Abstract
Background
Patients with acute respiratory distress syndrome (ARDS) risk lung collapse, severely altering the breath-to-breath respiratory mechanics. Model-based estimation of respiratory mechanics characterising patient-specific condition and response to treatment may be used to guide mechanical ventilation (MV). This study presents a model-based approach to monitor time-varying patient-ventilator interaction to guide positive end expiratory pressure (PEEP) selection.
Methods
The single compartment lung model was extended to monitor dynamic time-varying respiratory system elastance, Edrs, within each breathing cycle. Two separate animal models were considered, each consisting of three fully sedated pure pietrain piglets (oleic acid ARDS and lavage ARDS). A staircase recruitment manoeuvre was performed on all six subjects after ARDS was induced. The Edrs was mapped across each breathing cycle for each subject.
Results
Six time-varying, breath-specific Edrs maps were generated, one for each subject. Each Edrs map shows the subject-specific response to mechanical ventilation (MV), indicating the need for a model-based approach to guide MV. This method of visualisation provides high resolution insight into the time-varying respiratory mechanics to aid clinical decision making. Using the Edrs maps, minimal time-varying elastance was identified, which can be used to select optimal PEEP.
Conclusions
Real-time continuous monitoring of in-breath mechanics provides further insight into lung physiology. Therefore, there is potential for this new monitoring method to aid clinicians in guiding MV treatment. These are the first such maps generated and they thus show unique results in high resolution. The model is limited to a constant respiratory resistance throughout inspiration which may not be valid in some cases. However, trends match clinical expectation and the results highlight both the subject-specificity of the model, as well as significant inter-subject variability.
Keywords: Mechanical ventilation, PEEP, Time-varying elastance, Model-based methods, ARDS, Monitoring
Background
Acute respiratory distress syndrome (ARDS) [1] results in a stiffer lung [2]. ARDS patients are admitted to the intensive care unit (ICU) and require mechanical ventilation (MV) for breathing support. Positive end expiratory pressure (PEEP) is applied to aid recovery by improving gas exchange and maintaining recruited lung volume [3-6]. However, variation in a patient’s response to MV and the heterogeneity of ARDS means there is a need to determine optimal patient-specific PEEP [3,7].
ARDS involves alterations in a patient’s breath-to-breath respiratory mechanics. Modelling these alterations can potentially provide a non-invasive, patient-specific method to obtain clinically and physiologically useful information to guide treatment in real-time [8-11]. This approach can provide unique insight into disease progression and patient response to MV [12-15]. However, real-time monitoring of respiratory mechanics throughout MV treatment is, to date, limited in clinical application and impact [16].
Dynamic respiratory system elastance (Edrs) is a breath-specific time-varying lung elastance [17]. Dynamic elastance within a breath provides unique insight into a patient’s breathing pattern, revealing lung recruitment and overdistension [17,18]. In addition, identifying when minimum Edrs (maximum compliance) occurs during PEEP titration can help identify an optimal patient-specific PEEP to minimise work of breathing (WOB) and maximise recruitment without inducing further lung injury [19,20]. This work presents a novel method of visualising the time-varying respiratory elastance to provide a higher resolution metric to guide MV therapy.
Methods
Dynamic respiratory system elastance model
The equation of motion describing the airway pressure as a function of the resistive and elastic components of the respiratory system is defined as [21]:
| (1) |
where Paw is the airway pressure, t is time, Rrs is the series resistance of the conducting airway, Q is the air flow, Ers is an overall respiratory system elastance (1/compliance), V is the lung volume and P0 is the offset pressure.
During inspiration, a fully sedated patient will have a near constant chest wall elastance, Ecw. Thus, changes in the respiratory system elastance, Ers, are attributed directly to the patient’s lung elastance, Elung, as shown in Equation 2, thereby providing insight into patient condition and ARDS severity [2,21].
| (2) |
Equation 3 describes an integral-based method [22] used to estimate values of Ers and Rrs that best fit Equation 1. Integral-based parameter identification is similar to multiple linear regression, where using integrals significantly increases robustness to noise [17,22].
| (3) |
Respiratory resistance is assumed constant throughout a breath [17], but can vary with PEEP and time due to opening or closing of respiratory system airways [12,14,17,23]. Thus, once Rrs is determined for a particular breath using Equation 3, it is substituted into Equation 4 where dynamic lung elastance, Edrs, is defined as a time-varying lung elastance, such that Ers is effectively the average of Edrs.
| (4) |
Thus, Edrs can be determined from:
| (5) |
In this way, significantly more insight is gained into the respiratory elastance over the course of inspiration than can be provided by a single value of Ers.
During a PEEP increase, recruitment of new lung volume outweighs lung stretching provided that the global measure of Edrs decreases breath-to-breath [17]. Hence, the dynamic trajectory of Edrs captures the overall balance of volume (recruitment) and pressure (risk) within the lung.
Experimental data
Two experimental ARDS animal models are considered, each using three fully sedated pure pietrain piglets. The criterion for ARDS is limited to hypoxemia monitoring where the PaO2/FiO2 (PF ratio) is less than 300 mmHg.
1. Oleic Acid ARDS Models[24]: Each subject (Subjects 1–3) was sedated and ventilated through a tracheotomy under volume control (tidal volume, Vt = 8–10 ml/kg) with an inspired oxygen fraction (FiO2) of 0.5 and a respiratory rate of 20 breaths/min using an Engström CareStation ventilator (Datex, General Electric, Finland). ARDS was induced using oleic acid [25] and the arterial blood gas (ABG) was monitored half hourly. Once diagnosed with ARDS, each subject underwent a staircase recruitment manoeuvre (RM) with a PEEP level sequence of 5 – 10 – 15 – 20 – 15 – 10 – 5 cmH2O [26]. Breathing was maintained for approximately 10–15 breathing cycles at each PEEP level. Airway pressure and flow data were acquired using the Eview module provided with the ventilator. The data sampling rate was 25 Hz.
2. Lavage ARDS Models: After sedation and intubation via tracheotomy, the piglets (Subjects 4–6) were ventilated by intermittent positive pressure ventilation mode using a Drager Evita2 ventilator (Drager, Lubeck Germany). The ventilator was set to deliver a tidal volume of 8–10 ml/kg with a FiO2 of 0.5 at a respiratory rate of 20 breaths/min. Each subject underwent surfactant depletion using lavage methods [25]. The ABG was monitored and once diagnosed with ARDS, each subject underwent a staircase RM with PEEP settings at 1 – 5 – 10 – 15 – 20 – 15 – 10 – 5 – 1 mbar [26]. Breathing was maintained for approximately 10–15 breathing cycles at each PEEP level. Airway pressure and flow were measured using a 4700B pneumotachometer (Hans Rudolph Inc., Shawnee, KS) at a sampling rate of 200 Hz. Calibration was performed by matching pressure flow curve from the pneumotachometer to the peak inspiratory pressure (PIP), positive end expiratory pressure (PEEP), flow displayed in Drager Evita2 ventilator (Drager, Lubeck, Germany).
Airway pressure and flow rate data was analysed using MATLAB (The Mathworks, Natick, Massachusetts, USA). All experimental procedures, protocols and the use of data in this study were reviewed and approved by the Ethics Committee of the University of Liege Medical Faculty.
Visualisation of the dynamics (Elastance)
Dynamic respiratory system elastance (Edrs) varies within a breath as recruitment or overdistension occurs. Similarly, Edrs will evolve with time as recruitment is time dependent [27,28], disease state dependent [6,29] and MV dependent [28,30]. Arranging each breathing cycle’s Edrs curve such that it is bounded by the Edrs curve of the preceding breath and the subsequent breath leads to a three-dimensional, time-varying, breath-specific Edrs map. This method of visualisation gives new insight into how the breath-to-breath respiratory mechanics change with time over the course of treatment. In a similar manner, the corresponding change in airway pressure from PEEP to peak inspiratory pressure (PIP) is also displayed for each subject.
The dynamic elastance for each breath is calculated by dividing the numerator of Equation 5 by the volume vector. Therefore, at the very start of inspiration, when inspired volume is very small, Edrs approaches physiologically unrealistic values. Since overdistension is unlikely to occur at low volumes, the initial 20% of the inspiratory time for each breath is neglected for clarity. During this time, volume increases by approximately 0.04-0.06 L (less than 20% of the total inspired tidal volume) for each subject.
Results
Each subject has approximately 160 to 360 breathing cycles over the course of the RM. All breathing cycles are normalised to their total inspiratory time to provide clarity and to ensure consistency between breaths with different inspiratory times. Thus, each breath effectively begins at 0% and ends at 100% of the total inspiration time. The time-varying, breath-specific Edrs map of the RM for Subjects 1-6 are shown in Figures 1, 2, 3, 4, 5 and 6 respectively, where blue indicates low Edrs and red indicates high Edrs. The corresponding airway pressure and PEEP are shown in grey. The PF ratio for each subject is stated in the corresponding figure caption. Each figure is also provided with a MATLAB figure in a compressed zip file to permit rotation. The Edrs and Rrs for each subject over the course of the RM is also shown within each MATLAB figure file (see Additional file 1). The top view of all Edrs maps are shown in Additional file 2.
Figure 1.
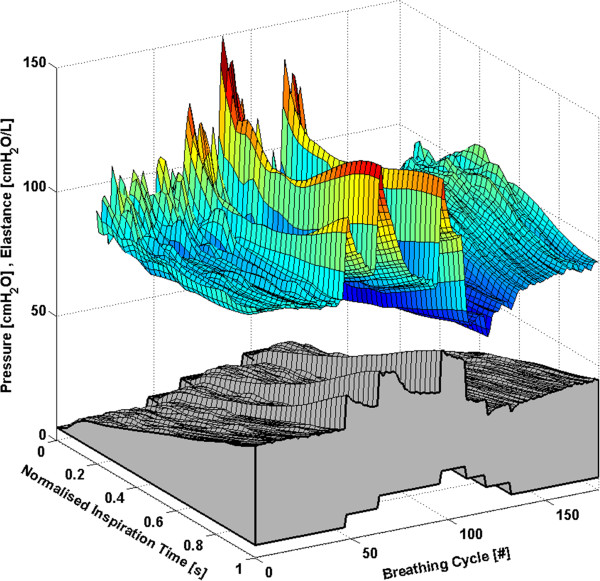
Variation in Edrsacross a normalised breath during a RM for Subject 1 (PaO2/FiO2 = 126.6 mmHg). The change in airway pressure for each normalised breathing cycle is shown in grey.
Figure 2.
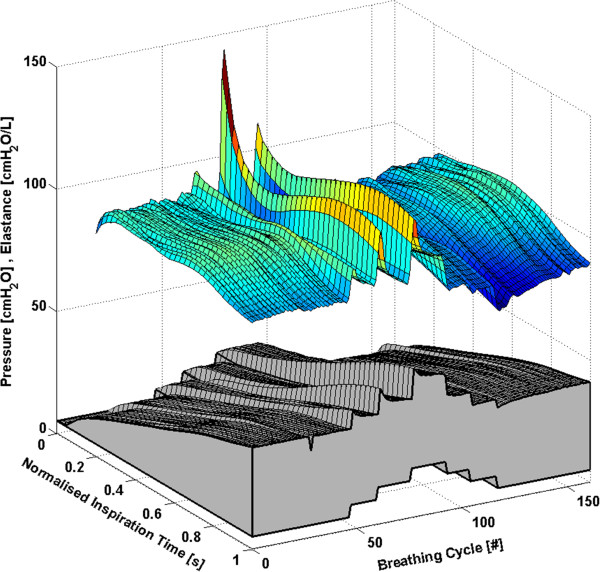
Variation in Edrsacross a normalised breath during a RM for Subject 2 (PaO2/FiO2 = 183.6 mmHg). The change in airway pressure for each normalised breathing cycle is shown in grey.
Figure 3.
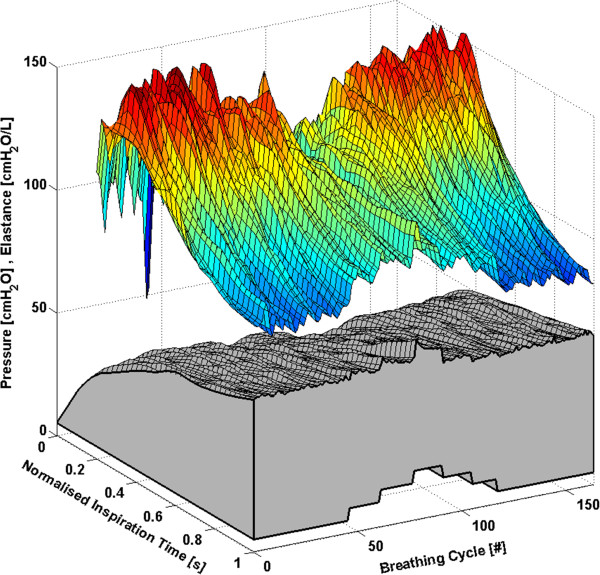
Variation in Edrsacross a normalised breath during a RM for Subject 3 (PaO2/FiO2 = 113.6 mmHg). The change in airway pressure for each normalised breathing cycle is shown in grey.
Figure 4.
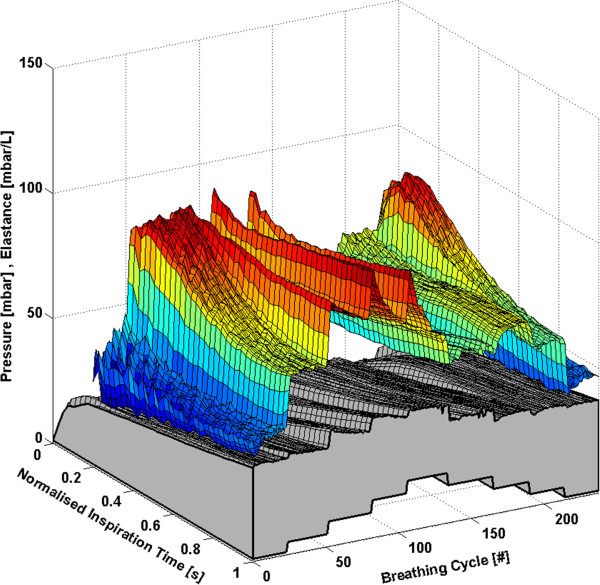
Variation in Edrsacross a normalised breath during a RM for Subject 4 (PaO2/FiO2 = 155.2 mmHg). The change in airway pressure for each normalised breathing cycle is shown in grey.
Figure 5.
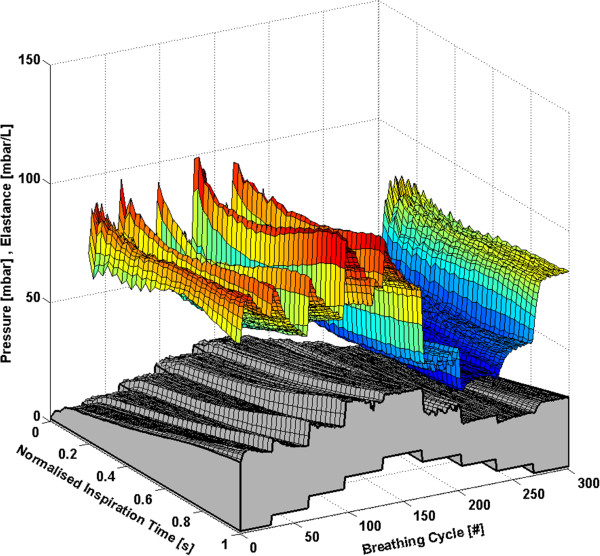
Variation in Edrsacross a normalised breath during a RM for Subject 5 (PaO2/FiO2 = 85.9 mmHg). The change in airway pressure for each normalised breathing cycle is shown in grey.
Figure 6.
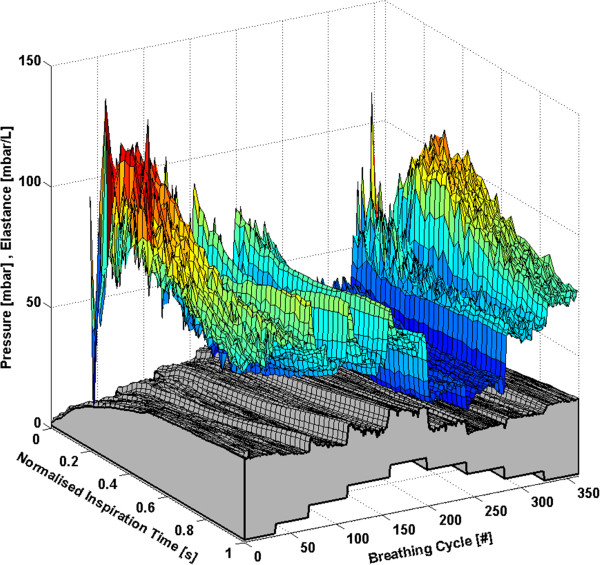
Variation in Edrsacross a normalised breath during a RM for Subject 6 (PaO2/FiO2 = 110.4 mmHg). The change in airway pressure for each normalised breathing cycle is shown in grey.
Discussion
General observations
All subjects showed, to some degree, an increase in Edrs immediately following a PEEP step increase of 5 cmH2O or 5 mbar. Each successive breath had a reduced peak Edrs indicating the time-dependent nature of recruitment and/or the lung’s viscoelastic properties, which cause hysteresis [31,32]. More specifically, there is a period of adaptation following an increase in PEEP that sees higher average Edrs, peak Edrs and PIP before the beneficial effect of lower Edrs is seen. Furthermore, the Edrs trajectory within a breath generally decreases during inspiration, suggesting in-breath recruitment. However, directly following a PEEP step increase, some subjects show a decreasing Edrs trajectory, followed by an increasing Edrs trajectory towards the end of inspiration. High elastance indicates serious potential for lung damage due to overstretching, and may not be captured by a single value of Ers[17,18]. Thus, as a result of this study, PEEP increments during a RM might be reduced to 1 cmH2O or 1 mbar, rather than increments of 5 cmH2O or 5 mbar, to avoid any damage due to the raised elastance in the early breaths and adaptation period following a PEEP increase. During a RM, smaller PEEP increments, each followed by a short period of stabilisation, may substantially reduce the peak of the Edrs spikes at the end of inspiration. However, it is important to note that the occurrence of lower respiratory elastance after stabilisation may also be a direct consequence of the initial high overdistension immediately following an increase in PEEP. This finding warrants further investigation where staircase recruitment is performed using smaller PEEP increments. Changes in ventilator pattern or mode to modify the Edrs trajectory also have potential to guide therapy.
The Edrs trend is significantly different between increasing and decreasing PEEP (using a non-parametric Wilcoxon rank sum test, p < 0.05 for each subject) where decreasing PEEP titration generally results in lower overall Edrs. When PEEP increases, recruitment, as well as potential lung overstretching occurs. However, as PEEP is reduced, the lung remains compliant and Edrs drops to an overall minimum. Equally, this phenomenon is seen where the opening pressure of collapsed alveoli is higher than the closing pressure [29,33]. Considering increasing and decreasing PEEP separately, a local Edrs minimum generally occurs at the same PEEP level, suggesting that optimum PEEP can be selected either way. Recruitment is a function of PEEP and time [28,30], and, equally, the ARDS affected lung is prone to collapse due to the instability of affected lung units [6,29]. Assuming that the severity of ARDS does not change within a short period, respiratory elastance during increasing PEEP titration is expected to reduce as time progresses to achieve stability. In contrast, respiratory elastance will increase with time during decreased PEEP to achieve stability. Hence, the authors hypothesise that PEEP can be titrated to a minimum elastance either way, provided a stabilisation period is given at each PEEP level to obtain a true minimal elastance.
The time-varying Edrs map is a higher resolution metric of dynamic adaptation to PEEP than a single Ers value. Selecting PEEP is a trade-off in minimising lung pressure and potential damage, versus maximising recruitment. Recruitment is a function of PEEP and time [28,30]. Therefore, true minimal Edrs can only be determined after a stabilisation period is provided at each PEEP level. Such a process could be readily automated and monitored in a ventilator. Setting PEEP at minimum elastance theoretically benefits ventilation by maximising recruitment, reducing work of breathing and minimising overdistension [12,14,15,34]. The PIP can be seen to follow the Edrs trend to some extent. However, it does not provide the same degree of resolution. In some cases PIP is seen to stabilise quickly or remain relatively constant following a change in PEEP, while Edrs continues to change significantly indicating the occurrence of significant lung dynamics not readily apparent from monitoring airway pressure alone. This result shows the greater sensitivity of using Edrs and that Edrs captures more relevant dynamics than airway pressure alone.
Oleic acid ARDS models
Subject 1
The response of Subject 1 to PEEP titration is seen in Figure 1. The Edrs drops to an overall minimum at a PEEP of 15 cmH2O, suggesting that maintaining this level of PEEP provides the optimal trade-off between maximising recruitment and reducing the risk of lung damage [12].
Subject 2
The response of Subject 2 to PEEP titration is seen in Figure 2 and is similar to that of Subject 1. However, the magnitude of the Edrs response to PEEP is reduced. The Edrs drops to an overall minimum at a PEEP of 15 cmH2O, implying optimal PEEP.
Subject 3
In Subject 3, Edrs rises to a maximum near the beginning of each breathing cycle before rapidly decreasing as seen in Figure 3. However, this trend is less pronounced at high PEEP levels. It is observed that Subject 3 had a more severe level of ARDS (PaO2/FiO2 = 113.6 mmHg) compared to Subject 1 (PaO2/FiO2 = 126.6 mmHg) or Subject 2 (PaO2/FiO2 = 183.6 mmHg), possibly resulting in the substantially different subject-specific response to PEEP titration. The airway pressure curves show an initial rapid increase followed by a more gradual increase. It is possible that a different flow profile may eliminate the initial rapid pressure increase and reduce the rise in Edrs. The most uniform elastance across a breath occurs at a PEEP of 15 cmH2O (at both increasing and decreasing PEEP), implying optimal PEEP.
Lavage ARDS models
Subject 4
Respiratory elastance increases significantly in Subject 4 when PEEP is increased from 1 mbar to 5 mbar as seen in Figure 4. The lowest elastance is encountered either side of the RM at a PEEP of 1 mbar. However, Edrs reaches a local minimum at a PEEP of 15 mbar during decreasing PEEP. Thus, in this case, minimal elastance would suggest that the subject should be ventilated at 1 mbar rather than 15 mbar. However, it is important to note that atelectasis occurs in ARDS patients [35], and clinically, ARDS patients should be ventilated at higher PEEP [1,36,37]. Thus, this Edrs map outlines a potential drawback of ventilation considering only minimum elastance. Clinicians should thus consider an alternate PEEP value when an unrealistically low PEEP is recommended by elastance.
Subject 5
The response of Subject 5 to PEEP titration is seen in Figure 5. The Edrs drops to an overall minimum at a PEEP of 10 mbar, implying optimal PEEP.
Subject 6
Unlike Subjects 4 and 5, the RM performed on Subject 6 was performed during an open chest surgery, thereby neglecting the effect of Ecw in Equation 2 and effectively capturing Elung directly. It was found that more noise was present in this trial when compared to closed chest ventilation performed on Subjects 1–5. The noise present in this data indicates that the chest wall may provide some form of damping to high frequency physiological or mechanical effects. It was observed that minimum elastance occurs at a decreasing PEEP of 10 mbar, as shown in Figure 6.
Limitations
The single compartment lung model used to derive Edrs does not capture some specific physiological aspects, such as cardiogenic oscillations or regional differences in mechanical properties [21]. Furthermore, the effects of non-linear flow or variations in airway resistance during a breath are also neglected [21]. The determination of Edrs accommodates whatever resistance value is chosen, such that the model perfectly fits the available pressure data. Hence, the assumption of constant resistance throughout a breath significantly impacts on the trends of Edrs. There is evidence to suggest that in some cases respiratory resistance can vary within a breath [23]. However, the effect of the resistive term is mathematically limited in its impact [17]. Since this analysis is predominantly based on the comparison of trends across PEEP values, where each subject is thus their own reference, the best validation is the ability to track clinically expected trends as shown here.
It is important to note that both ARDS animal models were different in many aspects and do not allow for a statistically significant comparison. More importantly, it was not able to fully justify PEEP optimisation based solely on minimal elastance. However, the main outcome of this research is that mapping of time-varying respiratory elastance of mechanically ventilated ARDS subjects can be monitored to provide a high resolution metric to describe disease state and physiological changes in response to PEEP. This outcome shows the robustness of both the model and the method of visualisation for application in the ICU. However, more inter-patient variability is present in patients admitted to the ICU. Thus, application of this monitoring technique warrants further investigation in both human and animal studies.
Selecting patient-specific optimal PEEP remains widely debatable with little consensus [36,37]. This study primarily provides a means to visualise respiratory system elastance continuously, thus allowing PEEP to be titrated to minimal elastance [12,14,15], and it was suggested that it can be done using either incremental or decremental phase of a staircase RM. However, this suggestion is limited to the protocol and data available. If only the incremental phase of the RM is available, PEEP titration can be performed during incremental stage, or vice-versa. If both incremental and decremental phase of the staircase RM are available, PEEP should be titrated during decreasing PEEP, as the incremental PEEP functions to recruit the collapsed lung [38,39].
A further limitation is that the findings of this research are solely based on observation of the Edrs map. The findings require further investigation together with additional imaging and monitoring tools such as in-vivo microscopy, computer tomography and/or electrical impedance tomography for validation. However, high resolution imaging technology is currently limited to regional investigation and clinically impractical for full and continuous monitoring [40-42]. Thus, the findings of this research are limited to comparisons with existing literature.
Conclusion
Visualisation of the dynamic respiratory elastance provides significantly more insight into dynamic lung behaviour than can be provided by a single value of Ers. Simultaneous monitoring of respiratory elastance across a breath and during a RM provides a new clinical perspective to guide therapy and provides unique subject-specific insight into the heterogeneous response to PEEP. The model is limited to a constant respiratory resistance throughout inspiration which may not be valid in some cases. However, trends match clinical expectation and the results highlight both the subject-specificity of the model, as well as significant inter-subject variability. Overall, further research is warranted to confirm the clinical potential of using this method in ARDS patients admitted to the ICU.
Abbreviations
ARDS: Acute respiratory distress syndrome; ICU: Intensive care unit; MV: Mechanical ventilation; PEEP: Positive end expiratory pressure; WOB: Work of breathing; PF: PaO2/FiO2; ABG: Arterial blood gas; RM: Recruitment manoeuvre; PIP: Peak inspiratory pressure.
Competing interests
The authors declare that they have no competing interests.
Authors’ contribution
EJD assisted in the development of the Edrs model, generated the Edrs maps, and drafted the manuscript. YSC participated in the implementation of the clinical trials, assisted in the development of the Edrs model, and helped to draft the manuscript. CP participated in the implementation of the clinical trials. GMS participated in the implementation and coordination of the study. BL and NJ participated in the implementation of the clinical trials. JGC participated in the implementation and coordination of the study, and helped to draft the manuscript. TD participated in the implementation and coordination of the study, implementation of the clinical trials, and helped to draft the manuscript. All authors read and approved the final manuscript.
Pre-publication history
The pre-publication history for this paper can be accessed here:
Supplementary Material
Subject 1 - Oleic acid ARDS. Subject 2 - Oleic acid ARDS. Subject 3 - Oleic acid ARDS. Subject 4 - Lavage ARDS. Subject 5 - Lavage ARDS. Subject 6 - Lavage ARDS.
Top view of E drs map for each subject.
Contributor Information
Erwin J van Drunen, Email: erwin.vandrunen@gmail.com.
Yeong Shiong Chiew, Email: yeongshiong.chiew@canterbury.ac.nz.
Christopher Pretty, Email: chris.pretty@canterbury.ac.nz.
Geoffrey M Shaw, Email: geoff.shaw@cdhb.govt.nz.
Bernard Lambermont, Email: b.lambermont@chu.ulg.ac.be.
Nathalie Janssen, Email: njanssen@chu.ulg.ac.be.
J Geoffrey Chase, Email: geoff.chase@canterbury.ac.nz.
Thomas Desaive, Email: tdesaive@ulg.ac.be.
Acknowledgement
The authors wish to thank Paul D. Docherty and members of mechanical ventilation research team in Center for Bio-Engineering, University of Canterbury and GIGA-Cardiovascular Sciences, University of Liege for supporting this work.
References
- The ARDS Definition Task Force A. Acute respiratory distress syndrome: the berlin definition. JAMA. 2012;307(23):2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- Gattinoni L, Pesenti A. The concept of “baby lung”. Intensive Care Med. 2005;31(6):776–784. doi: 10.1007/s00134-005-2627-z. [DOI] [PubMed] [Google Scholar]
- Amato MBP, Barbas CSV, Medeiros DM, Magaldi RB, Schettino GP, Lorenzi-Filho G, Kairalla RA, Deheinzelin D, Munoz C, Oliveira R, Takagaki TY, Carvalho CRR. Effect of a protective-ventilation strategy on mortality in the acute respiratory distress syndrome. N Engl J Med. 1998;338(6):347–354. doi: 10.1056/NEJM199802053380602. [DOI] [PubMed] [Google Scholar]
- The Acute Respiratory Distress Syndrome Network. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000;342(18):1301–1308. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- McCann UG, Schiller HJ, Carney DE, Gatto LA, Steinberg JM, Nieman GF. Visual validation of the mechanical stabilizing effects of positive end-expiratory pressure at the alveolar level. J Surg Res. 2001;99(2):335–342. doi: 10.1006/jsre.2001.6179. [DOI] [PubMed] [Google Scholar]
- Halter JM, Steinberg JM, Schiller HJ, DaSilva M, Gatto LA, Landas S, Nieman GF. Positive End-expiratory pressure after a recruitment maneuver prevents both alveolar collapse and recruitment/derecruitment. Am J Respir Crit Care Med. 2003;167(12):1620–1626. doi: 10.1164/rccm.200205-435OC. [DOI] [PubMed] [Google Scholar]
- Gattinoni L, Carlesso E, Brazzi L, Caironi P. Positive end-expiratory pressure. Curr Opin Crit Care. 2010;16(1):39–44. doi: 10.1097/MCC.0b013e3283354723. [DOI] [PubMed] [Google Scholar]
- Lauzon AM, Bates JH. Estimation of time-varying respiratory mechanical parameters by recursive least squares. J Appl Physiol. 1991;71(3):1159–1165. doi: 10.1152/jappl.1991.71.3.1159. [DOI] [PubMed] [Google Scholar]
- Sundaresan A, Yuta T, Hann CE, Geoffrey Chase J, Shaw GM. A minimal model of lung mechanics and model-based markers for optimizing ventilator treatment in ARDS patients. Comput Methods Programs Biomed. 2009;95(2):166–180. doi: 10.1016/j.cmpb.2009.02.008. [DOI] [PubMed] [Google Scholar]
- Ma B, Bates J. Modeling the complex dynamics of derecruitment in the lung. Ann Biomed Eng. 2010;38(11):3466–3477. doi: 10.1007/s10439-010-0095-2. [DOI] [PubMed] [Google Scholar]
- Sundaresan A, Chase JG. Positive end expiratory pressure in patients with acute respiratory distress syndrome - The past, present and future. Biomed Signal Process Control. 2011;7(2):93–103. [Google Scholar]
- Carvalho A, Jandre F, Pino A, Bozza F, Salluh J, Rodrigues R, Ascoli F, Giannella-Neto A. Positive end-expiratory pressure at minimal respiratory elastance represents the best compromise between mechanical stress and lung aeration in oleic acid induced lung injury. Crit Care. 2007;11(4):R86. doi: 10.1186/cc6093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucangelo U, Bernabè F, Blanch L. Lung mechanics at the bedside: make it simple. Curr Opin Crit Care. 2007;13(1):64–72. doi: 10.1097/MCC.0b013e32801162df. [DOI] [PubMed] [Google Scholar]
- Suarez-Sipmann F, Bohm SH, Tusman G, Pesch T, Thamm O, Reissmann H, Reske A, Magnusson A, Hedenstierna G. Use of dynamic compliance for open lung positive end-expiratory pressure titration in an experimental study. Crit Care Med. 2007;35:214–221. doi: 10.1097/01.CCM.0000251131.40301.E2. [DOI] [PubMed] [Google Scholar]
- Lambermont B, Ghuysen A, Janssen N, Morimont P, Hartstein G, Gerard P, D’Orio V. Comparison of functional residual capacity and static compliance of the respiratory system during a positive end-expiratory pressure (PEEP) ramp procedure in an experimental model of acute respiratory distress syndrome. Crit Care. 2008;12(4):R91. doi: 10.1186/cc6961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brochard L, Martin G, Blanch L, Pelosi P, Belda FJ, Jubran A, Gattinoni L, Mancebo J, Ranieri VM, Richard J-C, Gommers D, Vieillard-Baron A, Pesenti A, Jaber S, Stenqvist O, Vincent J-L. Clinical review: Respiratory monitoring in the ICU - a consensus of 16. Crit Care. 2012;16(2):219. doi: 10.1186/cc11146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiew YS, Chase JG, Shaw G, Sundaresan A, Desaive T. Model-based PEEP optimisation in mechanical ventilation. Biomed Eng Online. 2011;10(1):111. doi: 10.1186/1475-925X-10-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Z, Guttmann J, Moller K. Adaptive Slice Method: a new method to determine volume dependent dynamic respiratory system mechanics. Physiol Meas. 2012;33(1):51–64. doi: 10.1088/0967-3334/33/1/51. [DOI] [PubMed] [Google Scholar]
- Schranz C, Becher T, Schadler D, Weiler N, Moeller K. Congress for the German Swiss and Austrian Society for Biomedical Engineering (BMT2013) Graz, Austria: Springer; 2013. Model-based ventialtor settings in pressure controlled ventilation. [DOI] [PubMed] [Google Scholar]
- Esquinas Rodriguez A, Papadakos P, Carron M, Cosentini R, Chiumello D. Clinical review: helmet and non-invasive mechanical ventilation in critically ill patients. Crit Care. 2013;17(2):223. doi: 10.1186/cc11875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates JHT. Lung Mechanics: an inverse modeling approach. United States of America, New York: Cambridge University Press; 2009. [Google Scholar]
- Hann CE, Chase JG, Lin J, Lotz T, Doran CV, Shaw GM. Integral-based parameter identification for long-term dynamic verification of a glucose-insulin system model. Comput Methods Programs Biomed. 2005;77(3):259–270. doi: 10.1016/j.cmpb.2004.10.006. [DOI] [PubMed] [Google Scholar]
- Mols G, Kessler V, Benzing A, Lichtwarck‒Aschoff M, Geiger K, Guttmann J. Is pulmonary resistance constant, within the range of tidal volume ventilation, in patients with ARDS? Br J Anaesth. 2001;86(2):176–182. doi: 10.1093/bja/86.2.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiew YS, Chase JG, Lambermont B, Janssen N, Schranz C, Moeller K, Shaw G, Desaive T. Physiological relevance and performance of a minimal lung model - an experimental study in healthy and acute respiratory distress syndrome model piglets. BMC Pulmonary Medicine. 2012;12(1):59. doi: 10.1186/1471-2466-12-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballard-Croft C, Wang D, Sumpter LR, Zhou X, Zwischenberger JB. Large-animal models of acute respiratory distress syndrome. Ann Thorac Surg. 2012;93(4):1331–1339. doi: 10.1016/j.athoracsur.2011.06.107. [DOI] [PubMed] [Google Scholar]
- Hodgson CL, Tuxen DV, Bailey MJ, Holland AE, Keating JL, Pilcher D, Thomson KR, Varma D. A positive response to a recruitment maneuver with PEEP titration in patients with ARDS, regardless of transient oxygen desaturation during the maneuver. J Intensive Care Med. 2011;26(1):41–49. doi: 10.1177/0885066610383953. [DOI] [PubMed] [Google Scholar]
- Bates JHT, Irvin CG. Time dependence of recruitment and derecruitment in the lung: a theoretical model. J Appl Physiol. 2002;93(2):705–713. doi: 10.1152/japplphysiol.01274.2001. [DOI] [PubMed] [Google Scholar]
- Albert SP, DiRocco J, Allen GB, Bates JHT, Lafollette R, Kubiak BD, Fischer J, Maroney S, Nieman GF. The role of time and pressure on alveolar recruitment. J Appl Physiol. 2009;106(3):757–765. doi: 10.1152/japplphysiol.90735.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelosi P, Goldner M, McKibben A, Adams A, Eccher G, Caironi P, Losappio S, Gattinoni L, Marini JJ. Recruitment and derecruitment during acute respiratory failure: an experimental study. Am J Respir Crit Care Med. 2001;164:122–130. doi: 10.1164/ajrccm.164.1.2007010. [DOI] [PubMed] [Google Scholar]
- Barbas CSV, de Matos GFJ, Pincelli MP, da Rosa Borges E, Antunes T, de Barros JM, Okamoto V, Borges JB, Amato MBP, Ribeiro de Carvalho CR. Mechanical ventilation in acute respiratory failure: recruitment and high positive end-expiratory pressure are necessary. Curr Opin Crit Care. 2005;11(1):18–28. doi: 10.1097/00075198-200502000-00004. [DOI] [PubMed] [Google Scholar]
- Ganzert S, Moller K, Steinmann D, Schumann S, Guttmann J. Pressure-dependent stress relaxation in acute respiratory distress syndrome and healthy lungs: an investigation based on a viscoelastic model. Crit Care. 2009;13(6):R199. doi: 10.1186/cc8203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreassen S, Steimle KL, Mogensen ML, Serna JB, Rees S, Karbing DS. The effect of tissue elastic properties and surfactant on alveolar stability. J Appl Physiol. 2010;109(5):1369–1377. doi: 10.1152/japplphysiol.00844.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crotti S, Mascheroni D, Caironi P, Pelosi P, Ronzoni G, Mondino M, Marini JJ, Gattinoni L. Recruitment and derecruitment during acute respiratory failure. A clinical study. Am J Respir Crit Care Med. 2001;164(1):131–140. doi: 10.1164/ajrccm.164.1.2007011. [DOI] [PubMed] [Google Scholar]
- Zhao Z, Steinmann D, Frerichs I, Guttmann J, Moller K. PEEP titration guided by ventilation homogeneity: a feasibility study using electrical impedance tomography. Crit Care. 2010;14:R8. doi: 10.1186/cc8860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedenstierna G, Rothen HU. Atelectasis formation during anesthesia: causes and measures to prevent it. J Clin Monit Comput. 2000;16:329–335. doi: 10.1023/A:1011491231934. [DOI] [PubMed] [Google Scholar]
- Briel M, Meade M, Mercat A, Brower RG, Talmor D, Walter SD, Slutsky AS, Pullenayegum E, Zhou Q, Cook D, Brochard L, Richard J-CM, Lamontagne F, Bhatnagar N, Stewart TE, Guyatt G. Higher vs lower positive end-expiratory pressure in patients with acute lung injury and acute respiratory distress syndrome: Systematic review and meta-analysis. JAMA. 2010;303(9):865–873. doi: 10.1001/jama.2010.218. [DOI] [PubMed] [Google Scholar]
- Mercat A, Richard J-CM, Vielle B, Jaber S, Osman D, Diehl J-L, Lefrant J-Y, Prat G, Richecoeur J, Nieszkowska A, Gervais C, Baudot J, Bouadma L, Brochard L. Expiratory Pressure Study Group (Express) Positive end-expiratory pressure setting in adults with acute lung injury and acute respiratory distress syndrome: a randomized controlled trial. JAMA. 2008;299(6):646–655. doi: 10.1001/jama.299.6.646. [DOI] [PubMed] [Google Scholar]
- Huh J, Jung H, Choi H, Hong S-B, Lim C-M, Koh Y. Efficacy of positive end-expiratory pressure titration after the alveolar recruitment manoeuvre in patients with acute respiratory distress syndrome. Crit Care. 2009;13(1):R22. doi: 10.1186/cc7725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suarez-Sipmann F, Bohm S. Recruit the lung before titrating the right positive end-expiratory pressure to protect it. Crit Care. 2009;13(3):134. doi: 10.1186/cc7763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malbouisson LM, Muller J-C, Constantin J-M, Lu QIN, Puybasset L, Rouby J-J. the CTSASG. Computed tomography assessment of positive end-expiratory pressure-induced alveolar recruitment in patients with acute respiratory distress syndrome. Am J Respir Crit Care Med. 2001;163(6):1444–1450. doi: 10.1164/ajrccm.163.6.2005001. [DOI] [PubMed] [Google Scholar]
- Zhao Z, Steinmann D, Muller-Zivkovic D, Martin J, Frerichs I, Guttmann J, Moller K. A lung area estimation method for analysis of ventilation inhomogeneity based on electrical impedance tomography. J Xray Sci Technol. 2010;18:171–182. doi: 10.3233/XST-2010-0252. [DOI] [PubMed] [Google Scholar]
- Zhao Z, Moller K, Steinmann D, Frerichs I, Guttmann J. Evaluation of an electrical impedance tomography-based global inhomogeneity index for pulmonary ventilation distribution. Intensive Care Med. 2009;35:1900–1906. doi: 10.1007/s00134-009-1589-y. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Subject 1 - Oleic acid ARDS. Subject 2 - Oleic acid ARDS. Subject 3 - Oleic acid ARDS. Subject 4 - Lavage ARDS. Subject 5 - Lavage ARDS. Subject 6 - Lavage ARDS.
Top view of E drs map for each subject.


