in the affected entorhinal cortex of AD brain by immunofluorescence
We then studied if IL-33 and ST2 expression is co-localized with plaques and tangles by immunofluorescence staining in the entorhinal cortex of AD brains. We first performed immunofluorescence staining of IL-33 or ST2 followed by Thioflavin-S staining to detect APs (arrows) and NFTs (arrowheads) (Fig. 4A). The brain sections were first incubated either with monoclonal IL-33 and goat anti-mouse IgG Alexa Fluor conjugated 568 (red color) or with ST2 antibody and goat anti-rabbit IgG Alexa fluor conjugated 568 (red color) followed by Thioflavin-S staining (green color). Results showed that both IL-33 (red, arrows) and ST2 (red, arrows) expression was co-localized at the vicinity of APs (green, arrows) and NFTs (green, arrowheads) in the AD brain as shown in the merged pictures (Fig. 4A; yellow color, arrows = APs; arrowheads = NFTs). In higher magnification images obtained from different patient, we show the co-localization of IL-33 or ST2 expression (red, arrows) within and around Thioflavin-S-marked APs (green, arrow heads; Fig. 4B). Merged image (yellow color) show co-localization of IL-33 (arrows) or ST2 (arrows) with Thioflavin-S stained APs (arrowheads; Fig. 4B).
Fig. 4.
A) Immunofluorescence analysis of IL-33 and ST2 expression and their co-localization with plaques and tangles in affected entorhinal cortex of human AD brain (n = 10). We have first performed immunofluorescence staining of IL-33 or ST2 and then performed Thioflavin-S fluorescence to detect APs (arrows) and NFTs (arrowheads). The sections were stained with monoclonal IL-33 and goat anti-mouse IgG Alexa Fluor conjugated 568 (red color). Sections were also stained with ST2 and goat anti-rabbit IgG Alexa fluor conjugated 568 (red color). Following these IL-33 or ST2 staining, thioflavin–S staining (green color) was performed in these sections. Both IL-33 and ST2 expression was co-localized at the vicinity of APs (arrows) and NFTs (arrowheads) in the AD brain as shown in the merged picture (yellow color). Original magnification = 200×. B) In high magnification using brain from another patient, IL-33 expression was co-localized and also found concentrated near the center of APs in AD brain (top panel). ST2 expression was concentrated at the periphery of the plaques in AD brain (bottom panel). Both IL-33 and ST2 were co-localized with APs in the AD brain as shown in the merged picture (yellow color). Original magnification = 400×.
IL-33 and ST2 were co-localized with Aβ and AT8 labelled APs and NFTs, respectively by double immunofluorescence staining in the entorhinal cortex of AD brains
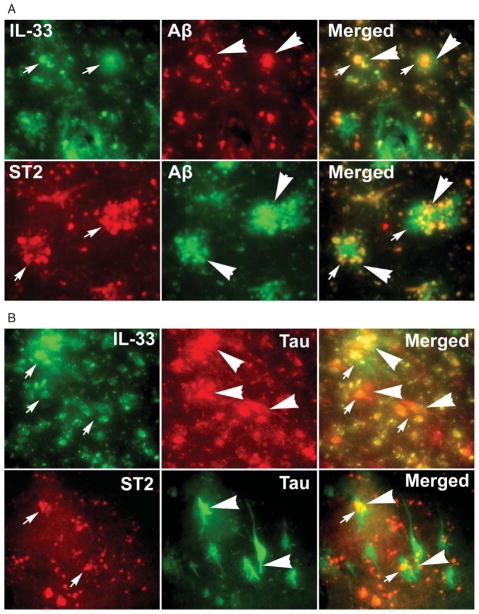
Next, we have performed (Fig. 5A) double immunofluorescence staining of IL-33 (green, arrows) or ST2 (red, arrows) and with amyloid plaques labeled with Aβ antibody (red or green arrowheads) staining in the entorhinal cortex of AD brain. Aβ staining indicates APs (arrow heads). Co-localization of IL-33 or ST2 with APs (arrow and arrow heads) is shown by the yellow color in merged images (Fig. 5A). Then we have also performed (Fig. 5B) double immunofluorescence staining of IL-33 (green, arrows) or ST2 (red, arrows) with phosphorylated tau with AT8 antibody (red or green, arrowheads) for NFTs in AD brain. AT8 Tau staining shows NFTs (arrow heads). Co-localization of IL-33 or STZ with NFTs (arrows and arrow heads) is shown by the yellow color in merged images (Fig. 5B).
Fig. 5.
IL-33 and ST2 were co-localized with Aβ and AT8 labelled APs and NFTs, respectively by double immunofluorescence staining in the entorhinal cortex of AD brains (n = 10). A) Double immunofluorescence staining of IL-33 (green, arrows) or ST2 (red, arrows) with amyloid plaques labeled with Aβ antibody (red or green arrowheads) staining in the AD brain. Co-localization of IL-33 or ST2 with APs is shown by the yellow color in merged images. Original magnifications = ×400. B) Double immunofluorescence staining of IL-33 (green, arrows) or ST2 (red, arrows) with phosphorylated tau with AT8 antibody (red or green, arrowheads) for NFTs antibody in AD brain. Co-localization of IL-33 or ST2 with NFTs is shown by the yellow color in merged images. Original magnification = ×400.
IL-33 expression in relation to glial cells in AD brain by immunofluorescence
We report elevated level of IL-33 (arrow heads) expression in the vicinity of APs surrounded by glial cells in the affected entorhinal cortex of AD brain (Fig. 6). Hypertrophic microglias were found within a smaller radius inside the Thioflavin-S-labeled APs compared to activated astrocytes. In AD cases, the microglia were highly concentrated in areas containing APs and NFTs. Microglia also underwent hypertrophy, with thicker processes and high expression of Iba-1 (Fig. 6, upper panel, arrows), as detected with Iba-1 primary antibody and goat anti-rabbit IgG Alexa Fluor 568 (red color). Astrocytes were detected with GFAP primary antibody and goat anti-mouse IgG Alexa Fluor 568 (lower panel, red color, arrows). In the AD brains, activated astrocytes were more heavily populated around plaques with dense cores. IL-33 and GFAP (Fig. 5, arrows) expression overlap at the center of these structures (merged figure).
Fig. 6.
Immunofluorescence localization of IL-33 in relation to astrocytes and microglia in the affected entorhinal cortex of human AD brain (n = 10). Sections were incubated with IL-33 primary antibody and then either with Iba-1 or GFAP antibody for the detection of microglia and astrocytes, respectively. Then these sections were incubated with secondary antibodies, Alexa Fluor 488 goat anti-rabbit IgG or Alexa Fluor 488 goat anti-mouse IgG. IL-33 expression (arrow heads) was seen at the lesional area of the entorhinal cortex. Upregulated expression of IL-33 (green) was localized in two patterns, dense and diffuse, similar to plaques. Upper panel: Monoclonal IL-33 antibody labeling shows the lesion infiltrated by hypertrophic microglia expressing Iba-1 (upper panel, red color, arrows). Areas of overlaps appear yellow color as shown in the merged figure. Lower panel: Polyclonal IL-33 antibody labeling shows the dense structure surrounded by a ring of astrocytes. Note the thickened processes of the activated astrocytes (arrows), which express high levels of GFAP (lower panel, red color, arrows). Original magnification = 400×.
Aβ1–42 induces the expression of IL-33 in mouse primary astrocytes
Since high IL-33 expression was detected at the vicinity of plaques, we have analyzed the expression of IL-33 by astrocytes after incubating for 72 h with Aβ1–42, which is a component of plaques (n = 3). Representative histograms show that Aβ1–42 increased the expression of IL-33 in the astrocytes when compared to untreated control astrocytes as determined by flow cytometry (Fig. 7).
Fig. 7.
Aβ1–42 induces the expression of IL-33 in mouse primary astrocytes. Astrocytes were incubated with Aβ1–42 (1 μM) for 72 h at 37°C in vitro. Then the expression of IL-33 was determined by flow cytometry using monoclonal anti-IL-33 phycoerythrin conjugated antibody (n = 3). Representative histograms show that Aβ1–42 increased the expression of IL-33 in the astrocytes when compared to untreated control astrocytes.
DISCUSSION
In the present study, we report that IL-33 is localized in the vicinity of APs and NFTs and is associated with glial cells that are thought to play key roles in plaque development during AD pathogenesis. We report that both IL-33 and ST2-positive cells were significantly increased in the AD brains when compared to non-AD brains. Further, we also have detected IL-33 receptor, ST2, associated with lesions indicating their expression in the glial cells. AD is characterized by the presence of APs, NFTs, and inflammatory responses. Previous report demonstrate increased level of IL-18 and also co-localization with APs in AD brains [23]. IL-18 is also known to induce Aβ production in SH-SY5Y cells [24]. Recent studies have shown that IL-33 exacerbates autoimmune diseases such as multiple sclerosis [25, 26]. The IL-33/ST2 signaling mechanism plays important roles in the neuroinflammatory diseases [27]. Several chemokines are secreted from the CNS lesions, attracting immune cells from across the blood-brain barrier or within the brain. Activated microglia and astrocytes are found in close association with plaques in AD brains [5, 28]. Activated glia reported to remove Aβ and therefore the activation of microglia and astrocytes may be beneficial for the AD patients at the early stages of disease [29]. Activated glial cells release several proinflammatory cytokines/chemokines and free radical oxygen species [30] and exacerbate neuroinflammatory reactions [31]. IL-33 has been shown to activate microglia and enhance phagocytosis [12] suggesting a protective role of IL-33, probably when the inflammatory responses are limited. However, prolonged and sustained inflammatory reaction with further upregulated expression of IL-33 and other neuroactive and inflammatory molecules may induce glial cells to cause cytotoxic effects and neuronal damage and thereby may increase the disease progression. Previous study has reported that incubation of rat mixed glial and neuronal culture with another IL-33 family member IL-1β, and TNF-α for 48 h caused decrease of neurons, indicating decreased neuronal survival or neurotoxic effect of IL-1β and TNF-α [32].
A previous study has shown decreased IL-33 in the brains of AD patients compared to control brains by mRNA and immunohistochemical analysis [33]. They reported that IL-33 is restricted to vascular capillaries in the brain and suggested IL-33 as a potential genetic determinant of AD [33]. They suggested that there is a clear evidence of an association between the risk of developing AD and the IL-33 gene; our present study has shown increased expression of IL-33 and ST2 in the vicinity of APs and NFTs in the affected entorhinal cortex of AD brains, as previously reported for IL-18 in AD brain [23]. They have used temporal anterior cortex (Brodmann area 38) for IL-33 immunohistochemistry and frontal cortex for IL-33 mRNA analysis. However, we have used entorhinal cortex (Brodmann’s area 28) for our IL-33 immunohistochemistry. AD pathogenesis starts first in the entorhinal cortex before the regions used in the above mentioned studies. Also genetic variants of IL-33 affect susceptibility to late onset AD in specific population [34]. Similar to our present findings, upregulated expression of other proinflammatory molecules in the affected region of AD brains has been previously reported [5, 11, 28]. Moreover, IL-33 has been shown to be elevated in the tissues of other inflammatory diseases including autoimmune diseases [18, 19, 35]. Proinflammatory molecules in the brain may contribute to the dysfunction in AD patients. IL-33 induces proliferation of microglia and enhances production of proinflammatory cytokines, such as IL-1β and TNF-α, as well as the anti-inflammatory cytokine IL-10 [12]. The proinflammatory molecules in turn, could also induce additional plaque deposition in the AD brain. The proinflammatory molecules such as TNF-α are neurotoxic in AD [28, 32]. One important IL-33 family member, IL-1β, is already known to activate glial cells in AD.
IL-33 is a signaling protein that is highly expressed in the CNS constitutively. In vitro and in vivo testing in animal models showed that binding of IL-33 to its receptor, a heterodimer consisting of IR-1RAcP and transmembrane ST2, activated inflammatory signaling pathways involving nuclear factor-kappa β and mitogen activated protein kinases pathways [36]. It has been previously reported that IL-33 expression increased in mouse glial cells in response to stimulation by pathogen-associated molecular pattern molecules and ATP [8]. A previous study has shown that IL-18 increased Aβ production and suggested Aβ may induce the synthesis of IL-18 [24]. Here, we have shown that Aβ increases the expression of IL-33 in mouse astrocytes. In order to understand the mechanism of how IL-33 expression could be increased in the vicinity of plaques, we have incubated mouse astrocytes with Aβ in vitro and demonstrate that Aβ increases astrocytic expression of IL-33, similar to the increased immunoreactivity for IL-33 that was seen adjacent to plaques in the AD brain specimens. Our recent study has shown that IL-33 induces the release of CCL2, TNF-α, and nitric oxide from mouse astrocytes in vitro [37]. Further our previous study also showed that incubation of mixed culture (astrocytes and neurons) or neuronal culture with IL-33 reduced the number of microtubule-associated protein 2–immunoreactive cells indicating neurodegeneration and neuronal death [37] similar to the neurotoxic effects of IL-1β or TNF-α reported previously [38].
The present study demonstrates increased expression of IL-33 and its receptor ST2 in the lesions of affected entorhinal cortex, and these lesions were penetrated by microglia and surrounded by astrocytes. The spatial relationship between glial cells, IL-33, and APs supports the link between inflammation and development of AD pathology. Microglia, the primary immune cells of the CNS is known to respond to chemokines signaling by expressing proinflammatory cytokines. These cells contain ST2, indicating sensitivity to IL-33 secreted from astrocytes. Astrocytes not only secrete IL-33 but also express ST2 receptors [12]. IL-33 may act as an autocrine and or paracrine signaling manner in glial cells and contribute to the neuroinflammation in AD brain. IL-33 levels may be determined in serum or cerebrospinal fluid from patients suspected of having AD. Its presence in AD also makes it a prospective therapeutic target. Our results show expression of IL-33 in the neuropathological lesions in the AD brain, and that IL-33 may exacerbate neuroinflammation in AD.
Acknowledgments
This work was supported by the Department of Veterans Affairs Merit Review award (to A.Z.) and by the National Institute of Neurological Disorders and Stroke grants NS073670 (to A.Z.).
Footnotes
Authors’ disclosures available online (http://www.j-alz.com/disclosures/view.php?id=2027).
References
- 1.Farfara D, Lifshitz V, Frenkel D. Neuroprotective and neurotoxic properties of glial cells in the pathogenesis of Alzheimer’s disease. J Cell Mol Med. 2008;12:762–780. doi: 10.1111/j.1582-4934.2008.00314.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Paris D, Ganey NJ, Laporte V, Patel NS, Beaulieu-Abdelahad D, Bachmeier C, March A, Ait-Ghezala G, Mullan MJ. Reduction of beta-amyloid pathology by celastrol in a transgenic mouse model of Alzheimer’s disease. J Nuroinflammation. 2010;7:17. doi: 10.1186/1742-2094-7-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carter MD, Simms GA, Weaver DF. The development of new therapeutics for Alzheimer’s disease. Clin Pharmacol Ther. 2010;88:475–486. doi: 10.1038/clpt.2010.165. [DOI] [PubMed] [Google Scholar]
- 4.Korczyn AD. Why have we failed to cure Alzheimer’s disease? J Alzheimers Dis. 2012;29:275–282. doi: 10.3233/JAD-2011-110359. [DOI] [PubMed] [Google Scholar]
- 5.Thangavel R, Stolmeier D, Yang X, Anantharam P, Zaheer A. Expression of glia maturation factor in neuropathological lesions of Alzheimer’s disease. Neuropathol Appl Neurobiol. 2011;38:572–581. doi: 10.1111/j.1365-2990.2011.01232.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Medeiros R, LaFerla FM. Astrocytes: conductors of the Alzheimer disease neuroinflammatory symphony. Exp Neurol. 2013;239:133–138. doi: 10.1016/j.expneurol.2012.10.007. [DOI] [PubMed] [Google Scholar]
- 7.Lee YJ, Han SB, Nam SY, Oh KW, Hong JT. Inflammation and Alzheimer’s disease. Arch Pharm Res. 2010;33:1539–1556. doi: 10.1007/s12272-010-1006-7. [DOI] [PubMed] [Google Scholar]
- 8.Hudson CA, Christophi GP, Gruber RC, Wilmore JR, Lawrence DA, Massa PT. Induction of IL-33 expression and activity in central nervous system glia. J Leukoc Biol. 2008;84:631–643. doi: 10.1189/jlb.1207830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Van Eldik LJ, Thompson WL, Ralay Ranaivo H, Behanna HA, Martin Watterson D. Glia proinflammatory cytokine upregulation as a therapeutic target for neurodegenerative diseases: function-based and target-based discovery approaches. Int Rev Neurobiol. 2007;82:277–296. doi: 10.1016/S0074-7742(07)82015-0. [DOI] [PubMed] [Google Scholar]
- 10.Stolmeier D, Thangavel R, Anantharam P, Khan MM, Kempuraj D, Zaheer A. Glia maturation factor expression in hippocampus of human Alzheimer’s disease. Neurochem Res. 2013;38:1580–1589. doi: 10.1007/s11064-013-1059-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zaheer S, Thangavel R, Sahu SK, Zaheer A. Augmented expression of glia maturation factor in Alzheimer’s disease. Neuroscience. 2011;194:227–233. doi: 10.1016/j.neuroscience.2011.07.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yasuoka S, Kawanokuchi J, Parajuli B, Jin S, Doi Y, Noda M, Sonobe Y, Takeuchi H, Mizuno T, Suzumura A. Production and functions of IL-33 in the central nervous system. Brain Res. 2011;1385:8–17. doi: 10.1016/j.brainres.2011.02.045. [DOI] [PubMed] [Google Scholar]
- 13.Moulin D, Donze O, Talabot-Ayer D, Mezin F, Palmer G, Gabay C. Interleukin (IL)-33 induces the release of pro-inflammatory mediators by mast cells. Cytokine. 2007;40:216–225. doi: 10.1016/j.cyto.2007.09.013. [DOI] [PubMed] [Google Scholar]
- 14.Kobori A, Yagi Y, Imaeda H, Ban H, Bamba S, Tsujikawa T, Saito Y, Fujiyama Y, Andoh A. Interleukin-33 expression is specifically enhanced in inflamed mucosa of ulcerative colitis. J Gastroenterol. 2010;45:999–1007. doi: 10.1007/s00535-010-0245-1. [DOI] [PubMed] [Google Scholar]
- 15.Llano DA, Li J, Waring JF, Ellis T, Devanarayan V, Witte DG, Lenz RA. Cerebrospinal Fluid Cytokine Dynamics Differ Between Alzheimer Disease Patients and Elderly Controls. Alzheimer Dis Assoc Disord. 2011;26:322–328. doi: 10.1097/WAD.0b013e31823b2728. [DOI] [PubMed] [Google Scholar]
- 16.Espinassous Q, Garcia-de-Paco E, Garcia-Verdugo I, Synguelakis M, von Aulock S, Sallenave JM, McKenzie AN, Kanellopoulos J. IL-33 enhances lipopolysaccharide-induced inflammatory cytokine production from mouse macrophages by regulating lipopolysaccharide receptor complex. J Immunol. 2009;183:1446–1455. doi: 10.4049/jimmunol.0803067. [DOI] [PubMed] [Google Scholar]
- 17.Theoharides TC, Zhang B, Kempuraj D, Tagen M, Vasiadi M, Angelidou A, Alysandratos KD, Kalogeromitros D, Asadi S, Stavrianeas N, Peterson E, Leeman S, Conti P. IL-33 augments substance P-induced VEGF secretion from human mast cells and is increased in psoriatic skin. Proc Natl Acad Sci U S A. 2010;107:4448–4453. doi: 10.1073/pnas.1000803107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li M, Li Y, Liu X, Gao X, Wang Y. IL-33 blockade suppresses the development of experimental autoimmune encephalomyelitis in C57BL/6 mice. J Neuroimmunol. 2012;247:25–31. doi: 10.1016/j.jneuroim.2012.03.016. [DOI] [PubMed] [Google Scholar]
- 19.Liew FY, Pitman NI, McInnes IB. Disease-associated functions of IL-33: the new kid in the IL-1 family. Nat Rev Immunol. 2010;10:103–110. doi: 10.1038/nri2692. [DOI] [PubMed] [Google Scholar]
- 20.Thangavel R, Kempuraj D, Stolmeier D, Anantharam P, Khan M, Zaheer A. Glia maturation factor expression in entorhinal cortex of Alzheimer’s disease brain. Neurochem Res. 2013;38:1777–1784. doi: 10.1007/s11064-013-1080-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zaheer A, Mathur SN, Lim R. Overexpression of glia maturation factor in astrocytes leads to immune activation of microglia through secretion of granulocyte-macrophage-colony stimulating factor. Biochem Biophys Res Commun. 2002;294:238–244. doi: 10.1016/S0006-291X(02)00467-9. [DOI] [PubMed] [Google Scholar]
- 22.Zaheer A, Yorek MA, Lim R. Effects of glia maturation factor overexpression in primary astrocytes on MAP kinase activation, transcription factor activation, and neurotrophin secretion. Neurochem Res. 2001;26:1293–1299. doi: 10.1023/a:1014241300179. [DOI] [PubMed] [Google Scholar]
- 23.Ojala J, Alafuzoff I, Herukka SK, van Groen T, Tanila H, Pirttila T. Expression of interleukin-18 is increased in the brains of Alzheimer’s disease patients. Neurobiol Aging. 2009;30:198–209. doi: 10.1016/j.neurobiolaging.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 24.Sutinen EM, Pirttila T, Anderson G, Salminen A, Ojala JO. Pro-inflammatory interleukin-18 increases Alzheimer’s disease-associated amyloid-beta production in human neuron-like cells. J Neuroinflammatio. 2012;9:199. doi: 10.1186/1742-2094-9-199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Christophi GP, Gruber RC, Panos M, Christophi RL, Jubelt B, Massa PT. Interleukin-33 upregulation in peripheral leukocytes and CNS of multiple sclerosis patients. Clin Immunol. 2012;142:308–319. doi: 10.1016/j.clim.2011.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Milovanovic M, Volarevic V, Radosavljevic G, Jovanovic I, Pejnovic N, Arsenijevic N, Lukic ML. IL-33/ST2 axis in inflammation and immunopathology. Immunol Res. 2012;52:89–99. doi: 10.1007/s12026-012-8283-9. [DOI] [PubMed] [Google Scholar]
- 27.Han P, Mi WL, Wang YQ. Research progress on interleukin-33 and its roles in the central nervous system. Neurosci Bull. 2011;27:351–357. doi: 10.1007/s12264-011-1025-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ruan L, Kang Z, Pei G, Le Y. Amyloid deposition and inflammation in APPswe/PS1dE9 mouse model of Alzheimer’s disease. Curr Alzheimer Rese. 2009;6:531–540. doi: 10.2174/156720509790147070. [DOI] [PubMed] [Google Scholar]
- 29.Koistinaho M, Lin S, Wu X, Esterman M, Koger D, Hanson J, Higgs R, Liu F, Malkani S, Bales KR, Paul SM. Apolipoprotein E promotes astrocyte colocalization and degradation of deposited amyloid-beta peptides. Nat Med. 2004;10:719–726. doi: 10.1038/nm1058. [DOI] [PubMed] [Google Scholar]
- 30.Sastre M, Klockgether T, Heneka MT. Contribution of inflammatory processes to Alzheimer’s disease: molecular mechanisms. Int j Dev Neurosi. 2006;24:167–176. doi: 10.1016/j.ijdevneu.2005.11.014. [DOI] [PubMed] [Google Scholar]
- 31.Meraz-Rios MA, Toral-Rios D, Franco-Bocanegra D, Villeda-Hernandez J, Campos-Pena V. Inflammatory process in Alzheimer’s Disease. Front Integr Neurosc. 2013;7:59. doi: 10.3389/fnint.2013.00059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marx CE, Jarskog LF, Lauder JM, Lieberman JA, Gilmore JH. Cytokine effects on cortical neuron MAP-2 immunoreactivity: implications for schizophrenia. Biol Psychiatry. 2001;50:743–749. doi: 10.1016/s0006-3223(01)01209-4. [DOI] [PubMed] [Google Scholar]
- 33.Chapuis J, Hot D, Hansmannel F, Kerdraon O, Ferreira S, Hubans C, Maurage CA, Huot L, Bensemain F, Laumet G, Ayral AM, Fievet N, Hauw JJ, DeKosky ST, Lemoine Y, Iwat-subo T, Wavrant-Devrieze F, Dartigues JF, Tzourio C, Buee L, Pasquier F, Berr C, Mann D, Lendon C, Alperovitch A, Kamboh MI, Amouyel P, Lambert JC. Transcriptomic and genetic studies identify IL-33 as a candidate gene for Alzheimer’s disease. Mol Psychiatry. 2009;14:1004–1016. doi: 10.1038/mp.2009.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yu JT, Song JH, Wang ND, Wu ZC, Zhang Q, Zhang N, Zhang W, Xuan SY, Tan L. Implication of IL-33 gene polymorphism in Chinese patients with Alzheimer’s disease. Neurobiol Aging. 2012;33:1014, e1011–1014. doi: 10.1016/j.neurobiolaging.2010.07.003. [DOI] [PubMed] [Google Scholar]
- 35.Pei C, Barbour M, Fairlie-Clarke KJ, Allan D, Mu R, Jiang HR. Emerging role of interleukin-33 in autoimmune diseases. Immunology. 2014;141:9–17. doi: 10.1111/imm.12174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schmitz J, Owyang A, Oldham E, Song Y, Murphy E, McClanahan TK, Zurawski G, Moshrefi M, Qin J, Li X, Gorman DM, Bazan JF, Kastelein RA. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity. 2005;23:479–490. doi: 10.1016/j.immuni.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 37.Kempuraj D, Khan MM, Thangavel R, Xiong Z, Yang E, Zaheer A. Glia Maturation Factor Induces Interleukin-33 Release from Astrocytes: Implications for Neurodegenerative Diseases. J Neuroimmune Pharmacol. 2013;8:643–650. doi: 10.1007/s11481-013-9439-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jana M, Anderson JA, Saha RN, Liu X, Pahan K. Regulation of inducible nitric oxide synthase in proinflammatory cytokine-stimulated human primary astrocytes. Free Radic Biol Med. 2005;38:655–664. doi: 10.1016/j.freeradbiomed.2004.11.021. [DOI] [PubMed] [Google Scholar]