Abstract
Background
Patients, identified to be at risk for but who have never experienced a potentially lethal cardiac arrhythmia, have the option of receiving an implantable cardioverter defibrillator (ICD) as prophylaxis against sudden cardiac death - a primary prevention indication. In Canada, there is no clear framework to support patients’ decision-making for these devices. Decision support, using a decision aid, could moderate treatment-related uncertainty and prepare patients to make well-informed decisions. Patient decision aids provide information on treatment options, risks, and benefits, to help patients clarify their values for outcomes of treatment options. The objectives of this research are: 1) develop a decision aid, 2) evaluate the decision aid, and 3) determine the feasibility of conducting a trial.
Methods/design
A development panel comprised of the core investigative team, health service researchers, decision science experts, cardiovascular healthcare practitioners, and ICD patient representatives will collaborate to provide input on the content and format of the aid. To generate probabilities to include in the aid, we will synthesize primary prevention ICD evidence. To obtain anonymous input about the facts and content, we will employ a modified Delphi process. To evaluate the draft decision aid will invite ICD patients and their families (n = 30) to rate its acceptability. After we evaluate the aid, to determine the feasibility, we will conduct a feasibility pilot randomized controlled trial (RCT) in new ICD candidates (n = 80). Participants will be randomized to receive a decision aid prior to specialist consultation versus usual care. Results from the pilot RCT will determine the feasibility of research processes; inform sample size calculation, measure decision quality (knowledge, values, decision conflict) and the influence of health related quality of life on decision-making.
Discussion
Our study seeks to develop a decision aid, for patients offered their first ICD for prophylaxis against sudden cardiac death. This paper outlines the background and methods of a pilot randomized trial which will inform a larger multicenter trial. Ultimately, decision support prior to specialist consultation could enhance the decision-making process between patients, physicians, and families, associated with life-prolonging medical devices like the ICD.
Trial registration
ClinicalTrials.gov: NCT01876173
Keywords: Decision aid, Feasibility trial, Implantable defibrillator, Decision-making, Shared decision-making
Background
Sudden cardiac death due to ventricular tachyarrhythmia is a serious cause of cardiovascular death in Canada [1], Europe [2,3], and the United States [4]. An innovative cardiovascular intervention to reduce the incidence of sudden cardiac death is the implantable cardioverter defibrillator (ICD). To restore a normal heart rhythm, the ICD delivers an internal shock, or anti-tachycardia pacing via an internally placed cardiovascular lead attached to the right ventricle of the heart. Evidence from randomized controlled trials (RCTs) and systematic reviews has demonstrated the effectiveness of ICDs for improving mortality and morbidity outcomes in patients with ischemic and non-ischemic disease, and heart failure [5-14]. Clinical practice guidelines provide current risk criteria to guide patient ICD candidacy [15]. Patients with or without ischemic disease, and reduced left ventricular function (among other criteria) may be offered an ICD for a primary prevention indication or sudden death prophylaxis. In addition, patients with heart failure may also be eligible for an ICD with the option of cardiac resynchronization, to augment ventricular efficiency and output [16]. Furthermore, indications for ICDs are broadening to include prophylaxis for genetically inherited channelopathies such as long QT-syndrome [16]. Estimates of the number of eligible Canadians range from 85,000 to 92,000 [16,17], with 3,700 candidates accruing annually.
Specialized electrophysiologists navigate the evidence associated with device options and risk-stratify their patients. However, for the patient and their families, the decision to receive an ICD may not be straightforward. Additional complexities surrounding ICD implantation, benefits, complications, and delivery of therapy exist, which warrant special attention to prepare patients to make informed, value-based choices. ICDs have benefits and risks associated with implantation, replacement, advisories, and psychosocial adjustment during the ICD lifetime [18-22]. These complex factors, balanced with the presence of competing mortality risks warrant structured, evidence-informed decision support. Decision support using a patient decision aid (PtDA) can moderate decisional conflict or treatment uncertainty and is used successfully in practice among several health treatment situations to improve patient outcomes [23]. A PtDA uses informational documents, or visual aids designed to prepare patients for decision-making about treatment options. Decision aids are intended to supplement rather than replace counselling from practitioners [24]. They are not passive informed consent materials, or educational interventions that are not geared to a specific decision. A 2011 Cochrane review of 86 studies found that when patients use decision aids they: a) improve their knowledge of the options; b) are helped to have more accurate expectations of possible benefits and harms; c) reach choices that are more consistent with their informed values; and d) participate more in decision making [23]. To date, the effectiveness of decision aids use in cardiovascular patients has been tested in atrial fibrillation [25,26], ischemic heart disease [27], and angiography [28,29]. To our knowledge, there is no research evaluating the use of a PtDA in patients who are offered an ICD for prophylaxis against sudden cardiac death in Canada.
Decision aids are used when there is more than one option. In the context of ICDs, this entails a choice between an elective prophylactic intervention versus continued optimal medical management - not death, but an annual risk of an arrhythmia. However, candidates who live with advancing heart failure may not comprehend the implications of living longer with their heart failure or the risks associated with ICD therapy if device shocks are received. At the point of decision-making for life-prolonging interventions, provision of time to consider end-of-life decisions may seem to be a contradiction. Nonetheless, this conversation and an introduction to what will be a future health decision are entrenched in the choice to receive an ICD [30]. Decision aids are embedded within the framework of shared decision-making, wherein together, patient, families, and physicians reach agreement on treatment-related decisions [31]. This approach stems from the concept of patient centered care [32]. In essence, shared decision-making encourages active engagement of patients during the process of decision-making and decision aids facilitate their participation. We have identified a need to provide better decision support for ICD patients during their decision-making process [30].
A considerable body of evidence gathered from ICD recipients suggests that psychosocial factors, including depression [33], anxiety [34-37] and personality [38,39] are associated with patient reported outcomes. Patients who receive shocks from the ICD report reductions in health-related quality of life (HRQL) [40,41]. Furthermore, the prevalence of depression or depressive symptoms is high both pre-ICD implantation and post-ICD implantation [42]. The influence of these factors at the time of health care decision-making is not known. We previously reported a high prevalence of depressive symptoms pre-implantation (30%), accompanied by poor pre-implantation mental-HRQL [43]. Poor mental-HRQL had a significant influence on early ICD acceptance in participants. This work highlights patient-specific factors known to influence patients’ HRQL, acceptance of, and adjustment to living with an ICD. Furthermore, a standard ICD does not alter or improve a patient’s underlying cardiovascular condition or symptoms. However, an ICD with added cardiac resynchronization (CRT) has a greater likelihood of improving heart failure symptoms and HRQL due to its ability to restore synchrony of the ventricles [11]. Differentiating what an ICD can and cannot do is vital for patients to consider at the time of decision-making.
Conceptual framework
The Ottawa Decision Support Framework (ODSF) [44] provides the conceptual framework for this research. The ODSF is rooted in evidence, concepts, and theories from psychology, decision analysis, decisional conflict, values, and self-efficacy [45]. Importantly, the ODSF emphasizes decision preparation using a framework that separates the effects of each decision support method [44]. The framework has three main elements (decisional needs, decision support, and decision quality) [45]. The framework asserts that participant’ decisional needs (perceptions of the decision, knowledge, unrealistic expectations of outcomes, uncertainty, values, support, and resources, personal and clinical characteristics), affect decision quality. Decision quality is defined as reaching a decision that is based on the best available evidence and patients’ informed values for outcomes of options. The second element; decision quality, affects behavior (for example, delays), health outcomes, emotions, costs, and use of services [45]. Clinical characteristics may include the health status of patients. The final element is decision support [45]. The aim of decision support when delivered through a decision aid, counselling, or coaching is to improve decision quality by addressing unresolved decisional needs. Treatment effects associated with decision support, framed within the ODSF have been empirically tested [44,46-51].
Methods
To meet the objectives of this research, the study will take place in three steps using a combination of quantitative and qualitative methods. In step one, we will draft, assess, and revise the decision aid contents using an inter-disciplinary panel of health services researchers, educators, practitioners, and ICD patients. In step two, we will invite patients and their family members who are experienced with the decision (have an ICD) to review and give feedback on the draft PtDA. Modifications based on patient and family feedback, will be made if necessary. Step three will employ a feasibility pilot randomized controlled trial in a single center, employing 1:1 allocation. The study setting is Hamilton Health Sciences (HHS), an academic tertiary care centre in Hamilton, ON, Canada. The center serves over one point four million residents in the regions of Brant, Burlington, Haldimand, Hamilton, Niagara, and Norfolk for cardiovascular procedures.
Step one: development of the decision aid
Development of the PtDA is guided by the ODSF and International Patient Decision Aid Standards (IPDAS) quality criteria [24,45]. Development includes, 1) synthesize evidence- based information about ICDs, 2) consider ways of presenting the ICD options (accepting or declining an ICD) ensuring good risk communication and addressing uncertainties, 3) determine the values associated with the benefits and risks that are important to patients, and 4) draft decision aid using a structured approach that can guide the patient through the decision-making process. In addition to IPDAS, development of the PtDA will be guided by components suggested in O’Connor and Edwards [52], and O’Connor and Jacobson [51].
A development panel comprised of the core investigative team, cardiovascular healthcare practitioners (heart failure, general cardiology, internal medicine, and nurse specialists), health service researchers, ICD patient representatives, and a decision aid expert, will collaborate to provide input on the information, content, and format of the aid. Early engagement of patient representatives and knowledge users is intentional as an integrated knowledge translation technique in order to build a foundation for effective knowledge translation and user uptake of the decision aid in a clinical context [53].
In order to inform the risk and benefit information for the ICD decision aid, the we will adapt and synthesize existing ICD practice guidelines, current meta-analyses of randomized trials, and systematic reviews specific to the primary prevention ICD indication population to develop probabilities to include in the aid [14,20,22,54-56].
The process of selecting final content to include in the ICD PtDA will include a survey using a Delphi process [57,58]. Electrophysiologists, nurses, decision science experts, stakeholders, and patients with ICDs will be invited to provide feedback on PtDA content. We will employ an electronic survey, utilizing confidential login procedures through a secure website administered at the study site. If requested by patient participants, anonymous paper versions will be provided. The Delphi process will seek to overcome some of the challenges found with making decisions in groups. The Delphi approach attempts to assess the extent of agreement and to resolve disagreement. The features include: 1) anonymity; achieved by use of a questionnaire, 2) iteration where the processes occur in ‘rounds’, 3) controlled feedback, showing the distribution of the group’s response, and 4) statistical group response using summary measures [57,58]. The process will take place in up to three rounds. Round 1: participants will be invited to provide opinions on the a) facts and information about ICDs, b) presentations of risks and benefits, and c) values clarification exercises, based on knowledge and experience. The opinions will be grouped under discreet headings drafted for circulation to all participants on a questionnaire; round 2: participants rank their agreement with statements that did not reach agreement in round 1; the rankings will be summarized and included in round 2. If agreement is not reached in round 2, the process will be repeated for a final round.
Step two: preliminary acceptability testing in patients with ICDs
To ensure the decision aid is acceptable, simple to complete, and clearly formatted for patient use, we will use a convenience sample of 30 patients from HHS with ICDs (primary prevention indication), and family members involved with the decision, to review the PtDA. In 2011, HHS received approximately 362 new ICD patient referrals. We will invite patients who are within one to six months of receiving the ICD. These patients will have the requisite experience with making ICD-related decisions, critical for informed and comprehensive assessment of the acceptability, feasibility, and formatting of the decision aid [59]. Exclusion criteria include 1) inability to understand the decision aid due to a language or visual impairment or 2) unstable cardiac condition. During scheduled outpatient ICD clinics, a physician or nurse specialist who is part of the healthcare team will introduce the study to patients. Patients who agree will receive an information package in the mail or if preferred, face to face. Demographics and medical history will be collected from the patients’ medical record and acceptability measures completed (see below).
Step two: decision aid acceptability outcomes
Participants will complete the Decision Support Acceptability Scale (DSTA). The tool is used frequently during decision aid development and preliminary evaluation [60]. The DSTA questionnaire is comprised of ten items addressing comprehensibility of the decision aid components, balance in the presentation of options, the amount of information, and overall suitability [60]. Responses will be reported descriptively in terms of proportions of positive or negative responses to each item. We will summarize patient and family member feedback, any issues for discussion or revisions will be brought to the development panel.
Step three: feasibility RCT testing
Primary purpose
Feasibility
Guided by the Ottawa Decision Support Framework [45] and the UK Medical Research Council recommendations [61], we will employ a feasibility pilot RCT (see Figure 1).
Figure 1.
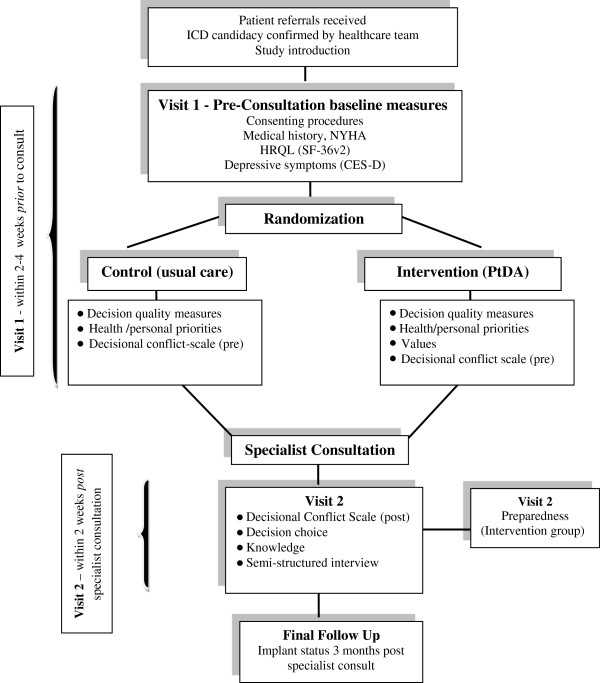
Feasibility pilot randomized controlled trial.
Feasibility endpoints will focus on processes (referral, recruitment rates, successful delivery of the decision aid, randomization procedures, the monitoring of resources (budget), and study management (trial coordination, human resources) [62]. We will assess the proportion of patients who complete the decision aid, percent of missing data, and questionnaire completion rates. Criteria for assessing the success of feasibility on key process outcomes include: 1) participant recruitment rate of at least 80% of all eligible patients, 2) successful delivery and completion of PtDA in 80% of consented patients prior to specialist consultation, 3) percentage of patients who complete PtDA of at least 80%, and 4) questionnaire completion rate of at least 80%.
Feasibility methodology
Participants, setting, and sample size
Participants will be patients referred for consultation for de novo ICDs for prophylaxis against sudden cardiac death. Exclusions are: 1) unable to understand the decision aid due to a language barrier or visual impairment, and 2) referral for an ICD with cardiac resynchronization or secondary prevention indication. To assess the feasibility of a conducting a larger trial, we will enroll 80 patients (40 per arm) over 10 months. The sample size for this pilot trial was based primarily on feasibility considerations. This pilot RCT will inform the estimates of effect sizes and variance for a larger trial.
Baseline visit
Members of the healthcare team will introduce the study to patients after ICD candidacy is determined but prior to their consultation in which the decision may be discussed/made. Introduction of the study will take place by mail or phone after candidates are contacted by the health care team to confirm the consultation appointment. Following the introduction to the study, the research assistant (RA) will either mail patients a study package, or arrange to meet patients for a baseline visit prior to the specialist consultation visit. At the time of the baseline visit, the RA will review study procedures, and obtain written consent. Following consent, the RA will collect baseline demographics, medical history, heart failure classification (NYHA), HRQL, and depressive symptoms. Once complete, the RA will randomize the patient.
Allocation
We will randomly allocate patients to intervention (PtDA prior to specialist consult) or usual care. We will use a secure electronic randomization system (http://www.randomize.net/). The allocation sequence is concealed. The research assistant will enroll participants and access the randomization system to determine group assignment following baseline data collection (see below).
Blinding
Because of the nature of the intervention, patients assigned to PtDA, and the RA, will not be blind (to decision aid or usual care). Data analysts will be blind to treatment assignment for outcomes measured in both groups (knowledge, HRQL, preference, health/personal priorities, decisional conflict pre/post, and decisional outcomes). Participants and the RA will not be aware of treatment assignment during baseline data collection.
Intervention
The intervention group will receive and use the PtDA with a trained research assistant prior to specialist consultation.
Usual care
The usual care group will receive standard HHS information upon arrival for their specialist consultation.
Visit one - pre consultation
Baseline measurements - (intervention and usual care)
To measure baseline psychosocial status and HRQL, we will use The Medical Outcomes Trust Short Form (SF-36v2), a generic HRQL scale used extensively in health care and ICD research. The reliability and validity of the SF-36v2 is well established [63]. The Center for Epidemiologic Studies Depression Scale will measure depressive symptoms (CES-D) [64]. The CES-D has demonstrated good reliability and validity across different community and clinical settings [65]. The NYHA [66] classifies functional abilities based on self reported symptom limitations; it has strong predictive utility and is used widely in practice. Participants will also rank their five priorities (health and personal).
Patients randomized to the intervention group will complete the PtDA and decision quality measures (values, decisional conflict, and knowledge). The usual care group will complete decision quality measures (decisional conflict, knowledge). Both groups will complete these measures prior to specialist consultation if feasible.
Visit two - post consultation
After confirming completion of the specialist consultation, the RA will contact participants for visit two. We will measure decisional conflict and knowledge in both groups (within two weeks post-consultation). Preparation for decision-making will be measured in the intervention group. Where feasible, and participants are in agreement, the RA will arrange to conduct a brief semi-structured interview to gain additional qualitative feedback about the PtDA and process.
Feasibility outcomes and measures
The primary feasibility outcomes will focus on processes (referral rates, recruitment rates, successful and timely delivery of the decision aid, randomization procedures, the monitoring of resources (budget), and study management (trial coordination, human resources) [62] (see above).
Decision quality measures (knowledge, values, preferences, decisional conflict)
To measure patients’ values, we will ask patients (intervention group) to rate the personal importance of benefits, harms, and preferences using a rating scale (0 to 10). Values item selections will be developed by the core panel based on ICD evidence [30,67], the Delphi, and clinical expertise. We will also measure all patients pre-consultation preferences (get a device, don’t get a device). Knowledge will be measured both pre and post consultation in both groups using five questions related to treatment, benefits, and risks. Initial reliability and validity testing of the knowledge test will take place during the feasibility testing. Finally, to measure decisional conflict, we will use the Decisional Conflict Scale [68,69]. The scale measures a person’s perception of the difficulty in making a decision, the extent to which they feel uncertain about treatment options, are knowledgeable about the risks and benefits of options, clear about personal values, and supported in decision making [68]; the scale has demonstrated good reliability (Cronbach’s alpha coefficients > 0.78) and predictive validity [69]. Higher scores indicate greater decisional conflict and patients experiencing decisional conflict are more likely to change their minds, delay decisions, express regret, and fail knowledge tests [69,70].
Decision preparation scale
Preparedness (intervention group), will be measured using the Preparation for Decision Making scale [59,71,72] which has ten items assessing patient’s perceptions of how useful a decision aid is in preparing them to communicate with their practitioner during consultation. The scale has demonstrated good internal consistency reliability (Cronbach’s alpha 0.92 to 0.96).
Evidence to inform the calculation of a sample size for a larger trial
The pilot RCT will inform estimates of effect sizes and variance for a larger trial. More specifically, we will identify clinically significant differences in the change scores of decision quality measures (Decisional Conflict) that correspond to acceptable levels of patient uncertainty about their course of action (treatment options).
Final follow-up
Three months after participants complete their baseline visit 1, the RA will confirm vital status, and device implant status from the health record.
Data collection, management and analysis
Results for feasibility outcomes including participant recruitment, referral rates, patient uptake of the decision aid, and missing data will determine feasibility of study processes. This will mainly be descriptive in nature. Continuous variables will be summarized using descriptive statistics and measures of central tendency (means, standard deviations) and categorical variables using percentages and frequencies. Assumptions of the normality of data will be assessed. When normality assumptions are not met, non-parametric tests will be applied [73]. To determine differences in decision quality (Decisional Conflict) each group’s scores will be compared using independent sample t-test at two time points. The first comparison will be within group (pre and post consultation) followed by 2) between group differences in the change scores using analysis of covariance (ANCOVA) that adjust for baseline scores [73]. All quantitative tests will be two-sided and conducted at the 5% level of significance. We will also use knowledge scores > 66% to predict decision choice using logistic regression techniques. To examine associations between baseline HRQL, depressive symptoms, and 1) decision choice, and 2) values, we will use regression analysis and calculation of odds ratios and 95% confidence intervals where appropriate. SAS 9.2 (Cary, NC, USA) will be used to conduct quantitative analyses.
Qualitative data (interviews, field notes) will be analyzed using interpretive descriptive analysis [74-76]. Interviews will be recorded, transcribed verbatim, and anonymized. NVivo (http://www.qsrinternational.com/contact.aspx) software will assist with the management and coding of findings. We will analyze patient interviews to identify themes surrounding patient perceived barriers to decision aid uptake.
Data management
To ensure data quality, all data will be collected via standardized forms and entered electronically via facsimile transmission to the data coordinating center using TeleForm (http://www.cardiff-teleform.com). All original paper forms will be stored at the study site (McMaster). Data integrity will be enforced by a variety of mechanisms. Data discrepancies and inquiries will be addressed individually and responses to each inquiry generated by the study site. Study data will be stored in locked cabinets. Access to data will be restricted.
External review of the PtDA
The decision aid will be uploaded for review via the Ottawa Decision Aid Library Inventory and, clinical experts external to the development process will be invited to review the decision aid as per IPDAS.
Discussion
This paper describes the protocol for the development of and feasibility testing of a decision aid for patients who are candidates for their first ICD as prophylaxis against sudden cardiac death associated with cardiac arrhythmias. Previously, in the Canadian ICD population, a need for decision support was revealed [30], thus the goal of this work is to develop this support. ICDs are life-prolonging medical devices and are an important cardiovascular treatment option for patients who are at risk for sudden cardiac death. Therefore, the provision and availability of decision support is imperative for patients and families to facilitate quality decision making (informed, deliberate, and value-based). We are developing a PtDA for a standard ICD when it is offered for a primary prevention indication. In Canada, this is an elective procedure whereby patients are referred to centers who offer specialized electrophysiology services. It is our intention to deliver the PtDA intervention prior to specialist consultation, thus the feasibility component of this work is significant for a future multi-center trial. To our knowledge, the provision of a PtDA prior to specialist consultation has not been tested in Canada.
A standard ICD cannot offer patients the potential to improve heart failure symptoms found with devices that have added cardiac resynchronization (CRT). It is important that patients who prioritize symptom reduction as an important health goal, who are not eligible for added CRT, understand this difference at the point of decision-making. An important ICD fact we will introduce in our PtDA is the potential for future deactivation of the ICD (or alternatively the option not to replace the ICD when the device battery approaches its end). Understanding long-term factors associated with an intervention such as the ICD early in the trajectory may assist patients and families with advanced directives planning where appropriate [77]. Moreover, we are engaging experienced ICD patients in the process of our PtDA development to ensure that our work is patient oriented and reflects the views of patients who are familiar with making this decision. Finally, we will determine the feasibility of conducting a larger trial. It is our goal to disseminate the PtDA and make it assessable to the public.
Ethics, consent, and dissemination
The study protocol (version 1; 20 March 2012) was approved by the Hamilton Integrated Research Ethics Board (#12-214), and registered at ClinicalTrials.gov. (NCT01876173). Informed consent will be obtained from all participants by a research assistant who is not involved with direct care in step two and step three of the study. Each participant will receive a signed copy of the consent/information sheet. If important protocol modifications are made communication to relevant parties (for example, investigators, Ethics Board, trial registry) will be undertaken. All personal information about potential and enrolled participants when stored electronically will be encrypted and password protected. Study data collection forms will not contain any personal identifiers, only study identification numbers. Participants in steps two and three will receive a gift card as a token of appreciation.
Trial status
Currently enrolling patients.
Abbreviations
ANCOVA: Analysis of covariance; CES-D: The center for epidemiologic studies depression scale; CRT: Cardiac resynchronization therapy; DSTA: Decision support acceptability scale; HRQL: Health-related quality of life; HHS: Hamilton health sciences; ICD: Implantable cardioverter defibrillator; IPDAS: International patient decision aid standards; NYHA: New York heart association; ODSF: Ottawa decision support framework; PtDA: Patient decision aid; RA: Research assistant; RCT: Randomized controlled trial; SF-36v2: The medical outcomes trust short form scale.
Competing interests
The authors of this protocol disclose no financial conflict or competing interests for this study.
Authors’ contributions
SLC participated in the design of the study, wrote the first draft of the manuscript, and applied for funding. SLC, MM, DS, JH, LT, HMA, GB participated in the design of the study, PtDA development panel, and critical review of this manuscript. All authors read and approved the final manuscript.
Contributor Information
Sandra L Carroll, Email: carroll@mcmaster.ca.
Michael McGillion, Email: MMcGill@mcmaster.ca.
Dawn Stacey, Email: Dawn.Stacey@uOttawa.ca.
Jeff S Healey, Email: Jeff.Healey@phri.ca.
Gina Browne, Email: Browneg@mcmaster.ca.
Heather M Arthur, Email: Arthurh@mcmaster.ca.
Lehana Thabane, Email: Thabanl@mcmaster.ca.
Acknowledgments
Dr. Sandra Carroll is supported by a Research Early Career Award from the Hamilton Health Sciences Foundation. Dr Lehana Thabane is the clinical trials mentor for the Canadian Institutes of Health Research.
Funding
The study has received funding from the Canadian Institutes of Health Research (Grant #119449). The funding source did not have a role in the design of the study.
References
- Tang AS, Ross H, Simpson CS, Mitchell LB, Dorian P, Goeree R. et al. Canadian cardiovascular society/Canadian heart rhythm society position paper on implantable cardioverter defibrillator use in Canada. Can J Cardiol. 2005;14:11A–18A. [PubMed] [Google Scholar]
- Byrne R, Constant O, Smyth Y, Callagy G, Nash P, Daly K. et al. Multiple source surveillance incidence and etiology of out-of-hospital sudden cardiac death in a rural population in the West of Ireland. Eur Heart J. 2008;14:1418–1423. doi: 10.1093/eurheartj/ehn155. [DOI] [PubMed] [Google Scholar]
- Priori SG, Aliot E, Blomstrom-Lundqvist C, Bossaert L, Breithardt G, Brugada P. et al. Task force on sudden cardiac death of the European society of cardiology. Eur Heart J. 2001;14:1374–1450. doi: 10.1053/euhj.2001.2824. [DOI] [PubMed] [Google Scholar]
- Lloyd-Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, De SG. et al. Heart disease and stroke statistics - 2010 update: a report from the American heart association.[erratum appears in circulation. 30 March 2010;121(12):e260 note: Stafford, Randall [corrected to Roger, Veronique L]] Circulation. 2010;14:e46–e215. doi: 10.1161/CIRCULATIONAHA.109.192667. [DOI] [PubMed] [Google Scholar]
- Connolly SJ, Hallstrom AP, Cappato R, Schron EB, Kuck KH, Zipes DP. et al. Meta-analysis of the implantable cardioverter defibrillator secondary prevention trials. AVID, CASH and CIDS studies. Antiarrhythmics versus implantable defibrillator study. Cardiac arrest study Hamburg. Canadian implantable defibrillator study. Eur Heart J. 2000;14:2071–2078. doi: 10.1053/euhj.2000.2476. [DOI] [PubMed] [Google Scholar]
- Moss AJ, Zareba W, Hall WJ, Klein H, Wilber DJ, Cannom DS. et al. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. New Engl J Med. 2002;14:877–883. doi: 10.1056/NEJMoa013474. [DOI] [PubMed] [Google Scholar]
- Bardy GH, Lee KL, Mark DB, Poole JE, Packer DL, Boineau R. et al. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N Engl J Med. 2005;14:225–237. doi: 10.1056/NEJMoa043399. [DOI] [PubMed] [Google Scholar]
- Kadish A, Dyer A, Daubert JP, Quigg R, Estes NA, Anderson KP. et al. Prophylactic defibrillator implantation in patients with nonischemic dilated cardiomyopathy. New Engl J Med. 2004;14:2151–2158. doi: 10.1056/NEJMoa033088. [DOI] [PubMed] [Google Scholar]
- Bristow MR, Saxon LA, Boehmer J, Krueger S, Kass DA, De MT. et al. Cardiac-resynchronization therapy with or without an implantable defibrillator in advanced chronic heart failure. N Engl J Med. 2004;14:2140–2150. doi: 10.1056/NEJMoa032423. [DOI] [PubMed] [Google Scholar]
- Moss AJ, Hall WJ, Cannom DS, Daubert JP, Higgins SL, Klein H. et al. Improved survival with an implanted defibrillator in patients with coronary disease at high risk for ventricular arrhythmia. Multicenter automatic defibrillator implantation trial investigators. New Engl J Med. 1996;14:1933–1940. doi: 10.1056/NEJM199612263352601. [DOI] [PubMed] [Google Scholar]
- Tang AS, Wells GA, Talajic M, Arnold MO, Sheldon R, Connolly S. et al. Cardiac-resynchronization therapy for mild-to-moderate heart failure. New Engl J Med. 2010;14:2385–2395. doi: 10.1056/NEJMoa1009540. [DOI] [PubMed] [Google Scholar]
- Moss AJ, Hall WJ, Cannom DS, Klein H, Brown MW, Daubert JP. et al. Cardiac-resynchronization therapy for the prevention of heart-failure events. New Engl J Med. 2009;14:1329–1338. doi: 10.1056/NEJMoa0906431. [DOI] [PubMed] [Google Scholar]
- Freemantle N, Tharmanathan P, Calvert MJ, Abraham WT, Ghosh J, Cleland JGF. Cardiac resynchronization for patients with heart failure due to left ventricular systolic dysfunction - a systematic review and meta-analysis. Eur J Heart Fail. 2006;14:433–440. doi: 10.1016/j.ejheart.2005.11.014. [DOI] [PubMed] [Google Scholar]
- Wells G, Parkash R, Healey JS, Talajic M, Arnold JM, Sullivan S. et al. Cardiac resynchronization therapy: a meta-analysis of randomized controlled trials. CMAJ. 2011;14:421–429. doi: 10.1503/cmaj.101685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein AE, DiMarco JP, Ellenbogen KA, Estes NA III, Freedman RA, Gettes LS. et al. ACC/AHA/HRS 2008 guidelines for device-based therapy of cardiac rhythm abnormalities: a report of the American college of cardiology/American heart association task force on practice guidelines (writing committee to revise the ACC/AHA/NASPE 2002 guideline update for implantation of cardiac pacemakers and antiarrhythmia devices) developed in collaboration with the American association for thoracic surgery and society of thoracic surgeons. J Am Coll Cardiol. 2008;14:e1–e62. doi: 10.1016/j.jacc.2008.02.032. [DOI] [PubMed] [Google Scholar]
- Fishman GI, Chugh SS, DiMarco JP, Albert CM, Anderson ME, Bonow RO. et al. Sudden cardiac death prediction and prevention: report from a national heart, lung, and blood institute and heart rhythm society workshop. Circ. 2010;14:2335–2348. doi: 10.1161/CIRCULATIONAHA.110.976092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson CS, Hoffmaster B, Dorian P. Downward delegation of implantable cardioverter defibrillator decision-making in a restricted-resource environment: the pitfalls of bedside rationing. Can J Card. 2005;14:595–599. [PubMed] [Google Scholar]
- Maisel WH, Moynahan M, Zuckerman BD, Gross TP, Tovar OH, Tillman DB. et al. Pacemaker and ICD generator malfunctions. JAMA. 2006;14:1901–1906. doi: 10.1001/jama.295.16.1901. [DOI] [PubMed] [Google Scholar]
- Gould PA, Krahn AD. for the Canadian Heart Rhythm Society Working Group on Device Advisories. Complications associated with implantable cardioverter-defibrillator replacement in response to device advisories. JAMA. 2006;14:1907–1911. doi: 10.1001/jama.295.16.1907. [DOI] [PubMed] [Google Scholar]
- Duray GZ, Schmitt J, Cicek-Hartvig S, Hohnloser SH, Israel CW. Complications leading to surgical revision in implantable cardioverter defibrillator patients: comparison of patients with single-chamber, dual-chamber, and biventricular devices. Europace. 2009;14:297–302. doi: 10.1093/europace/eun322. [DOI] [PubMed] [Google Scholar]
- Krahn AD, Lee DS, Birnie D, Healey JS, Crystal E, Dorian P. et al. Predictors of short-term complications after implantable cardioverter-defibrillator replacement/clinical perspective. Circ: Arrhythmia and Electrophysiology. 2011;14:136–142. doi: 10.1161/CIRCEP.110.959791. [DOI] [PubMed] [Google Scholar]
- Lee DS, Krahn AD, Healey JS, Birnie D, Crystal E, Dorian P. et al. Evaluation of early complications related to de novo cardioverter defibrillator implantation: insights from the Ontario ICD database. J Am Coll Cardiol. 2010;14:774–782. doi: 10.1016/j.jacc.2009.11.029. [DOI] [PubMed] [Google Scholar]
- Stacey D, Bennett CL, Barry MJ, Col NF, Eden KB, Holmes-Rovner M. et al. Decision aids for people facing health treatment or screening decisions. [Update of Cochrane database syst Rev. 2009;(3):CD001431; PMID: 19588325] Cochrane Database of Syst Rev. 2011;14:CD001431. doi: 10.1002/14651858.CD001431.pub3. [DOI] [PubMed] [Google Scholar]
- O’Connor AM. IPDAS Collaboration Background Document. 2005. http://www.ipdas.ohri.ca/IPDAS_Background.pdf.
- McAlister FA, Man-Son-Hing M, Straus SE, Ghali WA, Anderson D, Majumdar SR. et al. Impact of a patient decision aid on care among patients with nonvalvular atrial fibrillation: a cluster randomized trial. CMAJ. 2005;14:496–501. doi: 10.1503/cmaj.050091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Man-Son-Hing M, Laupacis A, O’Connor AM, Biggs J, Drake E, Yetisir E. et al. A patient decision aid regarding antithrombotic therapy for stroke prevention in atrial fibrillation. JAMA. 1999;14:737–743. doi: 10.1001/jama.282.8.737. [DOI] [PubMed] [Google Scholar]
- Morgan M, Deber R, Llewellyn-Thomas H, Gladstone P, Cusimano R, O’Rourke K. et al. Randomized, controlled trial of an interactive videodisc decision aid for patients with ischemic heart disease. J Gen Intern Med. 2000;14:685–693. doi: 10.1046/j.1525-1497.2000.91139.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein SJ, Skarupski KA, Grayson CE, Starling MR, Bates ER, Eagle KA. A randomized controlled trial of information-giving to patients referred for coronary angiography: effects on outcomes of care. Health Expect. 1998;14:50–61. doi: 10.1046/j.1369-6513.1998.00007.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwalm JD, Stacey D, Pericak D, Natarajan MK. Radial artery versus femoral artery access options in coronary angiogram procedures: randomized controlled trial of a patient-decision aid. Circ Cardiovasc Qual Outcomes. 2012;14:260–266. doi: 10.1161/CIRCOUTCOMES.111.962837. [DOI] [PubMed] [Google Scholar]
- Carroll SL, Strachan PH, de Laat S, Schwartz L, Arthur HM. Patients’ decision making to accept or decline an implantable cardioverter defibrillator for primary prevention of sudden cardiac death. Health Expect. 2013;14:69–79. doi: 10.1111/j.1369-7625.2011.00703.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charles C, Gafni A, Whelan T. Shared decision-making in the medical encounter: what does it mean? (or it takes at least two to tango) Soc Sci & Med. 1997;14:681–692. doi: 10.1016/S0277-9536(96)00221-3. [DOI] [PubMed] [Google Scholar]
- Edwards A, Elwyn G. Shared Decision-making. Achieving Evidence-based Patient Choice. Oxford: Oxford University Press; 2009. [Google Scholar]
- Dunbar SB, Langberg JJ, Reilly CM, Viswanathan B, McCarty F, Culler SD. et al. Effect of a psychoeducational intervention on depression, anxiety, and health resource use in implantable cardioverter defibrillator patients. Pacing Clin Electrophysiol. 2009;14:1259–1271. doi: 10.1111/j.1540-8159.2009.02495.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunbar SB, Jenkins LS, Hawthorne M, Kimble LP, Dudley WN, Slemmons M. et al. Factors associated with outcomes three months after implantable cardioverter defibrillator insertion. Heart Lung. 1999;14:303–315. doi: 10.1053/hl.1999.v28.a101052. [DOI] [PubMed] [Google Scholar]
- Pedersen SS, van Domburg RT, Theuns DAMJ, Jordaens L, Erdman RAM. Type D personality is associated with increased anxiety and depressive symptoms in patients with an implantable cardioverter defibrillator and their partners. Psychosom Med. 2004;14:714–719. doi: 10.1097/01.psy.0000132874.52202.21. [DOI] [PubMed] [Google Scholar]
- Pedersen SS, van Domburg RT, Theuns DAMJ, Jordaens L, Erdman RAM. Concerns about the implantable cardioverter defibrillator: a determinant of anxiety and depressive symptoms independent of experienced shocks. Am Heart J. 2005;14:664–669. doi: 10.1016/j.ahj.2004.06.031. [DOI] [PubMed] [Google Scholar]
- Sears SF, Todaro JF, Lewis TS, Sotile W, Conti JB. Examining the psychosocial impact of implantable cardioverter defibrillators: a literature review. Clinl Cardio. 1999;14:481–489. doi: 10.1002/clc.4960220709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen SS, Theuns DA, Muskens-Heemskerk A, Erdman RA, Jordaens L. Type-D personality but not implantable cardioverter-defibrillator indication is associated with impaired health-related quality of life three months post-implantation. Europace. 2007;14:675–680. doi: 10.1093/europace/eum041. [DOI] [PubMed] [Google Scholar]
- Sears SF, Lewis TS, Kuhl EA, Conti JB. Predictors of quality of life in patients with implantable cardioverter defibrillators. Psychosom: J Consult Liaison Psychiatry. 2005;14:451–457. doi: 10.1176/appi.psy.46.5.451. [DOI] [PubMed] [Google Scholar]
- Pedersen SS, Van Den Broek KC, Van Den Berg M, Theuns DA. Shock as a determinant of poor patient-centered outcomes in implantable cardioverter defibrillator patients: is there more to it than meets the eye? Pace-Pacing Clin Electrophysiol. 2010;14:1430–1436. doi: 10.1111/j.1540-8159.2010.02845.x. [DOI] [PubMed] [Google Scholar]
- Sears SF Jr, Conti JB. Quality of life and psychological functioning of ICD patients. Heart. 2002;14:488–493. doi: 10.1136/heart.87.5.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamphuis HC, de Leeuw JR, Derksen R, Hauer RN, Winnubst JA. Implantable cardioverter defibrillator recipients: quality of life in recipients with and without ICD shock delivery: a prospective study. Europace. 2003;14:381–389. doi: 10.1016/S1099-5129(03)00078-3. [DOI] [PubMed] [Google Scholar]
- Carroll SL, Markle-Reid M, Ciliska D, Connolly SJ, Arthur HM. Age and mental health predict early device-specific quality of life (QOL) in patients receiving prophylactic implantable defibrillators. Can J Card. 2012;14:502–507. doi: 10.1016/j.cjca.2012.01.008. [DOI] [PubMed] [Google Scholar]
- O’Connor AM, Tugwell P, Wells GA, Elmslie T, Jolly E, Hollingworth G. et al. A decision aid for women considering hormone therapy after menopause: decision support framework and evaluation. Patient Educ Couns. 1998;14:267–279. doi: 10.1016/S0738-3991(98)00026-3. [DOI] [PubMed] [Google Scholar]
- O’Connor AM. Ottawa Decision Support Framework. 2006. http://decisionaid.ohri.ca/odsf.html.
- O’Connor AM, Tugwell P, Wells GA, Elmslie T, Jolly E, Hollingworth G. et al. Randomized trial of a portable, self-administered decision aid for postmenopausal women considering long-term preventive hormone therapy. Med Decis Making. 1998;14:295–303. doi: 10.1177/0272989X9801800307. [DOI] [PubMed] [Google Scholar]
- Mitchell SL, Tetroe J, O’Connor AM. A decision aid for long-term tube feeding in cognitively impaired older persons. J Am Geriatr Soc. 2001;14:313–316. doi: 10.1046/j.1532-5415.2001.4930313.x. [DOI] [PubMed] [Google Scholar]
- Dales RE, O’Connor A, Hebert P, Sullivan K, McKim D, Llewellyn-Thomas H. Intubation and mechanical ventilation for COPD: development of an instrument to elicit patient preferences. Chest. 1999;14:792–800. doi: 10.1378/chest.116.3.792. [DOI] [PubMed] [Google Scholar]
- Cranney A, O’Connor AM, Jacobsen MJ, Tugwell P, Adachi JD, Ooi DS. et al. Development and pilot testing of a decision aid for postmenopausal women with osteoporosis. Patient Educ Couns. 2002;14:245–255. doi: 10.1016/S0738-3991(01)00218-X. [DOI] [PubMed] [Google Scholar]
- Fiset V, O’Connor AM, Evans W, Graham I, DeGrasse C, Logan J. Development and evaluation of a decision aid for patients with stage IV non-small cell lung cancer. Health Expect. 2000;14:125–136. doi: 10.1046/j.1369-6513.2000.00067.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metcalf KA, Poll A, O’Connor A, Gershman S, Armel S, Finch A. et al. Development and testing of a decision aid for breast cancer prevention for women with BRCA1 or BRCA2 mutation. Clin Genet. 2007;14:208–217. doi: 10.1111/j.1399-0004.2007.00859.x. [DOI] [PubMed] [Google Scholar]
- O’Connor AM, Edwards A. In: Evidence-based Patient Choice: Inevitable or Impossible? Edwards A, Elwyn G, editor. New York, NY: Oxford University Press; 2001. The role of decision aids in promoting evidence-based patient choice; pp. 220–242. [Google Scholar]
- Gagnon M. In: Knowledge Translation in Health Care: Moving from Evidence to Practice. Straus SE, Tetroe J, Graham ID, editor. Oxford: Wiley-Blackwell Publishing Ltd; 2009. Knowledge dissemination and exchange of knowledge; pp. 235–245. [Google Scholar]
- Theuns DA, Smith T, Hunink MG, Bardy GH, Jordaens L. Effectiveness of prophylactic implantation of cardioverter-defibrillators without cardiac resynchronization therapy in patients with ischemic or non-ischemic heart disease: a systematic review and meta-analysis. Europace. 2010;14:1564–1570. doi: 10.1093/europace/euq329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho C, Li H, Noorani H, Cimon K, Campbell K, Tang A, Birnie D. Implantable Cardiac Defibrillators for Primary Prevention of Sudden Cardiac Death in High Risk Patients: A Meta-analysis of Clinical Efficacy, and a Review of Cost-effectiveness and Psychosocial Issues, Technology report number 81. Ottawa: Canadian Agency for Drugs and Technologies in Health; 2007. [Google Scholar]
- Leon AR, Abraham WT, Curtis AB, Daubert JP, Fisher WG, Gurley J. et al. Safety of transvenous cardiac resynchronization system implantation in patients with chronic heart failure: combined results of over 2,000 patients from a multicenter study program. J Am Coll Cardiol. 2005;14:2348–2356. doi: 10.1016/j.jacc.2005.08.031. [DOI] [PubMed] [Google Scholar]
- Keeney S, Hasson F, McKenna HP. A critical review of the Delphi technique as a research methodology for nursing. Int Journ Nurs Stud. 2001;14:195–200. doi: 10.1016/S0020-7489(00)00044-4. [DOI] [PubMed] [Google Scholar]
- Hasson F, Keeney S, McKenna H. Research guidelines for the Delphi survey technique. J Adv Nurs. 2000;14:1008–1015. [PubMed] [Google Scholar]
- O’Connor AM, Jacobsen MJ. Workbook on Developing and Evaluating Patient Decision Aids. 2003. http://www.ohri.ca/decisionaid.
- O’Connor AM, Cranney A. User Manual - Acceptability. Ottawa Hospital Research Institute; 2002. pp. 1–5. http://decisionaid.ohri.ca/docs/develop/User_Manuals/UM_Acceptability.pdf, 1-6-2011. [Google Scholar]
- Medical Research Council Health Services and Public Health Research Board. A Framework for Development and Evaluation of RCT’s for Complex Interventions to Improve Health. UK: Medical Research Council; 2000. [Google Scholar]
- Thabane L, Ma J, Chu R, Cheng J, Ismaila A, Rios L. et al. A tutorial on pilot studies: the what, why and how. BMC Med Res Methodol. 2010;14:1. doi: 10.1186/1471-2288-10-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHorney CA, War JE Jr, Lu JFR, Sherbourne CD. The MOS 36-item short-form health survey (SF-36): III. Tests of data quality, scaling assumptions, and reliability across diverse patient groups. Med Care. 1994;14:40–66. doi: 10.1097/00005650-199401000-00004. [DOI] [PubMed] [Google Scholar]
- Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Appl Psych Meas. 1977;14:385–401. doi: 10.1177/014662167700100306. [DOI] [Google Scholar]
- Weissman MM, Sholomskas D, Pottenger M, Prusoff BA, Locke BZ. Assessing depressive symptoms in five psychiatric populations: a validation study. Am J Epidemiol. 1977;14:203–214. doi: 10.1093/oxfordjournals.aje.a112455. [DOI] [PubMed] [Google Scholar]
- The Criteria Committee of the New York Heart Association. Nomenclature and Criteria for Diagnosis of Diseases of the Heart and Great Vessels. 9. Boston, MA: Little Brown; 1994. [Google Scholar]
- Matlock DD, Nowels CT, Bekelman DB. Patient perspectives on decision making in heart failure. J Card Fail. 2010;14:823–826. doi: 10.1016/j.cardfail.2010.06.003. [DOI] [PubMed] [Google Scholar]
- O’Connor AM. User Manual-decisional Conflict Scale [Document on the internet] Ottawa: Ottawa Hospital Research Institute; © 1993 [updated 2010]: 16p. http://decisionaid.ohri.ca/docs/develop/User_Manuals/UM_Decisional_Conflict.pdf. [Google Scholar]
- O’Connor AM. Validation of a decisional conflict scale. Med Decis Making. 1995;14:25–30. doi: 10.1177/0272989X9501500105. [DOI] [PubMed] [Google Scholar]
- O’Connor AM, Bennett CL, Stacey D, Barry M, Col NF, Eden KB. et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev. 2009;14:CD001431. doi: 10.1002/14651858.CD001431.pub2. DOI: 10.1002/14651858.CD001431.pub2. [DOI] [PubMed] [Google Scholar]
- Bennett C, Graham ID, Kristjansson E, Kearing SA, Clay KF, O’Connor AM. Validation of a preparation for decision making scale. Patient Educ Couns. 2010;14:130–133. doi: 10.1016/j.pec.2009.05.012. [DOI] [PubMed] [Google Scholar]
- Graham ID, O’Connor AM. User Manual-preparation for Decision Making Scale. Ottawa Hospital Research Institute; 2010. pp. 1–3. http://decisionaid.ohri.ca/docs/develop/User_Manuals/UM_PrepDM.pdf, 1-6-2011. [Google Scholar]
- Norman GR, Streiner DL. Biostatistics: The Bare Essentials. 3. Hamilton: BC Decker Inc; 2008. [Google Scholar]
- Ryan GW, Bernard HR. Techniques to identify themes. Field Methods. 2003;14:85–109. doi: 10.1177/1525822X02239569. [DOI] [Google Scholar]
- Thorne S, Kirkham SR, O’Flynn-Magee K. The analytic challenge in interpretive description. Int J Qualitative Methods. 2004;14:1–11. [Google Scholar]
- Thorne S, Kirkham SR, MacDonald-Emes J. Interpretive description: a noncategorical qualitative alternative for developing nursing knowledge. Res Nurs Health. 1997;14:169–177. doi: 10.1002/(SICI)1098-240X(199704)20:2<169::AID-NUR9>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Garrett DD, Tuokko H, Stajduhar KI, Lindsay J, Buehler S. Planning for end-of-life care: findings from the Canadian study of health and aging. Can J Aging. 2008;14:11–21. doi: 10.3138/cja.27.1.11. [DOI] [PubMed] [Google Scholar]


