Abstract
Hyperbilirubinaemia is one of the most frequent problems in otherwise healthy newborn infants. Early discharge of the healthy newborn infants, particularly those in whom breastfeeding is not fully established, may be associated with delayed diagnosis of significant hyperbilirubinaemia that has the potential for causing severe neurological impairments. We present the shared Italian guidelines for management and treatment of jaundice established by the Task Force on hyperbilirubinaemia of the Italian Society of Neonatology.
The overall aim of the present guidelines is to provide an useful tool for neonatologists and family paediatricians for managing hyperbilirubinaemia.
Keywords: Hyperbilirubinaemia, Jaundice, Newborn, Discharge, Guidelines
Background
Hyperbilirubinaemia is a very common condition. The prevention, detection and management of jaundice remains a challenge especially because of early discharge of healthy late preterm and full term newborn infants [1-3]. Early discharge of the healthy newborn, particularly those in whom breastfeeding is not fully established, may be associated with delayed diagnosis of severe hyperbilirubinaemia [1,4,5]. A recent survey performed by Dani et al. [6] showed that the management of hyperbilirubinaemia is not homogeneous in Italy. Therefore the Task Force on hyperbilirubinaemia of the Italian Society of Neonatology drew up national guidelines for management of jaundice in the newborn and established a national registry of kernicterus and hyperbilirubinaemia in order to monitor the incidence of cases of kernicterus and severe hyperbilirubinaemia in Italy over time.
Focus of guidelines
The overall aim of this guidelines is to provide an useful tool for neonatologists and family paediatricians for managing hyperbilirubinaemia. These recommendations promote an approach based on the importance of universal systematic assessment for the risk of severe hyperbilirubinaemia, close follow-up, and prompt intervention when indicated. The statement fully applies to the care of infants at 35 or more weeks of gestation, while only some recommendations could be used for newborn infants with lower gestational age (GA).
Methods of statement development
Literature searches were last updated in September 2013. Guidelines from AAP [7-9], NICE [10,11], Canadian Pediatric Association [12], Nederland Neonatal Research Network [13], Norwegian Pediatric Society [14], Israel [15], New Zealand [16], Australia (Queensland) [17], India [18], Spain [19], Switzerland [20] were considered. Studies conducted in the neonatal units of Lazio were also evaluated to obtain recommendations more suitable for Italian population [21-23].
The hierarchy of evidence from the Centre for Evidence-Based-Medicine was applied (see Table 1) [24].
Table 1.
The hierarchy of evidence from the centre for evidence-based-medicine
| Level | Definition |
|---|---|
| 1a |
Systematic review (with homogeneity) of RCTs |
| 1b |
Individual RCT (with narrow confidence interval) |
| 2a |
Systematic review (with homogeneity) of cohort studies |
| 2b |
Individual cohort study |
| 3a |
Systematic review (with homogeneity) of case–control studies |
| 3b |
Individual case–control study |
| 4 |
Case-series (and poor quality cohort and case–control studies) |
| 5 | Expert opinion without explicit critical appraisal, or based on physiology, bench research or “first principles” |
Acute bilirubin encephalopathy
The main aim of these guidelines is to avoid cases of acute bilirubin encephalopathy (ABE) by establishing levels of serum bilirubin at risk of neurologic damage. A total serum bilirubin (TSB) of 20 mg/dl should not be exceeded in the first 96 hours of life in term newborns. After 96 hours of life a TSB of 25 mg/dl should be avoided (evidence level 2b), since the efficacy of Blood–brain-Barrier increases with postnatal age. The safe bilirubin level is lower for preterm infants: 12 mg/dl for babies with GA ≤ 30 weeks and 15 mg/dL for babies with GA 31–36 weeks [25,26].
Apart from TSB level, gestational age and postnatal age, some more risk factors for ABE should also be considered:
• Birth asphyxia
• Severe hypothermia (Tc < 36°C for > 6 hours)
• Respiratory failure (RDS, pneumonia, meconium aspiration syndrome)
• Prolonged acidosis (pH < 7.20 for > 6 hours)
• Severe hypoglycaemia (glucose < 45 mg/dl for > 12 hours)
• Severe haemolysis
• Sepsis and meningitis
• Drugs impairing bilirubin/albumin binding
Bilirubin measurement
All infants should be routinely monitored for the development of jaundice. The ability of physicians and other health care providers to recognise clinically significant jaundice and predict bilirubin levels based on the coephalo-caudal progression of jaundice is limited (evidence level 1b) [27-31].
Therefore each jaundiced newborn should receive a bilirubin measurement. Transcutaneous bilirubin (TcB) can be used as first step in order to reduce the number of invasive and painful blood sampling. BiliCheck TM (Respironics, Marietta, GA – USA) and JM-103 (Drager Medical Inc, Telford, Pennsylvania) are the two most used bilirubinometers in the studies conducted in the Italian population and both had good correlation with TSB values [21,22,32,33]. TSB measurement is always necessary when the level of bilirubin is high and for therapeutic decisions (evidence level 1b).
Prediction of hyperbilirubinaemia
The evaluation of jaundice is now facilitated by the availability of different nomograms for both serum [34] and transcutaneous bilirubin [21,35,36]. However, a nomogram could not perform well in one specific setting if the racial, genetic and environmental background of that setting are too different from that of the reference population. Furthermore, predictive tools should be developed in one sample and validated in another one. Hour-specific percentile based nomograms have been developed in cohorts of healthy Italian full term neonates or Italian neonates with ≥ 35 weeks’ gestational age using serial measurements of TcB and TSB (Tables 2 and 3; Figures 1 and 2) [21,23]. In a second phase the predictive ability of these nomograms has been evaluated. Sensitivity, specificity, positive and negative predictive value of percentiles in predicting significant hyperbilirubinaemia (defined as TSB > 17 mg/dL or need for phototherapy) are listed in Tables 4 and 5.
Table 2.
Values of TcB corresponding at the 50th and 75th percentile of the hour-specific nomogram
| h | 50th | 75th | h | 50th | 75th | h | 50th | 75th |
|---|---|---|---|---|---|---|---|---|
|
24 |
6, 3 |
7, 8 |
49 |
7, 7 |
10, 4 |
73 |
10 |
11, 7 |
| 25 |
6, 3 |
7, 8 |
50 |
7, 8 |
10, 4 |
74 |
10 |
11, 8 |
| 26 |
6, 4 |
7, 8 |
51 |
8 |
10, 5 |
75 |
10, 1 |
11, 9 |
| 27 |
6, 4 |
7, 9 |
52 |
8, 1 |
10, 5 |
76 |
10, 1 |
11, 9 |
| 28 |
6, 4 |
7, 9 |
53 |
8, 3 |
10, 6 |
77 |
10, 2 |
12 |
| 29 |
6, 5 |
7, 9 |
54 |
8, 4 |
10, 6 |
78 |
10, 2 |
12, 1 |
|
30 |
6, 5 |
7, 9 |
55 |
8, 6 |
10, 7 |
79 |
10, 3 |
12, 2 |
| 31 |
6, 6 |
8, 1 |
56 |
8, 7 |
10, 8 |
80 |
10, 4 |
12, 2 |
| 32 |
6, 6 |
8, 4 |
57 |
8, 9 |
11 |
81 |
10, 5 |
12, 3 |
| 33 |
6, 7 |
8, 6 |
58 |
9 |
11, 1 |
82 |
10, 5 |
12, 3 |
| 34 |
6, 7 |
8, 8 |
59 |
9, 2 |
11, 2 |
83 |
10, 6 |
12, 4 |
| 35 |
6, 8 |
9, 1 |
60 |
9, 3 |
11, 3 |
84 |
10, 7 |
12, 4 |
|
36 |
6, 8 |
9, 3 |
61 |
9, 4 |
11, 3 |
85 |
10, 7 |
12, 4 |
| 37 |
6, 9 |
9, 4 |
62 |
9, 5 |
11, 4 |
86 |
10, 8 |
12, 4 |
| 38 |
7, 1 |
9, 5 |
63 |
9, 6 |
11, 4 |
87 |
10, 8 |
12, 4 |
| 39 |
7, 2 |
9, 7 |
64 |
9, 6 |
11, 4 |
88 |
10, 8 |
12, 4 |
| 40 |
7, 3 |
9, 8 |
65 |
9, 7 |
11, 5 |
89 |
10, 9 |
12, 4 |
| 41 |
7, 5 |
9, 9 |
66 |
9, 8 |
11, 5 |
90 |
10, 9 |
12, 4 |
|
42 |
7, 5 |
10 |
67 |
9, 8 |
11, 5 |
91 |
10, 9 |
12, 5 |
| 43 |
7, 5 |
10, 1 |
68 |
9, 8 |
11, 5 |
92 |
10, 9 |
12, 5 |
| 44 |
7, 6 |
10, 1 |
69 |
9, 9 |
11, 6 |
93 |
10, 9 |
12, 6 |
| 45 |
7, 6 |
10, 2 |
70 |
9, 9 |
11, 6 |
94 |
10, 9 |
12, 6 |
| 46 |
7, 6 |
10, 2 |
71 |
9, 9 |
11, 6 |
95 |
10, 9 |
12, 7 |
| 47 |
7, 6 |
10, 3 |
72 |
9, 9 |
11, 6 |
96 |
10, 9 |
12, 7 |
| 48 | 7, 6 | 10, 3 |
Table 3.
Values of TSB corresponding at the 50th, 75th and 90th percentile of the hour-specific nomogram
| h | 50th | 75th | 90th | h | 50th | 75th | 90th | h | 50th | 75th | 90th |
|---|---|---|---|---|---|---|---|---|---|---|---|
|
24 |
6.1 |
7.5 |
8.9 |
49 |
9.0 |
10.3 |
11.9 |
73 |
10.0 |
11.7 |
13.2 |
| 25 |
6.2 |
7.7 |
9.1 |
50 |
9.1 |
10.4 |
12.0 |
74 |
10.0 |
11.8 |
13.3 |
| 26 |
6.4 |
7.8 |
9.2 |
51 |
9.1 |
10.4 |
12.1 |
75 |
10.1 |
11.8 |
13.3 |
| 27 |
6.5 |
8.0 |
9.3 |
52 |
9.2 |
10.5 |
12.2 |
76 |
10.1 |
11.8 |
13.4 |
| 28 |
6.7 |
8.2 |
9.5 |
53 |
9.2 |
10.6 |
12.3 |
77 |
10.2 |
11.9 |
13.4 |
| 29 |
6.8 |
8.3 |
9.6 |
54 |
9.3 |
10.7 |
12.4 |
78 |
10.2 |
11.9 |
13.5 |
|
30 |
7.0 |
8.5 |
9.7 |
55 |
9.3 |
10.8 |
12.5 |
79 |
10.3 |
12.0 |
13.5 |
| 31 |
7.2 |
8.6 |
9.9 |
56 |
9.3 |
10.8 |
12.5 |
80 |
10.3 |
12.1 |
13.6 |
| 32 |
7.3 |
8.7 |
10.1 |
57 |
9.3 |
10.9 |
12.6 |
81 |
10.4 |
12.1 |
13.7 |
| 33 |
7.5 |
8.9 |
10.2 |
58 |
9.4 |
10.9 |
12.7 |
82 |
10.5 |
12.2 |
13.7 |
| 34 |
7.7 |
9.0 |
10.5 |
59 |
9.4 |
11.0 |
12.8 |
83 |
10.6 |
12.3 |
13.8 |
| 35 |
7.9 |
9.1 |
10.6 |
60 |
9.5 |
11.0 |
12.9 |
84 |
10.6 |
12.4 |
13.8 |
|
36 |
8.0 |
9.2 |
10.8 |
61 |
9.5 |
11.1 |
12.9 |
85 |
10.6 |
12.4 |
13.9 |
| 37 |
8.1 |
9.3 |
10.8 |
62 |
9.5 |
11.1 |
12.9 |
86 |
10.7 |
12.5 |
14.0 |
| 38 |
8.2 |
9.4 |
10.9 |
63 |
9.5 |
11.2 |
12.9 |
87 |
10.7 |
12.5 |
14.1 |
| 39 |
8.3 |
9.5 |
10.9 |
64 |
9.6 |
11.2 |
13.0 |
88 |
10.7 |
12.5 |
14.2 |
| 40 |
8.4 |
9.6 |
11.0 |
65 |
9.6 |
11.3 |
13.0 |
89 |
10.8 |
12.6 |
14.3 |
| 41 |
8.5 |
9.7 |
11.1 |
66 |
9.6 |
11.3 |
13.0 |
90 |
10.8 |
12.6 |
14.4 |
|
42 |
8.6 |
9.8 |
11.1 |
67 |
9.6 |
11.4 |
13.0 |
91 |
10.9 |
12.7 |
14.5 |
| 43 |
8.7 |
9.9 |
11.2 |
68 |
9.6 |
11.4 |
13.1 |
92 |
11.0 |
12.9 |
14.6 |
| 44 |
8.7 |
9.9 |
11.3 |
69 |
9.7 |
11.5 |
13.1 |
93 |
11.2 |
13.0 |
14.7 |
| 45 |
8.8 |
10.0 |
11.5 |
70 |
9.8 |
11.6 |
13.1 |
94 |
11.3 |
13.2 |
14.8 |
| 46 |
8.9 |
10.1 |
11.6 |
71 |
9.8 |
11.7 |
13.2 |
95 |
11.4 |
13.4 |
14.9 |
| 47 |
8.9 |
10.2 |
11.7 |
72 |
9.9 |
11.7 |
13.2 |
96 |
11.5 |
13.5 |
15.0 |
| 48 | 9.0 | 10.2 | 11.8 |
Figure 1.
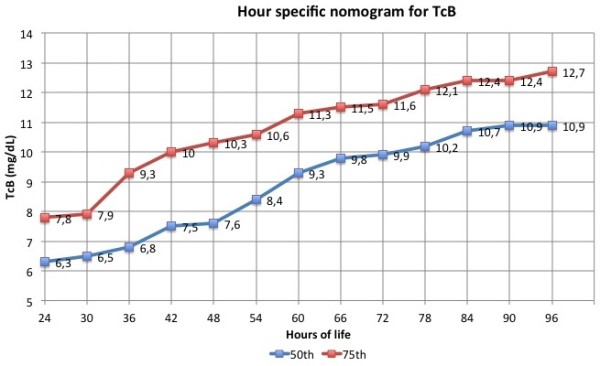
Depicts hour-specific percentile based nomograms for TcB.
Figure 2.
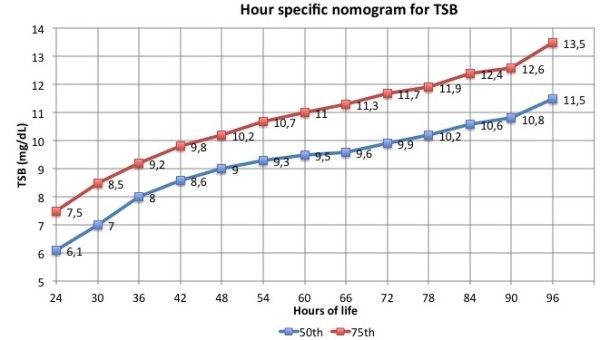
Depicts hour-specific percentile based nomograms for TSB.
Table 4.
Ability of TcB measurements over the 50th, the 75th and the 90th percentile of TcB nomogram to predict significant hyperbilirubinaemia, for designated time periods
| Hours of life | TP | FN | TN | FP | Sensitivity | Specificity | PPV | NPV |
|---|---|---|---|---|---|---|---|---|
|
24 - 48 hours |
|
|
|
|
|
|
|
|
| <50th percent |
27 |
0 |
161 |
460 |
100% |
25.9% |
5.5% |
100% |
| <75th percent |
25 |
2 |
429 |
192 |
92.6% |
69.1% |
11.5% |
99.5% |
| <90th percent |
14 |
13 |
564 |
57 |
51.9% |
90.8% |
19.7% |
97.7% |
|
49 - 72 hours |
|
|
|
|
|
|
|
|
| <50th percent |
21 |
0 |
410 |
615 |
100% |
40% |
3.3% |
100% |
| <75th percent |
21 |
0 |
702 |
323 |
100% |
68.5% |
6.1% |
100% |
| <90th percent |
18 |
3 |
888 |
137 |
85.7% |
86.6 |
11.6% |
99.7% |
|
73 - 96 hours |
|
|
|
|
|
|
|
|
| <50th percent |
7 |
0 |
246 |
220 |
100% |
52.8% |
3.1% |
100% |
| <75th percent |
7 |
0 |
348 |
118 |
100% |
74.7% |
5.6% |
100% |
| <90th percent | 5 | 2 | 409 | 57 | 71.4% | 87.8% | 8.1% | 99.5% |
Table 5.
Ability of TSB measurements over the 50th, the 75th and the 90th percentile of TSB nomogram to predict significant hyperbilirubinaemia, for designated time periods
| Hours of life | TP | FN | TN | FP | Sensitivity | Specificity | PPV | NPV |
|---|---|---|---|---|---|---|---|---|
|
24 - 48 hours |
|
|
|
|
|
|
|
|
| <50th percent |
24 |
1 |
357 |
263 |
96.0% |
57.6% |
8.4% |
99.7% |
| <75th percent |
22 |
3 |
479 |
141 |
88.0% |
77.3% |
13.5% |
99.4% |
| <90th percent |
12 |
13 |
576 |
44 |
48.0% |
92.9% |
21.4% |
97.8% |
|
49 - 72 hours |
|
|
|
|
|
|
|
|
| <50th percent |
23 |
0 |
657 |
368 |
100% |
64.1% |
5.9% |
100% |
| <75th percent |
22 |
1 |
860 |
165 |
95.7% |
83.9% |
11.8% |
99.9% |
| <90th percent |
17 |
6 |
975 |
50 |
73.9% |
95.1% |
25.4% |
99.4% |
|
73 - 96 hours |
|
|
|
|
|
|
|
|
| <50th percent |
7 |
0 |
322 |
145 |
100% |
69.0% |
4.6% |
100% |
| <75th percent |
7 |
0 |
409 |
58 |
100% |
87.6% |
10.8% |
100% |
| <90th percent | 6 | 1 | 446 | 21 | 85.7% | 95.5% | 22.2% | 99.8% |
On the base of these studies the Task Force on hyperbilirubinaemia of the Italian Society of Neonatology drew up the following recommendations for infants with GA ≥ 35 weeks (evidence level 1b).
Recommendations:
• All jaundiced newborn infants should be tested with a TcB measurement and the value should be plotted on the hour-specific nomogram for TcB measurements (Table 2).
• If the TcB measurement is > 75th percentile, a serum determination of bilirubin should be performed and the value should be plotted on the nomogram for TSB measurements (Table 3).
• Newborn infants with a TSB value < 50th percentile in the first 48 hours of live and babies with a value < 75th percentile after 48 hours of life are not at risk of hyperbilirubinaemia and do not require further evaluations.
• Newborn infants with a TSB value > 50th percentile in the first 48 hours of live and babies with a value > 75th percentile after 48 hours of life are at risk of hyperbilirubinaemia and should be tested again after 24 or 48 hours according to the hours of life and the presence of clinical risk factors.
At present, there are no nomograms able to predict hyperbilirubinaemia in infants with GA < 35 weeks. Therefore bilirubin measurements should be decided according to GA and treatment thresholds (evidence level 5).
Treatment: phototherapy
There is no reliable evidence to inform the choice of thresholds for starting phototherapy. For this reasons guidelines report different recommendations. In determining the bilirubin thresholds for treatment, the Italian Society of Neonatology considered as primary aim to choose a threshold allowing a large margin of safety, being the threshold for starting phototherapy less than that for performing exchange transfusion, and not so low to become unnecessary.
The thresholds for phototherapy are showed in a graph in which total bilirubin is plotted against age in hours. The groups of GA (GA < 30; 30–31; 32–34; 35–37; > 37) are represented by different lines (Figure 3).
Figure 3.
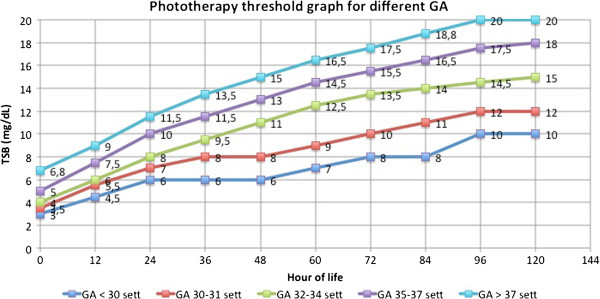
The graph shows the thresholds for phototherapy. Total bilirubin was plotted against age in hours. The groups of GA were represented with different lines.
Recommendations :
• Phototherapy should be administered for irradiating the most of the infant’s skin.
• Intensive phototherapy with an irradiance > 35 μW/cm2/nm is recommended. Daylight, blue light, conventional or blue LED phototherapy can be used (evidence level 1a).
• Fiberoptic phototherapy can be used even if it is less effective than conventional phototherapy and require more prolonged treatment (evidence level 1a) [37].
• Serum bilirubin should be tested 4–8 hours after the beginning of phototherapy or earlier if TSB < 3 mg/dL less than threshold for exchange transfusion. Subsequently, TSB should be assessed every 12–24 hours to monitor the treatment effectiveness.
• In case of treatment failure, multiple phototherapy should be started adding a fiberoptic device to the conventional treatment to increase the skin’s exposure (evidence level 1a) [37].
• Phototherapy should be discontinued once the bilirubin levels are below the threshold value for treatment on two consecutive measurements, 6–12 h apart. This would avoid keeping babies under phototherapy longer than necessary [38] (evidence level 1b).
• Serum bilirubin should be checked 12–24 hours after discontinuation of phototherapy the occurrence of post-phototherapy bilirubin rebound [39] (evidence level 4).
• Infants with either Rhesus or ABO haemolytic disease should be immediately treated with phototherapy once the diagnosis is made.
Hydration status should be monitored during phototherapy by daily weighing of the baby, assessing wept nappies and, if required, monitoring electrolytaemia. Breastfeeding should not be discontinued, because its interruption is associated with an increased frequency of stopping breastfeeding by one month (evidence level 2b) [40]. Italian Society of Neonatology suggests brief suspensions (up to 30 minutes) of phototherapy for allowing breastfeeding.
Breastfed babies should not be routinely supplemented with formula, water or dextrose water for the treatment of jaundice (evidence level 1b) [7,41]. The need for additional fluids during phototherapy should be considered only when the daily weight loss is higher then 5%, or when breast milk is not sufficient to permit the full feeding.
Exchange transfusion (ET)
Exchange Transfusion is recommended for newborn infants with TSB levels at risk of neurologic damage and with clinical signs and symptoms of ABE. However, available guidelines suggest different thresholds for ET, without differences between haemolytic and non haemolytic jaundice.
TSB threshold for ET in newborns with non haemolytic jaundice are generally 5–6 mg/dL higher than TSB threshold for phototherapy (Figure 4).
Figure 4.
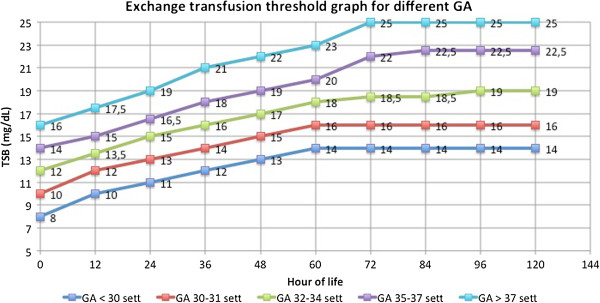
The graph shows the thresholds for exchange transfusion. Total bilirubin was plotted against age in hours. The groups of GA were represented with different lines.
The TSB threshold for ET in newborns with Rh or ABO haemolytic jaundice is lower than that of newborns with non haemolytic jaundice (Figure 5).
Figure 5.
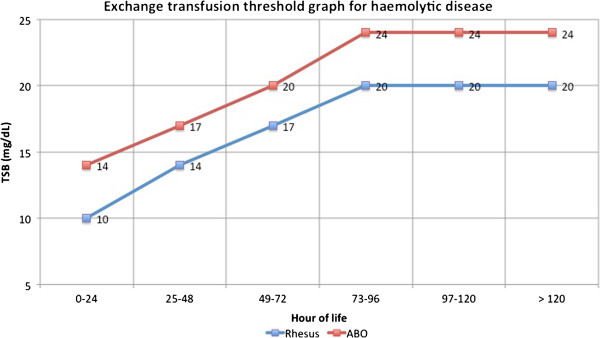
The graph shows the thresholds for exchange transfusion in case of haemolytic disease. Total bilirubin was plotted against age in hours.
The more conservative approach to the treatment of ABO haemolytic disease compared to Rh haemolytic disease is due to its less aggressive development.
Recommendations (see Additional file 1 ):
• ET should be performed only by trained personnel in a neonatal intensive care unit with fully monitoring and resuscitation capabilities.
• Heart rate, breath rate, SpO2 and body temperature should be monitored during ET and for 12–24 hours after the end of the procedure.
• The most common adverse effects of ET are thrombocytopenia, haemolysis, hypocalcaemia, hypotension, venous thrombosis, hypokalaemia and hypoglycaemia and should be carefully monitored.
• Measurement of TSB should be performed before ET and at the end of the procedure.
• As bilirubin levels may continue to rise after an ET we recommend that TSB is measured every 4–6 hours, and that phototherapy is continued.
• It is recommended to discontinue enteral feeding during ET and for 6 hours from the end of the procedure (evidence level 5).
• Infants underwent ET should be followed up for the development of anaemia after discharge.
Other therapies
Intravenous immunoglobulins (IVIG) act by preventing haemolysis. IVIG contain pooled IgG immunoglobulins extracted from the plasma of over 1000 blood donors. IVIG at a dose of either 500 mg/kg or 1 gr/kg over 2–4 hours reduce TSB in newborn infants with immune haemolytic jaundice [42,43] and reduce the need for ET (evidence level 1a). IVIG should be administered in the first hours of life to infants with Rhesus haemolytic disease or ABO haemolytic disease when the serum bilirubin continues to rise by more than 0.5 mg/dL per hour.
Italian registry of kernicterus and hyperbilirubinaemia
The Italian Society of Neonatology instituted the Italian Registry of Kernicterus and Hyperbilirubinaemia (RIKI: Registro Italiano del Kenicterus e dell’Iperbilirubinemia) for monitoring the incidence of kernicterus and severe hyperbilirubinaemia (TSB > 20 mg/dL or need for ET) in Italy. Each new case can be reported through the website of the Italian Society of Neonatology (http://www.neonatologia.it). The objective of this registry is to provide evidences of the guidelines effectiveness through the evaluation of severe hyperbilirubinaemia and kernicterus rate.
Conclusion
The development of national guidelines provide standardisation of the management of neonatal jaundice. Severe hyperbilirubinaemia in both healthy term or preterm newborn infants continues to carry the potential for complications from ABE. The careful assessment of involved risk factors and the systematic approach to the management of hyperbilirubinaemia are essential to decrease the occurrence of kernicterus.
Abbreviations
ABE: Acute bilirubin encephalopathy; B/A: Bilirubin/albumin ratio; ET: Exchange transfusion; GA: Gestational age; IVIG: Intravenous immunoglobulin; RBCs: Red blood cells; TSB: Total serum bilirubin; TcB: Transcutaneous bilirubin.
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
CR, CD e FR conceived this study as Task Force for hyperbilirubinaemia of Italian Society of Neonatology. EZ, SP and LC revised literature and collected data from Italian studies. CR, GB and EZ conducted the study on transcutaneous bilirubin and serum bilirubin as predictors of severe hyperbilirubinaemia. GB and CR prepared the draft of the paper. CD, FR and SP made critical revision of the article. All authors read and approved the final manuscript.
Supplementary Material
Exchange transfusion procedure.
Contributor Information
Costantino Romagnoli, Email: cromagnoli@rm.unicatt.it.
Giovanni Barone, Email: gbarone85@yahoo.it.
Simone Pratesi, Email: simone.pratesi@unifi.it.
Francesco Raimondi, Email: raimondi@unina.it.
Letizia Capasso, Email: letizia.capasso@gmail.com.
Enrico Zecca, Email: enrizecca@rm.unicatt.it.
Carlo Dani, Email: carlo.dani@unifi.it.
References
- Maisels MJ, Kring E. Length of stay, jaundice, and hospital readmission. Pediatrics. 1998;101:995–998. doi: 10.1542/peds.101.6.995. [DOI] [PubMed] [Google Scholar]
- Friedman MA, Spitzer AR. Discharge criteria for the term newborn. Pediatr Clin North Am. 2004;51:599–618. doi: 10.1016/j.pcl.2004.01.011. viii. [DOI] [PubMed] [Google Scholar]
- Mercier CE, Barry SE, Paul K, Delaney TV, Horbar JD, Wasserman RC, Berry P, Shaw JS. Improving newborn preventive services at the birth hospitalization: a collaborative, hospital-based quality-improvement project. Pediatrics. 2007;120:481–488. doi: 10.1542/peds.2007-0233. [DOI] [PubMed] [Google Scholar]
- Lee KS, Perlman M, Ballantyne M, Elliott I, To T. Association between duration of neonatal hospital stay and readmission rate. J Pediatr. 1995;127:758–766. doi: 10.1016/S0022-3476(95)70170-2. [DOI] [PubMed] [Google Scholar]
- Seidman DS, Stevenson DK, Ergaz Z, Gale R. Hospital readmission due to neonatal hyperbilirubinemia. Pediatrics. 1995;96:727–729. [PubMed] [Google Scholar]
- Dani C, Poggi C, Barp J, Romagnoli C, Buonocore G. Current Italian practices regarding the management of hyperbilirubinaemia in preterm infants. Acta Paediatr. 2011;100:666–669. doi: 10.1111/j.1651-2227.2011.02172.x. [DOI] [PubMed] [Google Scholar]
- American Academy of Pediatrics Subcommittee on H. Management of hyperbilirubinemia in the newborn infant 35 or more weeks of gestation. Pediatrics. 2004;114:297–316. doi: 10.1542/peds.114.1.297. [DOI] [PubMed] [Google Scholar]
- Maisels MJ, Bhutani VK, Bogen D, Newman TB, Stark AR, Watchko JF. Hyperbilirubinemia in the newborn infant > or =35 weeks’ gestation: an update with clarifications. Pediatrics. 2009;124:1193–1198. doi: 10.1542/peds.2009-0329. [DOI] [PubMed] [Google Scholar]
- Bhutani VK. Committee on F, Newborn, American Academy of P. Phototherapy to prevent severe neonatal hyperbilirubinemia in the newborn infant 35 or more weeks of gestation. Pediatrics. 2011;128:e1046–e1052. doi: 10.1542/peds.2011-1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Institute of Child Health and Clinical Excellence. 2011. http://pathways.nice.org.uk/pathways/neonatal-jaundice.
- Atkinson M, Budge H. Review of the NICE guidance on neonatal jaundice. Arch Dis Child Educ Pract Ed. 2011;96:136–140. doi: 10.1136/adc.2010.206755. [DOI] [PubMed] [Google Scholar]
- Newman J. Guidelines for detection, management and prevention of hyperbilirubinemia in term and late preterm newborn infants (35 or more weeks’ gestation) - Summary. Paediatr Child Health. 2007;12:401–418. doi: 10.1093/pch/12.5.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Imhoff DE, Dijk PH, Hulzebos CV. Bartrial study group NNRN. Uniform treatment thresholds for hyperbilirubinemia in preterm infants: background and synopsis of a national guideline. Early Hum Dev. 2011;87:521–525. doi: 10.1016/j.earlhumdev.2011.04.004. [DOI] [PubMed] [Google Scholar]
- Bratlid D, Nakstad B, Hansen TW. National guidelines for treatment of jaundice in the newborn. Acta Paediatr. 2011;100:499–505. doi: 10.1111/j.1651-2227.2010.02104.x. [DOI] [PubMed] [Google Scholar]
- Kaplan M, Merlob P, Regev R. Israel guidelines for the management of neonatal hyperbilirubinemia and prevention of kernicterus. J Perinatol. 2008;28:389–397. doi: 10.1038/jp.2008.20. [DOI] [PubMed] [Google Scholar]
- Newborn Service Clinical Guideline. Management of Neonatal Jaundice. http://www.adhb.govt.nz/newborn/guidelines/GI/Jaundice.htm.
- Queensland Maternity and Neonatal Clinical Guideline. Neonatal Jaundice: Prevention, Assessment and Management. 2009. http://www.health.qld.gov.au/qcg/
- Mishra S, Agarwal R, Deorari AK, Paul VK. Jaundice in the Newborn. AIIMS-NICU Protocols. 2007. http://www.newbornwhocc.org/
- Rodriguez Miguelez JM, Figueras Aloy J, Association Espanola de Pediatria. Ictericia Neonatal. http://www.aeped.es/documentos/protocolos-neonatologia.
- Arlettaz R, Blumberg A, Buetti L, Fahnenstich H, Mieth D, Roth-Kleiner M. Assessment and treatment of jaundice newborn infants 35 0/7 or more weeks of gestation. Swiss Soc Neonatology. 2007. pp. 1–4. http://www.neonet.ch/assets/pdf/2006_Bili-Empfehlungen_e_final.pdf.
- De Luca D, Romagnoli C, Tiberi E, Zuppa AA, Zecca E. Skin bilirubin nomogram for the first 96 h of life in a European normal healthy newborn population, obtained with multiwavelength transcutaneous bilirubinometry. Acta Paediatr. 2008;97:146–150. doi: 10.1111/j.1651-2227.2007.00622.x. [DOI] [PubMed] [Google Scholar]
- Romagnoli C, Tiberi E, Barone G, De Curtis M, Regoli D, Paolillo P, Picone S, Anania S, Finocchi M, Cardiello V, Zecca E. Validation of transcutaneous bilirubin nomogram in identifying neonates not at risk of hyperbilirubinaemia: a prospective, observational, multicenter study. Early Hum Dev. 2012;88:51–55. doi: 10.1016/j.earlhumdev.2011.07.001. [DOI] [PubMed] [Google Scholar]
- Romagnoli C, Tiberi E, Barone G, Curtis MD, Regoli D, Paolillo P, Picone S, Anania S, Finocchi M, Cardiello V, Giordano L, Paolucci V, Zecca E. Development and validation of serum bilirubin nomogram to predict the absence of risk for severe hyperbilirubinaemia before discharge: a prospective, multicenter study. Ital J Pediatr. 2012;38:6. doi: 10.1186/1824-7288-38-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oxford Centre for Evidence-Based Medicine. http://www.cebm.net/?o=1025.
- Maisels MJ, Watchko JF. Treatment of jaundice in low birthweight infants. Arch Dis Child Fetal Neonatal Ed. 2003;88(6):F459–F463. doi: 10.1136/fn.88.6.F459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watchko JF, Maisels MJ. Jaundice in low birthweight infants: pathobiology and outcome. Arch Dis Child Fetal Neonatal Ed. 2003;88(6):F455–F458. doi: 10.1136/fn.88.6.F455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyer VA, Ahn C, Sneed S. Accuracy of clinical judgment in neonatal jaundice. Arch Pediatr Adolesc Med. 2000;154:391–394. doi: 10.1001/archpedi.154.4.391. [DOI] [PubMed] [Google Scholar]
- De Luca D, Zecca E, Zuppa AA, Romagnoli C. The joint use of human and electronic eye: visual assessment of jaundice and transcutaneous bilirubinometry. Turk J Pediatr. 2008;50:456–461. [PubMed] [Google Scholar]
- Riskin A, Tamir A, Kugelman A, Hemo M, Bader D. Is visual assessment of jaundice reliable as a screening tool to detect significant neonatal hyperbilirubinemia? J Pediatr. 2008;152:782–787. doi: 10.1016/j.jpeds.2007.11.003. 787 e781-782. [DOI] [PubMed] [Google Scholar]
- Knudsen A. The influence of the reserve albumin concentration and pH on the cephalocaudal progression of jaundice in newborns. Early Hum Dev. 1991;25:37–41. doi: 10.1016/0378-3782(91)90204-G. [DOI] [PubMed] [Google Scholar]
- Knudsen A, Brodersen R. Skin colour and bilirubin in neonates. Arch Dis Child. 1989;64:605–609. doi: 10.1136/adc.64.4.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romagnoli C, Catenazzi P, Barone G, Giordano L, Riccardi R, Zuppa AA, Zecca E. BiliCheck vs JM-103 in identifying neonates not at risk of hyperbilirubinaemia. Ital J Pediatr. 2013;39:46. doi: 10.1186/1824-7288-39-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romagnoli C, Zecca E, Catenazzi P, Barone G, Zuppa AA. Transcutaneous bilirubin measurement: comparison of Respironics BiliCheck and JM-103 in a normal newborn population. Clin Biochem. 2012;45:659–662. doi: 10.1016/j.clinbiochem.2012.03.014. [DOI] [PubMed] [Google Scholar]
- Bhutani VK, Johnson L, Sivieri EM. Predictive ability of a predischarge hour-specific serum bilirubin for subsequent significant hyperbilirubinemia in healthy term and near-term newborns. Pediatrics. 1999;103:6–14. doi: 10.1542/peds.103.1.6. [DOI] [PubMed] [Google Scholar]
- Yu ZB, Dong XY, Han SP, Chen YL, Qiu YF, Sha L, Sun Q, Guo XR. Transcutaneous bilirubin nomogram for predicting neonatal hyperbilirubinemia in healthy term and late-preterm Chinese infants. Eur J Pediatr. 2011;170:185–191. doi: 10.1007/s00431-010-1281-9. [DOI] [PubMed] [Google Scholar]
- Varvarigou A, Fouzas S, Skylogianni E, Mantagou L, Bougioukou D, Mantagos S. Transcutaneous bilirubin nomogram for prediction of significant neonatal hyperbilirubinemia. Pediatrics. 2009;124:1052–1059. doi: 10.1542/peds.2008-2322. [DOI] [PubMed] [Google Scholar]
- Mills JF, Tudehope D. Fibreoptic phototherapy for neonatal jaundice. Cochrane Database Syst Rev. 2001;1:CD002060. doi: 10.1002/14651858.CD002060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barak M, Berger I, Dollberg S, Mimouni FB, Mandel D. When should phototherapy be stopped? A pilot study comparing two targets of serum bilirubin concentration. Acta Paediatr. 2009;98:277–281. doi: 10.1111/j.1651-2227.2008.01015.x. [DOI] [PubMed] [Google Scholar]
- Kaplan M, Kaplan E, Hammerman C, Algur N, Bromiker R, Schimmel MS, Eidelman AI. Post-phototherapy neonatal bilirubin rebound: a potential cause of significant hyperbilirubinaemia. Arch Dis Child. 2006;91:31–34. doi: 10.1136/adc.2005.081224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemper K, Forsyth B, McCarthy P. Jaundice, terminating breast-feeding, and the vulnerable child. Pediatrics. 1989;84:773–778. [PubMed] [Google Scholar]
- Martinez JC, Maisels MJ, Otheguy L, Garcia H, Savorani M, Mogni B, Martinez JC Jr. Hyperbilirubinemia in the breast-fed newborn: a controlled trial of four interventions. Pediatrics. 1993;91:470–473. [PubMed] [Google Scholar]
- Alcock GS, Liley H. Immunoglobulin infusion for isoimmune haemolytic jaundice in neonates. Cochrane Database Syst Rev. 2002;3:CD003313. doi: 10.1002/14651858.CD003313. [DOI] [PubMed] [Google Scholar]
- Miqdad AM, Abdelbasit OB, Shaheed MM, Seidahmed MZ, Abomelha AM, Arcala OP. Intravenous immunoglobulin G (IVIG) therapy for significant hyperbilirubinemia in ABO hemolytic disease of the newborn. J Matern Fetal Neonatal Med. 2004;16:163–166. doi: 10.1080/jmf.16.3.163.166. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Exchange transfusion procedure.


