Abstract
Background
Epidemiological evidence has suggested a link between beta2‐agonists and increases in asthma mortality. There has been much debate about possible causal links for this association, and whether regular (daily) long‐acting beta2‐agonists are safe.
Objectives
The aim of this review is to assess the risk of fatal and non‐fatal serious adverse events in trials that randomised patients with chronic asthma to regular salmeterol versus placebo or regular short‐acting beta2‐agonists.
Search methods
We identified trials using the Cochrane Airways Group Specialised Register of trials. We checked websites of clinical trial registers for unpublished trial data and FDA submissions in relation to salmeterol. The date of the most recent search was August 2011.
Selection criteria
We included controlled parallel design clinical trials on patients of any age and severity of asthma if they randomised patients to treatment with regular salmeterol and were of at least 12 weeks' duration. Concomitant use of inhaled corticosteroids was allowed, as long as this was not part of the randomised treatment regimen.
Data collection and analysis
Two authors independently selected trials for inclusion in the review. One author extracted outcome data and the second checked them. We sought unpublished data on mortality and serious adverse events.
Main results
The review includes 26 trials comparing salmeterol to placebo and eight trials comparing with salbutamol. These included 62,815 participants with asthma (including 2,599 children). In six trials (2,766 patients), no serious adverse event data could be obtained.
All‐cause mortality was higher with regular salmeterol than placebo but the increase was not significant (Peto odds ratio (OR) 1.33 (95% CI 0.85 to 2.08)). Non‐fatal serious adverse events were significantly increased when regular salmeterol was compared with placebo (OR 1.15 95% CI 1.02 to 1.29). One extra serious adverse event occurred over 28 weeks for every 188 people treated with regular salmeterol (95% CI 95 to 2606). There is insufficient evidence to assess whether the risk in children is higher or lower than in adults. We found no significant increase in fatal or non‐fatal serious adverse events when regular salmeterol was compared with regular salbutamol.
We combined individual patient data from the two largest studies (SNS: n=25,180 and SMART: n=26,355), as all the asthma‐related deaths in adults occurred in these studies. In patients who were not taking inhaled corticosteroids, compared to regular salbutamol or placebo, there was a significant increase in risk of asthma‐related death with regular salmeterol (Peto OR 6.15 95% CI 1.73 to 21.84). The confidence interval for patients who were taking inhaled corticosteroids is wide and cannot rule in or out an increase in asthma mortality in the presence of an inhaled corticosteroid (Peto OR 2.03 95% CI 0.82 to 5.00).
Authors' conclusions
In comparison with placebo, we have found an increased risk of serious adverse events with regular salmeterol. There is also a clear increase in risk of asthma‐related mortality in patients not using inhaled corticosteroids in the two large surveillance studies. Although the increase in asthma‐related mortality was smaller in patients taking inhaled corticosteroids at baseline, the confidence interval is wide, so we cannot conclude that the inhaled corticosteroids abolish the risks of regular salmeterol. The adverse effects of regular salmeterol in children remain uncertain due to the small number of children studied.
Keywords: Adult, Child, Humans, Adrenergic beta‐Agonists, Adrenergic beta‐Agonists/therapeutic use, Albuterol, Albuterol/administration & dosage, Albuterol/adverse effects, Albuterol/analogs & derivatives, Albuterol/therapeutic use, Anti‐Asthmatic Agents, Anti‐Asthmatic Agents/administration & dosage, Anti‐Asthmatic Agents/adverse effects, Asthma, Asthma/drug therapy, Asthma/mortality, Bronchodilator Agents, Bronchodilator Agents/administration & dosage, Bronchodilator Agents/adverse effects, Cause of Death, Placebos, Placebos/therapeutic use, Randomized Controlled Trials as Topic, Salmeterol Xinafoate
Plain language summary
Does daily treatment with salmeterol result in more serious adverse events compared with placebo or a salbutamol?
Asthma is a common condition that affects the airways – the small tubes that carry air in and out of the lungs. When a person with asthma comes into contact with an irritant (an asthma trigger), the muscles around the walls of the airways tighten, the airways become narrower, and the lining of the airways becomes inflamed and starts to swell. This leads to the symptoms of asthma ‐ wheezing, coughing and difficulty in breathing. They can lead to an asthma attack or exacerbation. People can have underlying inflammation in their lungs and sticky mucus or phlegm may build up, which can further narrow the airways. There is no cure for asthma; however there are medications that allow most people to control their asthma so they can get on with daily life.
Long‐acting beta2‐agonists, such as salmeterol, work by reversing the narrowing of the airways that occurs during an asthma attack. These drugs ‐ taken by inhaler ‐ are known to improve lung function, symptoms, quality of life and reduce the number of asthma attacks. However, there are concerns about the safety of long‐acting beta2‐agonists, particularly in people who are not taking inhaled corticosteroids to control the underlying inflammation. We did this review to take a closer look at the safety of people taking salmeterol daily compared to people on placebo or the short acting beta2‐agonist salbutamol.
There was no statistically significant difference in the number of people who died during treatment with salmeterol compared with placebo or salbutamol. Because so few people die of asthma, huge trials or observational studies are normally required to detect a difference in death rates from asthma. There were more non‐fatal serious adverse events in people taking salmeterol compared to those on placebo; for every 188 people treated with salmeterol for 28 weeks, one extra non‐fatal event occurred in comparison with placebo. There was no significant differences in serious adverse events in people on salmeterol compared to regular salbutamol.
In order to obtain individual patient data on asthma deaths, we looked separately at mortality in two large trials on over 51,000 patients who were not taking inhaled corticosteroids, and found that there was an increase in the number of asthma‐related deaths among people on salmeterol.
We conclude that, for patients whose asthma is not well‐controlled on moderate doses of inhaled corticosteroids, additional salmeterol can improve symptoms but this may be at the expense of an increased risk of serious adverse events and asthma related mortality. Salmeterol should not be used as a substitute for inhaled corticosteroids, and adherence with inhaled steroids should be kept under review if separate inhalers are used. Salmeterol should not be taken by people who are not taking regular inhaled steroids due to the increased risk of asthma‐related death.
Summary of findings
Summary of findings for the main comparison. Regular salmeterol compared to placebo for chronic asthma.
| Regular salmeterol compared to placebo for chronic asthma | ||||||
| Patient or population: patients with chronic asthma Settings: Intervention: regular salmeterol Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| placebo | Regular salmeterol | |||||
| All‐cause mortality ‐ adults and adolescents Follow‐up: mean 28 weeks | 2 per 10001 | 3 per 1000 (2 to 4) | OR 1.33 (0.85 to 2.08)2 | 29128 (10 studies) | ⊕⊕⊕⊝ moderate3 | Two studies (27,266 participants) reported that deaths had occurred, and 96% of these were in the SMART study. |
| All‐cause mortality ‐ children | See comment | See comment | Not estimable | 1126 (4) | See comment | There were no deaths in children during 793 patient years of observation |
| All‐cause non‐fatal serious adverse events ‐ adults and adolescents Follow‐up: mean 28 weeks | 34 per 10001 | 39 per 1000 (34 to 43) | OR 1.14 (1.01 to 1.28) | 30,196 (13 studies) | ⊕⊕⊕⊕ high | 92% of events occurred in the SMART study |
| All‐cause non‐fatal serious adverse events ‐ children Follow‐up: mean 31 weeks | 56 per 10001 | 72 per 1000 (46 to 108) | OR 1.3 (0.82 to 2.05) | 1333 (5 studies) | ⊕⊕⊕⊝ moderate3 | |
| Asthma‐related mortality ‐ adults and adolescents Follow‐up: mean 28 weeks | Medium risk population | OR 3.49 (1.31 to 9.31) | 29,128 (10 studies) | ⊕⊕⊕⊕ high | Mean risk of asthma death in placebo arm of trials was 2 in 10,000 and 9 in 10,000 for salmeterol arm of trials | |
| 0 per 10001 | 1 per 1000 (0 to 2) | |||||
| Asthma‐related non‐fatal serious adverse events ‐ adults and adolescents Follow‐up: mean 28 weeks | 9 per 10001 | 13 per 1000 (7 to 24) | OR 1.43 (0.75 to 2.71) | 3841 (12 studies) | ⊕⊕⊝⊝ low3,4 | |
| Asthma‐related non‐fatal serious adverse events ‐ children Follow‐up: mean 31 weeks | 33 per 10001 | 55 per 1000 (33 to 92) | OR 1.72 (1 to 2.98) | 1333 (5 studies) | ⊕⊕⊕⊝ moderate4 | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; OR: odds ratio | ||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate Very low quality: we are very uncertain about the estimate | ||||||
1 Assumed risks on control are taken from the mean risk in control arms of included trials 2 Risk ratios can be considered to be the same as odds ratios as event rates are very low 3 Few events were observed leading to wide CIs (including the possibilities of no effect and appreciable harm) 4 There was no independent assessment of the cause of serious adverse events, leading to possible ascertainment bias for disease‐specific outcomes
Summary of findings 2. Regular salmeterol compared to regular salbutamol for chronic asthma.
| Regular salmeterol compared to regular salbutamol for chronic asthma | ||||||
| Patient or population: patients with chronic asthma Settings: Intervention: regular salmeterol Comparison: regular salbutamol | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| regular salbutamol | Regular salmeterol | |||||
| All‐cause mortality ‐ adults and adolescents Follow‐up: mean 16 weeks | 2 per 10001 | 3 per 1000 (2 to 4) | OR 1.28 (0.79 to 2.05)2 | 26,463 (4 studies) | ⊕⊕⊕⊝ moderate3 | |
| All‐cause mortality ‐ children Follow‐up: mean 12 weeks | 3 per 10001 | 0 per 1000 (0 to 9) | OR 0.04 (0 to 2.97) | 1152 (3 studies) | ⊕⊕⊝⊝ low3 | A single death occurred in 273 patient‐years of observation in children |
| All‐cause non‐fatal serious adverse events ‐ adults and adolescents Follow‐up: mean 16 weeks | 43 per 10001 | 41 per 1000 (37 to 47) | OR 0.96 (0.85 to 1.1) | 27,002 (5 studies) | ⊕⊕⊕⊝ moderate4 | |
| All‐cause non‐fatal serious adverse events ‐ children Follow‐up: mean 12 weeks | 28 per 10001 | 38 per 1000 (20 to 71) | OR 1.37 (0.71 to 2.64) | 1181 (3 studies) | ⊕⊕⊕⊝ moderate3 | |
| Asthma‐related mortality ‐ adults and adolescents Follow‐up: mean 16 weeks | Medium risk population | OR 2.36 (0.78 to 7.16) | 26,463 (4 studies) | ⊕⊕⊕⊝ moderate3 | The mean risk of death from asthma was 2 per 10,000 on the regular salbutamol arms of the trials and 7 per 10,000 on the regular salmeterol arms. | |
| 0 per 10001 | 0 per 1000 (0 to 1) | |||||
| Asthma‐related non‐fatal serious adverse events ‐ adults and adolescents Follow‐up: mean 12 weeks | 17 per 10001 | 16 per 1000 (6 to 39) | OR 0.94 (0.37 to 2.34) | 1155 (3 studies) | ⊕⊕⊝⊝ low3,5 | |
| Asthma‐related non‐fatal serious adverse events ‐ children Follow‐up: mean 12 weeks | 23 per 10001 | 24 per 1000 (11 to 52) | OR 1.04 (0.47 to 2.31) | 1186 (3 studies) | ⊕⊕⊝⊝ low3,5 | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) CI: confidence interval; OR: odds ratio | ||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate Very low quality: we are very uncertain about the estimate | ||||||
1 Assumed risks on control are taken from the mean risk in control arms of included trials 2 Risk ratios can be considered to be the same as odds ratios as event rates are very low 3 Few events were observed leading to wide CIs (including the possibilities of no effect and appreciable harm) 4 The CI includes the possibility of no difference between treatments but is too wide to consider that the risks are equivalent 5 There was no independent assessment of the cause of serious adverse events, leading to possible bias in the ascertainment of disease‐specific outcomes
Background
Description of the condition
There is currently no universally accepted definition of the term "asthma". This is in part due to an overlap of asthmatic symptoms with those of other diseases such as chronic obstructive pulmonary disease (COPD), but is also due to the probable existence of more than one underlying pathophysiological process. There are, for example, wide variations in the age of onset, symptoms, triggers, associations with allergic disease and the type of inflammatory cell infiltrate seen in patients diagnosed with severe asthma (Miranda 2004). Patients with all forms and severity of disease will typically have intermittent symptoms of cough, wheeze and/or breathlessness. Underlying these symptoms there is a process of variable, at least partially reversible airway obstruction, airway hyper responsiveness and, in most cases, chronic inflammation.
Airway obstruction
Patients with a history of asthma demonstrate chronic changes within the airways including goblet cell hyperplasia, airway smooth muscle (ASM) hyperplasia and hypertrophy (Ebina 1993; Ordonez 2001; Woodruff 2004) and excess myofibroblasts with increased subepithelial collagen deposition (Brewster 1990). In the acute setting, in patients who have died of status asthmaticus airway obstruction is evident from air‐trapping and lung hyperinflation with mucus plugging of the small and large airways (Dunnill 1960; Kuyper 2003). There is also shedding of ciliated bronchial mucosal cells, inflammatory cell infiltrates and submucosal oedema with transudation of fluid into the bronchial lumen (Carroll 1993). It is more difficult to measure the degree of ASM contraction (bronchoconstriction) at post‐mortem studies although evidence for a role of bronchoconstriction in airway narrowing comes from other sources.
Airway hyper responsiveness
Patients with asthma typically display a degree of 'airway hyper responsiveness' to inhaled allergens (Cockcroft 2006), and to a variety of chemical stimuli including histamine, serotonin, bradykinin, prostaglandins, methacholine and acetylcholine as well as other triggers such as exercise, deep inhalation and inhalation of cold air (Boushey 1980). Bronchoconstriction is implicated as the primary effector mechanism of airway narrowing in these responses. This is because of both the short time frame of the response and because many of these stimuli typically either cause bronchoconstriction directly in vitro or promote bronchoconstriction through interference with the autonomic control of ASM. Further evidence comes from findings that this response can be abolished or diminished by bronchodilator medications such as atropine and beta2‐agonists (Phillips 1990; Simonsson 1967); although beta2‐agonists in particular may have additional mechanisms of action. Whether airway hyper responsiveness relates primarily to an abnormality of ASM, to increased ASM bulk (Wiggs 1990), to aberrant autonomic control or reflex pathways, or to physical damage to the airway epithelium remains to be established. Regular use of salbutamol has, however, been shown to increase airway hyper responsiveness to allergen exposure and produce tolerance to the protective effect of salbutamol against bronchoconstriction induced by both methacholine and allergens (Cockcroft 1993).
Inflammation
It has long been thought that the histological changes described above and the phenomenon of airway hyper responsiveness are due to a combined acute and chronic inflammatory response (Bousquet 2000). Patients with status asthmaticus have increased numbers of inflammatory cells including eosinophils and neutrophils, as well as a variety of pro‐inflammatory cytokines and chemokines found in bronchial alveolar lavage (Tonnel 2001). In patients with chronic asthma there is also evidence of increased eosinophil numbers (Bousquet 1990), inflammatory cell adhesion molecules (Vignola 1993) and some evidence of an association between the extent of inflammation, disease severity and hyperreactivity. This association has however been questioned on the background of a number of negative results (Brusasco 1998), although it is made difficult to prove by the lack of a consistent marker of a sequential and variable inflammatory response (Haley 1998).
Description of the intervention
Beta2‐agonists and mortality: an historical perspective
Time trend data and case control studies
Adrenaline was successfully used in the symptomatic treatment of asthma as far back as 1903 (Tattersfield 2006). Initially given subcutaneously, the inhaled route was tried in 1929 to reduce adverse effects but these remained a problem and in 1940 details of a new agent isoprenaline (isoproterenol) were published in Germany (Konzett 1940). Although isoprenaline was more selective for beta‐ as opposed to alpha‐adrenoreceptors, adverse effects including palpitations were still a major problem, particularly with oral administration (Gay 1949) and it first became available as atomizer spray for use in the UK in 1948 (Pearce 2001).
Prior to the 1940s, mortality rates from asthma in a number of countries were stable and low at less than one asthma death per 100,000 people per year (Pearce 2001; Figure 1). During the 1940s and 50s, there was a slight rise in mortality rates and concerns about a possible link to inhaled adrenaline were raised at an early stage (Benson 1948). However, the rise was small and the cause unclear and sales continued to increase with the introduction of aerosol or metered dose inhalers in the early 1960s. During this decade, there was an epidemic of asthma deaths in at least six countries including England, Wales and New Zealand (Figure 1). In all six countries the epidemics coincided with the licensing of an aerosol called 'Isoprenaline Forte', which contained five times the dose of isoprenaline per administration than the standard preparation (Stolley 1972). In other countries including the Netherlands where isoprenaline forte was introduced late and sales volumes low and in the US, where isoprenaline forte was not licensed, no increase in asthma mortality occurred. This was despite an approximate trebling in per capita alternative bronchodilator sales between 1962 and 1968 in the US (Stolley 1972). A detailed review of the epidemic in England and Wales concluded it was not due to changes in death certification, disease classification or an increase in asthma prevalence, but instead was most likely due to new methods of treatment (Speizer 1968). In England and Wales, mortality rates fell following health warnings about the overuse of inhalers and banning of over the counter sales in 1968. It was around this time that more selective beta2‐agonists such as terbutaline (Bergman 1969) and salbutamol (albuterol) (Cullum 1969) were being developed.
1.

Changes in asthma mortality (5‐34 age group) in three countries in relation to the introduction of isoprenaline forte in the UK and New Zealand and of fenoterol in New Zealand. (From Blauw 1995. With permission from the Lancet.)
In the late 1970s, a second epidemic of asthma deaths occurred in New Zealand (Figure 1). It was later shown that this epidemic coincided with the introduction and rising sales of fenoterol, a new short‐acting beta2‐agonist (Crane 1989; Figure 2). A significant association between mortality and fenoterol use was demonstrated in three consecutive case‐control studies, the latter studies addressing criticisms of the first (Crane 1989; Grainger 1991; Pearce 1990). Furthermore, the relative risk of asthma death in patients prescribed fenoterol increased markedly when analysis was restricted to subgroups defined by markers of severity, including previous hospital admission and use of oral corticosteroids. Following the publication of the first case control study, the fenoterol market share in New Zealand fell from 30% in 1988 to 3% in 1991 and by the early 1990s the mortality epidemic appeared to be over (Figure 2). During the gradual decline in mortality in New Zealand from its peak in 1979, total sales of alternative beta2‐agonists, including salbutamol, gradually rose and the use of inhaled corticosteroids also increased during the latter half of the 1980s (Pearce 2007).
2.

Inhaled fenoterol market share and annual asthma mortality in New Zealand in persons aged 5‐34.
The introduction of long‐acting beta2‐agonists
Given the relatively short‐lived action of beta2‐agonists such as salbutamol, in the late 1980s efforts were made to develop longer‐acting compounds. Subsequently, the long‐acting beta2‐agonists (LABAs), salmeterol and formoterol were released by GlaxoSmithKline (GSK) and Novartis, respectively. Both drugs cause bronchodilation that lasts for more than 12 hours, although formoterol has a faster onset of action (Kemp 1993; Ringdal 1998). Given previous concerns about the safety profile of some of the short acting beta2‐agonists, salmeterol and formoterol were subject to randomised controlled trials on larger numbers of patients. Using these trials, several Cochrane reviews have addressed the efficacy of LABAs in addition to inhaled corticosteroids (Ni Chroinin 2004; Ni Chroinin 2005), in comparison with placebo (Walters 2007), short‐acting beta2‐agonists (Walters 2002), leukotriene‐receptor antagonists (Ducharme 2006), and increased doses of inhaled corticosteroids (Greenstone 2005). The beneficial effects of LABAs on lung function, symptoms, quality of life and exacerbations requiring oral steroids have been demonstrated. However, with some studies demonstrating an associated increase in mortality, concerns about the safety profile of LABAs have heightened and there has been much debate about the potential protective role of inhaled corticosteroids.
How the intervention might work
We have outlined the pharmacology of beta2‐agonists in detail in Appendix 1. Since the early epidemics in asthma mortality, a number of potential mechanisms have been proposed to explain a relationship to the use of beta2‐agonists. We discuss these mechanisms in detail in Appendix 2; they include direct toxicity, tolerance, delay in seeking help and reduction in use of inhaled corticosteroids.
Why it is important to do this review
We have taken a different approach from Salpeter 2006, in that we have not assumed a class effect of long‐acting beta2‐agonists, but we have considered trials comparing regular salmeterol to placebo or regular salbutamol. We have chosen not to include results from trials on formoterol in this review, as there are known differences in the pharmacology properties of salmeterol and formoterol (Van Noord 1996); however formoterol is the subject of another ongoing review.
In view of the difficulty in ascertaining the causation of deaths and serious adverse events (SAEs), we have considered all‐cause fatal and non‐fatal SAEs as the main outcomes of this review, with asthma‐related and cardiovascular events as secondary outcomes.
Objectives
The aim of this review is to assess the risk of mortality and non‐fatal serious adverse events in trials which randomised patients with chronic asthma to salmeterol alone
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs) of parallel design, with or without blinding, in which salmeterol alone was randomly assigned to patients with chronic asthma. We excluded studies on people with acute asthma and exercise‐induced bronchospasm.
Types of participants
We included patients with a clinical diagnosis of asthma of any age group, unrestricted by disease severity, previous or current treatment.
Types of interventions
We included trials that randomised participants to receive inhaled salmeterol twice daily for a period of at least 12 weeks, at any dose and delivered by any device (metered dose inhalers (MDIs) with chlorofluorocarbons (CFCs) or hydrofluoroalkane (HFAs), or dry powder inhalers (DPIs). We included studies that used comparison groups with placebo or short‐acting beta2‐agonists, and co‐intervention with leukotriene receptor antagonists; inhaled or oral corticosteroids or theophylline was allowed as long as they were not part of the randomised intervention, and were therefore not systematically different between groups. We excluded studies that compared different doses of salmeterol or different delivery devices or propellants (with no placebo arm). We excluded studies in which salmeterol was randomised together with an inhaled steroid (in separate inhalers or a combined inhaler), but these studies were included in a separate review (Cates 2009).
Types of outcome measures
Primary outcomes
All‐cause mortality
All‐cause non‐fatal SAEs
Secondary outcomes
Asthma‐related mortality
Asthma‐related non‐fatal SAEs
Respiratory‐related mortality
Respiratory‐related non‐fatal SAEs
Cardiovascular‐related mortality
Cardiovascular‐related non‐fatal SAEs
Asthma‐related non‐fatal life‐threatening events (intubation or admission to intensive care)
Respiratory‐related non‐fatal life‐threatening events (intubation or admission to intensive care)
We did not sub‐divide outcomes according to whether the trial investigators considered them to be related to trial medication.
Search methods for identification of studies
Electronic searches
We identified trials using the Cochrane Airways Group Specialised Register of trials, which is derived from systematic searches of bibliographic databases including the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, EMBASE, CINAHL, AMED, and PsycINFO, and handsearching of respiratory journals and meeting abstracts (see Appendix 3 for details). All records in the Specialised Register coded as 'asthma' were last searched in August 2011 using the following terms:
(((beta* and agonist*) and (long‐acting or "long acting")) or ((beta* and adrenergic*) and (long‐acting or "long acting")) or (bronchodilat* and (long‐acting or "long acting")) or (salmeterol or formoterol or eformoterol or advair or symbicort or serevent or seretide or oxis)) AND (serious or safety or surveillance or mortality or death or intubat* or adverse or toxicity or complications or tolerability)
Searching other resources
We checked reference lists of all primary studies and review articles for additional references. We checked websites of clinical trial registers for unpublished trial data and FDA submissions in relation to salmeterol.
Data collection and analysis
Selection of studies
Both authors (CJC, MJC) independently assessed studies identified in the literature searches by examining titles, abstract and keywords fields. We obtained studies that potentially fulfilled the inclusion criteria in full text. We independently assessed these full text trial reports for inclusion. We resolved disagreements by consensus. We kept a record of decisions.
Data extraction and management
We extracted data using a prepared checklist before being entered into RevMan 2011. Data included characteristics of included studies (methods, participants, interventions, outcomes) and results of the included studies. We contacted authors of included studies for unpublished adverse event data, and searched manufacturers' websites for further details of adverse events. We recorded all‐cause SAEs (fatal and non‐fatal) and in view of the difficulty in deciding whether events were asthma related, we noted details of the cause of death and SAEs where they were available.
Assessment of risk of bias in included studies
Both authors assessed the included studies for bias protection (including sequence generation for randomisation, allocation concealment, blinding of participants and assessors, loss to follow‐up, completeness of outcome assessment and other possible bias prevention) as either high, low or unclear risk of bias in line with recommendation in the Cochrane Handbook of Systematic Reviews of Interventions (Higgins 2008). We resolved disagreements by consensus. We contacted study authors and sponsors to seek clarification where bias protection was unclear.
Assessment of heterogeneity
We explored heterogeneity on the basis of the subgroup and sensitivity analyses outlined below.
Assessment of reporting biases
We inspected funnel plots to assess publication bias.
Data synthesis
The outcomes of this review were dichotomous, and we recorded the number of participants with each outcome event, by allocated treated group. We planned to conduct the primary analysis, mortality, using risk difference, as many studies did not have any deaths in either arm. However, revisions to the Handbook (Higgins 2008) since the protocol was written advise against this approach. Therefore, the risk differences were only used to estimate the absolute impact of treatment. The Peto odds ratio has advantages when events are rare as no continuity correction for zero cells is required, and although it can perform less well with unbalanced treatment arms and large effect sizes, the updated review has now used Peto odds ratio for all primary analyses, and the Mantel‐Haenszel random effects model has been used for sensitivity analysis.
Subgroup analysis and investigation of heterogeneity
We conducted subgroup analyses on the basis of dose of salmeterol (usual dose versus high dose), age (adults versus children), severity of asthma, reported corticosteroid use at baseline, comparator used and ethnic groups. We compared subgroups using tests for interaction (Altman 2003).
Results
Description of studies
Results of the search
The initial search in October 2007 found 512 abstracts, which we reduced to 504 after removing duplicates. Of these, we identified 154 abstracts that related to salmeterol alone (three were from references listed in identified trials and review articles, and one was only published on the GSK website). We included 32 studies in the review (42 references) and excluded 118: 71 were less than 12 weeks in duration (including 26 single‐dose studies); eight were not RCTs; five were dose comparison studies; five are ongoing studies; four were on exercise‐induced bronchospasm; four compared with theophylline; three were propellant studies; three were cross‐over in design; and seven were for other reasons. We reached consensus on all the included studies after inspection of the full text from papers and websites; there was initial disagreement on six abstracts, five of which we subsequently included and one excluded. We identified additional references to the included studies and one unpublished study from other reviews (SLGA 3014).
A further search in July 2008 generated 48 new references, of which two were relevant to this review (Inoue 2007; Pascoe 2006), but we excluded both as they were short‐term crossover studies. We identified no further new studies by the latest searches in August 2009 (79 references) or August 2011 (191 references).
Included studies
There were 32 included studies which randomised 62,630 participants (Adinoff 1998; Boulet 1997; Boyd 1995; Britton 1992; Busse 1998; Chervinsky 1999; D'Alonzo 1994; D'Urzo 2001; Kavuru 2000; Kemp 1998a; Kemp 1998b; Lazarus 2001; Lenney 1995a; Lenney 1995b; Lundback 1993; Nathan 1999; Nathan 2006; Pearlman 1992; Pearlman 2004; Rosenthal 1999; Russell 1995; Shapiro 2000; Simons 1997; SLGA 3014; SLMF4002; SMART 2006; SNS 1993; Von Berg 1998; Wenzel 1998; Weinstein 1998; Wolfe 2000).
A total of 46,501 (74%) people completed treatment, encompassing more than 19,000 patient years studied. There were only seven studies on 2380 (Lenney 1995a; Lenney 1995b;Simons 1997; SLGA 3014; Von Berg 1998; Weinstein 1998) who completed a total of 921 patient years of treatment (this is not allowing for the nine‐month extension to the two Lenney studies). The remaining studies were in adults and adolescents with most studies randomising participants of 12 years and older; however many studies included few adolescent patients (for example 6% of all participants in SNS 1993).
Twenty‐six studies (34,781 participants) compared salmeterol to placebo and eight studies (28,532 participants) compared salmeterol to regular salbutamol. Two studies included both a placebo and a salmeterol arm (Pearlman 1992; SLGA 3014). The dose of salbutamol as a comparator varied between trials: four studies used salbutamol 200 µg four times daily (Boulet 1997; Pearlman 1992; SLGA 3014; SNS 1993); one study used the same regimen for the first three months, then 200 µg twice daily for the remaining nine months (Britton 1992); two studies used 200 µg twice daily (Lenney 1995a; Lenney 1995b); and one study used 400 µg of salbutamol delivered by diskhaler four times daily for the first three months and then twice daily for the following nine months (Lundback 1993).
The duration of trials ranged from 12 to 52 weeks, but in some of the 52‐week studies the data collection was separated into two periods. Four trials separated the first three months from the subsequent nine months of follow‐up (Britton 1992; Lenney 1995a; Lenney 1995b; Lundback 1993), whilst Von Berg 1998 separated data from the first and second six‐month periods.
The dose of salmeterol used was 50 µg twice daily (described as 42 µg delivered dose in the studies from the USA) in all studies except two which used 100 µg twice daily in participants with more severe asthma on high doses of inhaled steroids at study entry (Boyd 1995; SLMF4002). Three trials used either 25 µg or 50 µg twice daily in children (Lenney 1995a; Lenney 1995b; SLGA 3014).
Concurrent use of inhaled corticosteroids varied in the included studies from zero to 100%; Table 3 lists the inhaled steroid use by study. Only Rosenthal 1999 and Simons 1997 excluded patients who were using inhaled corticosteroids at baseline. Six trials withdrew inhaled corticosteroids from participants for the duration of the trial (Kavuru 2000; Lazarus 2001; Nathan 1999; Nathan 2006; Pearlman 2004; Shapiro 2000).
1. Baseline use of inhaled corticosteroids.
| Study ID | % patients |
| Adinoff 1998 | 64 |
| Boulet 1997 | 74 |
| Boyd 1995 | 100 |
| Britton 1992 | 64 |
| Busse 1998 | 67 |
| Chervinsky 1999 | 51 |
| D'Alonso 1994 | 21 |
| D'Urso 2001 | 93 |
| Kavuru 2000 | 26 ‐ Withdrawn |
| Kemp 1998a | 43 |
| Kemp 1998b | 100 |
| Lazarus 2001 | 53 ‐ Withdrawn |
| Lenney 1995 | 57 |
| Lundback 1993 | 31 |
| Nathan 1999 | 57 ‐ Withdrawn |
| Nathan 2006 | 100 ‐ Withdrawn |
| Pearlman 1992 | 25 |
| Pearlman 2004 | 37 ‐ Withdrawn |
| Rosenthal 1999 | 0 |
| Russell 1995 | 100 |
| Shapiro 2000 | 100 ‐ Withdrawn |
| Simons 1997 | 0 |
| SLGA3014 | 50 |
| SLMF4002 | 100 |
| SMART 2006 | 47 |
| SNS 1993 | 69 |
| Von Berg 1998 | 52 |
| Weinstein 1998 | 57 |
| Wenzel 1998 | 47 |
| Wolfe 2000 | 33 |
Risk of bias in included studies
All studies were double‐blind and unlikely to have been subject to selection bias; however lack of comprehensive reporting of fatal and non‐fatal SAEs make selective reporting a threat to the validity of this review (see Figure 3). It should however be noted that the missing data are from smaller studies, so 96% of patients in the trials had some information on SAEs.
3.

Risk of bias summary: review authors' judgments about each risk of bias item for each included study.
Allocation
There was very sparse information in the reports of the studies in journals or on the company website in terms of sequence generation and allocation concealment. Most of these studies were supported by GSK, and correspondence with the company indicated a high level of bias protection in sequence generation and allocation concealment for the studies that they sponsor, so it is unlikely that this area is a major concern.
Blinding
All included studies employed a double‐blind design.
Selective reporting
SAEs were not well reported in the journal publications, but were available from the controlled trial register on the GSK website for many of the included studies. The only trial that did not receive financial support from the manufacturers of salmeterol (GSK) was Lazarus 2001; the authors of this trial report have provided data on mortality and hospital admissions.
One study was unpublished (SLMF4002), but event information was available from the controlled trial register on the company website. A second unpublished trial had available data from FDA submission documents (SLGA 3014). The GSK trial register also included reports on the following studies Britton 1992 (SLGT02), D'Urzo 2001 (SLGQ94 [521/180]), Kavuru 2000 (SFCA 3002), Lenney 1995a (SLPT01/SMS40093), Lenney 1995b (SLPT02), Lenney 1995c, Nathan 2006 (SAS3004), Pearlman 2004 (SAS3003), Russell 1995 (SALMP/AH91/D89), Shapiro 2000 (SFCA 3003), SMART 2006 (SGLA5011), SNS 1993 (SNS‐D920619), Von Berg 1998 (SLGB3019[SLPT09]), Wolfe 2000 (SLGA3010 & SLGA3011).
Journal publications tend not to list mortality and all SAEs (often restricting reporting to frequent adverse events or those thought to be related to study medication). We could find no SAE data for six trials (Adinoff 1998; Boulet 1997; Chervinsky 1999; D'Alonzo 1994; Kemp 1998a; Pearlman 1992). These accounted for around 2,766 randomised patients, which represents 4% of the total randomised population. We have sought further information from GSK in relation to Adinoff 1998, Boulet 1997, Boyd 1995, Busse 1998, Chervinsky 1999, D'Alonzo 1994, Kemp 1998a, Kemp 1998b, Lenney 1995b (in relation to data from the final nine months), Lundback 1993 (in relation to data from the final nine months), Nathan 1999, Pearlman 1992, Rosenthal 1999, SNS 1993 (in relation to patients with any SAE), Weinstein 1998, and Wenzel 1998.
Although the trial identifiers have been confirmed for these studies, many do not have reports on the manufacturers trial website. At this time it has not been possible to obtain information on SAE numbers with the exception of Lenney 1995b (in relation to SAE data from the final nine months), but further information may be available later and can be included when the review is updated.
Other potential sources of bias
Only the two large surveillance studies SMART 2006 and SNS 1993 used independent outcome assessors. For this reason, the primary outcomes for this review are all‐cause mortality and SAEs, as these avoid subjective judgements on causation. SAEs thought to be related to the study medication are likely to be subject to bias and have not been included in this review.
Effects of interventions
Primary outcomes
All‐cause mortality
Events were sparse in the trials and the presence or absence of a mortality was not always reported in the paper publications.
Salmeterol versus placebo
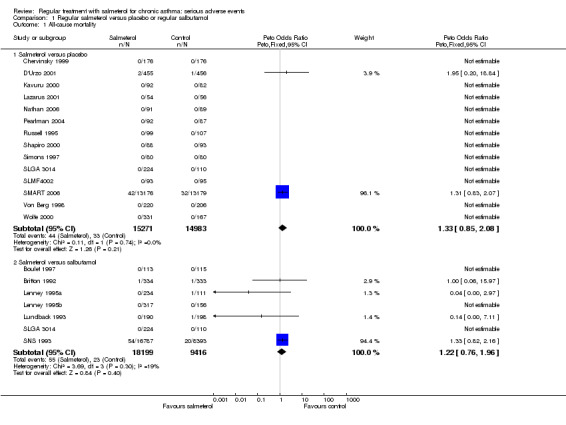
Data were available from the controlled trial register on the GSK website for 14 studies comparing salmeterol (N = 15,271) with placebo (N = 14,983); this represents 86% of the randomised patients for this comparison. Deaths only occurred in two of these trials in adults (D'Urzo 2001; SMART 2006), and overall there were 44 deaths on salmeterol and 33 on placebo. The pooled Peto OR was not statistically significant (1.33, 95% CI 0.85 to 2.08), Analysis 1.1 (see Figure 4). The confidence interval is almost identical using a Mantel‐Haenszel OR (1.33, 95% CI 0.85 to 2.10), and is the same for fixed‐effect and random‐effects models. The pooled risk difference has a point estimate of an increase of seven in 10,000, with a CI from five fewer deaths to 20 more deaths per 10,000 treated with salmeterol. There was no statistical heterogeneity in this outcome.
1.1. Analysis.
Comparison 1 Regular salmeterol versus placebo or regular salbutamol, Outcome 1 All‐cause mortality.
4.

Forest plot of comparison: 1 All‐cause Mortality, outcome: 1.1 Overall results.
Salmeterol versus salbutamol
Data were available from seven studies comparing salmeterol (N = 18,199) to salbutamol (N = 9,416), representing 93% of the randomised patients for this comparison. The larger numbers in the salmeterol arm are due to SNS 1993, in which twice as many patients were randomised to salmeterol in comparison to salbutamol. Three studies in adults (Britton 1992; Lundback 1993; SNS 1993) and one study in children (Lenney 1995a) contributed data to the outcome, although almost all the events came from the SNS study. The results were similar to those from the placebo comparison: 55 deaths occurred in the salmeterol arm compared to 23 in the salbutamol arm but again the difference was not statistically significant (Peto OR 1.22, 95% CI 0.76 to 1.96; Analysis 1.1; see Figure 4). The Mantel‐Haenszel odds ratio was very similar (1.23, 95% CI 0.75 to 2.02). The pooled risk difference was an increase of seven in 10,000, with a CI from six fewer deaths to 20 more deaths per 10,000 treated with salmeterol. There was some heterogeneity in this outcome (I2 = 19%) but sensitivity analysis using a fixed effect risk difference gave a very similar result, with a point estimate of six per 10,000 and a CI from eight fewer deaths to 19 more deaths per 10,000 treated with salmeterol.
Subgroup analyses
No subgroup analyses were possible for all‐cause mortality as the data were too sparse, and subgroup characteristics were not reported for this outcome.
SAEs (non‐fatal all cause)
An illustrative example of the definition of SAEs used in trials by GSK is shown in Appendix 4. Whilst the majority of SAEs result in hospitalisations, the number of patients with SAEs tends to be higher than the number who are admitted to hospital (see Analysis 1.22 for comparative data from SMART 2006). We have used information on patients with any SAE for these analyses (with the exception of SNS 1993 where this was not reported, so we have used hospital admissions and life‐threatening events).
1.22. Analysis.

Comparison 1 Regular salmeterol versus placebo or regular salbutamol, Outcome 22 All‐cause hospitalisation compared to SAE.
Salmeterol versus placebo
Combined data from adults and children
Data were available on non‐fatal SAEs from the GSK trial register and FDA submissions for 18 studies comparing salmeterol (N = 15,895) to placebo (N = 15,634); this represents 91% of the randomised patients for this comparison. The studies were largely on adults, but some data from 1333 children in five trials (Russell 1995; Simons 1997; SLGA 3014; Von Berg 1998; Weinstein 1998) contributed to the analysis. The overall result indicated an increased risk of SAEs with salmeterol (Peto OR 1.15, 95% CI 1.02 to 1.29) with low heterogeneity (I2 = 0), Figure 5. Random‐effects Mantel‐Haenzel modelling gave a very similar result (OR 1.14, 95% CI 1.01 to 1.28) and the CI was unchanged when Boyd 1995 was excluded in view of the higher dose of salmeterol (100 mcg bd) used in this trial.
5.

Forest plot of comparison: 1 Regular salmeterol versus placebo or regular salbutamol, outcome: 1.2 Non‐fatal serious adverse events (adults and children).
Since SAEs were rare in the studies (overall 3.6% of patients on placebo), the risk difference is small at 0.005 (95% CI 0.001 to 0.008); using the placebo arm of SMART 2006 for reference over a 28‐week period there would need to be 188 patients treated (95% CI 95 to 2606) for one extra SAE to occur, calculated by Visual Rx using the odds ratio and baseline risk of 3.6%. This is illustrated in Figure 6, which shows that for every thousand patients treated with salmeterol there are an extra five patients who will suffer a SAE, so that in comparison to 40 per thousand in the placebo group this rises to 45 per thousand on salmeterol.
6.

Serious adverse events with salmeterol in comparison to placebo. For 1000 patients given regular salmeterol for 28 weeks there would be 45 patients who suffer a serious adverse event, in comparison with 40 if all 1000 were given placebo.
Adults with non‐fatal SAEs
When the adult data comparing salmeterol with placebo were considered alone, the results showed a similar increase in risk which is also statistically significant (Peto OR 1.14, 95% CI 1.01 to 1.28; Figure 7). The result was unchanged using Mantel‐Haenszel fixed‐effect, but became borderline significant using random‐effects (OR 1.13, 95% CI 1.00 to 1.28).
7.

Forest plot of comparison: 1 Regular salmeterol versus placebo or regular salbutamol, outcome: 1.3 Non‐fatal serious adverse events in adults.
Children with non‐fatal SAEs
There were only 1333 children reported for this outcome so, although the direction of effect was the same as for the adults, the CI is wide (Peto OR 1.30, 0.82 to 2.85; Figure 8). The results in children include both the possibility of a greater risk than in adults, but also the possibility of no difference between salmeterol and placebo.
8.

Forest plot of comparison: 1 Regular salmeterol versus placebo or regular salbutamol, outcome: 1.4 Non‐fatal serious adverse events in children.
Salmeterol versus salbutamol
Combined data from adults and children
In contrast, the results from eight studies comparing salmeterol (N = 18,481) to salbutamol (N = 9,702), representing 98% of the randomised patients for this comparison, showed a reduction in the risk of SAEs with salmeterol which was not statistically significant (Peto OR 0.96, 95% CI 0.81 to 1.14). A test for interaction between the results comparing salmeterol with placebo and salbutamol was not significant. As it has not proved possible to obtain missing information in relation to the number of patients with any SAEs in SNS 1993, we used the number of hospitalisations or life/threatening events reported in the trial for the main analysis. We adopted this approach because the data collection form allowed more than one classification of events, and in contrast to the other studies in the review, the number of SAEs that did not result in hospitalisation was greater than in the hospitalisation category. We carried out a sensitivity analysis combining all non‐fatal SAEs (although this carries a risk that patients may have been more than once, which would lead to an over precise estimate) and this is shown in Analysis 1.5. This approach made very little difference to the pooled result (Peto OR 0.98, 95% CI 0.86 to 1.11).
1.5. Analysis.

Comparison 1 Regular salmeterol versus placebo or regular salbutamol, Outcome 5 Non‐fatal adverse events in adults and children (SNS sensitivity analysis).
Adults with non‐fatal SAEs
The results in adults do not differ from the overall result described above (Peto OR 0.94, 95% CI 0.79 to 1.11).
Children with non‐fatal SAEs
Again the number of children contributing to this outcome is very small, which makes the uncertainty around the results considerable. As with the previous comparison between salmeterol and placebo, it remains possible that the risk may be higher or lower in children than in adults.
We carried out a sensitivity analysis because separate results are reported from Lenney 1995a and Lenney 1995b for the first three months and the subsequent nine months of the trial. Since patients could have contributed adverse event data from both periods of these trials, we did not consider it safe to combine the results, and full 12‐month data were not available. Both pooled results are shown in Figure 8, and there is no significant difference shown in either period. For the first three months the pooled Peto OR was 1.37 (95% CI 0.71 to 2.64) and for the nine‐month follow‐up the pooled Peto OR was 1.17 (95% CI 0.71 to 1.94).
All‐cause SAEs (fatal and non‐fatal combined)
When fatal and non‐fatal SAEs are considered together the findings are very similar to those for the non‐fatal events (Analysis 1.6), with a significant increase in risk with regular salmeterol in comparison with placebo (Peto OR 1.16, 95% CI 1.03 to 1.30), but not in comparison with regular salbutamol.
1.6. Analysis.

Comparison 1 Regular salmeterol versus placebo or regular salbutamol, Outcome 6 Fatal and non‐fatal serious adverse events in adults and children.
Secondary outcomes
Mortality by cause of death
Asthma‐related mortality
The cause of death was only independently assessed in the two large surveillance studies (SMART 2006; SNS 1993). The results for asthma mortality in these studies are in Analysis 1.7; both studies found a similar increase in asthma mortality, but in the earlier SNS 1993 study this three‐fold increase in the risk of asthma death was not statistically significant, whilst in SMART 2006 a significant four‐fold increase in the risk of asthma death was found. In absolute terms the size of the risk difference for asthma mortality in SMART 2006 was very similar to that found for mortality of any cause; the point estimate for asthma related death being an increase of eight asthma deaths for every 10,000 patients treated with salmeterol for six months, with a CI from two more deaths to 14 more deaths per 10,000.
1.7. Analysis.

Comparison 1 Regular salmeterol versus placebo or regular salbutamol, Outcome 7 Asthma mortality.
The direction and size of the increase in asthma mortality is consistent between the two studies, and although there are differences in study design and the comparator used, if the results of the two studies are combined there is a significant increase in asthma mortality with regular salmeterol, (Peto OR 2.94, 95% CI 1.41 to 6.14).
Inhaled corticosteroid use in relation to asthma mortality
We were able to obtain unpublished data from GSK in relation to asthma mortality and the use of corticosteroids at baseline for each of the asthma‐related deaths in SNS 1993. This is shown together with similar published data from SMART 2006 in Analysis 1.8. In the subgroup of patients not taking inhaled corticosteroids at baseline the combined increase in asthma mortality is statistically significant (Peto OR 6.43, 95% CI 2.13 to 19.42). This indicates a clear increase in risk of asthma death when inhaled corticosteroids were not used when the two studies are considered together.
1.8. Analysis.

Comparison 1 Regular salmeterol versus placebo or regular salbutamol, Outcome 8 Asthma mortality (within study subgroups by ICS use at baseline).
For the subgroup taking inhaled corticosteroids at baseline, the increase in asthma mortality is smaller and not statistically significant (Peto OR 1.49, 95% CI 0.54 to 4.11). The test for interaction between inhaled corticosteroid use and asthma related deaths shows a relative OR of 0.23 (95% CI 0.05 to 1.04) and the CI includes the possibility of a 95% relative reduction in the risk of asthma death, but also a 4% relative increase in those on baseline inhaled corticosteroids. Moreover it should be pointed out that the CI for those on inhaled corticosteroids at baseline includes the overall three‐fold increase in mortality, so this finding cannot be interpreted as meaning that corticosteroids abolish any increased risk of asthma mortality from regular salmeterol.
Any corticosteroid use in relation to asthma mortality
Analysis 1.9 where the results of SMART 2006 & SNS 1993 are subgrouped according to any corticosteroid use, shows very similar results to Analysis 1.8. Whilst larger effects are shown in those not taking any form of steroid at baseline, the difference between subgroups is again not significant (Chi2= 1.96, df = 1, (P = 0.16)). As no details were provided in the report of SMART 2006 in relation to the number of patients taking oral corticosteroids at baseline, we assumed that the proportion is very small in comparison to those on inhaled corticosteroids. The information on baseline oral corticosteroid use in those who died has been extracted from Table 5 in the paper publication by Nelson et al.
1.9. Analysis.

Comparison 1 Regular salmeterol versus placebo or regular salbutamol, Outcome 9 Asthma mortality (within study subgroups by any steroid at baseline).
Cardiovascular‐related mortality
This outcome was particularly difficult to assess as it was not a primary outcome for SMART 2006 or SNS 1993, so was not subject to independent data verification. Moreover it is very difficult in practice to know which deaths should be included in this category. The only child who died in all the identified studies was in Lenney 1995a; the death is listed both under asthma attack and circulatory arrest. The pooled odds ratio was not statistically significant and small numbers of deaths and classification problems mean that there is wide uncertainty around the finding, see Analysis 1.10.
1.10. Analysis.

Comparison 1 Regular salmeterol versus placebo or regular salbutamol, Outcome 10 Cardiovascular mortality.
Asthma‐related SAEs
The reporting of disease‐specific SAEs on the GSK controlled trial register does not indicate how many patients suffered from more than one event. This represents a risk of over‐counting the number of patients suffering an asthma‐related SAE in the large studies, so we only included data for this outcome from studies which show individual events on the controlled‐trial web reports. The events in these studies are shown in Analysis 1.11. When salmeterol is compared to placebo the increase in asthma‐related SAEs is significant using Peto OR 1.59 (95% CI 1.05 to 2.41) but is not statistically significant using Mantel‐Haenszel random‐effects OR 1.48 (95% CI 0.97 to 2.27). In comparison with salbutamol the decrease in asthma‐related SAEs is not statistically significant (OR 0.99, 95% CI 0.54 to 1.81).
1.11. Analysis.

Comparison 1 Regular salmeterol versus placebo or regular salbutamol, Outcome 11 Adults and Children non‐fatal asthma‐related serious adverse events.
SAEs in the cardiovascular system
The reports of SAEs in the cardiovascular system suffer from the same difficulty of possible over counting of patients with events. However, in this case there are almost no data except from SMART 2006 and SNS 1993 so the reported events in these studies have been used and are shown in Analysis 1.12. No significant differences are shown comparing salmeterol to placebo or salbutamol.
1.12. Analysis.

Comparison 1 Regular salmeterol versus placebo or regular salbutamol, Outcome 12 Adults and children non‐fatal cardiovascular serious adverse events.
Within study subgroup analyses
D'Urzo 2001 reported the number of patients with severe asthma exacerbations (defined as hospitalisation, emergency department visit or use of oral prednisolone) in patients treated in primary care. They subgrouped according to the reported inhaled or oral corticosteroid consumption, and we found significant heterogeneity between subgroups. The direction of effect in the moderate dose subgroup was in favour of salmeterol (Peto OR 0.68, 95% CI 0.42 to 1.08). However in participants on oral corticosteroids or an inhaled daily dose of more than 1000 µg, the direction of effect reversed in favour of placebo (OR 1.75, 95% CI 0.99 to 3.11; Analysis 1.13). The Chi2 test for differences among the three subgroups returned P = 0.04, in keeping with the results of logistic regression analysis reported in the paper (P = 0.038). Very similar results were seen when the patients were stratified according to predicted peak flow (Analysis 1.14).
1.13. Analysis.

Comparison 1 Regular salmeterol versus placebo or regular salbutamol, Outcome 13 Proportion of participants with serious asthma exacerbations in relation to dose of ICS.
1.14. Analysis.

Comparison 1 Regular salmeterol versus placebo or regular salbutamol, Outcome 14 Proportion of participants with serious asthma exacerbations in relation to baseline PEF (% predicted).
SMART 2006 reported on numerous post‐hoc subgroups in relation to the primary outcome of the study (respiratory related deaths or life threatening events). The main results are shown in Analysis 1.15 to Analysis 1.21. Of these, the only comparison that had a statistically significant test for interaction was the post hoc comparison between African Americans and Causcasians (test for subgroup differences: Chi² = 5.50, df = 1 (P = 0.02); Analysis 1.16). Whilst it may be tempting to interpret the individual subgroup results according to their statistical significance (such as for the subgroup with PEF less than 60% predicted as shown in Analysis 1.20), this approach is not the correct way to test for interaction between PEF and salmeterol (Altman 2003); the test for interaction between subgroups does not indicate a significant difference between those with high and low % predicted PEF (test for subgroup differences: Chi² = 1.97, df = 1 (P = 0.16)).
1.15. Analysis.

Comparison 1 Regular salmeterol versus placebo or regular salbutamol, Outcome 15 Respiratory related deaths or life‐threatening events (SMART).
1.21. Analysis.

Comparison 1 Regular salmeterol versus placebo or regular salbutamol, Outcome 21 Respiratory related deaths or life‐threatening events (SMART subgrouped by study phase.
1.16. Analysis.

Comparison 1 Regular salmeterol versus placebo or regular salbutamol, Outcome 16 Respiratory related deaths or life‐threatening events (SMART subgrouped by race).
1.20. Analysis.

Comparison 1 Regular salmeterol versus placebo or regular salbutamol, Outcome 20 Respiratory related deaths or life‐threatening events (SMART subgrouped by baseline predicted PEF).
Discussion
Summary of main results
The difference in the risk of all‐cause mortality with regular salmeterol when compared with placebo does not reach statistical significance. Non‐fatal SAEs were significantly increased in comparison with placebo. These events were uncommon, such that in the largest study (SMART 2006) 3.6% of the placebo group suffered a SAE over 28 weeks; in comparison in the salmeterol arm of the trial 4.0% reported a SAE. One additional SAE was found to occur for every 188 people treated with regular salmeterol over 28 weeks, and the play of chance is compatible with one extra event for every 100 to nearly 3000 given regular salmeterol. This increase is largely derived from the studies in adults, and as the number of children studied is small there are insufficient data to ascertain how the effect in children compares to that found in adults.
We found no significant differences for all‐cause mortality or non‐fatal SAEs in trials comparing regular salmeterol with salbutamol.
Disease‐specific mortality was not easy to assess, but we found no significant differences in cardiovascular deaths. In contrast there was a consistent increase is asthma‐related mortality in both SMART 2006 and SNS 1993. The four‐fold increase in SMART 2006 was statistically significant whilst the three‐fold increase in SNS 1993 was not. The absolute increase in asthma‐related mortality in SMART 2006 was very similar to the size of the absolute increase in all‐cause mortality; the increase represents eight extra asthma deaths for every 10,000 patients treated for six months.
The combined results of SMART 2006 and SNS 1993 showed a significant in increase asthma mortality. This increase was most apparent in patients who did not take inhaled corticosteroids at baseline, but in patients who took inhaled corticosteroids at baseline the wide confidence interval failed to rule out the possibility of a three fold increase in asthma mortality.
Overall completeness and applicability of evidence
Although a very large number of adults given salmeterol were included in these studies, the rarity of mortality and SAEs means that there is still considerable uncertainty in relation to the size of the effects being investigated. This is a particular problem in children, where the numbers are smaller. There is insufficient evidence to be sure how the results in adults compare with those in children. It has proved difficult for the sponsor of these studies (GSK) to confirm data for some of the studies we identified, since they do not appear on their web‐based trial results register, and no data pertaining to adverse events were available for six of the included studies. The missing data are unlikely to alter the direction of the effects seen in this review, but could alter the point estimates and confidence intervals.
It is interesting to note that if this review had relied upon the information on SAEs published in papers, the results would have looked quite different (see Analysis 1.23). The published information does not indicate any significant increase in SAEs when regular salmeterol is compared to placebo in the 2117 patients included in this small subset of the included studies (Peto OR 1.13, 95% CI 0.65 to 1.95). If the analysis had been confined to SAEs that were thought to be drug related, the small number of events would not have provided sufficient information to draw any conclusions (Analysis 1.28).
1.23. Analysis.

Comparison 1 Regular salmeterol versus placebo or regular salbutamol, Outcome 23 Adults and children published non‐fatal serious adverse events.
1.28. Analysis.

Comparison 1 Regular salmeterol versus placebo or regular salbutamol, Outcome 28 Adults and children serious drug‐related adverse events.
Similar differences can be seen between published (Analysis 1.26) and complete data for adverse events (Analysis 1.25). The published drug related adverse events are shown in Analysis 1.27.
1.26. Analysis.

Comparison 1 Regular salmeterol versus placebo or regular salbutamol, Outcome 26 Adults and children published adverse events.
1.25. Analysis.

Comparison 1 Regular salmeterol versus placebo or regular salbutamol, Outcome 25 Adults and children all adverse events.
1.27. Analysis.

Comparison 1 Regular salmeterol versus placebo or regular salbutamol, Outcome 27 Adults and children all published drug‐related adverse events.
Quality of the evidence
All of the included studies were double blind, and are likely to have had adequate allocation concealment. However, only the two large surveillance studies had independent assessment of the cause of death. This should not be a threat to all‐cause mortality and all‐cause SAEs (primary outcomes). In contrast the ascertainment of disease‐specific outcomes may have been subject to bias, and the data were difficult to use as they were presented as numbers of events rather than number of patients who suffered an event.
Almost all of the studies included in this review were sponsored or supported by GSK, and the lack of published data from some of the studies is of concern.
Agreements and disagreements with other studies or reviews
The findings of this review are in agreement with the findings of Salpeter 2006, who reviewed the trial evidence in relation to asthma exacerbations leading to hospital admissions, life‐threatening events or death. However the Salpeter review combined studies on salmeterol and formoterol, and only considered placebo controlled studies. Our findings also match those presented to the FDA summarising the results of salmeterol trials carried out in the USA (FDA GSK USA Studies). The submission reported: "....analysis shows similar results for hospitalizations due to asthma for subjects receiving ICS compared with salmeterol plus ICS. For subjects not receiving concomitant ICS, there were a higher number of events in salmeterol recipients compared with placebo." The same submission also found a higher incidence of respiratory related SAEs in the salmeterol group compared to placebo and this is shown alongside our results in Analysis 1.29.
1.29. Analysis.

Comparison 1 Regular salmeterol versus placebo or regular salbutamol, Outcome 29 Adults and children non‐fatal asthma‐related serious adverse events (FDA data shown).
The impact of inhaled corticosteroids on adverse events in relation to long‐acting beta2‐agonists
We compared data provided by GSK in relation to the inhaled corticosteroid status of those patients who died from asthma in SNS 1993 to the findings of SMART 2006. Both studies showed a similar pattern, in that the main increase in asthma‐related mortality occurred in the subgroup of patients who were not on corticosteroids when they were recruited to the study Figure 12.
It is not possible to draw firm conclusions about the risks of salmeterol when used in conjunction with inhaled corticosteroids (Martinez 2006), as the patients in this review were not randomised to inhaled corticosteroid treatment and the test for interaction between the use of inhaled corticosteroids and salmeterol was not significant. We cannot therefore conclude that inhaled corticosteroids abolish the increased risk of asthma mortality in patients taking salmeterol, nor can we assume that mortality rates might not be even lower if inhaled corticosteroids were taken alone. Moreover, the findings of D'Urzo 2001 do not show consistency in the risk of severe asthma exacerbations with salmeterol and inhaled corticosteroids in primary care; beneficial effects of regular salmeterol with moderate doses of inhaled corticosteroids contrast with increased exacerbations in patients on high doses or maintenance oral corticosteroids. Whilst the interactions between inhaled corticosteroids and beta2‐agonists are complex, corticosteroids do not appear to prevent the development of tolerance during chronic beta2‐agonist treatment (Hancox 2006b).
The results of Lazarus 2001 provide support for the fact that salmeterol cannot be used to replace inhaled corticosteroids, because although there were no hospitalisations or deaths in the study, replacing triamcinolone with salmeterol did not maintain asthma control; there was a significant increase in exacerbations on salmeterol or placebo in comparison to triamcinolone. Compliance with inhaled corticosteroids is often poor as demonstrated in Sovani 2008, and there is a risk that patients who are using salmeterol and an inhaled steroid in separate inhalers may default on the latter without immediate deterioration in their asthma symptoms.
For patients whose asthma is not well‐controlled on moderate doses of inhaled corticosteroids, additional salmeterol can give symptomatic benefit, but this may be at the expense of an increased risk of SAEs and asthma‐related mortality, risks which are not clearly abolished by inhaled corticosteroids. Therefore, the risks as well as the benefits of regular salmeterol should be discussed with patients; the drug should be discontinued if no symptomatic benefit is achieved and the manufacturers' advice not to increase the dose of salmeterol during exacerbations should be made clear. Salmeterol should not be used as a substitute for inhaled corticosteroids, and adherence with inhaled steroids should be kept under review if separate inhalers are used. Combining the medications in a single inhaler prevents patients taking salmeterol alone. Observational data from Delea 2008 indicated use of salmeterol and fluticasone in a single inhaler resulted in higher use of inhaled corticosteroids in comparison to patients prescribed two separate inhalers.
Authors' conclusions
Implications for practice.
In comparison with placebo, we have found an increased risk of serious adverse events with regular salmeterol. There is also a clear increase in risk of asthma‐related mortality in patients not using inhaled corticosteroids in the two large surveillance studies. Although the increase in asthma‐related mortality was smaller in patients taking inhaled corticosteroids at baseline, the CI is wide, so it cannot be concluded that inhaled corticosteroids abolish the risks of regular salmeterol. The adverse effects of regular salmeterol in children remain uncertain due to the small number of children studied.
Implications for research.
Data on SAEs should be more fully reported in medical journals. In view of the increasing use of salmeterol in combination with inhaled corticosteroids, further studies investigating the impact of salmeterol alone on SAEs in adults may not be feasible, but studies using a combination of salmeterol and inhaled steroids should collect and fully report data on fatal and non‐fatal SAEs. The evidence base for assessing the risks and benefits of salmeterol in children is currently weak.
What's new
| Date | Event | Description |
|---|---|---|
| 11 April 2013 | Amended | NIHR acknowledgement inserted |
History
Protocol first published: Issue 1, 2007 Review first published: Issue 3, 2008
| Date | Event | Description |
|---|---|---|
| 17 August 2011 | New search has been performed | New search in August 2011 but no new included studies. Minor edits made and plain language summary rewritten. |
| 24 August 2009 | New search has been performed | New search in August 2009 but no new studies found. New Summary of Findings tables added to show separate results for adults and children. Russell 1995 was previously wrongly classified as an adult study and this has now been corrected. The total number of participants in the review has been altered to reflect those who were randomised to one of the treatment arms included in this review. There is no change in the conclusions of the review. |
| 20 December 2008 | Amended | Primary analysis changed to Peto Odds Ratio. Typographic errors in the number of trials and participants contributing to the mortality and non‐fatal serious adverse events have been corrected to match the results in Figures 5 and 7. The results are unchanged. NIHR Programme Grant Support has been acknowledged as external funding. |
| 2 July 2008 | New search has been performed | A new search was carried out on July 1st 2008 but no new studies have been included. Additional figures have been added comparing the results of published adverse events and the complete data set available to us at present. This is included in an updated discussion section. The risk of bias tables have also been amended to reflect all the data available for serious adverse events in each study (previously the risk of selective reporting was classified according to the paper publications). The ongoing GSK study has now been excluded as it is a single dose study. An error in the data entry for SNS in Figure 7 has been corrected. No changes have been made to the conclusions of the review. |
Acknowledgements
We thank Toby Lasserson, Susan Hansen and Elizabeth Stovold of the Cochrane Airways Group for their assistance in searching for trials and obtaining the abstracts and full reports, and John White for editing the review. We also thank Steve Yancey and Richard Follows from GSK for their help in obtaining data, and Anne Tattersfield for helpful comments. We also thank the authors of Lazarus 2001 for providing additional information from their study.
CRG Funding Acknowledgement: The National Institute for Health Research (NIHR) is the largest single funder of the Cochrane Airways Group.
Disclaimer: The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the NIHR, NHS or the Department of Health.
Appendices
Appendix 1. Pharmacology of beta2‐agonists
Beta2‐agonists are thought to cause bronchodilation primarily through binding beta2‐adrenoceptors on airways smooth muscle (ASM), with subsequent activation of both membrane‐bound potassium channels and a signalling cascade involving enzyme activation and changes in intracellular calcium levels following a rise in cyclic adenosine monophosphate (cAMP) (Barnes 1993). However, beta2‐adrenoceptors are also expressed on a wide range of cell types where beta2‐agonists may have a clinically significant effect including airway epithelium (Morrison 1993), mast cells, post capillary venules, sensory and cholinergic nerves and dendritic cells (Anderson 2006). Beta2‐agonists will also cross‐react to some extent with other beta‐adrenoceptors including beta1‐adrenoceptors on the heart.
The in vivo effect of any beta2‐agonist will depend on a number of factors relating to both the drug and the patient. The degree to which a drug binds to one receptor over another is known as selectivity, which can be defined as absolute binding ratios to different receptors in vitro, whilst functional selectivity is measured from downstream effects of drugs in different tissue types in vitro or in vivo. All of the beta2‐agonists described thus far are more beta2 selective than their predecessor isoprenaline in vitro. However, because attempts to differentiate selectivity between the newer agents are confounded by so many factors, it is difficult to draw conclusions about in vitro selectivity studies and probably best to concentrate on specific adverse side‐effects in human subjects at doses which cause the same degree of bronchoconstriction. The potency of a drug refers to the concentration that achieves half the maximal receptor activation of which that drug is capable but it is not very important clinically as for each drug, manufacturers will alter the dose to try to achieve a therapeutic ratio of desired to undesired effects. In contrast efficacy refers to the ability of a drug to activate its receptor independent of drug concentration. Drugs that fully activate a receptor are known as full agonists and those that partially activate a receptor are known as partial agonists. Efficacy also is very much dependent on the system in which it is being tested and is affected by factors including the number of receptors available and the presence of other agonists and antagonists. Therefore, whilst salmeterol acts as a partial agonist in vitro, it causes a similar degree of bronchodilation to the strong agonist formoterol in stable asthmatic patients (Van Noord 1996), presumably because there are an abundance of well‐coupled beta2‐adrenoceptors available with few downstream antagonising signals. In contrast, with repetitive dosing formoterol is significantly better than salmeterol at preventing methacholine‐induced bronchoconstriction (Palmqvist 1999). These differences have led to attempts to define the 'intrinsic efficacy' of a drug independent of tissue conditions (Hanania 2002), as shown in Table 5. The clinical significance of intrinsic efficacy remains unclear.
2. Intrinsic efficacy of beta‐agonists.
| Drug | Intrinsic efficacy (%) |
| Isoprenaline, adrenaline | 100 |
| Fenoterol | 42 |
| Formoterol | 20 |
| Salbutamol | 4.9 |
| Salmeterol | < 2 |
Adapted from Hanania 2002. The authors acknowledge that it is difficult to determine the intrinsic efficacy of salmeterol given its high lipophilicity
Appendix 2. Possible mechanisms of increased asthma mortality with beta2‐agonists
Direct toxicity
This hypothesis states that direct adverse effects of beta2‐agonists are responsible for an associated increase in mortality and most research in the area has concentrated on effects detrimental to the heart. Whilst it is often assumed that cardiac side‐effects of beta2‐agonists are due to cross‐reactivity with beta1‐adrenoceptors (i.e. poor selectivity), it is worth noting that human myocardium also contains an abundance of beta2‐adrenoceptors capable of triggering positive chronotropic and inotropic responses (Lipworth 1992). Indeed, there is good evidence that cardiovascular side effects of isoprenaline and other beta2‐agonists including salbutamol are mediated predominantly via cardiac beta2‐adrenoceptors thus making the concept of in vitro selectivity less relevant (Arnold 1985; Hall 1989). Generalised beta2‐adrenoceptor activation can also cause hypokalaemia and it has been proposed that, through these and other actions beta2‐agonists may predispose to life‐threatening dysrhythmias or cause other adverse cardiac effects (Brown 1983).
During the 1960s epidemic, most deaths occurred in patients with severe asthma and it was originally assumed that asthma and its sequelae, including hypoxia, were the primary cause of death. However, mucus plugging and hypoxia does not preclude a cardiac event as the final cause of death, and one might expect those with severe asthma to take more doses of a prescribed inhaler. As noted by Speizer and Doll, most deaths in the 1960s were in the 10‐19 age group and “at these ages children have begun to act independently and may be particularly prone to misuse a self‐administered form of treatment” (Speizer 1968). If toxicity were related to increasing doses of beta2‐agonists one might expect most deaths to occur in hospital where high doses are typically used and this was not the case. One possible explanation for this anomaly was provided by animal experiments in which large doses of isoprenaline caused little ill effect in anaesthetised dogs with normal arterial oxygenation whereas much smaller doses caused fatal cardiac depression and asystole (although no obvious dysrhythmia) when hypoxic (Collins 1969; McDevitt 1974). It has been hypothesised therefore that such events would be less likely in hospital where supplemental oxygen is routinely given. The clinical relevance of these studies remains unclear although there is some evidence of a synergistic effect between hypoxia and salbutamol use in asthmatic patients in reducing total peripheral vascular resistance (Burggraaf 2001) – another beta2 mediated effect which could be detrimental to the heart during an acute asthma attack through a reduction in diastolic blood pressure. Other potential mechanisms of isoprenaline toxicity include a potential increase in mucous plugging and worsening of ventilation perfusion mismatch despite bronchodilation (Pearce 1990).
Further concerns about a possible toxic effect of beta2‐agonists were raised during the New Zealand epidemic in the 1970s. In 1981 Wilson et al who first reported the epidemic reviewed 22 fatal cases of asthma and noted “In 16 patients death was seen to be sudden and unexpected. Although all were experiencing respiratory distress, most were not cyanosed and the precipitate nature of their death suggested a cardiac event, such as an arrest, inappropriate to the severity of their respiratory problem” (Wilson 1981). In humans, fenoterol causes significantly greater chronotropic, inotropic and electrocardiographic side effects than salbutamol in asthmatic patients (Wong 1990). Interestingly, across the same parameters fenoterol also causes more side‐effects than isoprenaline (Burgess 1991).
In patients with mild asthma and without a bronchoconstrictor challenge, salmeterol and salbutamol cause a similar degree of near maximal bronchodilation at low doses (Bennett 1994). However, whilst as a one off dose salbutamol is typically used at 2 to 4 times the concentration of salmeterol, the dose equivalences for salmeterol versus salbutamol in increasing heart rate and decreasing potassium concentration and diastolic blood pressure were 17.7, 7.8 and 7.6 respectively (i.e. salmeterol had a greater effect across all parameters). Given the lower intrinsic efficacy of salmeterol (Table 5), these results highlight the importance of in vivo factors; one possible explanation for the difference is the increased lipophilicity of salmeterol compared to salbutamol contributing to higher systemic absorption (Bennett 1994).
When comparing increasing actuations of standard doses of formoterol and salmeterol inhalers in stable asthmatic patients, relatively similar cardiovascular effects are seen at lower doses (Guhan 2000). However, at the highest doses (above those recommended by the manufacturers) there were trends towards an increase in systolic blood pressure with formoterol; in comparison there was a trend towards a decrease in diastolic blood pressure and an increase in QTc interval with salmeterol although no statistical analysis of the difference was performed. In contrast in asthmatic patients with methacholine‐induced bronchoconstriction there was no significant difference between salmeterol and formoterol in causing increased heart rate and QTc interval although formoterol caused significantly greater bronchodilation and hypokalaemia (Palmqvist 1999). Whilst there is good evidence of cardiovascular and metabolic side‐effects with increasing doses of beta2‐agonists, it is a little difficult to envisage serious adverse effects of this nature when using LABAs at manufacturer‐recommended preventative doses. However, it is possible that some patients choose to use repeated doses of LABAs during exacerbations.
Tolerance
In this setting, the term tolerance refers to an impaired response to beta2‐agonists in patients who have been using regular beta2‐agonist treatment previously (Haney 2006). Tolerance is likely to result from a combination of reduced receptor numbers secondary to receptor internalisation and reduced production and also uncoupling of receptors to downstream signalling pathways following repeated activation (Barnes 1995). This phenomenon is likely to explain the beneficial reduction in systemic side effects seen with regular use of beta2‐agonists including salbutamol after 1 to 2 weeks (Lipworth 1989). However, the same effect on beta2‐adrenoceptors in the lung might be expected to produce a diminished response to the bronchodilating activity of beta2‐agonists following regular use. In patients with stable asthma, whilst there is some evidence of tolerance to both salbutamol (Nelson 1977) and terbutaline (Weber 1982) other studies have been less conclusive (Harvey 1982; Lipworth 1989). However, evidence of tolerance to short and long‐acting beta2‐agonists in both protecting against and reducing bronchoconstriction is much stronger in the setting of an acute bronchoconstrictor challenge with chemical, allergen and 'natural' stimuli (Haney 2006; Lipworth 1997).
Studies comparing salmeterol and formoterol have shown that both cause tolerance compared to placebo but there was no significant difference between the drugs (Van der Woude 2001). There also appears to be little difference in the tolerance induced by regular formoterol and regular salbutamol treatment (Hancox 1999; Jones 2001). To the authors' knowledge no studies have looked specifically at the degree of tolerance caused by isoprenaline and fenoterol in the setting of acute bronchoconstriction. Tolerance to bronchodilation has been shown to clearly occur with addition of inhaled corticosteroids to salmeterol and formoterol (Lee 2003) and terbutaline (Yates 1996). There is conflicting evidence as to whether high dose steroids can reverse tolerance in the acute setting (Jones 2001; Lipworth 2000).
At first glance the toxicity and tolerance hypotheses might appear incompatible as systemic and cardiovascular tolerance ought to protect against toxicity in the acute setting and there is good evidence that such tolerance occurs in stable asthmatic patients (Lipworth 1989). However, whilst this study showed that changes in heart rate and potassium levels were blunted by previous beta2‐agonist use, they were not abolished; furthermore, at the doses studied these side effects appear to follow an exponential pattern (Lipworth 1989). In contrast, in the presence of bronchoconstrictor stimuli the bronchodilator response to beta2‐agonists follows a flatter curve (Hancox 1999; Wong 1990) and as previously discussed this curve is shifted downwards by previous beta2‐agonist exposure (Hancox 1999). Thus, it is theoretically possible that in the setting of an acute asthmatic attack and strong bronchoconstricting stimuli, bronchodilator tolerance could lead to repetitive beta2‐agonist use and ultimately more systemic side effects than would otherwise have occurred. Of course, other sequelae of inadequate bronchodilation including airway obstruction will be detrimental in this setting.
Whilst the tolerance hypothesis is often cited as contributing towards the asthma mortality epidemics it is difficult to argue that reduced efficacy of a drug can cause increased mortality relative to a time when that drug was not used at all. However, tolerance to the bronchodilating effect of endogenous circulating adrenaline is theoretically possible and there is also evidence of rebound bronchoconstriction when stopping fenoterol (Sears 1990), which may be detrimental. Furthermore, it appears that regular salbutamol treatment can actually increase airway responsiveness to allergen (Cockcroft 1993), a potentially important effect that could form a variant of the toxicity hypothesis. Differences between beta2‐agonists in this regard are unclear, but the combination of rebound hyperresponsiveness and tolerance of the bronchodilator effect with regular beta2‐agonist exposure has been recently advocated as a possible mechanism to explain the association between beta2‐agonists and asthma mortality (Hancox 2006a).
Other explanations
Confounding by severity
Historically, this hypothesis has been used extensively to try to explain the association between mortality and the use of fenoterol during the 1970s New Zealand epidemic and is still quoted today (Pearce 2007). The hypothesis essentially relies on the supposition that patients with more severe asthma are more likely to take either higher doses of beta2‐agonists or a particular beta2‐agonist (such as fenoterol) thereby explaining the association. This hypothesis was carefully ruled out in the three case‐control studies by comparing the association between fenoterol and mortality in patients with varying severity of disease (Crane 1989; Grainger 1991; Pearce 1990). Furthermore, the hypothesis cannot explain the overall increase in mortality in the 1960s and 1970s, nor can it explain any significant increase in mortality (whether taking inhaled steroids or not) from randomised controlled trial data.
The delay hypothesis
This hypothesis accepts that beta2‐agonists or a particular beta2‐agonist cause an increased risk of mortality but indirectly by causing patients to delay before getting medical help and further treatments including high dose steroids and oxygen. There is evidence that both salmeterol and formoterol can reduce awareness of worsening underlying inflammation (Bijl‐Hofland 2001; McIvor 1998). It is difficult to rule out the delay hypothesis in either explaining or contributing towards both the asthma mortality epidemics and an association with regular use of LABAs. There is evidence that beta2‐agonists with higher intrinsic efficacy are more effective at relieving bronchoconstriction in the acute setting and could paradoxically cause patients to delay seeking medical help for longer (Hanania 2007). For the delay hypothesis to explain the increase in mortality during the 1960s and 1970s, one has to imply that hospital treatment of asthma when mortality rates were low during the earlier years of the 20th century was effective. It is difficult to say exactly how effective such treatment is likely to have been.
Reduced corticosteroid treatment
A slight but significant variation of the delay hypothesis suggests that patients who have separate beta2‐agonists and corticosteroid inhalers may choose to take less corticosteroid because of better symptom control from the inhaled beta2‐agonists and it is reduced corticosteroid treatment that contributes to a rise in mortality. It is rather difficult to see how this hypothesis explains the epidemics of asthma deaths in the 1960s and 1970s relative to the 1920s and 30s (Figure 1), given that corticosteroids were not used for the treatment of asthma in the earlier decades. If this hypothesis were to explain increased mortality from more recent randomised controlled trial data, one would not expect to see an increase in mortality in those taking LABAs alone.
Appendix 3. Sources and search methods for the Cochrane Airways Group Specialised Register (CAGR)
Electronic searches: core databases
| Database | Frequency of search |
| MEDLINE (Ovid) | Weekly |
| EMBASE (Ovid) | Weekly |
| CENTRAL (the Cochrane Library) | Quarterly |
| PSYCHINFO (Ovid) | Monthly |
| CINAHL (EBSCO) | Monthly |
| AMED (EBSCO) | Monthly |
Handsearches: core respiratory conference abstracts
| Conference | Years searched |
| American Academy of Allergy, Asthma and Immunology (AAAAI) | 2001 onwards |
| American Thoracic Society (ATS) | 2001 onwards |
| Asia Pacific Society of Respirology (APSR) | 2004 onwards |
| British Thoracic Society Winter Meeting (BTS) | 2000 onwards |
| Chest Meeting | 2003 onwards |
| European Respiratory Society (ERS) | 1992, 1994, 2000 onwards |
| International Primary Care Respiratory Group Congress (IPCRG) | 2002 onwards |
| Thoracic Society of Australia and New Zealand (TSANZ) | 1999 onwards |
MEDLINE search strategy used to identify trials for the CAGR
Asthma search
1. exp Asthma/
2. asthma$.mp.
3. (antiasthma$ or anti‐asthma$).mp.
4. Respiratory Sounds/
5. wheez$.mp.
6. Bronchial Spasm/
7. bronchospas$.mp.
8. (bronch$ adj3 spasm$).mp.
9. bronchoconstrict$.mp.
10. exp Bronchoconstriction/
11. (bronch$ adj3 constrict$).mp.
12. Bronchial Hyperreactivity/
13. Respiratory Hypersensitivity/
14. ((bronchial$ or respiratory or airway$ or lung$) adj3 (hypersensitiv$ or hyperreactiv$ or allerg$ or insufficiency)).mp.
15. ((dust or mite$) adj3 (allerg$ or hypersensitiv$)).mp.
16. or/1‐15
Filter to identify RCTs
1. exp "clinical trial [publication type]"/
2. (randomised or randomised).ab,ti.
3. placebo.ab,ti.
4. dt.fs.
5. randomly.ab,ti.
6. trial.ab,ti.
7. groups.ab,ti.
8. or/1‐7
9. Animals/
10. Humans/
11. 9 not (9 and 10)
12. 8 not 11
The MEDLINE strategy and RCT filter are adapted to identify trials in other electronic databases
Appendix 4. Definition of serious adverse events (SAEs)
A serious adverse event (SAE) is any adverse event occurring at any dose that results in any of the following outcomes:
Death
A life threatening adverse event
Inpatient hospitalisation or prolongation of existing hospitalisation
A disability/incapacity
A congenital anomaly in the offspring of a subject who received medication
Important medical events that may not result in death, be life‐threatening, or require hospitalisation may be considered a serious adverse event when, based upon appropriate medical judgment, they may jeopardise the patient or subject and may require medical or surgical intervention to prevent one of the outcomes listed in this definition. Examples of such medical events include allergic bronchospasm requiring intensive treatment in an emergency room or at home, blood dyscrasias or convulsions that do not result in inpatient hospitalisation, or the development of medication dependency or medication abuse.
Clarifications
'Occurring at any dose' does not imply that the subject is receiving study medication.
Life‐threatening means that the subject was, in the view of the investigator, at immediate risk of death from the event as it occurred. This definition does not include an event that, had it occurred in a more severe form, might have caused death.
Hospitalisation for elective treatment of a pre‐existing condition that did not worsen during the study is not considered an AE.
Complications that occur during hospitalisation are AEs. If a complication prolongs hospitalisation, the event is a SAE.
'Inpatient' hospitalisation means the subject has been formally admitted to a hospital for medical reasons. This may or may not be overnight. It does not include presentation at a casualty or emergency room.
With regard to criterion number 6 above, medical and scientific judgment should be used in deciding whether prompt reporting is appropriate in this situation.
Events or outcomes not qualifying as SAEs
The events or outcomes identified as asthma exacerbations will be recorded in the asthma exacerbations page of the case report form (CRF) page if they occur. However, these individual events or outcomes, as well as any sign, symptom, diagnosis, illness, and/or clinical laboratory abnormality that can be linked to any of these events or outcomes, are not reported to GW as SAEs even though such event or outcome may meet the definition of SAE, unless the following conditions apply:
the investigator determines that the event or outcome qualifies as a SAE under criterion number 6 of the SAE definition (see Section 7.2, Definition of a SAE), or the event or outcome is in the investigator’s opinion of greater intensity, frequency or duration than expected for the individual subject, or death occurring for any reason during a study, including death due to a disease‐related event.
Data and analyses
Comparison 1. Regular salmeterol versus placebo or regular salbutamol.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 All‐cause mortality | 20 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 1.1 Salmeterol versus placebo | 14 | 30254 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.33 [0.85, 2.08] |
| 1.2 Salmeterol versus salbutamol | 7 | 27615 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.22 [0.76, 1.96] |
| 2 Non‐fatal serious adverse events (adults and children) | 25 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 2.1 Salmeterol versus placebo | 18 | 31529 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.15 [1.02, 1.29] |
| 2.2 Salmeterol versus salbutamol | 8 | 28183 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.96 [0.81, 1.14] |
| 3 Non‐fatal serious adverse events in adults | 18 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 3.1 Salmeterol versus placebo in adults | 13 | 30196 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.14 [1.01, 1.28] |
| 3.2 Salmeterol versus salbutamol in adults | 5 | 27002 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.94 [0.79, 1.11] |
| 4 Non‐fatal serious adverse events in children | 7 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 4.1 Salmeterol versus placebo in children | 5 | 1333 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.30 [0.82, 2.05] |
| 4.2 Salmeterol versus salbutamol (from first 3 months of Lenney) | 3 | 1181 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.37 [0.71, 2.64] |
| 4.3 Salmeterol versus salbutamol (data from Lenney 9 month trial extensions) | 3 | 1130 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.17 [0.71, 1.94] |
| 5 Non‐fatal adverse events in adults and children (SNS sensitivity analysis) | 25 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 5.1 Salmeterol versus placebo | 18 | 31529 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.15 [1.02, 1.29] |
| 5.2 Salmeterol versus salbutamol | 8 | 28188 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.98 [0.86, 1.11] |
| 6 Fatal and non‐fatal serious adverse events in adults and children | 25 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 6.1 Salmeterol versus placebo | 18 | 31529 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.16 [1.03, 1.30] |
| 6.2 Salmeterol versus salbutamol | 8 | 28183 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.99 [0.84, 1.16] |
| 7 Asthma mortality | 2 | 51535 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.94 [1.41, 6.14] |
| 7.1 Salmeterol versus placebo | 1 | 26355 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 3.49 [1.31, 9.31] |
| 7.2 Salmeterol versus salbutamol | 1 | 25180 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.36 [0.78, 7.16] |
| 8 Asthma mortality (within study subgroups by ICS use at baseline) | 2 | 46650 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.90 [1.38, 6.13] |
| 8.1 ICS used at baseline | 2 | 26969 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.49 [0.54, 4.11] |
| 8.2 Not on ICS at baseline | 2 | 19681 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 6.43 [2.13, 19.42] |
| 9 Asthma mortality (within study subgroups by any steroid at baseline) | 2 | 47550 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.95 [1.41, 6.14] |
| 9.1 ICS or oral steroids used at baseline | 2 | 27869 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.03 [0.82, 5.00] |
| 9.2 Not any steroids at baseline | 2 | 19681 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 6.15 [1.73, 21.84] |
| 10 Cardiovascular mortality | 19 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 10.1 Salmeterol versus placebo | 13 | 29920 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.75 [0.32, 1.77] |
| 10.2 Salmeterol versus salbutamol | 6 | 27281 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.22 [0.64, 2.34] |
| 11 Adults and Children non‐fatal asthma‐related serious adverse events | 22 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 11.1 Salmeterol versus placebo | 17 | 5174 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.59 [1.05, 2.41] |
| 11.2 Salmeterol versus salbutamol | 6 | 2341 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.99 [0.54, 1.81] |
| 12 Adults and children non‐fatal cardiovascular serious adverse events | 21 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 12.1 Salmeterol versus placebo | 15 | 30579 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.98 [0.73, 1.31] |
| 12.2 Salmeterol versus placebo (US trial results) | 1 | 9275 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.90 [0.27, 2.97] |
| 12.3 Salmeterol versus salbutamol | 5 | 26794 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.06 [0.67, 1.68] |
| 13 Proportion of participants with serious asthma exacerbations in relation to dose of ICS | 1 | 911 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.99 [0.71, 1.39] |
| 13.1 ICS under 500 mcg per day | 1 | 165 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.04 [0.42, 2.61] |
| 13.2 ICS 500 to 1000 mcg per day | 1 | 537 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.68 [0.42, 1.08] |
| 13.3 ICS over 1000 mcg per day or oral corticosteroids | 1 | 209 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.75 [0.99, 3.11] |
| 14 Proportion of participants with serious asthma exacerbations in relation to baseline PEF (% predicted) | 1 | 911 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.97 [0.70, 1.35] |
| 14.1 PEF > 80% predicted | 1 | 531 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.00 [0.62, 1.61] |
| 14.2 PEF 60% to 80% predicted | 1 | 210 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.59 [0.32, 1.10] |
| 14.3 PEF < 60% predicted | 1 | 170 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.70 [0.85, 3.38] |
| 15 Respiratory related deaths or life‐threatening events (SMART) | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 15.1 Salmeterol vs placebo | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 16 Respiratory related deaths or life‐threatening events (SMART subgrouped by race) | 1 | 23327 | Odds Ratio (IV, Fixed, 95% CI) | 1.40 [0.88, 2.21] |
| 16.1 Caucasians | 1 | 18642 | Odds Ratio (IV, Fixed, 95% CI) | 1.04 [0.62, 1.76] |
| 16.2 African Americans | 1 | 4685 | Odds Ratio (IV, Fixed, 95% CI) | 3.95 [1.48, 10.53] |
| 17 Respiratory related deaths or life‐threatening events (SMART subgrouped by baseline ICS use) | 1 | 26355 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.39 [0.91, 2.12] |
| 17.1 ICS at baseline | 1 | 12265 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.21 [0.66, 2.22] |
| 17.2 No ICS at baseline | 1 | 14090 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.58 [0.87, 2.85] |
| 18 Respiratory related deaths or life‐threatening events (SMART subgrouped by race and ICS (Caucasians)) | 1 | 18642 | Odds Ratio (IV, Fixed, 95% CI) | 1.04 [0.62, 1.76] |
| 18.1 ICS at baseline | 1 | 9223 | Odds Ratio (IV, Fixed, 95% CI) | 0.88 [0.42, 1.84] |
| 18.2 No ICS at baseline | 1 | 9419 | Odds Ratio (IV, Fixed, 95% CI) | 1.24 [0.60, 2.58] |
| 19 Respiratory related deaths or life‐threatening events (SMART subgrouped by race and ICS (African Americans)) | 1 | 4685 | Odds Ratio (IV, Fixed, 95% CI) | 3.82 [1.42, 10.28] |
| 19.1 ICS at baseline | 1 | 1781 | Odds Ratio (IV, Fixed, 95% CI) | 2.92 [0.79, 10.81] |
| 19.2 No ICS at baseline | 1 | 2904 | Odds Ratio (IV, Fixed, 95% CI) | 5.47 [1.21, 24.74] |
| 20 Respiratory related deaths or life‐threatening events (SMART subgrouped by baseline predicted PEF) | 1 | 25714 | Odds Ratio (IV, Fixed, 95% CI) | 1.42 [0.92, 2.20] |
| 20.1 PEF > 60% | 1 | 21026 | Odds Ratio (IV, Fixed, 95% CI) | 1.04 [0.57, 1.93] |
| 20.2 PEF =< 60% | 1 | 4688 | Odds Ratio (IV, Fixed, 95% CI) | 1.96 [1.05, 3.66] |
| 21 Respiratory related deaths or life‐threatening events (SMART subgrouped by study phase | 1 | 26355 | Odds Ratio (IV, Fixed, 95% CI) | 1.39 [0.90, 2.14] |
| 21.1 Phase 1 | 1 | 15342 | Odds Ratio (IV, Fixed, 95% CI) | 1.46 [0.87, 2.46] |
| 21.2 Phase 2 | 1 | 11013 | Odds Ratio (IV, Fixed, 95% CI) | 1.25 [0.59, 2.67] |
| 22 All‐cause hospitalisation compared to SAE | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 22.1 Patients with hospitalisation in SMART | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 22.2 Patients with SAE in SMART | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 23 Adults and children published non‐fatal serious adverse events | 11 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 23.1 Salmeterol versus placebo | 8 | 2117 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.13 [0.65, 1.95] |
| 23.2 Salmeterol versus salbutamol | 3 | 26107 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.99 [0.83, 1.19] |
| 24 Hospitalisations for asthma (FDA data) | 1 | 6043 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.14 [1.16, 3.93] |
| 24.1 Salmeterol versus placebo (FDA US trials asthma hospitalisations) | 1 | 6043 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.14 [1.16, 3.93] |
| 25 Adults and children all adverse events | 17 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 25.1 Salmeterol versus placebo | 12 | 4017 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.15 [1.00, 1.33] |
| 25.2 Salmeterol versus salbutamol | 5 | 2130 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.93 [0.77, 1.13] |
| 26 Adults and children published adverse events | 6 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 26.1 Salmeterol versus placebo | 5 | 1726 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.07 [0.87, 1.32] |
| 26.2 Salmeterol versus salbutamol | 1 | 667 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.87 [0.63, 1.20] |
| 27 Adults and children all published drug‐related adverse events | 11 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 27.1 Salmeterol versus placebo | 10 | 2755 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.38 [1.04, 1.85] |
| 27.2 Salmeterol versus salbutamol | 4 | 766 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.04 [0.73, 1.48] |
| 28 Adults and children serious drug‐related adverse events | 14 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 28.1 Salmeterol versus placebo | 10 | 3103 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.92 [0.32, 2.65] |
| 28.2 Salmeterol versus salbutamol | 4 | 1463 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.63 [0.13, 3.07] |
| 29 Adults and children non‐fatal asthma‐related serious adverse events (FDA data shown) | 23 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 29.1 Salmeterol versus placebo | 17 | 5174 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.59 [1.05, 2.41] |
| 29.2 Salmeterol versus placebo (US trial results) | 1 | 9275 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.07 [1.36, 3.13] |
| 29.3 Salmeterol versus salbutamol | 6 | 2341 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.99 [0.54, 1.81] |
1.2. Analysis.

Comparison 1 Regular salmeterol versus placebo or regular salbutamol, Outcome 2 Non‐fatal serious adverse events (adults and children).
1.3. Analysis.

Comparison 1 Regular salmeterol versus placebo or regular salbutamol, Outcome 3 Non‐fatal serious adverse events in adults.
1.4. Analysis.

Comparison 1 Regular salmeterol versus placebo or regular salbutamol, Outcome 4 Non‐fatal serious adverse events in children.
1.17. Analysis.

Comparison 1 Regular salmeterol versus placebo or regular salbutamol, Outcome 17 Respiratory related deaths or life‐threatening events (SMART subgrouped by baseline ICS use).
1.18. Analysis.

Comparison 1 Regular salmeterol versus placebo or regular salbutamol, Outcome 18 Respiratory related deaths or life‐threatening events (SMART subgrouped by race and ICS (Caucasians)).
1.19. Analysis.

Comparison 1 Regular salmeterol versus placebo or regular salbutamol, Outcome 19 Respiratory related deaths or life‐threatening events (SMART subgrouped by race and ICS (African Americans)).
1.24. Analysis.

Comparison 1 Regular salmeterol versus placebo or regular salbutamol, Outcome 24 Hospitalisations for asthma (FDA data).
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Adinoff 1998.
| Methods | Study design: parallel group 4 groups, multi centre, 27 in USA (majority primary care). 4‐6 weeks initially, 2 groups weaned from non‐steroidal asthma medications, then 36 weeks treatment Randomisation: yes, randomised in blocks 5:5:5:1 Blinding: double blind, double dummy, matching devices Withdrawals/drop outs: 75 withdrawals in total, 61 protocol violations, 5 lack efficacy Compliance: not assessed | |
| Participants | N = randomised 386/completed 311, adult/adolescents, M = 203, F = 183; mean age 36.5 (range 12‐85) Baseline severity: stable asthma, severity not stated. Mean FEV1 66% predicted Inclusion: diagnosis asthma by ATS criteria, baseline FEV1 50%‐80% predicted, > 15% FEV1 reversibility to SABA Exclusion: non‐smoker > 1 yr, history life threatening asthma, requiring > 2 canisters SABA/month, oral steroids or RTI < 1 month | |
| Interventions | Intervention: salmeterol 42 µg bd versus (with most patients weaning off nonsteroidal medications during the course of the study) Comparison: placebo bd (half the patients randomised to be weaned off nonsteroidal maintenance treatment) Device: MDI Treatment period: 2‐6 weeks weaning, 36 weeks maintenance Rescue: albuterol prn Co‐interventions: baseline ICS 64%, cromones 20%, theophylline 77% (only ICS were not weaned in any group) | |
| Outcomes | No website data found for this study. Further information awaited from GSK Paper only reports "no deaths or serious drug‐related adverse events occurred" | |
| Notes | Funding: grant from GSK | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind, double dummy, matching devices |
| Selective reporting (reporting bias) | High risk | Not found in GSK trial register |
Boulet 1997.
| Methods | Setting: multicentre study (14 sites in Canada) Length of intervention period: 12 weeks (2 weeks run‐in and 1 week run‐out) Randomisation: no details Allocation concealment: no details Design: parallel group Masking: double‐blind Excluded: 300 enrolled, 228 randomised (all included in the analysis) | |
| Participants | 228 adults and adolescents randomised (12 years old or more). Mean age 38 years. M/F (%): 57/53; mean FEV1 66%; previous ICS use 73% Inclusion criteria: medical history of mild‐to‐moderate asthma that required daily pharmacotherapy for at least 6 months. Prebronchodilator FEV1 50%‐80% with 15% reversibility following 200 µg salbutamol. Symptoms of asthma on 4/7 in last week of run‐in period | |
| Interventions | Run‐in period: 2 weeks LABA: salmeterol 50 µg bd with placebo inhaler in between SABA: salbutamol 200 µg qid Device: MDI Treatment period: 12 weeks Co‐interventions: ICS use 73%; ICS and cromoglycate allowed if enrolled on these for at least one month and the dose was constant through the study | |
| Outcomes | No website data found for this study; further information awaited from GSK "Adverse events were monitored throughout the study" The paper reports frequent adverse events but does not report fatal or non‐fatal adverse events | |
| Notes | Funding: sponsored by GSK | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind |
| Selective reporting (reporting bias) | High risk | Not found in GSK trial register |
Boyd 1995.
| Methods | Multi‐centre parallel group study. Double‐blind placebo controlled (use of identical placebo) | |
| Participants | 119 symptomatic asthmatic adults. All patients were on inhaled corticosteroids. Mean age 47 years. FEV1 66% predicted at baseline. Most patients took ICS 1000‐2000 µg/day at baseline. At least 15% improvement from baseline in lung function following inhaled salbutamol and at least 2 acute asthma exacerbations in the preceding 18 months were required for inclusion Exclusion criteria: ‐ concurrent uncontrolled systemic disease ‐ having received treatment for an acute respiratory infection in the last 2 weeks ‐ or had a FEV1 < 40% predicted |
|
| Interventions | 12 week duration; salmeterol 100 µg twice daily compared with placebo (diskhaler) | |
| Outcomes | No website data found for this study; further information awaited from GSK Paper reports seven patients in each treatment group suffered a serious adverse event. Salmeterol 5 respiratory, one leg injury, one abdominal pain and fever. Placebo 4 respiratory tract and 3 hospitalised for unrelated surgical procedures. No mention of fatal events | |
| Notes | Funding: sponsored by GSK | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Sequence generation by computer generated random numbers and allocation concealment using numbered coded inhalers supplied by pharmacy |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind placebo controlled (use of identical placebo) |
| Selective reporting (reporting bias) | Low risk | Not found in GSK trial register, but all cause serious adverse events were described by treatment group in the paper |
Britton 1992.
| Methods | A randomised, double‐blind, parallel‐group study 62 centres in 13 European countries: Austria (2), Belgium (3), Denmark (6), Finland (4), France (3), Germany (6), Ireland (5), Italy (5), Netherlands (3), Norway (4), Sweden (9), Switzerland (2), United Kingdom (10) Sequence generation: unknown Allocation concealment: unknown 06 Apr 1988 to 19 Oct 1989 | |
| Participants | Patient characteristics: median age 49 years (range 18 to 81). FEV1 2.14 L. 23% were in no steroids, 27% ICS under 1000 µg/day, 36% ICS over 1000 µg/day and 14% on regular oral steroids Asthmatic subjects > 18 years of age with either FEV1 or daily PEFR > 50% of predicted values, reversibility in FEV1. 15% 15 minutes following the inhalation of salbutamol 200 µg, and on > 4 of 7 days in the second week of run‐in period 2 either asthma symptom scores for > 2 or a diurnal variation in PEFR. 15% were eligible for study participation. Subjects were excluded if they required a maintenance dose of oral prednisolone > 20 mg/day, had a clinical or laboratory history of serious systemic disease, or were treated with beta‐receptor antagonists | |
| Interventions | Salmeterol 50 µg (25 µg/actuation) bd via MDI (N = 334); salbutamol 200 µg (100 µg/actuation) QID via MDI (N = 333) Co‐interventions: 23% were on no steroids, 27% ICS under 1000 µg/day, 36% ICS over 1000 µg/day and 14% on regular oral steroids Subjects entered 2 consecutive 1‐week run‐in periods during which they used a salbutamol inhaler when necessary for the relief of symptoms. Subjects were then randomised to receive either salbutamol 200 µg QID or salmeterol 50 µg bd, along with a placebo inhaler bd for 3 months. At the end of 3 months, subjects randomised to salmeterol 50 µg bd continued the same treatment for a further 9 months. Subjects randomised to salbutamol had their dosage reduced to 200 µg bd. All subjects also received salbutamol inhalers to be used when necessary for the relief of symptoms during the study | |
| Outcomes | Paper reports "Two patients died during the year of the study, one from a stroke caused by a glioma in the left midbrain after 10 months of salmeterol and one from septicaemia and renal failure following an oesophagectomy for an adenocarcinoma after six months treatment with salbutamol" Website: SLGT02. Data on the initial 3 month period on‐therapy. One fatal adverse event in salmeterol group (glioma) and one in salbutamol group (adenocarcinoma). Non‐fatal serious adverse events occurred in 33 subjects in the salmeterol group and 39 in the salbutamol group |
|
| Notes | Funding: sponsored by GSK | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind |
| Selective reporting (reporting bias) | Low risk | Full SAE data on GSK trials register |
Busse 1998.
| Methods | Study design: parallel group, multicentre (55), USA, 2 weeks run in/ 12 weeks treatment Randomisation: randomised treatment, no method given Blinding: double‐blind, double dummy, matching devices Withdrawals/drop outs: 95 withdrawals, 47 active, 48 placebo | |
| Participants | N = randomised 538, completed 443. M = 51; F = 68, adults, mean age 36 (range 12‐80) Baseline severity: moderate persistent asthma. FEV1 62% Inclusion: non‐smoking. Diagnosis asthma by ATS criteria, used SABA daily during 6 weeks or more. Baseline FEV1 40‐80% predicted. > 15% FEV1 reversibility to SABA. Symptomatic in run in ASS >= 2 Exclusion: URTI/LRTI < 4 weeks, COPD, CF, unstable asthma, pregnancy, lactation, Use of OS < 1 month | |
| Interventions | Long‐acting beta agonist: salmeterol 42 µg bd Placebo: bd Device: MDI Treatment period: 12 weeks Rescue: Salbutamol 100 µg prn Co‐interventions: ICS 65%, cromones 7%, theophyllines 30% ‐ all fixed doses | |
| Outcomes | No website data found for this study. Further information awaited from GSK Paper reports "Serious adverse events (asthma exacerbation, deep vein thrombosis, respiratory tract infection) developed in 2 patients treated with salmeterol; however, these were not considered by the investigator to be potentially related to treatment." Drug‐related adverse events shown in Table 3 | |
| Notes | Funding: sponsored by GSK | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind, double dummy, matching devices |
| Selective reporting (reporting bias) | Low risk | Not found in GSK trial register, but all‐cause serious adverse events reported in paper |
Chervinsky 1999.
| Methods | Study design: parallel group, multicentre (18), USA, 2‐4 weeks run in/52 weeks treatment and 2 week run out. Randomisation: randomised treatment, using computer generated randomisation schedule Blinding: double blind, matching devices Withdrawals/drop outs: 87 withdrawals, 45 active, 42 placebo | |
| Participants | N = randomised 352, completed 265. M = 179; F = 173, adults, mean age 30 (range 12‐67) Baseline severity: mild persistent asthma that required pharmacotherapy. FEV1 62%. ICS use 56% placebo, 45% salmeterol Inclusion: non‐smoking. Diagnosis asthma by ATS criteria. Baseline FEV1 70%‐90% predicted. > 15% FEV1 reversibility to SABA. BHR 20% with methacholine 7.5 mg/ml or less. Exclusion: URTI/LRTI/ < 6 weeks, abnormal ECG, ongoing passive exposure to tobacco smoke | |
| Interventions | Long‐actingbeta agonist: salmeterol 50 µg bd Placebo: bd Device: DPI Treatment period: 52 weeks Co‐interventions: ICS use 56% placebo, 45% salmeterol. All concomitant treatment 60% and 47% respectively | |
| Outcomes | No website data found for this study. Further information awaited from GSK Paper reports no deaths in the study. The main focus of this study was on cardiovascular adverse events, no reporting of all serious adverse events in the paper | |
| Notes | Funding: sponsored by GSK | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Randomised treatment , using computer generated randomisation schedule |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind, matching devices |
| Selective reporting (reporting bias) | High risk | Not found in GSK trial register |
D'Alonzo 1994.
| Methods | Study design: three treatment parallel group, multicentre (11) study in USA, 1‐2 weeks run in/12 weeks treatment Randomisation: yes, but method not given Blinding: double‐blind, double dummy, with 2 matching inhalers Withdrawals: 42/322 , by groups ‐ 15 in salmeterol, 16 in albuterol, 11 in placebo Confounders: differential rates of ICS and cromone use in regular and prn group, use of theophyllines in run in period | |
| Participants | N =322: albuterol = 108, placebo = 108, salmeterol = 106 Age: means ‐ albuterol 31 (14), placebo 28 (12), salmeterol 29 (12) Severity of asthma: baseline FEV1 65% predicted Inclusion: diagnosis asthma by ATS criteria, requiring daily drug treatment for > 6 months. Baseline FEV1 50%‐70% predicted, > 15% FEV1 reversibility to SABA Exclusion: smokers | |
| Interventions | Long‐acting beta agonist: salmeterol 42 µg bd
Short‐acting beta agonist: albuterol 180 µg qid Placebo: qid Device: MDI Period:12 weeks Rescue: albuterol 90 µg prn Co‐interventions: ICS ‐ used by 20% on placebo , 24% on albuterol, 21% on salmeterol Oral steroids ‐ not at randomisation Cromones ‐ used by 9% on placebo , 6% on albuterol, 10% on salmeterol Theophyllines ‐ used only during run in by 46% on placebo, 50% on albuterol, 43% on salmeterol Ooral beta agonists ‐ not permitted |
|
| Outcomes | No website data found for this study. Further information awaited from GSK Paper reports common adverse events thought to be related to treatment, but no serious adverse event data | |
| Notes | Funding: sponsored by GSK | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind, double dummy, with 2 matching inhalers |
| Selective reporting (reporting bias) | High risk | Not found in GSK trial register, and no SAE data in paper |
D'Urzo 2001.
| Methods | A multicenter, randomised, double‐blind, parallel‐group trial to evaluate the long‐term efficacy and safety of inhaled salmeterol 50 µg bd compared to short‐acting beta2‐agonists as‐needed in adult patients with asthma 15 December 1994 – 30 September 1996 | |
| Participants | Location: 253 centres in Canada 911 subjects 18 years of age and older with documented asthma who required regular anti‐inflammatory treatment and used inhaled beta2‐agonists to control symptoms more than twice daily whom the physician believed would benefit from a long‐acting beta2‐agonist were included in this study. Females of child‐bearing potential could participate if they were using adequate contraceptive methods, were not pregnant or lactating, and did not plan to become pregnant during the study. Exclusions included subjects with serious pulmonary or systemic disease, subjects who had received prior salmeterol, subjects receiving beta‐blockers, and subjects with known hypersensitivity to salmeterol. | |
| Interventions | 24 weeks of one of the following double‐blind study treatments: Salmeterol 50 µg (2 puffs 25 µg) via MDI (CFC) bd (N = 455) Placebo 2 puffs via MDI bd (N = 456) Co‐interventions: 93% patients were on ICS and 5% oral steroids (165 under 500 µg/day, 535 on 500 to 1000 µg/day, and 209 on more than 1000 µg per day or prednisone) | |
| Outcomes | Paper publication reports "During the course of the study there were three deaths reported: two in the salmeterol group (one with congestive heart failure, and one with undetermined cause at autopsy, although the subject had a history of abdominal pain) and one in the placebo group (myocardial infarction leading to anoxic encephalopathy). All deaths were judged to be unrelated to study medication" Website: SLGQ94 (521/180). Salmeterol group: 2 fatal SAEs (chronic obstructive lung disease/congestive heart failure and abdominal pain). Placebo arm: 1 fatal SAE (respiratory distress, also secondary lung edema, myocardial infarction and encephalopathy anoxia). Salmeterol 24 non‐fatal SAEs, and placebo 22 non‐fatal SAEs | |
| Notes | Funding: sponsored by GSK | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind |
| Selective reporting (reporting bias) | Low risk | Full SAE data on GSK trials register |
FDA GSK USA Studies.
| Methods | This document presents the combined results of GSK studies from the USA in which salmeterol was compared with placebo in randomised controlled trials. Trials lasted from one to 52 weeks | |
| Participants | 63 trials involving 17,000 patients with chronic asthma included in Phase II to IV clinical trials in the USA (but results have only been used in this review for patients randomised to salmeterol without ICS) | |
| Interventions | Salmeterol (with or without additional randomised inhaled corticosteroids) versus placebo | |
| Outcomes | Respiratory and cardiac serious adverse events | |
| Notes | Full details are available from http://www.fda.gov/ohrms/dockets/ac/05/briefing/2005‐4148B1_01_01‐GSK‐Serevent.pdf | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | No details of individual studies given |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No details of individual studies given |
| Selective reporting (reporting bias) | Unclear risk | No details of individual studies given |
Kavuru 2000.
| Methods | Setting: multicentre study, USA Length of intervention period: 12 weeks Randomisation: yes (method not stated) Allocation concealment: not stated Design: parallel group Masking: double blind Excluded: yes Withdrawals: stated | |
| Participants | 527 adults and adolescents screened, 356 randomised (four arm study; placebo: N = 82; salmeterol: N = 92), age range: 12‐67; mean baseline FEV1 (% predicted): placebo: 64; salmeterol: 64. Therapy @ baseline: placebo: ICS ‐ 55%, SAL ‐ 66%
Patient characteristics: mean age 36 years, FEV1 64% predicted
Inclusion criteria: >/= 12 years of age; medical history of asthma (ATS criteria); at least 6 months duration; FEV1 between 40%‐85% predicted; >/= 15% reversibility post SABA; participants treated with ICS prior to enrolment had to have been treated with BDP 252‐420 µg/d (6‐10 puffs/d), TAA 600‐1000 µg/d (6‐10 puffs/d); flunisolide: 1000 µg/d (250 QID); fluticasone propionate 176 µg/d (44 QID) for at least 1 month prior to enrolment without change in regimen; If participants using SAL, they had to do so for at least 1 week prior to screening, and demonstrated FEV1 </= 85% predicted post SABA and not received concurrent ICS for 1 month prior to screening; provision of signed consent Exclusion criteria: female participants had negative pregnancy test, and were sterile, post‐menopausal or using acceptable birth control measures; history of life‐threatening asthma; hypersensitivity to ICS or beta‐agonists; smoking history (> 10 pack years); use of oral, inhaled (external to guidelines above) or IM corticosteroid therapy, intranasal corticosteroids (except for 'Flonase'); use of OCS in previous 6 months; use of over the counter medicines that might affect course of asthma; abnormal chest x‐ray; abnormal ECG; significant concurrent disease. During screening period, participants not eligible if they had > 3 awakenings requiring asthma during 7 days immediately preceding randomisation. ICS patients not eligible if using > 12 puffs SABA per day for more than 3 days, SAL patients not eligible using > 6 puffs SABA per day for more than 3 days |
|
| Interventions | Salmeterol 50 µg bd versus placebo Inhaler device: Diskus. Single‐blind run‐in with a placebo (2 weeks) Co‐interventions: baseline ICS 26%. Group 1 (250 participants) all had ICS before enrolment, Group 2 (86 participants) had not used ICS for at least a month but had used salmeterol for at least a week. Protocol suggests that none of the salmeterol or placebo groups continued on ICS as this was a randomised treatment for the other arms | |
| Outcomes | Serious adverse events reported in paper: salmeterol ‐ one asthma exacerbation and one fever. Placebo ‐ one asthma exacerbation. (None were considered related to study medication by the investigator.) Website SFCA 3002 ‐ SAE data: no fatal SAE. One asthma SAE confirmed in salmeterol and placebo groups. The one patient with fever appears to have also had D & V, abdominal discomfort, weight loss, dizziness and anaemia | |
| Notes | Funding: supported by a grant from GSK | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind |
| Selective reporting (reporting bias) | Low risk | Full information on GSK trials register |
Kemp 1998a.
| Methods | Study design: two Parallel group, multicentre studies, 15 centres USA, 12 weeks. Randomisation: yes, no method stated Blinding: double‐blind, double dummy, matching inhalers Withdrawals/drop outs: 41 described after randomisation Compliance: > 70% reported for all but 7 subjects | |
| Participants | N = 451 randomised (149 salmeterol, 150 albuterol, 152 placebo). Adults, M = 262 F = 189 mean age 31 (SD 14) Baseline severity: persistent/symptomatic FEV1 65% predicted; 43% on ICS at baseline Inclusion: diagnosis asthma by ATS criteria. Baseline FEV1 50%‐80% predicted, > 15% FEV1 reversibility to SABA. Requiring daily drug treatment for > 6 months. Exclusion: smoking. | |
| Interventions | Long‐acting beta agonist: salmeterol 50 µg bd Short‐acting beta agonist: albuterol 180 µg qid Placebo: qui Device: MDI & dry powder device Treatment period: 12 weeks Rescue: albuterol 100 µg prn Co‐interventions: ICS 55%‐70 %. Cromones 7%‐12% | |
| Outcomes | No website data found for this study. Further information awaited from GSK Paper only reports drug‐related adverse events; no serious adverse event reporting | |
| Notes | Funding: sponsored by GSK | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind, double dummy, matching inhalers |
| Selective reporting (reporting bias) | High risk | Not found in GSK trial register, and all cause SAE data absent from paper |
Kemp 1998b.
| Methods | Design: parallel‐group, multicenter (44 centres in USA) | |
| Participants | 506 symptomatic asthmatic adults and adolescents. Mean age 42 years, baseline FEV1 63% predicted
Eligibility criteria: average daytime symptom score of 1 on a 0 to 3‐point scale over a 2‐week screening period
‐ use of a short‐acting bronchodilator on a daily basis
‐ FEV1 of 40% to 80% predicted
‐ >= 15% improvement from baseline in FEV1 following inhaled albuterol
‐ use of one of the following inhaled corticosteroids on a daily basis at a fixed dose that is within package insert guidelines for a minimum of 6 weeks prior to the screening visit: beclomethasone (300‐900 µg/day), flunisolide (1000‐2000 µg/day), triamcinolone (600‐1600 µg/day) Exclusion criteria: concurrent tobacco use ‐ oral corticosteroid therapy ‐ immunotherapy requiring dosage change ‐ inability to withdraw asthma/allergy medication before PFTs at screening or clinic visits throughout the study ‐ cystic fibrosis, COPD, any significant uncontrolled disease state other than asthma ‐ any other significant illness ‐ pregnancy or lactation ‐ contraindication to study medications ‐ unstable asthma requiring albuterol >= 12 puffs/day or 12 puffs for > 3 days/week ‐ hospitalisation for asthma within 3 months ‐ mechanical ventilation during an asthma exacerbation within 2 years or > 2 albuterol (or equivalent) inhalers/month within 3 months of screening |
|
| Interventions | 12 weeks of treatment with salmeterol 50 µg twice daily or placebo. Usual ICS continued | |
| Outcomes | No website data found for this study. Further information awaited from GSK Paper reports that "One salmeterol treated patient experienced a serious adverse event (respiratory failure) that the investigators judged to be possibly due to the study drug." No report of fatal events | |
| Notes | Funding: sponsored by GSK | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Randomised by computer generated sequence, (assignment by opaque consecutive numbered envelopes) |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind, identical placebo |
| Selective reporting (reporting bias) | High risk | Not found in GSK trial register, and all cause SAE data absent from paper |
Lazarus 2001.
| Methods | Setting: multicentre study (6 sites in USA). Feb 1997 to Jan 1999 Length of intervention period: 16 weeks (6 weeks run‐in and run‐out) Randomisation: stratified online randomisation Allocation concealment: random block size. Remote allocation Design: parallel group Masking: triple blind Excluded: 422 enrolled. 361 completed run‐in. 339 eligible for run in. 175 patients who were not well controlled during run in were entered into the SLIC study (Lemanske 2001). 164 randomised in this trial Withdrawals: 12 withdrawn in the salmeterol and placebo arms during the randomised treatment period (placebo 5 withdrew consent. Salmeterol 5 withdrew consent, 1 adverse event. TAA 1 lost to follow‐up) | |
| Participants | 164 adults and adolescents randomised. Total of 16 participants aged less than 18 years old. Mean age 31 years
M/F (%): 35/65; Caucasian: 70%; mean FEV1 93%; previous ICS use 53% Inclusion criteria: ATS definition of asthma who met recommended treatment criteria for an ICS |
|
| Interventions | Run‐in period: 6 weeks, during which all patients received triamcinolone 400 µg bd. Enrolled if they were objectively defined as well controlled. LABA: salmeterol 42 µg bd Placebo: placebo 2 puffs bd Device: MDI Treatment period: 16 weeks Co‐interventions: nil | |
| Outcomes | One serious non‐fatal adverse event (not asthma related) occurred in the salmeterol group. There was no mention of deaths in the paper but the authors confirmed none had occurred (data in paper publication and confirmed by study authors) | |
| Notes | Funding from National Heart, Lung, and Blood Institute | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Stratified online randomisation. Random block size. Remote allocation |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Triple‐blind |
| Selective reporting (reporting bias) | Low risk | Data obtained from authors |
Lenney 1995a.
| Methods | This was a multi‐centre, multi‐national, double‐blind, randomised, 3‐limbed, parallel‐group study This study was divided into a 3‐month safety and efficacy period followed by a 9‐month safety only period. This is the report of the 3‐month efficacy and safety study Study Period: 01 Aug 1990 to 28 Mar 1991 | |
| Participants | This study was conducted in 42 centres in 9 countries: Germany (4); Ireland (1); Israel (6); Italy (5); Portugal (2); South Africa (5); Spain (2); Switzerland (1); United Kingdom (16) Subjects aged 4 to 16 years with a history of RAO, who demonstrated at least 15% reversibility of either PEFR or FEV1 15 minutes following a standard dose of salbutamol (200ìg), and who required regular or, as required, inhaled beta2‐agonist treatment for control of their asthma symptoms prior to the start of study. Subjects who required a change in asthma medication, or who had had an acute lower respiratory tract infection or a hospital admission for their airways disease in the four weeks preceding the study and subjects who required a maintenance dose of oral steroids were excluded from the study | |
| Interventions | The objectives of the study were to compare the efficacy and safety of inhaled salmeterol 25 µg and 50 µg bd with salbutamol 200 µg bd delivered from pressurised inhalers over a 3‐month period Subjects were randomly assigned to receive 1 of the following 3 treatments via pressurised inhalers: salmeterol 25 µg bd (N = 122), salmeterol 50 µg bd (N = 130), or salbutamol 200 µg bd (N = 122). Prior to randomisation subjects completed a 2‐week run‐in period where they took salbutamol, as needed (prn). Co‐interventions: 58% were on ICS at baseline | |
| Outcomes | Paper makes no mention of serious adverse events or mortality Website: SLPT01 (first 3 months). No fatal SAEs. Non‐fatal SAEs 5 with salmeterol 25 µg, 9 with salmeterol 50 µg, 8 with salbutamol 200 µg. SMS40093 provides data on the 9‐month follow‐up period in which there was one patient on salbutamol who died of an asthma attack with circulatory arrest | |
| Notes | Funding: sponsored by GSK | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind |
| Selective reporting (reporting bias) | Low risk | Full SAE data on GSK trials register |
Lenney 1995b.
| Methods | This was a multicenter, multinational, double‐blind, randomised, 3‐limbed parallel‐group study This study was divided into a 3‐month safety and efficacy period followed by a 9‐month safety only period. This is the report of the 3‐month efficacy and safety study 12 Sep 1990 to 10 Apr 1991 | |
| Participants | This study was conducted in 54 centres in 13 countries: Austria (3); Belgium (8); Denmark (6); Finland (2); France (5); Germany (2); Ireland (1); Italy (3); Netherlands (4); Norway (4); Sweden (11); Switzerland (1); United Kingdom (4) Subjects aged 4 to 16 years of age with a history of RAO were recruited into the study. Subjects demonstrated at least 15% reversibility of either PEFR or FEV1 15 minutes following a standard dose of salbutamol (200 µg). Subjects required regular or, as required, inhaled beta2‐agonist treatment for control of their asthma symptoms prior to the start of study. Subjects had an asthma symptom score of 2 or more on at least five days/nights of the run‐in or had diurnal variation in PEFR of > 15% on at least 7 days of the run‐in or had required symptomatic bronchodilator use on at least 7 days in the run‐in period Subjects were excluded if they had required a change in asthma medication or had been admitted to hospital for their airways disease or had experienced an acute lower respiratory tract infection in the 4 weeks preceding the study. Also excluded were patients requiring maintenance oral steroids or beta‐adrenergic antagonists Mean age 10 years | |
| Interventions | Subjects were randomly assigned to receive 1 of the following 3 treatments: salmeterol 25 µg bd (N = 157), salmeterol 50 µg bd (N = 160), or salbutamol 200 µg bd (N = 156) via the Diskhaler® dry powder inhaler Co‐interventions: 58% were on ICS at baseline | |
| Outcomes | Paper makes no mention of serious adverse events or mortality Website: SLPT02. No fatal SAE. Subjects with non‐fatal SAE: 4 salmeterol 25 µg, 6 salmeterol 50 µg, 2 salbutamol 200 µg. The number withdrawn due to adverse events were 5, 2, and 1 respectively 9‐month data provided for these patients by GSK | |
| Notes | Funding: sponsored by GSK | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind |
| Selective reporting (reporting bias) | Low risk | Full SAE data on GSK trials register |
Lundback 1993.
| Methods | A randomised, double‐blind, parallel‐group, multi‐centre study Double‐blind double‐dummy design | |
| Participants | Location: 42 centres in 11 European countries: Austria; Belgium; Denmark; Finland; France; Germany; Italy; Portugal; Sweden; Switzerland; United Kingdom Subjects aged .18 years with mild to moderate RAO defined by FEV1 or mean PEFR of .50% of predicted values, .15% reversibility of FEV1 after salbutamol, and either a total asthma symptom score .2 or diurnal variation in PEFR .15% on four of the seven days prior to randomisation. Key exclusion criteria were: treatment with maintenance prednisolone > 20 mg daily; lower respiratory tract infection, hospitalisation for any aspect of reversible airways disease, course of prednisolone > 20mg daily within previous 28 days; pregnancy or lactation, treatment with beta‐adrenoreceptor antagonists; hypersensitivity to beta‐adrenoreceptor agonists | |
| Interventions | Salmeterol 50 µg Rotadisk™ bd (N = 190); salbutamol 400 µg Rotadisk QID (N = 198), and as needed salbutamol metered‐dose inhaler (MDI) 100 µg/actuation for the first three months, then salbutamol dropped to 400 µg bd for the final 9 months (which remained blinded). Double dummy used for the initial 3 months Inhaled corticosteroids were used by 57% of patients in each group, with another 7% taking oral and inhaled steroids and 4% taking oral steroids alone | |
| Outcomes | Paper reports 12‐month follow‐up: "Twenty‐seven patients (12 receiving salmeterol and 15 receiving salbutamol) experienced serious adverse events requiring admission to hospital. Seventeen of these events involved exacerbations of asthma, of which seven occurred in patients receiving salmeterol and 10 in patients receiving salbutamol. One patient in the salbutamol group died as a result of myocardial infarction, which was considered unlikely to be related to the study drug."
Website: SLGT06. This is only the first three months of treatment. No fatal SAEs, 5 subjects with non‐fatal SAEs in salmeterol group (4 with asthma exacerbation and 1 depressive syndrome). Salbutamol group 15 subjects with non‐fatal SAE (9 with asthma exacerbation, 2 cough, 1 TIA, 1 fracture femur, 1 diverticulitis, 1 surgery) No GSK website data for the 9 month follow‐up study. 12‐month data used for this analysis |
|
| Notes | Funding: sponsored by GSK | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind, double‐dummy design |
| Selective reporting (reporting bias) | Low risk | 9‐month follow‐up data missing from GSK website but SAE data for full 12 months available from paper |
Nathan 1999.
| Methods | Parallel group, multicentre (25) USA, 2 week run‐in/26 weeks treatment | |
| Participants | 386 adult/adolescents. Mean age 30, with a diagnosis of asthma by ATS criteria for at least 3/12, baseline FEV1: 65%‐90% predicted. > 15% FEV1 reversibility Exclusion: exacerbation asthma, 1 month, use of ICS/OS < 6mths, smoker, requiring > 12 puffs/day rescue SABA in run in 4/7 | |
| Interventions | Salmeterol 50 mcg twice daily versus placebo Device: MDI, no spacer allowed Treatment period: 26 weeks Co‐interventions: none permitted (no ICS allowed for the previous 6 months) | |
| Outcomes | No website data found for this study. Further information awaited from GSK Paper reports one patient in each group experienced a serious adverse event (but all judged unrelated to study drug). Salmeterol one overdose and major depressive disorder, placebo one depression. No published information on any deaths | |
| Notes | Funding: grant from GSK | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind, double dummy |
| Selective reporting (reporting bias) | Low risk | Not found in GSK trial register, but all‐cause SAE data reported in paper |
Nathan 2006.
| Methods | Parallel group multicentre study over 12 weeks | |
| Participants | 365 adults and adolescents randomised. Age range: 12‐82 years, mean FEV1 68% predicted. Inclusion criteria: FP440‐660 µg/d for at least 3 months prior to study entry; FEV1 40%‐85%; reversibility >= 15% | |
| Interventions | Combination HFA FP/SAL 110/42 bd (220/84) versus CFC SAL 42 bd (84) versus CFC FP 110 bd (220) versus HFA placebo. Inhaler devices: MDI. Run‐in: 2 weeks This review only includes data from the salmeterol and placebo arms Co‐interventions: ICS at usual dose was an inclusion criterion, but appears to have been withdrawn in the salmeterol and placebo arms of the study | |
| Outcomes | The paper publication mentions one drug‐related SAE (an upper GI bleed from the placebo group) Website: SAS30004. No fatal SAE. One SAE on salmeterol (viral meningitis) and one on placebo (upper GI bleed) | |
| Notes | Funding: sponsored by GSK | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind |
| Selective reporting (reporting bias) | Low risk | Full SAE data on GSK trials register |
Pearlman 1992.
| Methods | Parallel‐group (3), multicentre (8) in USA | |
| Participants | N = randomised 234; salmeterol N = 78; albuterol (salbutamol) N = 77; placebo N = 79 Patients over 12 years old with asthma (as defined by the American Thoracic Society) and non‐smokers. At least 6 months of daily therapy before the start of the study. FEV1 50%‐80% predicted values | |
| Interventions | Long‐acting beta agonist: salmeterol 42 µg bd Short acting beta agonist: albuterol (salbutamol) 180 µg qid Placebo: qid Device: MDI Period treatment: 12 weeks Rescue: albuterol 90 mcg prn Co‐interventions: ICS 25%, chromones 10% | |
| Outcomes | No website data found for this study. Further information awaited from GSK No information on serious adverse events reported in the paper | |
| Notes | Funding: grant from GSK | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind, placebo controlled using 2 identical inhalers |
| Selective reporting (reporting bias) | High risk | Not found in GSK trial register, and all‐cause SAE data not in paper |
Pearlman 2004.
| Methods | Setting: Multicentre study, USA Length of intervention period: 12 weeks Randomisation: yes (method not reported) Allocation concealment: not stated Design: parallel group Masking: double blind Excluded: not stated Withdrawals: not stated (ITT) | |
| Participants | N = 360. Salmeterol arm N = 92, placebo arm N = 87
Study population: males and females 12 years of age or older, with a diagnosis of asthma using the American Thoracic Society definition were screened. All subjects were required to have a FEV1 of 40% to 85% predicted normal and > 15% reversibility following 2 puffs of VENTOLIN at screening. The study population was stratified according to whether or not subjects were treated with inhaled corticosteroids or inhaled beta2‐agoinsts at screening (salmeterol or short‐acting beta2‐agonists only). Subjects treated with inhaled corticosteroids must have been treated for at least 3 months prior to visit 1 and receiving a daily dose of: 252‐336 µg beclomethasone dipropionate, 600‐800 µg triamcinolone acetonide, 1000 µg flunisolide, 400‐600 µg budesonide, 176 µg fluticasone propionate inhalation aerosol or 200 µg FP inhalation powder for at least one month prior to visit 1 with no change in regimen. Eligible subjects using only, as‐needed, short‐acting beta‐agonist therapy were required to have received treatment for at least one week prior to visit 1 and have a 7‐day total symptom score > 7 for the 7 days prior to visit 2. Eligible subjects using salmeterol at baseline were required to have received salmeterol and as‐needed, short‐acting beta2‐agonists only for at least one week prior to visit 1 No details on distribution between the groups provided. Participants described as symptomatic. Baseline medication: prn SABA alone: 142; SAL: 84; ICS: 134 (37%) |
|
| Interventions | Salmeterol 42 µg bd via CFC‐MDI versus placebo via HFA‐MDI. Inhaler device: MDI. 2 week run‐in. Duration 12 weeks Co‐interventions: as ICS is part of the randomised treatment for 2 arms of this study, we have assumed that the salmeterol and placebo arms did not take ICS | |
| Outcomes | Paper reports no serious drug‐related adverse events Website: SAS3003. No fatal or non‐fatal SAE in the salmeterol or placebo group | |
| Notes | Funding: sponsored by GSK | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double blind |
| Selective reporting (reporting bias) | Low risk | Full SAE data on GSK trials register |
Rosenthal 1999.
| Methods | 2 parallel group studies, multi‐centre (31) USA, 2 week run in/24 weeks treatment/4 weeks run out | |
| Participants | 408 adult/adolescents with mean age 28 Inclusion: clinical diagnosis asthma, baseline FEV1 > 70% predicted, > 15% FEV1 reversibility to SABA. BHR PD20 < 512 µg or 7.5 mg/mL methacholine Exclusion: URTI/LRTI < 6 weeks hospitalisation < 12 weeks serious uncontrolled systemic disease | |
| Interventions | Salmeterol 50 µg twice daily or placebo (given by MDI) Co‐interventions: none permitted (patients were not using inhaled corticosteroids) | |
| Outcomes | No website data found for this study. Further information awaited from GSK The paper publication states that there were seven events requiring hospitalisation in the salmeterol group (respiratory arrest due to alcohol, tonsillitis, mononucleosis, asthma exacerbation with bronchitis, knee ligament tear, fractured leg and anaphylaxis due to acne treatment). It is not reported how many patients suffered a serious adverse event. One patient in the placebo group was hospitalised for status asthmaticus due to pneumonia | |
| Notes | Funding: supported by a research grant from GSK | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double blind, placebo controlled, blinded devices |
| Selective reporting (reporting bias) | High risk | Not found in GSK trial register, and paper does not report number of patients with SAE |
Russell 1995.
| Methods | Multi‐centre, double‐blind, placebo‐controlled, parallel‐group study 14 March 1992 to 14 March 1993 | |
| Participants | 78 centres in the United Kingdom Male and female asthmatic children aged 4 to 16 years, inclusive, who had been taking at least 200 µg daily of BDP or equivalent for at least 6 months and who were symptomatic and demonstrated a mean PEF of 90% or less of predicted normal on at least 4 days of baseline. Subjects excluded were those receiving oral 2‐adrenoceptor agonist therapy or maintenance oral corticosteroid therapy or those who had a short course of oral corticosteroids in the two weeks prior to the start of the baseline period. Also excluded were those who had received newly prescribed asthma therapy, or had changed asthma therapy in the two weeks prior to the start of the baseline period | |
| Interventions | The study was designed to assess the efficacy and safety of inhaled salmeterol xinafoate (SAL) 50 µg bd when added to the existing therapy for moderate to severe asthmatic children Subjects remained on their usual prophylactic therapy of at least the maximum licensed dose of BDP or equivalent during the 2‐week baseline period, and their usual beta2‐adrenoceptor agonist therapy was replaced by commercially available salbutamol 200 µg Diskhaler (Ventodisks) to be used as required for symptomatic relief. Subjects were then randomised to receive either salmeterol 50 µg bd via the Diskhaler (N = 99) or matching placebo bd (N = 107) in addition to their usual prophylactic therapy for 12 weeks Co‐interventions: 100% ICS as this was an inclusion criterion | |
| Outcomes | Paper publication reported serious adverse events in 10 patients on salmeterol and 13 on placebo Website: SALMP/AH91/D89. No fatal SAEs. Salmeterol 10 subjects had non‐fatal SAE, placebo 13 subjects | |
| Notes | Funding: sponsored by GSK | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double blind |
| Selective reporting (reporting bias) | Low risk | Full SAE data on GSK trials register |
Shapiro 2000.
| Methods | Setting: multicentre study, USA | |
| Participants | 349 adults and adolescents randomised (four treatment arm study; placebo: 93; salmeterol: 88. Data from 13 participants excluded from the analysis due to poor procedure at one site) Inclusion criteria: >/= 12 years; ATS defined asthma of >/= 6 mo duration requiring pharmacotherapy for at least 6 months; FEV1 between 40% and 85% predicted; >/= 15% increase in FEV1 30 minutes after 2 puffs of albuterol; use of ICS 12 weeks prior to the study Exclusion criteria: females with negative pregnancy tests; life‐threatening asthma; hypersensitivity to sympathomimetic drugs/steroids; smoking within previous year; smoking history of > 10 pack years; use of oral/injectable steroid therapy within 1 month of study; use of daily oral steroids within 6 months prior to the study; use of any prescription or over the counter medication that could have affected asthma or course of treatment; abnormal CXR; clinically significant abnormal 12‐lead ECGs history of concurrent disease |
|
| Interventions | Salmeterol 50 µg bd via Diskus inhaler versus placebo. Duration: 12 weeks Co‐interventions: baseline 100% ICS (as this was an inclusion criterion). However the ICS appears to have been withdrawn from the salmeterol and placebo arms that we considered | |
| Outcomes | 49% completed study in salmeterol arm and 28% in placebo arm Paper reports "no serious drug‐related adverse events. Two patients treated with salmeterol withdrew from the study because of adverse events; however, these adverse events were considered by the investigator to be unrelated to study drug (bilateral subcapsular cataracts and postsurgical infection)." Website: SFCA3003: no fatal adverse events. No serious adverse events in placebo arm; three in salmeterol arm (asthma exacerbation, post surgical infection and chest pain) | |
| Notes | Funding: sponsored by GSK | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double blind |
| Selective reporting (reporting bias) | Low risk | Full SAE data on GSK trials register |
Simons 1997.
| Methods | Parallel group multicentre study from Canada, 2 week run in/52 weeks treatment/2 week run out | |
| Participants | 241 children. Mean age 9.3 mths with mild‐moderate persistent asthma Inclusion criteria: clinical stable asthma, atopic, well controlled, baseline FEV1 > 70% predicted, > 10% FEV1 reversibility to SABA. PC20 methacholine < 8 mg/ml Exclusion: use of ICS/OS for > 4 weeks previously, OS/ICS within 3 mths exacerbation < 12 weeks | |
| Interventions | Salmeterol 50 µg twice daily versus placebo (Diskus). ICS was not permitted as it was used as a third comparison arm in this study | |
| Outcomes | Effect on growth reported as adverse event, but no information in paper on fatal or non‐fatal serious adverse events Website: SMS40065 (521/120 [SLPT10]). No fatal SAE. Salmeterol 7 subjects with non‐fatal SAE, placebo 3 subjects with non‐fatal SAE | |
| Notes | Funding: supported by a grant from GSK | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double blind |
| Selective reporting (reporting bias) | Low risk | Full SAE data on GSK trials register |
SLGA 3014.
| Methods | Parallel group, multicentre (36) USA, 12‐week treatment | |
| Participants | 449 children aged 4 to 11 years (mean age 8 years) Inclusion: diagnosis asthma by ATS criteria, Baseline FEV1/PEF 45%‐80% predicted Exclusion: not mentioned but reported as similar to Weinstein 1998 (URTI/LRTI/hospitalisation < 4 weeks, OS, anticholinergics, theophyllines, antihistamines, tobacco exposure, serious uncontrolled systemic disease). |
|
| Interventions | 4 arm study. Salmeterol 50 µg twice daily, salmeterol 25 µg twice daily, albuterol 200 µg four times daily versus placebo (all using dry powder devices ‐ diskus/rotahaler) Co‐interventions: ICS around 50%, cromones 25%, immunotherapy continued at same dose | |
| Outcomes | No website information or paper publication Information from FDA submission: no deaths reported during this trial. Serious adverse events were reported in eight patients, one albuterol patient, three 25 µg Diskus patients and four 50 µg Diskus patients. The albuterol patient and three Diskus patients experienced asthma exacerbations. |
|
| Notes | Funding: sponsored by GSK. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind, double dummy |
| Selective reporting (reporting bias) | Low risk | Mortality and SAE reported in FDA submission, results not found on GSK trial register |
SLMF4002.
| Methods | Multicentre, randomised, double‐blind, placebo‐controlled study November 1998 to January 2000 | |
| Participants | 56 centres in France Main inclusion criteria for entry in run‐in period: males and non‐pregnant, non‐breastfeeding females, aged 18 years or over with asthma currently controlled with ICS (beclometasone 800 to 1200 µg/day or equivalent, or fluticasone 500 µg/day) without change with the last three months; bronchodilator treatment in the previous month as a short‐acting beta‐2 agonist (or beta‐2 agonist in combination with anticholinergics) Main inclusion criteria for randomisation were a controlled asthma confirmed during the run‐in period, with, during the last week: morning PEF 80% of predicted value, and bronchodilator requirement less than 3 times within one day or within several days, and no more than one day with PEF variation > 20% Main exclusion criteria were: a hospitalisation for asthma exacerbation in the previous year or a lower respiratory tract infection in the previous month or, in the previous month, a treatment with long acting beta‐2 agonist, fixed‐dose of short‐acting beta‐2 agonist or an anticholinergic, an anti‐leukotriene, or theophylline, or, in the last three months, a treatment with corticosteroids by general route, or an extended release corticosteroid | |
| Interventions | Salmeterol 50 µg/inhalation (N = 93), or matching placebo (N = 95) twice daily during 6 months The primary objective was to evaluate the efficacy of sal 100 µg per day in helping maintenance of asthma control when ICS dosage is halved in subjects who were receiving 1000 µg/day of beclometasone or equivalent Co‐interventions: ICS 100% as this was an inclusion criterion but the dose was halved as part of the protocol | |
| Outcomes | Unpublished Website: SLMF4002. No subjects with fatal SAE. Non‐fatal SAE in 1 subject in the salmeterol group and 4 subjects in the placebo group | |
| Notes | Funding: sponsored by GSK | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double blind |
| Selective reporting (reporting bias) | Low risk | Full SAE data on GSK trials register |
SMART 2006.
| Methods | 28 week parallel group study in 6163 centres in the USA | |
| Participants | Randomised: 26,355. Completed 19,128 (73%). Mean age: 39 years Patient characteristics at inclusion: mean PEFR: 355 L/min. Baseline ICS use 47% Inclusion criteria: at least 12 years of age; diagnosis of asthma (clinical investigator); receiving current prescription of asthma medication Exclusion criteria: prior use of LABA; pregnancy or lactation; concurrent disease that may pose a risk to the participant; sensitivity to long‐acting beta‐agonists; current ß‐blocker use | |
| Interventions | Salmeterol 50 µg twice daily verus placebo (MDI) Co‐interventions: ICS (47%), methylxanthines, leukotriene agents | |
| Outcomes | Respiratory deaths and life‐threatening events Mortality (total, asthma‐related, respiratory‐related) All cause hospitalisation Combined all‐cause death and life‐threatening events, asthma‐related death and life‐threatening events Subgroups identified (post hoc): Caucasian/African American participants; ICS at baseline/no ICS at baseline; predicted PEF above or below 60% Paper report: "Overall, 1093 subjects (4% in each treatment group) had serious adverse events during the study" Website SGLA5011: 580 fatal and non‐fatal SAEs with salmeterol and 513 with placebo |
|
| Notes | Funding: sponsored by GSK | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Blinding of participants, personnel and outcomes: placebo MDI used. Morbidity and Mortality Review Committee were blinded to study treatment |
| Selective reporting (reporting bias) | Low risk | Full SAE data on GSK trials register |
SNS 1993.
| Methods | Parallel group, double‐blind 16 week study in UK Primary Care (3516 GPs) | |
| Participants | 25,180 adults and adolescents (age range 12 to > 60 years) Inclusion: clinical diagnosis of asthma requiring regular bronchodilator Exclusion: beta blocker use, serious uncontrolled diseases , pregnancy | |
| Interventions | Salmeterol 50 µg twice with placebo at noon and early evening versus salbutamol 200 µg four times daily (given by MDI) Co‐interventions: ICS 69%, OS 4.7%, theophyllines, chromones, ipratropium ‐ stable doses | |
| Outcomes | Deaths, admission to hospital/life‐threatening events. Other serious adverse events and withdrawals. Each outcome was subdivided into respiratory related to asthma and respiratory unrelated to asthma and non‐respiratory causes. If patients suffered more than one adverse event they were counted under the most severe category Results expressed as absolute incidence and relative risks Website: SNS‐D920619. Data on all mortality and serious adverse events | |
| Notes | Funding: sponsored by GSK. No published inhaled corticosteroid usage in relation to outcomes, but data obtained from GSK for baseline steroid use in those with asthma‐related deaths | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Computer generated random code, blocks of 6. Prenumbered treatment packs given to the patients as they were allocated the next consecutive study number |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double blind, double dummy, matching devices. Independent consultants who reviewed deaths possibly related to asthma were blinded to treatment group |
| Selective reporting (reporting bias) | Low risk | Full SAE data on GSK trials register |
Von Berg 1998.
| Methods | Parallel group, multicentre (57) in 11 countries, 2 week run in and 52 week treatment with 2 week period off treatment at 6mths to assess BHR | |
| Participants | 426 children, mean age 10 years (range 5‐15)
Inclusion: clinically diagnosed asthma, > 15% FEV1 reversibility to SABA or diurnal variation PEF > 15%, am PEF< 85% best in run in Exclusion: URTI/LRTI/hospitalisation/changed asthma medication < 4 weeks, requiring OS/anticholinergics/methylxanthines at entry |
|
| Interventions | Salmeterol 50 µg twice daily versus placebo (diskhaler) Co‐interventions: ICS 50%, cromones 22% | |
| Outcomes | Paper publication mentions frequent and drug‐related adverse events but no report of serious adverse events Website SLGB3019 (SLPT09): no fatal adverse events over the entire study period. Data averaged from the two periods so 18 in salmeterol group and 13 in placebo group on average over each six months | |
| Notes | Funding: sponsored by GSK | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double blind |
| Selective reporting (reporting bias) | Low risk | Full SAE data on GSK trials register |
Weinstein 1998.
| Methods | Parallel group, multicentre (11) USA, 1‐2 week run in on single blind placebo/12 week treatment | |
| Participants | 207 children, mean age 8.4 years (range 4‐11) Inclusion: diagnosis asthma by ATS criteria, Baseline FEV1 50%‐80% predicted, > 15% FEV1 reversibility to SABA Exclusion: URTI/LRTI/hospitalisation < 4 weeks, OS, anticholinergics, theophyllines, antihistamines, tobacco exposure, serious uncontrolled systemic disease | |
| Interventions | Salmeterol 50 µg twice daily versus placebo Co‐interventions: ICS 57%, cromones 32%, immunotherapy continued at same dose | |
| Outcomes | Paper reports "Four salmeterol‐ and five placebo‐treated patients experienced serious adverse events requiring hospitalisation. These included asthma exacerbation (four salmeterol, two placebo), appendicitis (two placebo), and kidney obstruction and dehydration (one placebo)" No website data for this trial on GSK site (SLD390). Listed on FDA site submission from GSK | |
| Notes | Funding: sponsored by GSK | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double blind, double dummy |
| Selective reporting (reporting bias) | Low risk | Not found in GSK trial register, but SAE data given in paper |
Wenzel 1998.
| Methods | Parallel group, multicentre, 40, USA. 12 weeks | |
| Participants | 539 adults/adolescents with mean age: 35.4 (range 12 to 83) Inclusion: diagnosis asthma by ATS criteria, requiring daily bronchodilator treatment > 6 weeks. Baseline FEV1 40% to 80% predicted, > 15% FEV1 reversibility to SABA Exclusion: CF, COPD, current smokers, hospitalised by exacerbation asthma < 6 weeks. Use of theophyllines or cromones | |
| Interventions | Salmeterol 50 µg twice daily with placebo twice daily versus salbutamol 200 µg four times daily Co‐interventions: ICS 46% (no other details regarding co‐interventions) | |
| Outcomes | No website data found for this study. Further information requested from GSK Paper report: "Three patients experienced serious adverse events....Two patients treated with salmeterol experienced status asthmaticus; one event was considered to be potentially related to the study drug while the other was not. The other serious adverse event that occurred in one patient treated with albuterol was antisocial behavior and was not considered to be related to study drug" | |
| Notes | Funding: supported by a grant from GSK | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double blind, double dummy, matching devices |
| Selective reporting (reporting bias) | Low risk | Not found in GSK trial register, but all‐cause SAE data published in paper |
Wolfe 2000.
| Methods | Parallel group. Combined data from two studies with 3 arms comparing 2 forms of salmeterol (powder and aerosol) and placebo, multicentre (27) USA. 2 week run in period/12‐week treatment period | |
| Participants | 498 adults/ adolescents, with mean age 33 years (range 12‐79) Inclusion : diagnosis asthma by ATS criteria 6 mths requiring pharmacotherapy. Baseline FEV1 > 85% predicted, > 15% FEV1 reversibility to SABA Exclusion : URTI/LRTI/exacerbation < 6 weeks, smoker or > 10 year pack history | |
| Interventions | Salmeterol 50 µg twice daily via accuhaler or MDI versus placebo in same device Co‐interventions: ICS > 30% all groups, chromones continued at same dose | |
| Outcomes | Paper publication "Overall there were no statistically significant between‐group differences in the incidence of drug‐related adverse events" Website SLGA3010: no subjects with fatal SAE, non‐fatal SAE in 5 subjects on salmeterol (3 asthma and 2 bronchitis) and one on placebo (pneumonia) Website SLGA3011: no subjects with fatal SAE, non‐fatal SAE in 1 subject on salmeterol (cholecystitis) and none on placebo | |
| Notes | Funding: sponsored by GSK | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double blind, double dummy |
| Selective reporting (reporting bias) | Low risk | Full SAE data on GSK trials register |
ATS: American Thoracic Society bd: Bis die (Latin for 'twice a day') BDP: beclomethasone dipropionate BHR: bronchial hyperresponsiveness CF: cystic fibrosis CFC: chlorofluorocarbon COPD: chronic obstructive pulmonary disease CXR: chest x‐ray D & V: diarrhoea and vomiting DPI: dry powder inhaler ECG: electrocardiogram FDA: Food & Drug Administration FP: fluticasone propionate GI: gastrointestinal HFA: hydrofluoroalkane ICS: inhaled corticosteroids IM: intramuscular LABA: long‐acting beta2‐agonist LRTI: lower respiratory tract infection MDI: metered dose inhaler OCS: oral corticosteroid PEF: peak expiratory flow PEFR: peak expiratory flow rate PFT: pulmonary function test prn: pro re nata (Latin for 'taken as needed') QID: Quarter in die (Latin for 'four times each day') RAO: reversible airways obstruction RTI: respiratory tract infection SAE: serious adverse event SAL: salmeterol TAA: triamcinolone acetonide TIA: transient ischemic attack URTI: upper respiratory tract infection
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Bagnato 1996 | Single dose study |
| Beach 1992 | Single dose study |
| Beach 1993 | 6 week duration |
| Blake 1999 | Single dose study |
| Bons 1992 | Overview of other studies |
| Booth 1993 | 8 week duration |
| Boulet 1997b | 8 week duration |
| Bousquet 1996 | 8 week duration |
| Brambilla 1994 | 2 week duration |
| Bronsky 1994 | 1 week duration |
| Bronsky 1999 | Single dose study |
| Busse 1999 | 4 week duration |
| Cartier 1993 | Single dose study |
| Castle 1992 | Not RCT |
| Cazzola 2002 | Patients with COPD |
| Ceugniet 1997 | Single dose |
| Cheung 1992 | 8 week duration |
| Chopra 2005 | Comparison of propellants |
| Cloosterman 2001 | Formoterol not salmeterol used |
| Crompton 1999 | 6 week duration |
| D'Alonzo 1995 | Review of D'Alonzo 1994 and Pearlman 1992 |
| D'Urzo 1998 | Not RCT |
| Dahl 1991 | 4 week duration |
| De Oliveira 1998 | 4 week duration |
| Dekhuijzen 2006 | Not RCT |
| Demirkan 2000 | Comparison of delivery devices |
| Deykin 2007 | Combination therapy with salmeterol and LRTA |
| Edelman 2000 | 8 week duration |
| Eryonucu 2005 | Single dose study |
| Faurschou 1994 | 3 week duration |
| Faurschou 1996 | 6 week duration |
| Fjellbirkeland 1994 | 2 week duration |
| Fuller 1995 | Not RCT (overview of safety data) |
| GlaxoSmithKline 2005 | Single dose study |
| Gongora 1991 | Single dose study |
| Gotz 1995 | Crossover study |
| Gustafsson 1994 | Comparison of delivery devices |
| Harper 2001 | 5 week study duration |
| Hermansson 1995 | 4 week duration |
| Inoue 2007 | Single dose crossover study |
| Jartti 1998 | 4 week duration |
| Jenkins 1991 | Not RCT (time series data of exacerbations) |
| Johnson 1994 | 6 week duration |
| Kemp 1993 | Single dose study |
| Kirby 1995 | Not on asthma (healthy volunteers) |
| Kraemer 1997 | Comparison of delivery devices |
| Kurihara 1993 | Comparison of delivery devices |
| Langley 1998 | 4 week duration |
| Langton Hewer 1995 | 8 week treatment period |
| Lemaigre 2006 | Single dose study |
| Lemanske 2001 | Patients randomised to salmeterol and TAA and then TAA was withdrawn in one arm |
| Lotvall 1998 | Not asthma |
| Lurie 2005 | Not RCT |
| Martin 1999 | 3 week duration |
| Meier 1997 | Not RCT (cohort study) |
| Mikawa 1993 | 2 week duration |
| Miyamoto 1993a | 4 week duration |
| Miyamoto 1993b | 4 week duration |
| Miyamoto 1993c | Single dose |
| Miyamoto 1993d | Single dose |
| Miyamoto 1993e | 4 week duration |
| Morgan 1994 | Single dose study |
| Muir 1992 | 4 week duration |
| Nathan 1995 | Review of D'Alonzo 1994 and Pearlman 1992 |
| Nelson 1999 | 4 week duration |
| Nelson 2001 | Comparison with LRTA |
| Nishima 1993 | Single dose |
| Nishiyama 2006 | Comparison with tulobuterol |
| Norhaya 1999 | 4 week duration, cross‐over |
| Nutini 1998 | Comparison with theophylline |
| Orgel 1985 | Single dose study |
| Ortiz 2002 | Single dose study |
| Paggiaro 1996 | Comparison with theophylline |
| Palmer 1992 | Dose response study |
| Pascoe 2006 | Crossover study |
| Pastorello 1998 | Comparison with theophylline |
| Pearlman 1999 | 4 week duration |
| Peslis 1994 | Comparison with fenoterol |
| Peters 2000 | Short‐term study in hospitalised patients |
| Pohunek 2004 | Single dose study |
| Pollard 1997 | Comparison to theophylline |
| Prieto 2002 | 6 week duration |
| Revill 1998 | Exercise‐induced bronchospasm |
| Rhee 1997 | 6 week duration |
| Ringbaek 1996 | 4 week duration |
| Ringdal 1995 | 4 week duration, propellant comparison |
| Ritz 1997 | Short term study on patients in ITU |
| Roberts 1999 | 6 week duration |
| Sano 1993 | Single dose study |
| Schaanning 1996 | Exercise‐induced bronchospasm |
| Shaheen 1994 | Single dose, propellant comparison |
| Shepherd 1991 | Overview of other studies |
| SLGL24 | 8 week randomised treatment period |
| Stahl 1999 | 6 week duration |
| Starke 1996 | 4 week duration, cross‐over |
| Storms 2004 | 4 week duration |
| Szczeklik 1998 | Single dose study |
| Taguchi 1993 | 8 week duration |
| Taylor 1992 | Single dose study |
| Taylor 1998 | Cross‐over study |
| Taylor 2000a | Not RCT |
| Taylor 2000b | 2 week cross‐over study |
| Thompson 1994 | No control arm for open label extension reported in this abstract |
| Tomac 1996 | 4 week duration |
| Ukena 1997 | Cross‐over study, comparison with theophylline |
| Ullman 1990 | 2 week duration |
| Venables 1992 | 4 week duration, cross‐over |
| Verberne 1993 | Single dose study |
| Verberne 1998 | All patients randomised to ICS and salmeterol |
| Verini 1998 | 1 week duration |
| Villaran 1999 | Exercise‐induced bronchospasm |
| Weersink 1997 | 6 week duration |
| Weiner 2003 | Single dose study |
| Weinstein 1997 | 1 week duration |
| Wiegand 1999 | Comparison with theophylline |
| Wilding 1997 | Cross‐over study |
| Williams 1998 | Not RCT (case control study) |
| Zarkovic 1998 | Cross‐over study |
| Zimmermann 2003 | Exercise‐induced bronchospasm |
ICS: inhaled corticosteroids; ITU: intensive therapy unit/intensive treatment unit; LRTA: leukotriene receptor antagonist; TAA:
Differences between protocol and review
Risk difference was not used as the primary metric for analysis of rare events, due to new advice in the latest revision of the Handbook (Higgins 2008). We have added sensitivity analysis to the review to investigate the impact of considering drug‐related SAEs, paper publication SAEs, and combining SAEs with all other minor events. The primary analysis has now been carried out using Peto odds ratios in order to avoid the need for continuity corrections for zero cells.
Contributions of authors
CJC: Conception of the idea, study selection and data collection, statistical analysis, and co‐writing of the review MJC: Background information (including Appendices), study selection and data collection and co‐writing of the review
Sources of support
Internal sources
St George's University of London, UK.
External sources
-
NIHR Cochrane Programme Grant, UK.
Funding for time to work on this review
Declarations of interest
None known.
Edited (no change to conclusions)
References
References to studies included in this review
Adinoff 1998 {published data only}
- Adinoff A, Schwartz H, Rickard K, Yancey S, Swearingen B. Salmeterol compared with current therapies in chronic asthma. Journal of Family Practice 1998;47(4):278‐84. [PubMed] [Google Scholar]
Boulet 1997 {published data only}
- Boulet LP, Laviolette M, Boucher S, Knight A, Hébert J, Chapman KR. A twelve week comparison of salmeterol and salbutamol in the treatment of mild‐to‐moderate asthma: a Canadian multicentre study. Journal of Allergy and Clinical Immunology 1997;99(1 Pt 1):13‐21. [DOI] [PubMed] [Google Scholar]
Boyd 1995 {published data only}
- Boyd G. Salmeterol xinafoate in asthmatic patients under consideration for maintenance oral corticosteroid therapy. European Respiratory Journal 1995;8:1494‐98. [PubMed] [Google Scholar]
Britton 1992 {published data only}
- Britton MG, Earnshaw JS, Palmer JBD. A twelve month comparison of salmeterol with salbutamol in asthmatic patients. European Respiratory Journal 1992;5(9):1062‐7. [PubMed] [Google Scholar]
- SLGT02. Inhaled GR33343G In Reversible Airways Obstruction – Efficacy Over 3 Months and Safety Over 12 Months. http://ctr.gsk.co.uk/Summary/salmeterol/III_SLGT02.pdf.
Busse 1998 {published data only}
- Busse WW, Casale TB, Murray JJ, Petrocella V, Cox F, Rickard K. Efficacy, safety, and impact on quality of life of salmeterol in patients with moderate persistent asthma. American Journal of Managed Care 1998;4(11):1579‐87. [PubMed] [Google Scholar]
Chervinsky 1999 {published data only}
- Chervinsky P, Goldberg P, Galant S, Wang Y, Arledge T, Welch MB, et al. Long‐term cardiovascular safety of salmeterol powder pharmacotherapy in adolescent and adult patients with chronic persistent asthma: a randomized clinical trial. Chest 1999;115(3):642‐8. [DOI] [PubMed] [Google Scholar]
D'Alonzo 1994 {published data only}
- D'Alonzo GE. Efficacy of inhaled salmeterol in the treatment of asthma. European Respiratory Review 1995;5:128‐32. [Google Scholar]
- D'Alonzo GE, Nathan RA, Henchowicz MD. Salmeterol xinafoate as maintenance therapy compared with albuterol in patients with asthma. JAMA 1994;271(18):1412‐6. [PubMed] [Google Scholar]
D'Urzo 2001 {published data only}
- D'Urzo AD, Chapman KR, Cartier A, Hargreave FE, Fitzgerald M, Tesarowski D. Effectiveness and safety of salmeterol in non‐specialist practice settings. Chest 2001;119(3):714‐9. [DOI] [PubMed] [Google Scholar]
- SLGQ94 (521/180). A multicenter, randomized, double‐blind, parallel‐group trial to evaluate the long‐term efficacy and safety of inhaled salmeterol 50ìg BID compared to short‐acting â2‐agonists as‐needed in adult patients with asthma. http://ctr.gsk.co.uk/Summary/salmeterol/studylist.asp.
FDA GSK USA Studies {published data only}
- GlaxoSmithKline. Safety of Long‐Acting Beta‐Agonist Bronchodilators. http://www.fda.gov/ohrms/dockets/ac/05/briefing/2005‐4148B1_01_01‐GSK‐Serevent.pdf 2005.
Kavuru 2000 {published and unpublished data}
- Edwards T, Gross G, Mitchell D, Chervinsky P, Woodring A, Baitinger L, et al. The salmeterol xinafoate/fluticasone propionate dry powder combination product via diskus(r) inhaler improves asthma control compared to salmeterol xinafoate or fluticasone propionate dry powder alone. American Journal of Respiratory and Critical Care Medicine 1998;157(3 Suppl):A414. [Google Scholar]
- Kavuru M, Melamed J, Gross G, Laforce C, House K, Prillaman B, et al. Salmeterol and fluticasone propionate combined in a new powder inhalation device for the treatment of asthma: a randomized, double‐blind, placebo‐controlled trial. Journal of Allergy and Clinical Immunology 2000;106(6 (pt 1)):1108‐16. [DOI] [PubMed] [Google Scholar]
- Nathan R, Woodring A, Baitinger L, Prillaman B, Faris M, House K, et al. The salmeterol/fluticasone propionate Diskus combination decreases the incidence of exacerbations compared to treatment with salmeterol or fluticasone propionate alone. European Respiratory Journal 1999;14:123S. [Google Scholar]
- SFCA3002. A randomized, double‐blind, parallel‐group trial evaluating safety and efficacy of salmeterol 50mcg BID and fluticasone propionate 100mcg BID individually and in combination and placebo in subjects with asthma. http://ctr.gsk.co.uk 2005.
Kemp 1998a {published data only}
- Kemp J, Wolfe J, Grady J, LaForce C, Stahl E, Arlidge T, et al. Salmeterol powder compared with albuterol aerosol as maintenance therapy for asthma in adolescent and adult patients. Clinical Therapeutics 1998;20(2):270‐82. [DOI] [PubMed] [Google Scholar]
- Wolfe J, LaForce C, Chervinsky P, Galant S, Lumry W, Noonan M, et al. Inhaled salmeterol powder compared with albuterol aerosol given regularly or as needed for asthma. Annals of Allergy, Asthma & Immunology 1995;74:52. [Google Scholar]
Kemp 1998b {published data only}
- Kemp JP, Cook DA, Incaudo GA, Corren J, Kalberg C, Emmett A, et al. Salmeterol improves quality of life in patients with asthma requiring inhaled corticosteroids. Journal of Allergy and Clinical Immunology 1998;101:188‐95. [DOI] [PubMed] [Google Scholar]
Lazarus 2001 {published data only}
- Lazarus SC, Boushey HA, Fahy JV, Chinchilli VM, Lemanske RF Jr, Sorkness CA, et al. Long‐acting {beta}2‐agonist monotherapy vs continued therapy with inhaled corticosteroids in patients with persistent asthma: a randomized controlled trial. JAMA 2001;285(20):2583‐93. [DOI] [PubMed] [Google Scholar]
Lenney 1995a {published data only}
- Lenney W, Pedersen S, Boner AL, Ebbutt A, Jenkins MM. Efficacy and safety of salmeterol in childhood asthma. European Journal of Pediatrics 1995;154:983‐90. [DOI] [PubMed] [Google Scholar]
- SLPT01. A Multicentre, Randomized, Double‐Blind, 3‐Limbed, Parallel‐Group Study Comparing the Efficacy and Tolerability of Salmeterol Xinafoate 25ìg and 50ìg BID With Salbutamol 200ìg BID Given via Pressurized Inhalers in Children With Reversible Airways Obstruction. (3‐Month Efficacy and Safety Study.). http://ctr.gsk.co.uk/Summary/salmeterol/III_SLPT01.pdf.
- SMS40093 (SLPT01). A multi‐centre, randomised, double blind, three limbed, parallel‐group study comparing the efficacy and tolerability of salmeterol xinafoate 25ìg and 50ìg bd with salbutamol 200ìg bd given via pressurised inhalers in children with reversible airways obstruction. (Results of the final nine months safety phase.). http://ctr.gsk.co.uk/Summary/salmeterol/studylist.asp.
Lenney 1995b {published data only}
- Lenney W, Pedersen S, Boner AL, Ebbutt A, Jenkins MM. Efficacy and safety of salmeterol in childhood asthma. European Journal of Pediatrics 1995;154:983‐90. [DOI] [PubMed] [Google Scholar]
- SLPT02. A Multi‐centre, Randomised, Double‐Blind, 3‐Limbed, Parallel‐Group Study Comparing the Efficacy and Tolerability of Dry Powder Formulations of Salmeterol Xinafoate 25ìg and 50ìg Twice Daily With Salbutamol 200ìg Twice Daily Given in Children With Reversible Airways Obstruction. http://ctr.gsk.co.uk/Summary/salmeterol/III_SLPT02.pdf.
Lundback 1993 {published data only}
- Lundback B, Rawlinson DW, Palmer JB. Twelve month comparison of salmeterol and salbutamol as dry powder formulations in asthmatic patients. European Study Group. Thorax 1993;48(2):148‐53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SLGT06. Inhaled GR33343G in Reversible Airways Obstruction – Efficacy and Safety Over Three Months: A Double‐Blind, Parallel‐Group Study Comparing Dry Powder Formulations of Inhaled GR33343G (50mcg) Administered Twice a Day and Inhaled Salbutamol (400mcg) Administered Four Times a Day. http://ctr.gsk.co.uk/Summary/salmeterol/studylist.asp.
Nathan 1999 {published data only}
- Nathan R, Pinnas J, Schwartz H, Grossman J, Yancey S, Emmett A, et al. A six‐month, placebo‐controlled comparison of the safety and efficacy of salmeterol or beclomethasone for persistent asthma. Annals of Allergy 1999;82(6):521‐9. [DOI] [PubMed] [Google Scholar]
Nathan 2006 {published and unpublished data}
- Edin HM, Payne E, Herrle MR, Schoaf L, Mather DB, Scott CA, et al. Salmeterol/fluticasone propionate combination via HFA MDI improves quality of life. Journal of Allergy and Clinical Immunology 2001;107(2):S246. [Google Scholar]
- Nathan RA, Mitchell D, Condemi J, Heller A, Schoaf L, Herrle M, et al. Cardiovascular and hypothalmic‐pituitary‐adrenal axis safety of fluticasone propionate/salmeterol HFA MDI in adolescent and adult patients with asthma. American Journal for Respiratory and Critical Care Medicine 2001;163(5):A863. [Google Scholar]
- Nathan RA, Rooklin A, Schoaf L, Scott C, Ellsworth A, House K, et al. Efficacy and tolerability of fluticasone propionate/salmeterol administered twice daily via hydrofluoroalkane 134a metered‐dose inhaler in adolescent and adult patients with persistent asthma: a randomized, double‐blind, placebo‐controlled, 12‐week study. Clinical Therapeutics 2006;28(1):73‐85. [DOI] [PubMed] [Google Scholar]
- Pearlman DS, Kent E, Lanz MJ, Peden D, Baitinger L, Herrle M, et al. Fluticasone propionate/salmeterol HFA MDI has a rapid onset of effect in asthmatics treated with short‐ or long‐acting beta‐agonists (BA) or inhaled corticosteroids (ICS). Amercian Journal of Respiratory and Critical Care Medicine 2001;163(5):A865. [Google Scholar]
- Rooklin A, Elkayam D, Weiler J, Windom H, Schoaf L, Scott C, et al. The fluticasone propionate/salmeterol HFA MDI is significantly more efficacious in treating asthma than placebo HFA MDI, fluticasone propionate CFC MDI or salmeterol CFC MDI. Journal of Allergy and Clinicial Immunology 2001;107(2):100s. [Google Scholar]
- SAS30004. A randomized, double‐blind, placebo‐controlled, parallel‐group 12‐week trial evaluating the safety and efficacy of the salmeterol/fluticasone propionate combination in GR106642X MDI, 50/250 mcg BID, and salmeterol in propellant 11/12 MDI, 50mcg BID, fluticasone propionate in propellant 11/12 MDI, 250 mcg BID, and placebo propellant GR106642X MDI in adult and adolescent subjects with asthma. http://ctr.gsk.co.uk 2005.
Pearlman 1992 {published data only}
- Arledge T, Liddle D, Stahl E, Rossing T. Salmeterol does not cause tolerance during long term asthma therapy. Journal of Allergy and Clinical Immunology 1996;98(6 Pt 1):1116‐9. [DOI] [PubMed] [Google Scholar]
- Nathan R, Seltzer J, Kemp J, Chervinsky P, Alexander W, Liddle R, et al. Safety of salmeterol in the maintenance treatment of asthma. Annals of Allergy, Asthma & Immunology 1995;75(3):243‐8. [PubMed] [Google Scholar]
- Pearlman DS. Long‐acting beta2‐agonist salmeterol compared with albuterol in maintenance asthma therapy. Annals of Allergy, Asthma & Immunology 1995;75(2):180‐4. [PubMed] [Google Scholar]
- Pearlman DS, Chervinsky P, LaForce C, Seltzer JM, Southern DL, Kemp JP, et al. A comparison of salmeterol with albuterol in the treatment of mild‐to‐moderate asthma. New England Journal of Medicine 1992;327(20):1420‐5. [DOI] [PubMed] [Google Scholar]
- Pearlman DS, Liddle R. Controlling asthma symptoms: salmeterol compared with salbutamol in large‐scale multicentre studies. European Respiratory Review 1994;4(21):301‐5. [Google Scholar]
Pearlman 2004 {published and unpublished data}
- Pearlman DS, Peden D, Condemi JJ, Weinstein S, White M, Baitinger L, et al. Efficacy and safety of fluticasone propionate/salmeterol HFA 134A MDI in patients with mild‐to‐moderate persistent asthma. Journal of Asthma 2004;41(8):797‐806. [DOI] [PubMed] [Google Scholar]
- SAS30003. A stratified, randomised, double‐blind, placebo‐controlled, parallel‐group, 12‐week trial evaluating the safety and efficacy of the salmeterol/fluticasone propionate combination in HFA 134a MDI, 42/88 mcg BID, and salmeterol in propellant 11/12 MDI, 42 mcg BID, fluticasone propionate in propellant 11/12 MDI, 88 mcg BID, and placebo propellant HFA 134a MDI in adult and adolescent subjects with asthma. http://ctr.gsk.co.uk 2005.
- Weinstein SF, Pearlman DS, Condemi JJ, Herrle MR, Scott CA, Payne JE, et al. Superior efficacy of the fluticasone propionate/salmeterol 88/42 mcg HFA‐MDI combination product versus the individual components in asthmatics previously treated with either short‐ or long‐acting beta2‐agonists or inhaled corticosteroids. Journal of Allergy & Clinial Immunology 2001;107(2):S102. [Google Scholar]
Rosenthal 1999 {published data only}
- Rosenthal R, Busse W, Kemp J, Baker J, Kalberg C, Emmett A, et al. Effect of long‐term salmeterol therapy compared with as‐needed albuterol use on airway hyperresponsiveness. Chest 1999;116(3):595‐602. [DOI] [PubMed] [Google Scholar]
Russell 1995 {published data only}
- Russell G, Williams DA, Weller P, Price JF. Salmeterol xinafoate in children on high dose inhaled steroids. Annals of Allergy, Asthma & Immunology 1995;75(5):423‐8. [PubMed] [Google Scholar]
- SALMP/AH91/D89. A phase III, multi‐centre, double‐blind, placebo controlled, parallel group study assessing the efficacy and safety of inhaled salmeterol xinafoate (Serevent™) 50 micrograms BD via the Diskhaler™ when added to the existing treatment of moderate to severe asthmatic children.. http://ctr.gsk.co.uk/Summary/salmeterol/studylist.asp.
Shapiro 2000 {published data only}
- SFCA3003. A randomized, double‐blind, parallel‐group trial evaluating safety and efficacy of salmeterol 50 mcg BID and fluticasone propionate 250 mcg BID individually and in combination and placebo in subjects with asthma. http://ctr.gsk.co.uk/Summary/fluticasone_salmeterol/III_SFCA3003.pdf 2005.
- Shapiro G, Lumry W, Wolfe J, Given J, White M, Woodring A, et al. Combined salmeterol 50 mcg and fluticasone propionate 250 mcg in the Diskus device for the treatment of asthma. American Journal of Respiratory and Critical Care Medicine 2000;161:527‐34. [DOI] [PubMed] [Google Scholar]
Simons 1997 {published data only}
- Malozowski S, Stadel BV, Pian LP. Comparison of beclomethasone, salmeterol, and placebo in children with asthma. New England Journal of Medicine 1998;339(10):704‐5. [DOI] [PubMed] [Google Scholar]
- SMS40065 (521/120 [SLPT10]). Efficacy and safety of long‐term inhaled salmeterol and beclomethasone dipropionate in corticosteroid‐naïve children with mild to moderate, chronic, stable asthma. http://ctr.gsk.co.uk/Summary/salmeterol/studylist.asp.
- Simons FE. A comparison of beclomethasone, salmeterol, and placebo in children with asthma. Canadian Beclomethasone Dipropionate‐Salmeterol Xinafoate Study Group. New England Journal of Medicine 1997;337(23):1659‐65. [DOI] [PubMed] [Google Scholar]
- Simons FER, Dolovich J, Moote DW, Mazza JA, Lyttle B, Becker AB, et al. A one year placebo controlled study of the efficacy and safety of beclomethasone DP versus salmeterol in glucocorticoid naive children with asthma. Journal of Allergy and Clinical Immunology 1997;99:S402. [Google Scholar]
SLGA 3014 {unpublished data only}
- GlaxoSmithKline. Serevent submission to the FDA. http://www.fda.gov/cder/foi/nda/98/20692S1,2_Serevent_medr_P2.pdf 2001.
SLMF4002 {published data only}
- SLMF 4002. Efficacy and safety of salmeterol in patients with asthma controlled with inhaled corticosteroids. http://ctr.gsk.co.uk/Summary/salmeterol/IV_SLMF4002.pdf.
SMART 2006 {published data only}
- GlaxoSmithKline. Safety of Long‐Acting Beta‐Agonist Bronchodilators. http://www.fda.gov/ohrms/dockets/ac/05/briefing/2005‐4148B1_01_01‐GSK‐Serevent.pdf 2005.
- Knobil K, Yancey S, Kral K, Rickard K. Salmeterol Multicentre Asthma Research Trial (SMART): results from an interim analysis. Chest 2003;124:335s. [Google Scholar]
- Nelson HS, Weiss ST, Bleecker ER, Yancey SW, Dorinsky PM, the SMART Study Group. The Salmeterol Multicenter Asthma Research Trial: a comparison of usual pharmacotherapy for asthma or usual pharmacotherapy plus salmeterol. Chest 2006;129(1):15‐26. [DOI] [PubMed] [Google Scholar]
- Rickard KA. 2003 safety alert ‐ serevent (salmeterol xinafoate). www.fda.gov 2003.
- SLGA5011 SMART. SMART: a double‐blind, randomized, placebo‐controlled surveillance study of asthma event outcomes in subjects receiving either usual pharmacotherapy of asthma or usual pharmacotherapy plus salmeterol 42mcg twice daily. http://www.ctr.gsk.co.uk 2006.
- Wooltorton E. Salmeterol (Serevent) asthma trial halted early. Canadian Medical Association Journal 2003;168(6):738. [PMC free article] [PubMed] [Google Scholar]
SNS 1993 {published data only}
- Castle W, Fuller R, Hall J. Serevent nationwide surveillance study:Comparison of salmeterol with salbutamol in asthmatic patients who require regular bronchodilator treatment. BMJ 1993;306:1034‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SNS‐D920619. Serevent Nationwide Surveillance (SNS) Trial. http://ctr.gsk.co.uk/Summary/salmeterol/IV_SNS_D920619.pdf.
Von Berg 1998 {published data only}
- SLGB3019 (SLPT09). A multicentre, randomised, double‐blind, parallel group study comparing the efficacy and safety of inhaled salmeterol xinafoate 50 mcg bd with that of salbutamol 200 mcg to use “as required” from Diskhalers for twelve months in children with asthma. http://ctr.gsk.co.uk/Summary/salmeterol/III_SLGB3019.pdf.
- Berg A, Blic J, Rosa M. Regular salbutamol xinafoate compared with salbutamol as required in children with moderate asthma. American Journal of Respiratory and Critical Care Medicine 1996;4:A76. [Google Scholar]
- Berg A, Blic J, Rosa M, Kaad PH, Moorat A. A comparison of regular salmeterol vs 'as required' salbutamol therapy in asthmatic children. Respiratory Medicine 1998;92(2):292‐9. [DOI] [PubMed] [Google Scholar]
Weinstein 1998 {published data only}
- GlaxoSmithKline. Safety of Long‐Acting Beta‐Agonist Bronchodilators. www.fda.gov/ohrms/dockets/ac/05/briefing/2005‐4148B1_01_01‐GSK‐Serevent.pdf 2005.
- Pearlman DS, Bronsky E, Chervinsky P. Inhaled salmeterol powder compared with placebo administered over 12 weeks to children with mild to moderate asthma. American Journal of Respiratory and Critical Care Medicine 1996;153(4):A76. [Google Scholar]
- Weinstein S, Pearlman D, Bronsky E, Byrne A, Arledge T, Liddle R, et al. Efficacy of salmeterol xinafoate powder in children with chronic persistent asthma. Annals of Allergy, Asthma & Immunology 1998;81(1):51‐8. [DOI] [PubMed] [Google Scholar]
Wenzel 1998 {published data only}
- Cox F, Goodwin B, Wenzel S, Rickard K, Kalberg C, Emmett A. Asthma specific quality of life in patients treated with salmeterol or albuterol. Journal of Allergy and Clinical Immunology 1996;97:256. [Google Scholar]
- Goodwin R, Cox F, Lumry W, Rickard K, Kalberg C, Emmett A. The impact of salmeterol versus albuterol on disease specific quality of life in mild to moderate asthmatics. Journal of Allergy and Clinical Immunology 1996;97 (Pt 3):256. [Google Scholar]
- Wenzel SE, Lumry W, Manning M, Kalberg C, Cox F, Emmett A, et al. Efficacy, safety, and effects on quality of life of salmeterol versus albuterol in patients with mild to moderate persistent asthma. Annals of Allergy 1998;80(6):463‐70. [DOI] [PubMed] [Google Scholar]
Wolfe 2000 {published and unpublished data}
- SLGA3010. A randomized, double‐blind, double‐dummy, comparative clinical trial of salmeterol 50 mcg via the Diskus and salmeterol 50 mcg via the metered‐dose inhaler versus placebo for 12 weeks in adolescent and adult subjects with mild‐to‐moderate asthma. http://ctr.gsk.co.uk/Summary/salmeterol/III_SLGA3010.pdf.
- SLGA3011. A randomized, double‐blind, double‐dummy, comparative clinical trial of salmeterol 50 mcg via the Diskus and salmeterol 50 mcg via the metered‐dose inhaler versus placebo for twelve weeks in adolescent and adult subjects with mild‐to‐moderate asthma.. http://ctr.gsk.co.uk/Summary/salmeterol/III_SLGA3011.pdf.
- Wolfe J, Kreitzer S, Chervinsky P, Lawrence M, Wang Y, Reilly D, et al. Comparison of powder and aerosol formulations of salmeterol in the treatment of asthma. Annals of Allergy 2000;84(3):334‐40. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Bagnato 1996 {published data only}
- Bagnato GF, Mileto A, Gulli S, Oriti S, Cesare E, Cinquegrani M, et a, Acute cardiovascular effects of salmeterol in subjects with stable bronchial asthma. Monaldi Archives for Chest Disease 1996;51(4):275‐8. [PubMed] [Google Scholar]
Beach 1992 {published data only}
- Beach JR, Young CL, Stenton SC, Avery AJ, Walters EH, Hendrick DJ. A comparison of the speeds of action of salmeterol and salbutamol in reversing methacholine‐induced bronchoconstriction. Pulmonary Pharmacology 1992;5(2):133‐5. [DOI] [PubMed] [Google Scholar]
Beach 1993 {published data only}
- Beach JR, Young CL, Harkawat R, Gardiner PV, Avery AJ, Coward GA, et al. Effect on airway responsiveness of six weeks treatment with salmeterol. Pulmonary Pharmacology 1993;6(2):155‐7. [DOI] [PubMed] [Google Scholar]
Blake 1999 {published data only}
- Blake K, Pearlman DS, Scott C, Wang Y, Stahl E, Arledge T. Prevention of exercise‐induced bronchospasm in pediatric asthma patients: a comparison of salmeterol powder with albuterol. Annals of Allergy, Asthma & Immunology 1999;82(2):205‐11. [DOI] [PubMed] [Google Scholar]
Bons 1992 {published data only}
- Bons J, Pappo M. Salmeterol and prolonged treatment of asthma: international clinical data. Revue des Maladies Respiratoires 1992;9 Suppl 1:R15‐8. [PubMed] [Google Scholar]
Booth 1993 {published data only}
- Booth H, Fishwick K, Harkawat R, Devereux G, Hendrick DJ, Walters EH. Changes in methacholine induced bronchoconstriction with the long acting beta 2 agonist salmeterol in mild to moderate asthmatic patients. Thorax 1993;48(11):1121‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Boulet 1997b {published data only}
- Boulet LP, Turcotte H, Boutet M, Dube J, Gagnon M, Laviolette M. Influence of salmeterol on chronic and allergen‐induced airway inflammation in mild allergic asthma: a pilot study. Current Therapeutic Research, Clinical & Experimental 1997;58(4):240‐59. [Google Scholar]
Bousquet 1996 {published data only}
- Bousquet J, Aubert B, Bons J. Comparison of salmeterol with disodium cromoglycate in the treatment of adult asthma. Annals of Allergy, Asthma & Immunology 1996;76(2):189‐94. [DOI] [PubMed] [Google Scholar]
Brambilla 1994 {published data only}
- Brambilla C, Chastang C, Georges D, Bertin L. Salmeterol compared with slow‐release terbutaline in nocturnal asthma. A multicenter, randomized, double‐blind, double‐dummy, sequential clinical trial. French Multicenter Study Group. Allergy 1994;49(6):421‐6. [DOI] [PubMed] [Google Scholar]
Bronsky 1994 {published data only}
- Bronsky EA, Kemp JP, Orgel HA, Bierman CW, Tinkelman DG, As A, et al. A 1‐week dose‐ranging study of inhaled salmeterol in patients with asthma. Chest 1994;105(4):1032‐7. [DOI] [PubMed] [Google Scholar]
Bronsky 1999 {published data only}
- Bronsky EA, Pearlman DS, Pobiner BF, Scott C, Wang Y, Stahl E. Prevention of exercise‐induced bronchospasm in pediatric asthma patients: a comparison of two salmeterol powder delivery devices. Pediatrics 1999;104(3 Pt 1):501‐6. [DOI] [PubMed] [Google Scholar]
Busse 1999 {published data only}
- Busse W, Nelson H, Wolfe J, Kalberg C, Yancey SW, Rickard KA. Comparison of inhaled salmeterol and oral zafirlukast in patients with asthma. Journal of Allergy and Clinical Immunology 1999;103(6):1075‐80. [DOI] [PubMed] [Google Scholar]
Cartier 1993 {published data only}
- Cartier A, Ghezzo H, L'Archeveque J, Trudeau C, Malo JL. Duration and magnitude of action of 50 and 100 micrograms of inhaled salmeterol in protecting against bronchoconstriction induced by hyperventilation of dry cold air in subjects with asthma. Journal of Allergy and Clinical Immunology 1993;92(3):488‐92. [DOI] [PubMed] [Google Scholar]
Castle 1992 {published data only}
- Castle WM. Review of salmeterol international safety data base. European Respiratory Journal 1992;5(Suppl 15):318S. [Google Scholar]
Cazzola 2002 {published data only}
- Cazzola M, Califano C, Perna F, D'Amato M, Terzano C, Matera MG, et al. Acute effects of higher than customary doses of salmeterol and salbutamol in patients with acute exacerbation of COPD. Respiratory Medicine 2002;96(10):790‐5. [DOI] [PubMed] [Google Scholar]
Ceugniet 1997 {published data only}
- Ceugniet F, Cauchefer F, Fragneaud C, Evano‐Celli I. Prophylactic treatment of exercise‐induced asthma in children: salmeterol or sodium cromoglycate single dose before exercise?. Annales De Pediatrie 1997;44(9):625‐34. [Google Scholar]
Cheung 1992 {published data only}
- Cheung D, Timmers MC, Zwinderman AH, Bel EH, Dijkman JH, Sterk PJ. Long‐term effects of a long‐acting beta 2‐adrenoceptor agonist, salmeterol, on airway hyperresponsiveness in patients with mild asthma. New England Journal of Medicine 1992;327(17):1198‐203. [DOI] [PubMed] [Google Scholar]
Chopra 2005 {published data only}
- Chopra N, Williams M, Rimmer M, Kahl L, Jenkins M, Teams SMOaSIS. Salmeterol HFA is as effective as salmeterol CFC in children and adults with persistent asthma. Respiratory Medicine 2005;99(Suppl 1):S1‐10. [DOI] [PubMed] [Google Scholar]
Cloosterman 2001 {published data only}
- Cloosterman SG, Bijl‐Hofland ID, Herwaarden CL, Akkermans RP, Elshout FJ, Folgering HT, et al. A placebo‐controlled clinical trial of regular monotherapy with short‐acting and long‐acting beta(2)‐agonists in allergic asthmatic patients. Chest 2001;119(5):1306‐15. [DOI] [PubMed] [Google Scholar]
Crompton 1999 {published data only}
- Crompton GK, Ayres JG, Basran G, Schiraldi G, Brusasco V, Eivindson A, et al. Comparison of oral bambuterol and inhaled salmeterol in patients with symptomatic asthma and using inhaled corticosteroids. American Journal of Respiratory and Critical Care Medicine 1999;159(3):824‐8. [DOI] [PubMed] [Google Scholar]
D'Alonzo 1995 {published data only}
- D'Alonzo GE. Efficacy of inhaled salmeterol in the treatment of asthma. European Respiratory Review 1995;5(27):128‐32. [Google Scholar]
D'Urzo 1998 {published data only}
- D'Urzo A, Chapman KR, Cartier A, Fitzgerald M, Hargreave FE, Tesarowski D. Safety and efficacy of salmeterol in a post‐marketing surveillance trial in asthmatics. American Journal of Respiratory and Critical Care Medicine 1998;157(3 Suppl):A402. [Google Scholar]
Dahl 1991 {published data only}
- Dahl R, Earnshaw JS, Palmer JB. Salmeterol: a four week study of a long‐acting beta‐adrenoceptor agonist for the treatment of reversible airways disease. European Respiratory Journal 1991;4(10):1178‐84. [PubMed] [Google Scholar]
Dekhuijzen 2006 {published data only}
- Dekhuijzen PNR. Caution recommended in prescribing long‐acting beta2‐adrenergic agonists to patients with asthma. Nederlands Tijdschrift voor Geneeskunde 2006;150(16):889‐91. [PubMed] [Google Scholar]
Demirkan 2000 {published data only}
- Demirkan K, Tolley E, Mastin T, Soberman J, Burbeck J, Self T. Salmeterol administration by metered‐dose inhaler alone vs metered‐dose inhaler plus valved holding chamber. Chest 2000;117(5):1314‐8. [DOI] [PubMed] [Google Scholar]
De Oliveira 1998 {published data only}
- Oliveira MA, Jardim JR, Faresin SM, Lucas SR, Nery LE. Efficacy of and tolerance to salmeterol compared to salbutamol in patients with bronchial asthma. Revista Da Associacao Medica Brasileira 1998;44(3):169‐75. [PubMed] [Google Scholar]
Deykin 2007 {published data only}
- Deykin A, Wechsler ME, Boushey HA, Chinchilli VM, Kunselman SJ, Craig TJ, et al. Combination therapy with a long‐acting beta‐agonist and a leukotriene antagonist in moderate asthma. American Journal of Respiratory & Critical Care Medicine 2007;175(3):228‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Edelman 2000 {published data only}
- Edelman JM, Turpin JA, Bronsky EA, Grossman J, Kemp JP, Ghannam AF, et al. Oral montelukast compared with inhaled salmeterol to prevent exercise‐induced bronchoconstriction. A randomized, double‐blind trial. Annals of Internal Medicine 2000;132(2):97‐104. [DOI] [PubMed] [Google Scholar]
Eryonucu 2005 {published data only}
- Eryonucu B, Uzun K, Guler N, Tuncer M, Sezgi C. Comparison of the short‐term effects of salmeterol and formoterol on heart rate variability in adult asthmatic patients. Chest 2005;128(3):1136‐9. [DOI] [PubMed] [Google Scholar]
Faurschou 1994 {published data only}
- Faurschou P, Engel AM, Haanaes OC. Salmeterol in two different doses in the treatment of nocturnal bronchial asthma poorly controlled by other therapies. Allergy 1994;49(10):827‐32. [DOI] [PubMed] [Google Scholar]
Faurschou 1996 {published data only}
- Faurschou P, Steffensen I, Jacques L. Effect of addition of inhaled salmeterol to the treatment of moderate‐to‐severe asthmatics uncontrolled on high‐dose inhaled steroids. European Respiratory Study Group. European Respiratory Journal 1996;9(9):1885‐90. [DOI] [PubMed] [Google Scholar]
Fjellbirkeland 1994 {published data only}
- Fjellbirkeland L, Gulsvik A, Palmer JB. The efficacy and tolerability of inhaled salmeterol and individually dose‐titrated, sustained‐release theophylline in patients with reversible airways disease. Respiratory Medicine 1994;88(8):599‐607. [DOI] [PubMed] [Google Scholar]
Fuller 1995 {published data only}
- Fuller R. Safety of salmeterol in the treatment of asthma. European Respiratory Review 1995;5(27):133‐7. [Google Scholar]
GlaxoSmithKline 2005 {published data only}
- GlaxoSmithKline. A double‐blind evaluation of efficacy, safety, and tolerability of several single doses of salmeterol hydroxynaphthoate (GR33343G) compared with albuterol and placebo in asthmatic patients. GlaxoSmithKline Clinical Trial Register 2005.
Gongora 1991 {published data only}
- Gongora HC, Wisniewski AF, Tattersfield AE. A single‐dose comparison of inhaled albuterol and two formulations of salmeterol on airway reactivity in asthmatic subjects. American Review of Respiratory Disease 1991;144(3 Pt 1):626‐9. [DOI] [PubMed] [Google Scholar]
Gotz 1995 {published data only}
- Gotz MH. The efficacy and safety of inhaled salmeterol xinafoate (50 mcg bd) compared with salbutamol (200 mcg prn) in children with asthma. European Respiratory Journal 1995;8 Suppl 19:517S. [Google Scholar]
Gustafsson 1994 {published data only}
- Gustafsson PM, Von BA, Jenkins MM. Salmeterol 50 mcg twice daily in the treatment of mild‐to‐moderate asthma in childhood ‐ a comparison of two inhalation devices. European Journal of Clinical Research 1994;5:63‐73. [Google Scholar]
Harper 2001 {published data only}
- Harper TB, Stevens A, Clements D, Vandermeer A, Reisner C. Cardiac safety of salmeterol in 2 to 4 year old subjects with asthma. Journal of Allergy and Clinical Immunology 2001;107(2):S108. [Google Scholar]
Hermansson 1995 {published data only}
- Hermansson BA, Jenkins RJ. A 4‐week comparison of salmeterol and terbutaline in adult asthma. Allergy 1995;50(7):551‐8. [DOI] [PubMed] [Google Scholar]
Inoue 2007 {published data only}
Jartti 1998 {published data only}
- Jartti TT, Kaila TJ, Tahvanainen KU, Kuusela TA, Vanto TT, Valimaki IA. Altered cardiovascular autonomic regulation after salmeterol treatment in asthmatic children. Clinical Physiology 1998;18(4):345‐53. [DOI] [PubMed] [Google Scholar]
Jenkins 1991 {published data only}
- Jenkins MM, Hilton CJ, Kock JC, Palmer JB. Exacerbations of asthma in patients on salmeterol. Lancet 1991;337(8746):913‐4. [DOI] [PubMed] [Google Scholar]
Johnson 1994 {published data only}
- Johnson ME. A multicentre study to compare the efficacy and safety of salmeterol xinafoate and nedocromil sodium via metered‐dose inhalers in adults with mild‐to‐moderate asthma. European Journal of Clinical Research. 1994;5:75‐85. [Google Scholar]
Kemp 1993 {published data only}
- Kemp JP, Bierman CW, Cocchetto DM. Dose‐response study of inhaled salmeterol in asthmatic patients with 24‐hour spirometry and Holter monitoring. Annals of Allergy 1993;70(4):316‐22. [PubMed] [Google Scholar]
Kirby 1995 {published data only}
- Kirby SM, Smith J, Ventresca GP. Salmeterol inhaler using a non‐chlorinated propellant, HFA134a: systemic pharmacodynamic activity in healthy volunteers. Thorax 1995;50(6):679‐81. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kraemer 1997 {published data only}
- Kraemer R, Dhank B. Long term efficacy and tolerability of salmeterol (50 µG BD) administered by pressurised inhaler propelled by propellants 11 and 12 or by an alternative propellant, GR106642X for a period of 12 months in children with reversible airways obstruction. European Respiratory Journal 1997;10(Suppl 25):222S. [Google Scholar]
Kurihara 1993 {published data only}
- Kurihara K, Mikawa H, Baba M, Ichikawa K, Masuda K, Yamada T, et al. Clinical study of salmeterol xinafoate (SN408) dry powder: comparative study between salmeterol xinafoate aerosol in children with bronchial asthma. Rinsho Iyaku (Journal of Clinical Therapeutics and Medicines) 1993;9(10):2473‐87. [Google Scholar]
Langley 1998 {published data only}
- Langley SJ, Masterson CM, Batty EP, Woodcock A. Bronchodilator response to salbutamol after chronic dosing with salmeterol or placebo. European Respiratory Journal 1998;11(5):1081‐5. [DOI] [PubMed] [Google Scholar]
Langton Hewer 1995 {published data only}
- Langton Hewer S, Hobbs J, French D, Lenney W. Pilgrims progress: the effect of salmeterol in older children with chronic severe asthma. Respiratory Medicine 1995;89:435‐40. [DOI] [PubMed] [Google Scholar]
Lemaigre 2006 {published data only}
- Lemaigre V, Bergh O, Smets A, Peuter S, Verleden GM. Effects of long‐acting bronchodilators and placebo on histamine‐induced asthma symptoms and mild bronchus obstruction. Respiratory Medicine 2006;100(2):348‐53. [DOI] [PubMed] [Google Scholar]
Lemanske 2001 {published data only}
- Lemanske RF Jr, Sorkness CA, Mauger EA, Lazarus SC, Boushey HA, Fahy JV, et al. Inhaled corticosteroid reduction and elimination in patients with persistent asthma receiving salmeterol: a randomized controlled trial. JAMA 2001;285(20):2594‐603. [DOI] [PubMed] [Google Scholar]
Lotvall 1998 {published data only}
- Lotvall J, Lunde H, Svedmyr N. Onset of bronchodilation and finger tremor induced by salmeterol and salbutamol in asthmatic patients. Japanese Journal of Urology 1998;89(1):43‐9. [DOI] [PubMed] [Google Scholar]
Lurie 2005 {published data only}
- Lurie P, Wolfe SM. Misleading data analyses in salmeterol (SMART) study. Lancet 2005;366(9493):1261‐2. [DOI] [PubMed] [Google Scholar]
Martin 1999 {published data only}
- Martin RJ, Kraft M, Beaucher WN, Kiechel F, Sublett JL, LaVallee N, et al. Comparative study of extended release albuterol sulfate and long‐acting inhaled salmeterol xinafoate in the treatment of nocturnal asthma. Annals of Allergy, Asthma, & Immunology 1999;83(2):121‐6. [DOI] [PubMed] [Google Scholar]
Meier 1997 {published data only}
- Meier CR, Jick H. Drug use and pulmonary death rates in increasingly symptomatic asthma patients in the UK. Thorax 1997;52(7):612‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mikawa 1993 {published data only}
- Mikawa H, Mayumi M, Kimata H, Baba M, Ichikawa K, Matsumoto S, et al. Clinical study of salmeterol xinafoate (SN408) aerosol: comparative study by multiple administration in children with bronchial asthma. Rinsho Iyaku (Journal of Clinical Therapeutics and Medicines) 1993;9(Suppl 4):179‐99. [Google Scholar]
Miyamoto 1993a {published data only}
- Miyamoto T, Takishima T, Makino S, Kabe J, Takahashi T, Yamakido M, et al. Clinical study of salmeterol xinafoate (SN408) aerosol: double‐blind parallel study between procaterol tablet in patients with bronchial asthma. Rinsho Iyaku (Journal of Clinical Therapeutics and Medicines) 1993;9(11):2671‐99. [Google Scholar]
Miyamoto 1993b {published data only}
- Miyamoto T, Takishima T, Makino S, Kabe J, Takahashi T, Yamakido M, et al. Clinical study of salmeterol xinafoate (SN408) aerosol: examination on optimal dose by multiple administration in patients with bronchial asthma. Rinsho Iyaku (Journal of Clinical Therapeutics and Medicines) 1993;9(Suppl 4):81‐111. [Google Scholar]
Miyamoto 1993c {published data only}
- Miyamoto T, Takahashi T, Kabe J, Makino S. Clinical study of salmeterol xinafoate (SN408) aerosol: examination on bronchodilation by single administration in patients with bronchial asthma: examination on bronchodilation by single administration in patients with bronchial asthma. Rinsho Iyaku (Journal of Clinical Therapeutics and Medicines) 1993;9(Suppl 4):23‐48. [Google Scholar]
Miyamoto 1993d {published data only}
- Miyamoto T, Takahashi T, Kabe J, Makino S. Clinical study of salmeterol xinafoate (SN408) dry powder: examination on bronchodilation by single administration in patients with bronchial asthma. Rinsho Iyaku (Journal of Clinical Therapeutics and Medicines) 1993;9(Suppl 4):219‐41. [Google Scholar]
Miyamoto 1993e {published data only}
- Miyamoto T, Makino S, Kabe J, Takahashi T, Nakashima M. Clinical study of salmeterol xinafoate (SN408) aerosol: comparative study by multiple administration in patients with bronchial asthma. Rinsho Iyaku (Journal of Clinical Therapeutics and Medicines) 1993;9:49‐79. [Google Scholar]
Morgan 1994 {published data only}
- Morgan M. A comparison of the efficiency of salmeterol xinofoate with oral salbutamol controlled release against exercise induced asthma in adult asthmatics. National Research Register (https://portal.nihr.ac.uk/Pages/NRRArchiveSearch.aspx) 1994. [N0123137694]
Muir 1992 {published data only}
- Muir JF, Georges D. Usefulness of salmeterol in nocturnal asthma: a comparative study with theophylline‐ketotifen association. A French multicenter group. Revue des Maladies Respiratoires 1992;9(Suppl 1):R23‐6. [PubMed] [Google Scholar]
Nathan 1995 {published data only}
- Nathan RA, Seltzer JM, Kemp JP, Chervinsky P, Alexander WJ, Liddle R, et al. Safety of salmeterol in the maintenance treatment of asthma. Annals of Allergy, Asthma & Immunology 1995;75(3):243‐8. [PubMed] [Google Scholar]
Nelson 1999 {published data only}
- Nelson HS, Berkowitz RB, Tinkelman DA, Emmett AH, Rickard KA, Yancey SW. Lack of subsensitivity to albuterol after treatment with salmeterol in patients with asthma. American Journal of Respiratory and Critical Care Medicine 1999;159(5 Pt 1):1556‐61. [DOI] [PubMed] [Google Scholar]
Nelson 2001 {published data only}
- Nelson HS, Nathan RA, Kalberg C, Yancey SW, Rickard KA. Comparison of inhaled salmeterol and oral zafirlukast in asthmatic patients using concomitant inhaled corticosteroids. Medscape General Medicine 2001;3(4):3. [PubMed] [Google Scholar]
Nishima 1993 {published data only}
- Nishima S, Odajima H, Origasa H. Clinical study of salmeterol xinafoate (SN408) dry powder: bioequivalency study between salmeterol xinafoate aerosol in children with bronchial asthma by single dose crossover method. Rinsho Iyaku (Journal of Clinical Therapeutics and Medicines) 1993;9(9):2133‐46. [Google Scholar]
Nishiyama 2006 {published data only}
- Nishiyama O, Taniguchi H, Kondoh Y, Kimura T, Kato K, Kume H, et al. Comparison of the effects of tulobuterol patch and salmeterol in moderate to severe asthma. Clinical and Experimental Pharmacology and Physiology 2006;33(11):1016‐21. [DOI] [PubMed] [Google Scholar]
Norhaya 1999 {published data only}
- Norhaya MR, Yap TM, Zainudin BMZ. Addition of inhaled salmeterol to inhaled corticosteroids in patients with poorly controlled nocturnal asthma. Respirology 1999;4(1):77‐81. [DOI] [PubMed] [Google Scholar]
Nutini 1998 {published data only}
- Nutini S, Martini T, Righi R. Long‐term treatment of asthmatic patients with salmeterol vs slow‐release theophylline. Respiratory Medicine 1998;92(4):683‐90. [DOI] [PubMed] [Google Scholar]
Orgel 1985 {published data only}
- Orgel HA, Kemp JP, Tinkelman DG, Webb DR, Jr. Bitolterol and albuterol metered‐dose aerosols: comparison of two long‐acting beta 2‐adrenergic bronchodilators for treatment of asthma. Journal of Allergy and Clinical Immunology 1985;75(1 Pt 1):55‐62. [DOI] [PubMed] [Google Scholar]
Ortiz 2002 {published data only}
- Ortiz G, Menendez R. The effects of inhaled albuterol and salmeterol in 2‐ to 5‐year‐old asthmatic children as measured by impulse oscillometry. Journal of Asthma 2002;39(6):531‐6. [DOI] [PubMed] [Google Scholar]
Paggiaro 1996 {published data only}
- Paggiaro PL, Giannini D, Di FA, Testi R. Comparison of inhaled salmeterol and individually dose‐titrated slow‐ release theophylline in patients with reversible airway obstruction. European Respiratory Journal 1996;9(8):1689‐95. [DOI] [PubMed] [Google Scholar]
Palmer 1992 {published data only}
- Palmer JB, Stuart AM, Shepherd GL, Viskum K. Inhaled salmeterol in the treatment of patients with moderate to severe reversible obstructive airways disease ‐ a 3‐month comparison of the efficacy and safety of twice‐daily salmeterol 100 micrograms with salmeterol 50 micrograms. Respiratory Medicine 1992;86(5):409‐17. [DOI] [PubMed] [Google Scholar]
Pascoe 2006 {published data only}
- Pascoe S, Knowles J, Glasbrenner M, Duvauchelle T, Fuhr R, Brookman J. Efficacy and safety of therapeutic and supratherapeutic doses of indacaterol compared to salmeterol and salbutamol in mild to moderate asthma [Abstract]. European Respiratory Journal 2006; Vol. 28, issue Suppl 50:207s [P1238].
Pastorello 1998 {published data only}
- Pastorello EA, Mauro M, Incorvaia C. Comparison of efficacy and safety of inhaled salmeterol and slow‐release oral theophylline in patients with moderate/severe asthma. Internista 1998;6(2):101‐7. [Google Scholar]
Pearlman 1999 {published data only}
- Pearlman DS, Stricker W, Weinstein S, Gross G, Chervinsky P, Woodring A, et al. Inhaled salmeterol and fluticasone: a study comparing monotherapy and combination therapy in asthma. Annals of Allergy, Asthma, & Immunology 1999;82(3):257‐65. [DOI] [PubMed] [Google Scholar]
Peslis 1994 {published data only}
- Peslis N, Weber HH, Kettner J. Salmeterol and fenoterol ‐ comparison of efficacy and safety in patients with reversible obstructive airways disease. European Respiratory Journal 1994;7(Suppl 18):112s. [Google Scholar]
Peters 2000 {published data only}
- Peters JI, Shelledy DC, Jones AP Jr, Lawson RW, Davis CP, LeGrand TS. A randomized, placebo‐controlled study to evaluate the role of salmeterol in the in‐hospital management of asthma. Chest 2000;118(2):313‐20. [DOI] [PubMed] [Google Scholar]
Pohunek 2004 {published data only}
- Pohunek P, Matulka M, Rybnicek O, Kopriva F, Honomichlova H, Svobodova T. Dose‐related efficacy and safety of formoterol (Oxis) Turbuhaler compared with salmeterol Diskhaler in children with asthma. Pediatric Allergy and Immunology 2004;15(1):32‐9. [DOI] [PubMed] [Google Scholar]
Pollard 1997 {published data only}
- Pollard SJ, Spector SL, Yancey SW, Cox FM, Emmett A. Salmeterol versus theophylline in the treatment of asthma. Annals of Allergy, Asthma, & Immunology 1997;78(5):457‐64. [DOI] [PubMed] [Google Scholar]
Prieto 2002 {published data only}
- Prieto L, Gutierrez V, Torres V, Uixera S, Marin J. Effect of salmeterol on seasonal changes in airway responsiveness and exhaled nitric oxide in pollen‐sensitive asthmatic subjects. Chest 2002;122(3):798‐805. [DOI] [PubMed] [Google Scholar]
Revill 1998 {published data only}
- Revill SM, Morgan MD. The cardiorespiratory response to submaximal exercise in subjects with asthma following pretreatment with controlled release oral salbutamol and high‐dose inhaled salmeterol. Respiratory Medicine 1998;92(8):1053‐8. [DOI] [PubMed] [Google Scholar]
Rhee 1997 {published data only}
- Rhee YK. A comparison of salmeterol with salbutamol inhalation in treatment of mild to moderate asthma. Tuberculosis and Respiratory Diseases 1997;44(4):815‐21. [Google Scholar]
Ringbaek 1996 {published data only}
- Ringbaek TJ, Soes‐Petersen U, Christensen M, Iversen ET, Rasmussen FV. Salmeterol improves the control of disease in patients with moderate asthma. A comparative study of inhaled salmeterol 50 mg and salbutamol depot tablets 8 mg, both administered twice daily. Ugeskrift for Laeger 1996;158(27):3940‐3. [PubMed] [Google Scholar]
Ringdal 1995 {published data only}
- Ringdal N, Bateman E, Laitinen L, Sykes AP. Efficacy and safety of salmeterol 50 mcg via a pressurised inhaler formulated with GR106642X. American Journal of Respiratory and Critical Care Medicine 1995;151(4 Pt 2):A57. [Google Scholar]
Ritz 1997 {published data only}
- Ritz M, Thorens JB, Arnold‐Ketterer M, Chevrolet JC. Effects of inhaled salmeterol and salbutamol (albuterol) on morning dips compared in intensive care patients recovering from an acute severe asthma attack. Intensive Care Medicine 1997;23(12):1225‐30. [DOI] [PubMed] [Google Scholar]
Roberts 1999 {published data only}
- Roberts JA, Bradding P, Britten KM, Walls AF, Wilson S, Gratziou C, et al. The long‐acting beta2‐agonist salmeterol xinafoate: effects on airway inflammation in asthma. European Respiratory Journal 1999;14(2):275‐82. [DOI] [PubMed] [Google Scholar]
Sano 1993 {published data only}
- Sano Y, Miyamoto Y, Arai Y, Tanaka Y, Akiyama N, Origasa H. Clinical study of salmeterol xinafoate (SN408) dry powder: bioequivalency study between salmeterol xinafoate aerosol in patients with bronchial asthma by single dose crossover method. Rinsho Iyaku (Journal of Clinical Therapeutics and Medicines) 1993;9(9):2105‐18. [Google Scholar]
Schaanning 1996 {published data only}
- Schaanning J, Vilsvik J, Henriksen AH, Bratten G. Efficacy and duration of salmeterol powder inhalation in protecting against exercise‐induced bronchoconstriction. Annals of Allergy, Asthma, & Immunology 1996;76(1):57‐60. [DOI] [PubMed] [Google Scholar]
Shaheen 1994 {published data only}
- Shaheen MZ, Ayres JG, Benincasa C. Incidence of acute decreases in peak expiratory flow following the use of metered‐dose inhalers in asthmatic patients. European Respiratory Journal 1994;7(12):2160‐4. [DOI] [PubMed] [Google Scholar]
Shepherd 1991 {published data only}
- Shepherd GL, Jenkins WJ, Alexander J. Asthma exacerbations in patients taking regular salmeterol, or salbutamol for symptoms. Lancet 1991;337(8754):1424. [DOI] [PubMed] [Google Scholar]
SLGL24 {published data only}
- SLGL24. A double‐blind, randomised, parallel group study to investigate the efficacy of inhaled salmeterol 50 mcg administered twice daily compared with salbutamol 200 mcg administered four times daily in patients with poorly controlled nocturnal asthma. http://ctr.gsk.co.uk/Summary/salmeterol/studylist.asp.
Stahl 1999 {published data only}
- Stahl E, Svensson K, Crompton GK. Bambuterol is a more cost‐effective treatment strategy than salmeterol in asthmatic patients with nocturnal symptoms. Journal of Medical Economics 1999;2:117‐22. [Google Scholar]
Starke 1996 {published data only}
- Starke ID, Luce P. The efficacy and safety of inhaled salmeterol 50 microg bd in older patients with reversible airflow obstruction. Age and Ageing 1996;25(1):67‐71. [DOI] [PubMed] [Google Scholar]
Storms 2004 {published data only}
- Storms W, Chervinsky P, Ghannam AF, Bird S, Hustad CM, Edelman JM, et al. A comparison of the effects of oral montelukast and inhaled salmeterol on response to rescue bronchodilation after challenge. Respiratory Medicine 2004;98(11):1051‐62. [DOI] [PubMed] [Google Scholar]
Szczeklik 1998 {published data only}
- Szczeklik A, Dworski R, Mastalerz L, Prokop A, Sheller JR, Nizankowska E, et al. Salmeterol prevents aspirin‐induced attacks of asthma and interferes with eicosanoid metabolism. American Journal of Respiratory and Critical Care Medicine 1998;158(4):1168‐72. [DOI] [PubMed] [Google Scholar]
Taguchi 1993 {published data only}
- Taguchi O, Ibata H, Suzuki S, Miyamoto T. Clinical study of salmeterol xinafoate (SN408) in a regular inhalation therapy in patients with bronchial asthma: comparative evaluation with azelastine regarding its effect on bronchial hyperresponsiveness. Rinsho Iyaku (Journal of Clinical Therapeutics and Medicines) 1993;9(11):2711‐23. [Google Scholar]
Taylor 1992 {published data only}
- Taylor IK, O'Shaughnessy KM, Choudry NB, Adachi M, Palmer JB, Fuller RW. A comparative study in atopic subjects with asthma of the effects of salmeterol and salbutamol on allergen‐induced bronchoconstriction, increase in airway reactivity, and increase in urinary leukotriene E4 excretion. Journal of Allergy & Clinical Immunology 1992;89(2):575‐83. [DOI] [PubMed] [Google Scholar]
Taylor 1998 {published data only}
- Taylor DR, Town GI, Herbison GP, Boothman‐Burrell D, Flannery EM, Hancox B, et al. Asthma control during long‐term treatment with regular inhaled salbutamol and salmeterol published [erratum appears in Thorax 1999 Feb;542:188]. Thorax 1998;53(9):744‐52. [DOI] [PMC free article] [PubMed] [Google Scholar]
Taylor 2000a {published data only}
- Taylor DR, Drazen JM, Herbison GP, Yandava CN, Hancox RJ, Town GI. Asthma exacerbations during long term beta agonist use: influence of beta2 adrenoceptor polymorphism. Thorax 2000;55(9):762‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Taylor 2000b {published data only}
- Taylor DR, Hancox RJ, McRae W, Cowan JO, Flannery EM, McLachlan CR, et al. The influence of polymorphism at position 16 of the beta2‐adrenoceptor on the development of tolerance to beta‐agonist. Journal of Asthma 2000;37(8):691‐700. [DOI] [PubMed] [Google Scholar]
Thompson 1994 {published data only}
- Thompson P, Bellesis M. Long‐term safety and efficacy of salmeterol in nocturnal asthmatics. Australian & New Zealand Journal of Medicine 1994;24:459. [Google Scholar]
Tomac 1996 {published data only}
- Tomac N, Tuncer A, Saraclar Y, Adalioglu G. Efficacy of salmeterol in the treatment of childhood asthma. Acta Paediatrica Japonica 1996;38(5):489‐94. [DOI] [PubMed] [Google Scholar]
Ukena 1997 {published data only}
- Ukena D, Koper I, Braun H, Leutz M, Schlimmer P, Sybrecht GW. Therapy of nocturnal asthma: salmeterol versus nocturnal administration of retard theophylline‐‐comparison of effectiveness and tolerance. Pneumologie 1997;51(3):317‐23. [PubMed] [Google Scholar]
Ullman 1990 {published data only}
- Ullman A, Hedner J, Svedmyr N. Inhaled salmeterol and salbutamol in asthmatic patients. An evaluation of asthma symptoms and the possible development of tachyphylaxis. American Review of Respiratory Disease 1990;142(3):571‐5. [DOI] [PubMed] [Google Scholar]
Venables 1992 {published data only}
- Venables T. A multicentre study in general practice to compare the efficacy and tolerability of inhaled salmeterol xinafoate and terbutaline in the treatment of asthma. British Journal of Clinical Research 1992;3:125‐36. [Google Scholar]
Verberne 1993 {published data only}
- Verberne AA, Hop WC, Bos AB, Kerrebijn KF. Effect of a single dose of inhaled salmeterol on baseline airway caliber and methacholine‐induced airway obstruction in asthmatic children. Journal of Allergy and Clinical Immunology 1993;91(1 Pt 1):127‐34. [DOI] [PubMed] [Google Scholar]
Verberne 1998 {published data only}
- SLGB4014 (SLPT15). Placebo controlled study during one year comparing the addition of salmeterol with an increase of the dose of the inhaled corticosteroid in asthmatic children already on treatment with inhaled corticosteroids. http://ctr.gsk.co.uk/Summary/salmeterol/IV_SLGB4014.pdf.
- Verberne AA, Frost C, Roorda RJ, Laag H, Kerrebijn KF. One year treatment with salmeterol compared with beclomethasone in children with asthma. The Dutch Paediatric Asthma Study Group. American Journal of Respiratory & Critical Care Medicine 1997;156(3 Pt 1):688‐95. [DOI] [PubMed] [Google Scholar]
Verini 1998 {published data only}
- Verini M, Verrotti A, Greco R, Chiarelli F. Comparison of the bronchodilator effect of inhaled short‐ and long‐acting beta2‐agonists in children with bronchial asthma. A randomised trial. Clinical Drug Investigation 1998;16(1):19‐24. [DOI] [PubMed] [Google Scholar]
Villaran 1999 {published data only}
- Villaran C, O'Neill SJ, Helbling A, Noord JA, Lee TH, Chuchalin AG, et al. Montelukast versus salmeterol in patients with asthma and exercise‐induced bronchoconstriction. Montelukast/Salmeterol Exercise Study Group. Journal of Allergy & Clinical Immunology 1999;104(3 Pt 1):547‐53. [DOI] [PubMed] [Google Scholar]
Weersink 1997 {published data only}
- Weersink EJ, Zomeren EH, Koeter GH, Postma DS. Treatment of nocturnal airway obstruction improves daytime cognitive performance in asthmatics. American Journal of Respiratory & Critical Care Medicine 1997;156(4 Pt 1):1144‐50. [DOI] [PubMed] [Google Scholar]
Weiner 2003 {published data only}
- Weiner P, Magadle R, Beckerman M, Berar‐Yanay N. The effect of time on the perception of dyspnea following inhaled long‐acting bronchodilator. Harefuah 2003;142(5):342‐4, 398. [PubMed] [Google Scholar]
Weinstein 1997 {published data only}
- Weinstein S, Chervinsky P, Pollard SJ, Bronsky EA, Nathan RA, Prenner B, et al. A one‐week dose‐ranging study of inhaled salmeterol in children with asthma. Journal of Asthma 1997;34(1):43‐52. [DOI] [PubMed] [Google Scholar]
Wiegand 1999 {published data only}
- Wiegand L, Mende CN, Zaidel G, Zwillich CW, Petrocella VJ, Yancey SW, et al. Salmeterol vs theophylline: sleep and efficacy outcomes in patients with nocturnal asthma. Chest 1999;115(6):1525‐32. [DOI] [PubMed] [Google Scholar]
Wilding 1997 {published data only}
- Wilding P, Clark M, Coon JT, Lewis S, Rushton L, Bennett J, et al. Effect of long term treatment with salmeterol on asthma control: A double blind, randomised crossover study. BMJ 1997;314(7092):1441‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Williams 1998 {published data only}
- Williams C, Crossland L, Finnerty J, Crane J, Holgate S, Pearce N, et al. Case‐control study of salmeterol and near‐fatal attacks of asthma. Thorax 1998;53(1):7‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zarkovic 1998 {published data only}
- Zarkovic J, Gotz MH, Holgate ST, Taak NK. Effect of long‐term regular salmeterol treatment in children with moderate asthma. Clinical Drug Investigation 1998;15(3):169‐175. [Google Scholar]
Zimmermann 2003 {published data only}
- Zimmermann T, Gulyas A, Bauer CP, Steinkamp G, Trautmann M. Salmeterol versus sodium cromoglycate for the protection of exercise induced asthma in children‐‐a randomised cross‐over study. European Journal of Medical Research 2003;8(9):428‐34. [PubMed] [Google Scholar]
Additional references
Altman 2003
- Altman DG, Bland JM. Statistics notes: interaction revisited: the difference between two estimates. BMJ 2003;326(7382):219. [DOI] [PMC free article] [PubMed] [Google Scholar]
Anderson 2006
Arnold 1985
- Arnold JMO, Oconnor PC, Riddell JG, Harron DWG, Shanks RG, McDevitt DG. Effects of the beta‐2‐adrenoceptor antagonist ici‐118,551 on exercise tachycardia and isoprenaline‐induced beta‐adrenoceptor responses in man. British Journal of Clinical Pharmacology 1985; Vol. 19, issue 5:619‐30. [DOI] [PMC free article] [PubMed]
Barnes 1993
Barnes 1995
Bennett 1994
- Bennett JA, Smyth ET, Pavord ID, Wilding PJ, Tattersfield AE. Systemic effects of salbutamol and salmeterol in patients with asthma. Thorax 1994; Vol. 49, issue 8:771‐4. [DOI] [PMC free article] [PubMed]
Benson 1948
Bergman 1969
Bijl‐Hofland 2001
- Bijl‐Hofland ID, Cloosterman SG, Folgering HT, Elshout FJ, Weel C, Schayck CP. Inhaled corticosteroids, combined with long‐acting beta(2)‐agonists, improve the perception of bronchoconstriction in asthma. American Journal of Respiratory and Critical Care Medicine 2001; Vol. 164, issue 5:764‐9. [DOI] [PubMed]
Boushey 1980
Bousquet 1990
Bousquet 2000
Brewster 1990
Brown 1983
Brusasco 1998
- Brusasco V, Crimi E, Pellegrino R. Airway hyperresponsiveness in asthma: not just a matter of airway inflammation. Thorax 1998; Vol. 53, issue 11:992‐8. [DOI] [PMC free article] [PubMed]
Burgess 1991
- Burgess CD, Windom HH, Pearce N, Marshall S, Beasley R, Siebers RWL, et al. Lack of evidence for beta‐2 receptor selectivity ‐ a study of metaproterenol, fenoterol, isoproterenol, and epinephrine in patients with asthma. American Review of Respiratory Disease 1991; Vol. 143, issue 2:444‐6. [DOI] [PubMed]
Burggraaf 2001
- Burggraaf J, Westendorp RGJ, In't Veen J, Schoemaker RC, Sterk PJ, Cohen AF, et al. Cardiovascular side effects of inhaled salbutamol in hypoxic asthmatic patients. Thorax 2001; Vol. 56, issue 7:567‐9. [DOI] [PMC free article] [PubMed]
Carroll 1993
Cates 2009
- Cates CJ, Lasserson TJ, Jaeschke R. Regular treatment with salmeterol and inhaled steroids for chronic asthma: serious adverse events. Cochrane Database of Systematic Reviews 2009, Issue 3. [DOI: 10.1002/14651858.CD006922.pub2] [DOI] [PubMed] [Google Scholar]
Cockcroft 1993
Cockcroft 2006
Collins 1969
- Collins JM, McDevitt DG, Shanks RG, Swanton JG. Cardio‐toxicity of isoprenaline during hypoxia. British Journal of Pharmacology 1969;36(1):36‐45. [DOI] [PMC free article] [PubMed] [Google Scholar]
Crane 1989
Cullum 1969
- Cullum VA, Farmer JB, Jack D, Levy GP. Salbutamol ‐ a new selective beta‐adrenoceptive receptor stimulant. British Journal of Pharmacology 1969;35(1):141‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
Delea 2008
- Delea TE, Hagiwara M, Stanford RH, Stempel DA. Effects of fluticasone propionate/salmeterol combination on asthma‐related health care resource utilization and costs and adherence in children and adults with asthma. Clinical Therapeutics 2008;30(3):560‐71. [DOI] [PubMed] [Google Scholar]
Ducharme 2006
- Ducharme FM, Lasserson TJ, Cates CJ. Long‐acting beta‐2 agonists versus anti‐leukotrienes as add‐on therapy to inhaled corticosteroids for chronic asthma (Cochrane Review). Cochrane Database of Systematic Reviews 2006, Issue 4. [DOI: 10.1002/14651858.CD003137.pub3] [DOI] [PubMed] [Google Scholar]
Dunnill 1960
- Dunnill MS. The pathology of asthma, with special reference to changes in the bronchial mucosa. Journal of Clinical Pathology 1960; Vol. 13, issue 1:27‐33. [DOI] [PMC free article] [PubMed]
Ebina 1993
Gay 1949
Grainger 1991
- Grainger J, Woodman K, Pearce N, Crane J, Burgess C, Keane A, et al. Prescribed fenoterol and death from asthma in New Zealand, 1981‐7 ‐ a further case‐control study. Thorax 1991; Vol. 46, issue 2:105‐11. [DOI] [PMC free article] [PubMed]
Greenstone 2005
- Greenstone IR, Ni Chroinin M, Masse V, Danish A, Magdalinos H, Zhang X, et al. Combination of inhaled long‐acting beta2‐agonists and inhaled steroids versus higher dose of inhaled steroids in children and adults with persistent asthma (Cochrane Review). Cochrane Database of Systematic Reviews 2005, Issue 4. [DOI: 10.1002/14651858.CD005533] [DOI] [PubMed] [Google Scholar]
Guhan 2000
- Guhan AR, Cooper S, Oborne J, Lewis S, Bennett J, Tattersfield AE. Systemic effects of formoterol and salmeterol: a dose‐response comparison in healthy subjects. Thorax 2000; Vol. 55, issue 8:650‐6. [DOI] [PMC free article] [PubMed]
Haley 1998
Hall 1989
Hanania 2002
Hanania 2007
Hancox 1999
Hancox 2006a
Hancox 2006b
Haney 2006
Harvey 1982
- Harvey JE, Tattersfield AE. Airway response to salbutamol ‐ effect of regular salbutamol inhalations in normal, atopic, and asthmatic subjects. Thorax 1982; Vol. 37, issue 4:280‐7. [DOI] [PMC free article] [PubMed]
Higgins 2008
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.0 [updated February 2008]. The Cochrane Collaboration, 2008. Available from www.cochrane‐handbook.org.
Jones 2001
Konzett 1940
- Konzett H. Neue broncholytisch hochwirksame Körper der Adrenalinreihe. Naunyn‐Schmiedeberg's Archives of Pharmacology 1940; Vol. 197:27‐40.
Kuyper 2003
Lee 2003
- Lee DKC, Jackson CM, Currie GP, Cockburn WJ, Lipworth BJ. Comparison of combination inhalers vs inhaled corticosteroids alone in moderate persistent asthma. British Journal of Clinical Pharmacology 2003; Vol. 56, issue 5:494‐500. [DOI] [PMC free article] [PubMed]
Lipworth 1989
Lipworth 1992
- Lipworth BJ, McDevitt DG. Inhaled beta‐2‐adrenoceptor agonists in asthma ‐ help or hindrance. British Journal of Clinical Pharmacology 1992; Vol. 33, issue 2:129‐38. [DOI] [PMC free article] [PubMed]
Lipworth 1997
- Lipworth BJ. Airway subsensitivity with long‐acting beta 2‐agonists: is there cause for concern?. Drug Safety 1997;16(5):295‐308. [DOI] [PubMed] [Google Scholar]
Lipworth 2000
Martinez 2006
McDevitt 1974
- McDevitt DG, Shanks RG, Swanton JG. Further observations on cardiotoxicity of isoprenaline during hypoxia. British Journal of Pharmacology 1974; Vol. 50, issue 3:335‐44. [DOI] [PMC free article] [PubMed]
McIvor 1998
Miranda 2004
Morrison 1993
Nelson 1977
Ni Chroinin 2004
- Ni Chroinin M, Greenstone IR, Ducharme FM. Addition of inhaled long‐acting beta2‐agonists to inhaled steroids as first line therapy for persistent asthma in steroid‐naive adults. Cochrane Database of Systematic Reviews 2004, Issue 4. [DOI: 10.1002/14651858.CD005307] [DOI] [PubMed] [Google Scholar]
Ni Chroinin 2005
- Ni Chroinin M, Greenstone IR, Danish A, Magdolinos H, Masse V, Zhang X, et al. Long‐acting beta2‐agonists versus placebo in addition to inhaled corticosteroids in children and adults with chronic asthma. Cochrane Database of Systematic Reviews 2005, Issue 4. [DOI: 10.1002/14651858.CD005535] [DOI] [PubMed] [Google Scholar]
Ordonez 2001
Palmqvist 1999
Pearce 1990
- Pearce N, Grainger J, Atkinson M, Crane J, Burgess C, Culling C, et al. Case‐control study of prescribed fenoterol and death from asthma in New Zealand, 1977‐81. Thorax 1990; Vol. 45, issue 3:170‐5. [DOI] [PMC free article] [PubMed]
Pearce 2001
- Pearce N BR, Crane J, Burgess C. Epidemiology of asthma mortality. In Asthma and Rhinitis. Oxford: Blackwell Scientific 2001 2001:56‐69.
Pearce 2007
- Pearce N. Adverse Reactions: The Fenoterol Story. 1st Edition. Auckland: Auckland University Press, 2007:215. [Google Scholar]
Phillips 1990
- Phillips GD, Finnerty JP, Holgate ST. Comparative protective effect of the inhaled beta‐2‐agonist salbutamol (albuterol) on bronchoconstriction provoked by histamine, methacholine, and adenosine 5'‐monophosphate in asthma. Journal of Allergy and Clinical Immunology 1990; Vol. 85, issue 4:755‐62. [DOI] [PubMed]
RevMan 2011 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.1. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2011.
Ringdal 1998
Salpeter 2006
- Salpeter SR, Buckley NS, Ormiston TM, Salpeter EE. Meta‐analysis: effect of long‐acting beta‐agonists on severe asthma exacerbations and asthma‐related deaths. Annals of Internal Medicine 2006;144(12):904‐12. [DOI] [PubMed] [Google Scholar]
Sears 1990
Simonsson 1967
- Simonsso BG, Jacobs FM, Nadel JA. Role of autonomic nervous system and the cough reflex in the increased responsiveness of airways in patients with obstructive airway disease. Journal of Clinical Investigation 1967; Vol. 46, issue 11:1812‐8. [DOI] [PMC free article] [PubMed]
Sovani 2008
- Sovani MP, Whale CI, Oborne J, Cooper S, Mortimer K, Ekström T, et al. Poor adherence with inhaled corticosteroids for asthma: can using a single inhaler containing budesonide and formoterol help?. British Journal of General Practice 2008; Vol. 58:37‐43. [DOI] [PMC free article] [PubMed]
Speizer 1968
- Speizer FE, Doll R, Heaf P. Observations on recent increase in mortality from asthma. British Medical Journal 1968; Vol. 1, issue 5588:335‐9. [DOI] [PMC free article] [PubMed]
Stolley 1972
Tattersfield 2006
Tonnel 2001
Van der Woude 2001
- Woude HJ, Winter TH, Aalbers R. Decreased bronchodilating effect of salbutamol in relieving methacholine induced moderate to severe bronchoconstriction during high dose treatment with long acting β2 agonists. Thorax 2001; Vol. 56, issue 7:529‐35. [DOI] [PMC free article] [PubMed]
Van Noord 1996
- Noord JA, Smeets JJ, Raaijmakers JAM, Bommer AM, Maesen FPV. Salmeterol versus formoterol in patients with moderately severe asthma: onset and duration of action. European Respiratory Journal 1996;9(8):1684‐8. [DOI] [PubMed] [Google Scholar]
Vignola 1993
Walters 2002
- Walters EH, Walters JAE, Gibson PW. Regular treatment with long acting beta agonists versus daily regular treatment with short acting beta agonists in adults and children with stable asthma. Cochrane Database of Systematic Reviews 2002, Issue 3. [DOI: 10.1002/14651858.CD003901] [DOI] [PMC free article] [PubMed] [Google Scholar]
Walters 2007
- Walters EH, Gibson PG, Lasserson TJ, Walters JAE. Long‐acting beta2‐agonists for stable chronic asthma. Cochrane Database of Systematic Reviews 2007, Issue 1. [DOI: 10.1002/14651858.CD001385.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]


