Abstract
Humans parturition involves interaction of hormonal, neurological, mechanical stretch and inflammatory pathways and the placenta plays a crucial role. The paraoxonases (PONs 1–3) protect against oxidative damage and lipid peroxidation, modulation of endoplasmic reticulum stress and regulation of apoptosis. Nothing is known about the role of PON2 in the placenta and labour. Since PON2 plays a role in oxidative stress and inflammation, both features of labour, we hypothesised that placental PON2 expression would alter during labour. PON2 was examined in placentas obtained from women who delivered by cesarean section and were not in labour and compared to the equivalent zone of placentas obtained from women who delivered vaginally following an uncomplicated labour. Samples were obtained from 12 sites within each placenta: 4 equally spaced apart pieces were sampled from the inner, middle and outer placental regions. PON2 expression was investigated by Western blotting and real time PCR. Two PON2 forms, one at 62 kDa and one at 43 kDa were found in all samples. No difference in protein expression of either isoform was found between the three sites in either the labour or non-labour group. At the middle site there was a highly significant decrease in PON2 expression in the labour group when compared to the non-labour group for both the 62 kDa form (p = 0.02) and the 43 kDa form (p = 0.006). No spatial differences were found within placentas at the mRNA level in either labour or non-labour. There was, paradoxically, an increase in PON2 mRNA in the labour group at the middle site only. This is the first report to describe changes in PON2 in the placenta in labour. The physiological and pathological significance of these remains to be elucidated but since PON2 is anti-inflammatory further studies are warranted to understand its role.
Introduction
Pregnancy is characterized by a complex interplay of inflammatory events regulated by both the innate and acquired immune systems. In humans parturition involves interaction of hormonal, neurological, mechanical stretch and inflammatory pathways and the placenta plays a crucial role [1]–[4]. The paraoxonases (PON) are multifaceted and pleiotropic enzymes encoded by three highly conserved genes (PON1, PON2, and PON3) located on chromo- some 7q21.3–22.1 [5]. They have multifunctional roles and are involved in various biochemical pathways. These include protection against oxidative damage and lipid peroxidation, modulation of endoplasmic reticulum stress, regulation of cell proliferation/apoptosis contribution to innate immunity and detoxification of reactive molecules and bioactivation of drugs [5]–[6]. Phylogenetic analysis has shown that PON1 and PON3 arose from gene duplication of the ancestral PON2 gene [5]–[6]. PON1 and PON3 are circulating proteins associated with high-density lipoproteins whereas PON2 is expressed in many tissues and is cell associated [7]. Research in the PON family has increased greatly in the last few years, particularly in the cardiovascular field [8].
Nothing is known about the role of PON2 in the placenta or whether it plays a role in labour. However since PON2 plays a role in oxidative stress and inflammation, both features of labour, we hypothesised that placental PON2 expression would alter during labour. Thus the aim of this study was to examine the spatial expression of PON2 in placentas obtained from women who delivered by cesarean section and were not in labour and to compare the expression of each zone with the equivalent zone of placentas obtained from women who delivered vaginally following an uncomplicated labour.
Methods
Ethics statement
Human term placentae were collected from pregnant women at the Southern General Hospital, Glasgow. All ethics protocols were followed as per Declaration of Helsinki. The study was approved by the West of Scotland research ethics service Signed patient consent was obtained prior to delivery. Patients were handed an information sheet telling them about the study before being handed the consent sheet. The information and consent sheets were also approved by the ethics committee. All signed consent sheets were stored incase of the need for audit.
Patients studied
Placentae were collected from: (i) women who had uncomplicated pregnancies and delivered at term either vaginally (labour group, n = 6) or by caesarean section (non-labour group, n = 6). The labour group were all spontaneous labour and were a tight group (labour time minimum 3 hours maximum 8 hours). All placentas were free of infection, confirmed by the pathology report of every placenta. The non-labour group were all definitely without labour. All were planned Caesarean sections performed for obstetric reasons: breach presentation (2) previous caesarean section (2) or maternal request (2). The groups studied had no underlying maternal conditions such as hypertension, preeclampsia, diabetes or gestational diabetes or any other medical disorders. There was no fetal pathology such as fetal growth restriction. Patient demographics were compared with the student t-test. There was no significant difference in maternal age (non-labour 28.33±5.71 versus labour 26±2.28 years), placental weight (non-labour 594.7±110.5 versus labour 589.5±75 g), birth weight (non-labour 3443±537 versus labour 3719±347 g), number of primigravid (non-labour n = 2 versus labour n = 4) or number of smokers (n = 0 labour versus n = 2 non-labour).
Placental sampling
For each patient (6 patients per group), placental samples (∼1 cm3) were obtained from three sites by taking measurements from the cord insertion point: inner third closest to cord insertion point (inner zone), middle of placenta (middle zone) and outer third of placenta (outer zone) of placenta. Within each zone four separate samples were obtained representing the four quadrants as previously described [9]–[11]. Placentas had a central cord insertion. Samples were rinsed and immediately flash frozen in liquid nitrogen. For this study we had performed a power analysis using G*Power 3.1 for Macintosh and based the numbers on previously published work with the same samples [9]–[11].
Chemicals
All chemicals were purchased from Sigma-Aldrich (U.K.) unless stated otherwise.
Tissue Homogenizing For Western Blot
Placental samples were recovered from storage at −70°C and ground to a fine powder in liquid nitrogen using a mortar and pestle. The frozen powder was then homogenised in the presence of protease inhibitors as previously described [9]–[11]. Placenta homogenates were spun at 5000 g for 10 min at 4°C to remove debris then supernatants were collected, divided into aliquots and stored at −70°C. Protein concentrations were determined by Bradford analysis using bovine serum albumin as a standard.
Western Blotting
Western blotting was performed as described previously [9]–[11] with some modifications. A volume containing 50 µg of each sample was separated by SDS-PAGE electrophoresis on 10% sodium dodecyl sulfate-polyacrylamide resolving gels. Pre-stained low range molecular weight markers (BioRad, UK) were loaded onto each gel. Transfer of proteins to Hybond ECL nitrocellulose membranes (Amersham Pharmacia Biotech, UK) was performed at 22V and 200 mA for 30 min. Membranes were blocked in 5% goat serum (Serotec) in TBSTB buffer (20 mM TRIS pH 7.5, 0.5 M NaCl, 0.4% Tween and 0.25% bovine serum albumin) for 1 h at room temperature (RT). Primary antibodies were pre-absorbed in 5% human serum in TBSTB at RT during the blocking process. Membranes were incubated for 1 h at RT with primary antibody solution. The PON2 (mouse monoclonal antibody) was obtained from Santa Cruz Biotechonolgy, USA (catalogue number sc-373981) and used at concentration of 1∶200. Membranes were washed and then incubated for 1 h at RT with horseradish peroxidase conjugated goat anti-mouse secondary antibody (antibody (SantaCruz (sc-2005) diluted 1∶1000 in TBSTB. Membranes were rinsed with TBSTB (2×5 min) and once with distilled water. The same samples were exposed to a β-actin antibody (Sigma) to confirm even protein loading as shown previously [9]–[11]. Immunologically reactive proteins were visualised and quantified as described previously [9]–[11]. A standard curve was performed for different blot exposures and densitometry was performed when bands were on the linear part of the loading graph as described previously [9]–[11]. For each group of experiments the same loading control placenta sample was added to every gel and the densitometry units were normalized to that. We previously confirmed that this method of analysis gives similar findings to other quantitative methods of densitometry [9]–[11].
Quantitative Real Time-Polymerase Chain Reaction (RT-PCR)
Total RNA was isolated using the RNeasy Midi Kit (Qiagen, 75142). RNA (100 ng) was reverse transcribed into cDNA. Buffers and primers were obtained from the QuantiTect Kit (Qiagen, 205310) and GoScript reverse transcriptase from Promega (A501C). PON2 expression was analyzed by RT-PCR using validated TaqMan Gene Expression assays with StepOnePlus (Applied Biosystems). β-actin was used as an endogenous control. A positive control human placenta cDNA (Primer design) was used. The relative target gene levels were calculated by comparative CT (ΔΔCT).
Statistical analysis
Patients details were compared using the student t-test. Statistical analysis was performed using Graphpad prism 5 on a PC. For Western blot analysis and RT-PCR analysis statistical analysis was performed using the Friedman test for non-parametric data.
Results
Experiment 1
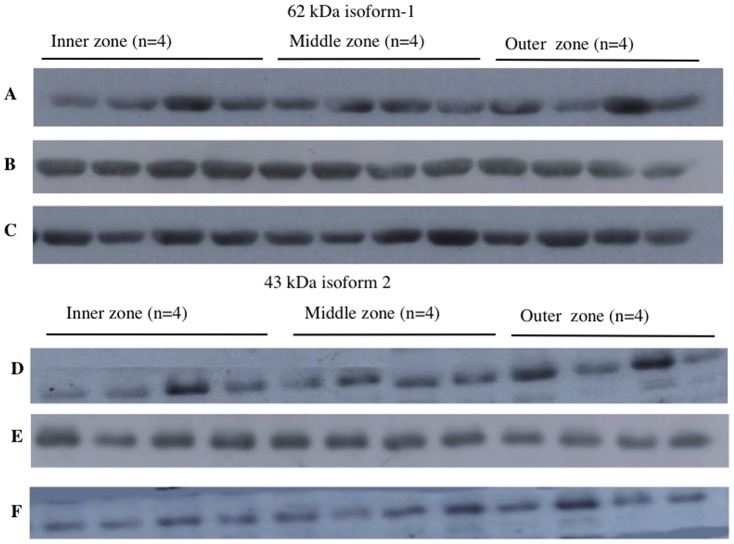
This experiment was designed to test if there was a spatial difference in expression of PON2 within individual placentas obtained from women who were not in labour. Two main bands were detected in all placenta samples, one at 62 kDa and one at 43 kDa. These are named as isoform 1 62 kDa and isoform 43 kDa throughout. Examples of Western blots showing PON2 (isoform 1, 62 kDa) expression for 3 different placentas (all non-labour) are shown in Figure 1 (blots A-C). Examples of Western blots showing PON2 (isoform 2, 43 kDa) expression for the same three placentas are also shown in Figure 1 (blots D–F). Friedman test analysis showed there was no difference in expression of either isoform between the three sites (inner, middle, outer) within individual placentas. The graphs for blots A–C (isoform 1, 62 kDa) are shown in Figure 2 (A–C) and the graphs for blots D–F (isoform 2, 43 kDa) are shown in Figure 2 (D–F).
Figure 1. Western blots showing PON2 expression in inner, middle and outer zones of three individual placentas (non-labour group).
Four quadrants were sampled in each zone. A, B and C show the 62Figure 1. D, E and F show the 43 kDa isoform 2 for the 3 placentas also shown in Figure 1.
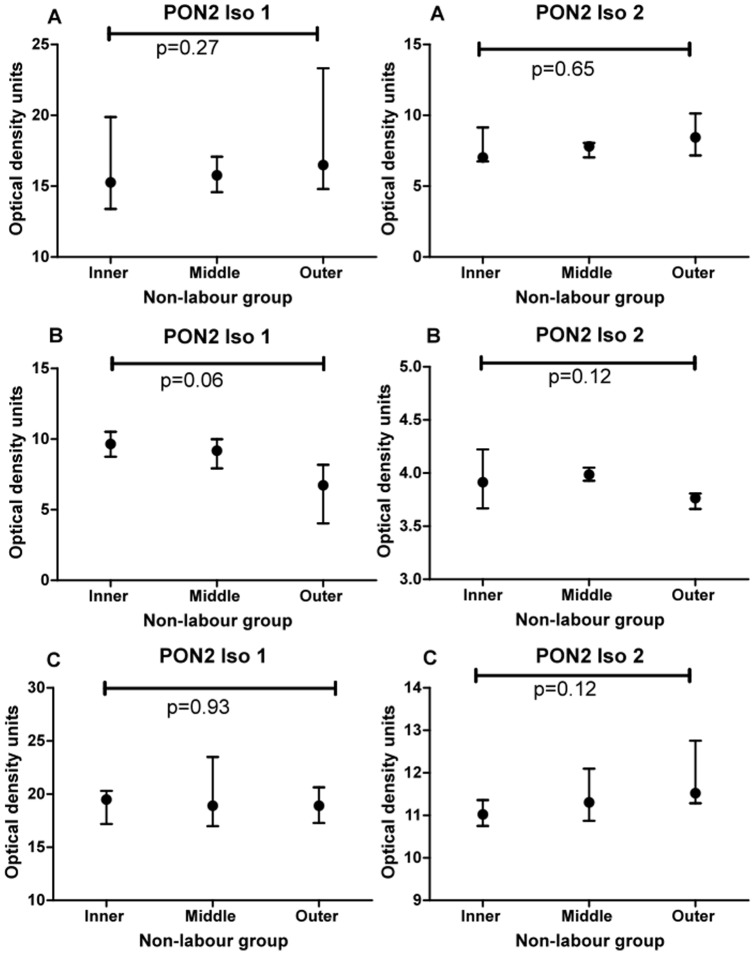
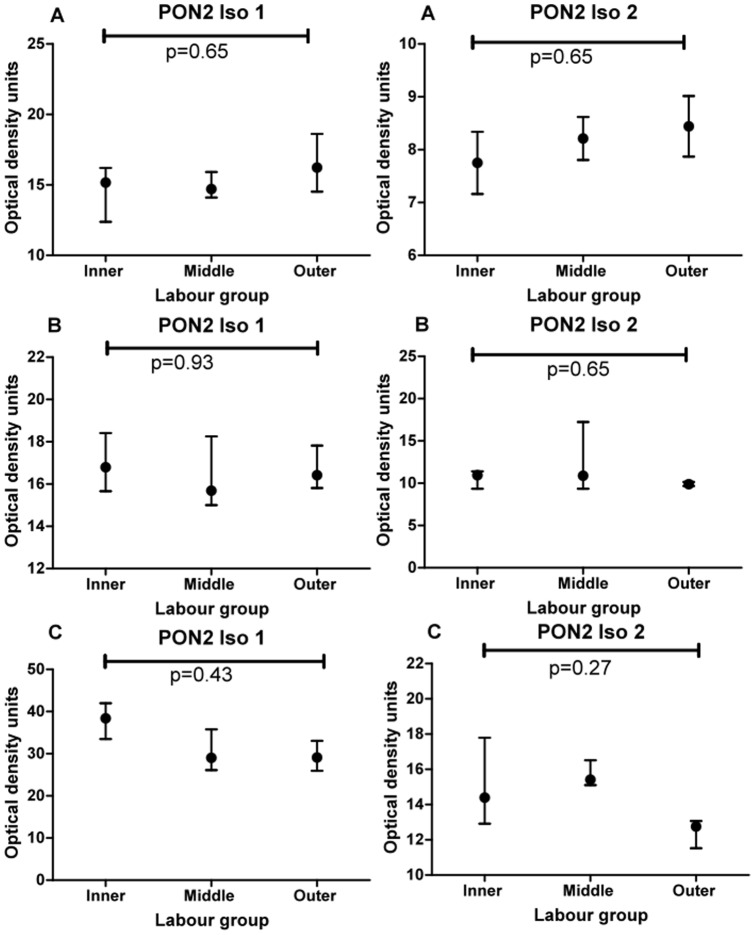
Figure 2. Graphs show median and interquartile range for PON2 isoforms 1 and 2 for the combined 4 quadrants sampled in the inner, middle and outer zones of three different placentas (A, B, C non-labour group) shown in Figure 1.
Comparison between zones was performed using Friedman analysis.
Experiment 2
This experiment was designed to test if there was a spatial difference in expression of PON2 within individual placentas obtained from women who were in labour. Two isoforms (62 kDa and 43 kDa) were also identified in all placenta samples. Examples of Western blots showing PON2 (isoform 1, 62 kDa) expression for 3 different placentas (labour) are shown in Figure 3 (blots A–C). Examples of Western blots showing PON2 (isoform 2, 43 kDa) expression for the same three placentas are also shown in Figure 3 (blots D–F). Friedman test analysis showed there was also no difference in expression of either isoform between the three sites (inner, middle, outer). The graphs for blots A–C (isoform 1, 62 kDa) are shown in Figure 4 (A–C) and the graphs for blots (isoform 2, 43 kDa) are shown in Figure 4 (D–F). In summary for experiment 1 and 2, there was no spatial difference in expression of either PON2 isoform within individual placentas both in non-labour and in labour groups.
Figure 3. Western blots showing PON2 expression in inner, middle and outer zones of three individual placentas (labour group).
Four quadrants were sampled in each zone. A, B and C show the 62 kDa isoform 1. D, E and F show the 43 kDa isoform 2.
Figure 4. Graphs show median and interquartile range for the 4 quadrants sampled in the inner, middle and outer zones of each of the three placentas (labour group) shown in Figure 3.
Comparison between zones was performed using Friedman analysis.
Experiment 3
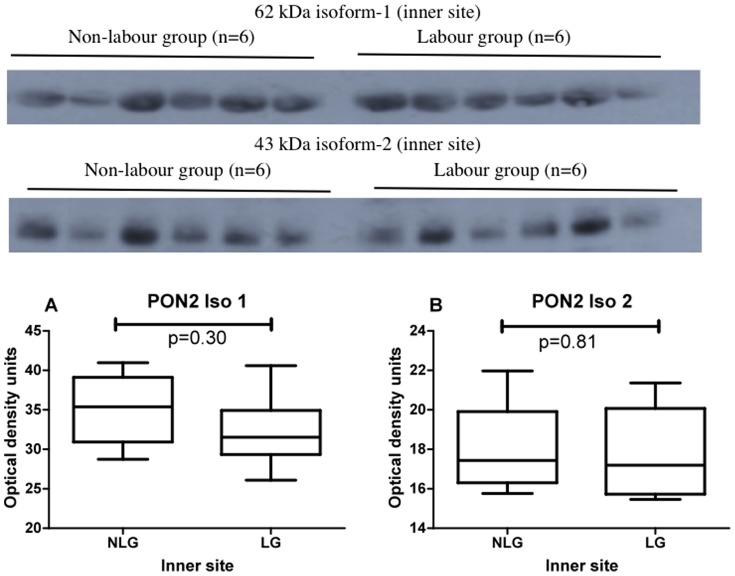
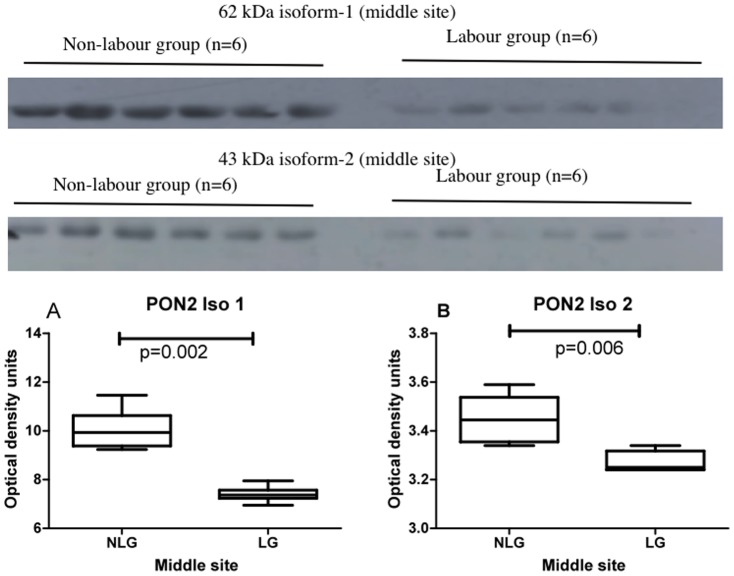
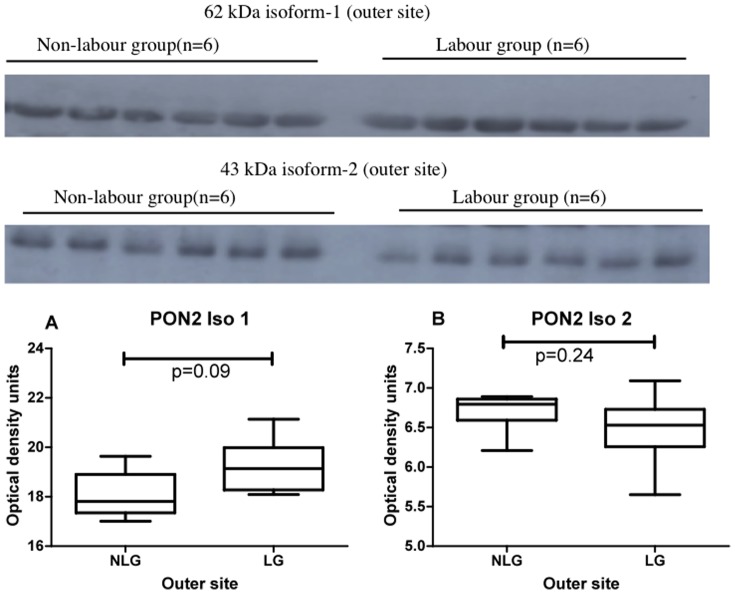
This experiment was designed to test if there was any difference in PON2 expression between labour and non-labour groups at the inner, middle or outer areas of placentas. Western blots showing placental PON2 expression in non-labour and labour at the inner site are shown in Figure 5 (upper panel 62 kDa isoform 1; lower panel 43 kDa isoform 2). The graphs and statistical analysis are shown below the blots. No differences were found. Western blots showing placental PON2 expression in non-labour and labour at the middle site are shown in Figure 6 (upper panel 62 kDa isoform 1; lower panel 43 kDa isoform 2). The graphs and statistical analysis are shown below the blots. There was a highly significant decrease in PON2 expression in the labour group when compared to the non-labour group for both the 62 kDa isoform 1, (p = 0.02) and the 43 kDa isoform 2, (p = 0.006). Western blots showing placental PON2 expression in non-labour and labour at the outer placental site are shown in Figure 7 (upper panel 62 kDa isoform 1; lower panel 43 kDa isoform 2). The graphs and statistical analysis are shown below the blots. No differences were found.
Figure 5. Western blots showing PON2 62(upper panel) and PON2 43 kDa isoform 2 (lower panel) expression in the inner placental site in non-labour and labour (n = 6 in each group).
The graphs show the box and whiskers analysis of the blots. Comparison between zones was performed using Friedman analysis. Graph A, 62; Graph B, 43 kDa isoform 2. NLG non-labour group, LG labour group.
Figure 6. Western blots showing PON2 62(upper panel) and PON2 43 kDa isoform 2 (lower panel) expression in the middle placental site in non-labour and labour (n = 6 in each group).
The graphs show the box and whiskers analysis of the blots. Comparison between zones was performed using Friedman analysis. Graph A, 62; Graph B, 43 kDa isoform 2. NLG non-labour group, LG labour group.
Figure 7. Western blots showing PON2 62(upper panel) and PON2 43 kDa isoform 2 (lower panel) expression in the outer site in non-labour and labour (n = 6 in each group).
The graphs show the box and whiskers analysis of the blots. Comparison between zones was performed using Friedman analysis. Graph A, 62; Graph B, 43 kDa isoform 2. NLG non-labour group, LG labour group.
Experiment 4 was designed to test if there were any differences in PON2 mRNA expression within individual placentas at different zones in either labour or non-labour. Figure 8 shows the PON2 RQ values in the inner, middle and outer zones for non labour (A) and labour (B). Just as for both PON2 protein isoforms, no spatial differences were found at the mRNA level in either labour or non-labour.
Figure 8. RQ values for mRNA measurements in inner, middle and outer placental sites within individual placentas.
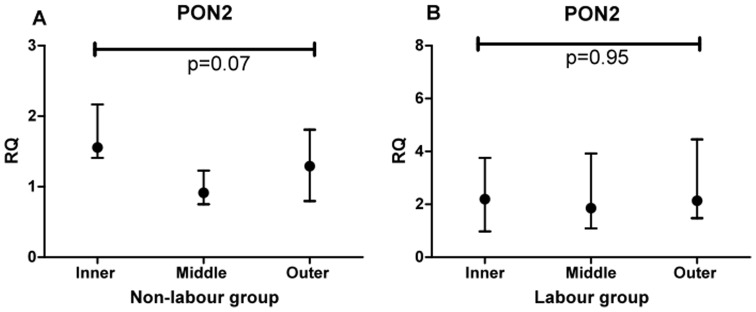
A non-labour and B labour. Comparison between zones was performed using Friedman analysis.
The final experiment 5 tested whether there was any difference between labour and non-labour at the inner, middle our outer sites at the mRNA level. The results are shown in Figure 9. As for PON2 protein no differences were found between labour and non-labour at the inner or outer placental sites. There was, paradoxically, an increase in PON2 mRNA in the labour group at the middle site.
Figure 9. RQ values for mRNA measurements in inner, middle and outer placental sites for labour compared with non-labour.
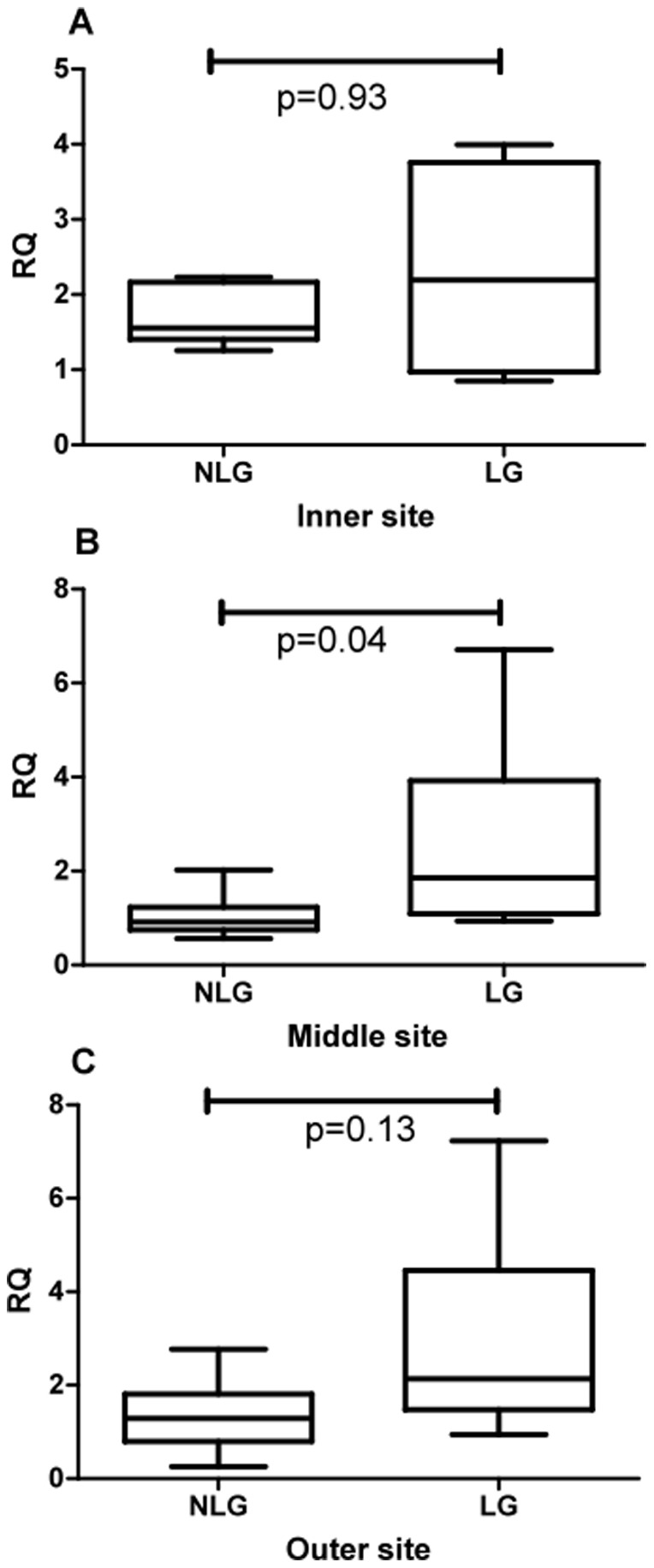
Comparison between zones was performed using Friedman analysis.
Discussion
PON2 and present findings
The PON family form dimers and trimers and can be glycosylated; this may, in part, explain the range of different molecular weights reported to date [12]. In the present study two main bands were detected with the PON2 antibody. The smaller band of around 43 kDa is the range reported for PON2. The larger band around 62 kDa has been described in liver lysates; reported in the PON2 antibody datasheet (http://www.novusbio.com/PON2-Antibody_NBP1-52005.html). This larger band was successfully blocked by incubation with the immunizing peptide showing it is a form of PON2. The reduced placental expression of PON2 protein expression found in labour in the middle site would be likely to promote inflammatory processes. Thus it is possible that a reduction in PON2 protein may help to initiate or promote labour but this is speculative. Why PON2 mRNA was found to be increased may seem paradoxical but it may be that the increased mRNA is a separate response to oxidative stress as a result of myometrial contractions. The reduction in PON2 protein could also be due to processes other than reducing mRNA synthesis, such as increased protein degradation. PONs are glycosylated with high-mannose-type sugars, which are important for protein stability but are not essential for their enzymatic activities [12]. Whether alterations in glycosylation of PON2 could affect PON2 expression in the placenta would require future research.
Link to oxidative stress and ischemia-reperfusion injury
Oxidative stress occurs when the production of reactive oxygen species overwhelms the intrinsic anti-oxidant defenses. At the moment it is not clear whether the observed reduction in PNO2 protein expression in the placenta in the labour group is a consequence of labour itself or, alternatively, contributed to labour via endocrine, hemodynamic or other processes. Since human patients have been used it is difficult to separate these effects. It is known however that contraction of the uterus leads to ischemic-reperfusion injury that can alter placental protein expression [13]. Furthermore Doppler ultrasound studies have demonstrated an inverse relationship between uterine artery resistance and the intensity of uterine contractions during labour [14]. It has been shown in chronically instrumented pregnant rhesus monkeys that placental blood flow is almost completely stopped during sustained myometrial contractions and that this occurs as a result of compression of the arcuate and spiral arteries [15], [16]. The closest human model to this was performed on patients prior to termination of pregnancy at 17–20 weeks of gestation [17]. During oxytocin-induced contractions, a 50% reduction in flow into the intervillous space, as well as a fall in entry sites and volume, was found compared to when no contractions occurred. This suggests that intermittent perfusion of the intervillous space would lead to an ischemic-reperfusion injury of the placenta. Reactive oxygen species and the oxidant/antioxidant balance can be affected as a result [17]. In keeping with this labour has been reported to be associated with placental alterations in several pathways linked to oxidative stress [18]–[24] Other studies looking at heat shock proteins, Mn-SOD, Cu/Zn-SOD and peroxidation of lipids also show an association between labour and placental oxidative stress [9]–[10], [13].
The biochemical events associated with labour involve increased interleukin-1β and prostaglandin synthesis [24]. The later stages of gestation are likely to be associated with more fluctuations in blood flow as demand by the placenta and fetus is maximal. COX-2 increases in mouse and rat placental trophoblast with gestation [25]–[26]. In vitro studies also suggest that hypoxia-reoxygenation increases COX-2 which may in turn play a role in augmentation or even initiation of labour [26]. Furthermore human placental trophoblast show activation of the NF-κB and COX-2 during labour. Increase in levels of cleaved caspase-3 and cleaved caspase-9 confirm evidence of placental apoptosis during labour [13]. As will be discussed below PON2 expression and activity can affect these biochemical events.
PONS
The paroxonases were named so because the substrate for PON1 is paroxon which is the active metabolite of the organophosphorus insecticide parathion. PON2 and 3 lack this esterase activity despite the similar nomenclature. PON1, 2 and 3 are lactonases and PON2 has the highest activity of the three PONs [5], [12], [27]–[29]. PON1 is mainly synthesized by the liver. It associates with high-density lipoprotein (HDL) in the circulation [30]. PON1 hydrolyses several substrates; these include organophosphate insecticides and nerve gases, lipid hydroperoxides, lactones and thiolactones [12], [31]–[33]. PON1 is a potent anti-atherosclerotic enzyme [34]–[38]. PON1 and PON3 proteins are present in plasma and reside in the high-density lipoprotein fraction and protect against oxidative stress by hydrolyzing certain oxidized lipids in lipoproteins, macrophages, and atherosclerotic lesions [37]–[40]. Paraoxonases are important detoxifying and anti-oxidative enzymes with roles being described in organophosphate poisoning, diabetes, obesity, cardiovascular diseases, innate immunity and with atherosclerosis [41]–[45]
PON2 and cell death
Endoplasmic reticulum (ER) stress activates the unfolded protein response (UPR) pathway and pro-apoptotic CHOP protein in the presence of overwhelming ER stress, [46]–[48]. Mitochondria also play a key role in cell death via production of excess reactive oxygen species (ROS) [49]. It has been shown that human PON2 diminished not only ROS but also ER stress-induced apoptosis in vascular cells [50]. PON2 is expressed in several tissues with antioxidant properties. It is capable of preventing cell-mediated oxidative modification of low density lipoprotein and ER stress-induced apoptosis. [7], [9], [51]. PON2 is not present in serum lipoprotein fractions but exists as an intracellular protein found in almost every tissue, particularly at the perinuclear region, ER and mitochondria. [7], [50], [52]. Natural substrates remain unknown albeit PON2, as part of the innate immune system, appears involved in the defense against infections by the human pathogen Pseudomonas aeruginosa [52]. Several studies demonstrated that PON2 protected macrophages, vascular and other cells against oxidative stress, whereas its downregulation reversed this effect [7], [50], [53].
PON2 has been shown to be overexpressed in several cancers and it has been suggested that this may be due to the fact that PON2 confers resistance to apoptosis as well as oxidative stress [54]. It has been shown that during ER stress, high levels of PON2 lowered redox-triggered induction of pro-apoptotic CHOP particularly via the JNK pathway, which prevented mitochondrial cell death signaling [54]. Apart from CHOP, PON2 also diminished intrinsic apoptosis as it prevented mitochondrial superoxide formation, cardiolipin peroxidation, cytochrome c release, and caspase activation. Oxidized lipids can also induce proinflammatory genes, such as TNF-α and MCP-1, via NF-κB activation [55]. Therefore one possibility is that less placental PON2 would result in more oxidized lipids and more NF-κB activation which would promote labour. Macrophages harvested from PON2-/- mice are more susceptible to cellular oxidative stress than wild-type macrophages [56]. PON2 also inhibits the development of atherosclerosis in mice, via mechanisms involving the reduction of oxidative stress [30], [45], [57]. One study investigated the distribution of PON1 and 2 mRNA in 24 human tissues, using gene expression panels. PON2 but not PON1 was identified in placenta [58].
The mechanism by which PON2 modulates ROS production is still unclear [59]–[62]. Lactones have been suggested to be the natural substrates of PON2 and PON2 lactonase activity has been shown to correlate with this enzyme's biological antioxidant properties.
Genetics studies
Studies have shown increased risk of coronary artery disease, carotid atherosclerosis and stroke in patients with low paraoxonase activity PON1 and 2 alleles [63]. Specific gene polymorphisms for PON1 and PON2 have been reported in children born pre-term. [63]. There is a lack of data in this new field. However term labour results from a physiological reduction in the influence of endocrine signals and other factors that act to inhibit myometrial contractility, in conjunction with the activation of pro-inflammatory biochemical pathways that precede myometrial activation and contraction. Premature activation of inflammatory mediators is a major feature of the pathophysiology of preterm labour and in particularly very preterm labour where it is often induced by infection [3]. Whether PON2 may be involved in pre-term labour is worthy of investigation.
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. All data are included in the manuscript.
Funding Statement
This work was supported by a scholarship to S. Alwarfaly from the Funded by the Libyan Cultural Affairs- administered By University of Glasgow. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Petraglia F, Imperatore A, Challis JR (2010) Neuroendocrine mechanisms in pregnancy and parturition. Endocrin Rev 31: 783–816. [DOI] [PubMed] [Google Scholar]
- 2. Challis JRG, Mathews SG, Gibb W, Lye SJ (2000) Endocrine and Paracrine Regulation of Birth at Term and Preterm. Endocrin Rev 21: 514–550. [DOI] [PubMed] [Google Scholar]
- 3.MacIntyre DA, Sykes L, Teoh TG, Bennett PR (2012) Prevention of preterm labour via the modulation of inflammatory pathways. J Mat Fetal Neonatal Med Suppl 1:17–20. [DOI] [PubMed]
- 4.Keelan JA, Blumenstein M, Helliwell RJ, Sato TA, Marvin KW, et al.. (2003) Cytokines, prostaglandins and parturition—a review. Placenta Suppl A:S33–46. [DOI] [PubMed]
- 5. Primo-Parma SL, Sorenson RC, Teiber J, La Du BN (1996) The human serum paraoxonase/arylesterase gene (PON1) is one member of a multigene family. Genomics 33: 498–509. [DOI] [PubMed] [Google Scholar]
- 6. Draganov DI, La Du BN (2004) Pharmacogenetics of paraoxonase: a brief review. Naunyn Schmiedebergs Arch Pharmacol 369: 78–88. [DOI] [PubMed] [Google Scholar]
- 7. Ng CJ, Wadleigh DJ, Gangopadhyay A, Hama S, Grijalva V, et al. (2001) Paraoxonase-2 is a ubiquitously expressed protein with antioxidant properties and is capable of preventing cell-mediated oxidative modification of low density lipoprotein. J Biol Chem 276: 4444–4449. [DOI] [PubMed] [Google Scholar]
- 8. Martinelli1 N, Consoli L, Girelli D, Grison E, Corrocher R, et al (2012) Paraoxonases: Ancient Substrate Hunters and Their Evolving Role in Ischemic Heart Disease. Adv Clin Chem 59: 65–100. [DOI] [PubMed] [Google Scholar]
- 9. Abdulsid A, Fletcher A, Lyall F (2013) Heat Shock Protein 27 Is Spatially Distributed in the Human Placenta and Decreased during Labor. PLoS ONE 8(8): e71127. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 10. Abdulsid A, Lyall F (2014) Heat shock protein 27 expression is spatially distributed in human placenta and selectively regulated during preeclampsia. J Reprod Immunol 101: 89–95. [DOI] [PubMed] [Google Scholar]
- 11. Abdulsid A, Hanretty K, Lyall F (2013) Heat shock protein 70 expression is spatially distributed in human placenta and selectively upregulated during labor and preeclampsia. PLoS One 2013; 8(1): e54540. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 12. Draganov DI, Teiber JF, Speelman A, Osawa Y, Sunahara R, et al. (2005) Human paraoxonases (PON1, PON2, and PON3) are lactonases with overlapping and distinct substrate specificities. J Lipid Res 46: 1239–1247. [DOI] [PubMed] [Google Scholar]
- 13. Cindrova-Davies T, Yung H-W, Johns J, Spasic-Boskovic O, Korolchuk S, et al. (2007) Oxidative stress, gene expression, and protein changes induced in the human placenta during labour. Am J Pathol 171: 1168–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Brar HS, Platt LD, DeVore GR, Horenstein J, Medearis AL (1988) Qualitative assessment of maternal uterine and fetal umbilical artery blood flow and resistance in laboring patients by Doppler velocimetry. Am J Obstet Gynecol 158: 952–956. [DOI] [PubMed] [Google Scholar]
- 15.Ramsey E, Donner M (1980). Placental vasculature and circulation. Anatomy, Physiology, Radiology, Clinical Aspects Atlas and Textbook. Stuttgart: Georg Thieme Publishers Stuttgart).
- 16. Borell U, Fernstroem I, Ohlson L, Wiqvist N (1965) Influence of uterine contractions on the uteroplacental blood flow at term. Am J Obstet Gynecol 93: 44–57. [DOI] [PubMed] [Google Scholar]
- 17. McCord JM (1993) Human disease, free radicals, and the oxidant/antioxidant balance Clin Biochem. 26: 351–357. [DOI] [PubMed] [Google Scholar]
- 18. Many A, Roberts JM (1997) Increased xanthine oxidase during labour—implications for oxidative stress. Placenta 199718: 725–726. [DOI] [PubMed] [Google Scholar]
- 19. Lee KJ, Shim SH, Kang KM, Kang JH, Park DY, et al. (2010) Global gene expression changes induced in the human placenta during labor. Placenta 31: 698–704. [DOI] [PubMed] [Google Scholar]
- 20. Peng H-H, Kao C-C, Chang S-D, Chao A-S, Chag Y-L (2011) The effects of labor on differential gene expression in parturient women. Kaohsiung J Med Sci 27: 494–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sitras V, Paulssen H, Grønaas H, Vårtun A, Acharya G (2008) Gene expression profile in labouring and non-labouring human placenta near term Mol Hum Rep. 14: 61–65. [DOI] [PubMed] [Google Scholar]
- 22. Elliott CL, Kelly RW, Critchley HOD, Rilet SC, Calder AA (1998) Regulation of interleukin 8 production in the term human placenta during labor and by antigestagens. Am J Obs Gyn 179: 215–20. [DOI] [PubMed] [Google Scholar]
- 23. Lim R, Riley C, Barker G, Rice GE, Lappas M (2012) Human labour is associated with decreased cytoplasmic FoxO4. Placenta 33: 52–59. [DOI] [PubMed] [Google Scholar]
- 24. Allport VC, Pieber D, Slater DM, Newton R, White JO, et al. (2001) Human labour is associated with nuclear factor-κB activity which mediates cyclo-oxygenase-2 expression and is involved with the 'functional progesterone withdrawal. Mol. Hum. Reprod 7: 581–586. [DOI] [PubMed] [Google Scholar]
- 25. Xu Y, Knipp GT, Cook TJ (2005) Expression of cyclooxygenase isoforms in developing rat placenta, human term placenta, and BeWo humantrophoblast model. Mol Pharm 2: 481–490. [DOI] [PubMed] [Google Scholar]
- 26. Burdon C, Mann C, Cindrova-Davies T, Ferguson-Smith AC, Burton GJ (2007) Oxidative Stress and the Induction of Cyclooxygenase Enzymes and Apoptosis in the Murine Placenta. Placenta 28: 724–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Stoltz DA, Ozer EA, Ng CJ, Yu JM, Reddy ST, et al. (2007) Paraoxonase-2 deficiency enhances Psudomonas aeruginosa quorum sensing in murine tracheal epithelia. Am J Physiol Lung Cell Mol Physiol 292: L852–L860. [DOI] [PubMed] [Google Scholar]
- 28. Teiber JF, Horke S, Haines DC, Chowdhary PK, Xiao J, et al. (2008) Dominant role of paraoxonases in inactivation of the Pseudomonas aeruginosa quorum-sensing signal N-(3-oxododecanoyl)-L- homoserine lactone. Infect. Immun 76: 2512–2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Horke S, Witte I, Wilgenbus P, Altenhofer S, Krueger MLiH, et al. (2010) Protective effect of paraoxonase-2 against ER stress-induced apoptosis is lost upon disturbance of calcium-homeostasis. Biochem J 426: 73–83. [DOI] [PubMed] [Google Scholar]
- 30.Deakin S, Leviev I, Gomaraschi M, Calabresi L, Franceschini G, et al. (2002) Enzymatically active paraoxonase 1 is located at the external membrane of producing cells and released by a high affinity, saturable, desorption mechanism. J Biol Chem 277: ; 4301–4308. [DOI] [PubMed] [Google Scholar]
- 31.Furlong CE (2008) Paraoxonase: an historical perspective. In The Paraoxonases: Their Role in Disease Development and Xenobiotics Metabolism. (Mackness, B., Mackness, M., Aviram, M., Paragh, G., eds.). pp. 3–32, Springer, Dordrect.
- 32.Mackness MI, Arrol S, Durrington PN (1991) Paraoxonase prevents accumulation of lipoperoxides in low-density lipoprotein. FEBS Lett 286: ; 152–154. [DOI] [PubMed] [Google Scholar]
- 33.Jakubowski H (2000) Calcium-dependent human serum homocysteine thiolactone hydrolase. J Biol Chem 275: ; 3957–3962. [DOI] [PubMed] [Google Scholar]
- 34.Mackness MI, Arrol S, Abbott CA, Durrington PN (1993) Protection of low-density lipoprotein against oxidative modification by high-density lipoprotein associated paraoxonase. Atherosclerosis 104: ; 129–135. [DOI] [PubMed] [Google Scholar]
- 35.Aviram M, Hardak E, Vaya J, Mahmood S, Milo S, et al. (2000) Human serum paraoxonase (PON1) Q and R selectively decrease lipid peroxides in human coronary and carotid atherosclerotic lesions: PON1 esterase and peroxidase-like activities. Circulation 101: ; 2510–2517. [DOI] [PubMed] [Google Scholar]
- 36.Watson AD, Berliner JA. Hama SYLa, Du BN, Fault KF, et al. (1995) Protective effect of high density lipoprotein associated paraoxonase—inhibition of the biological activity of minimally oxidised low-density lipoprotein. J Clin Invest 96: ; 2882–2891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sierksma A, van der Gaag MS, van Tol A, James RW, Hendriks HF. Kinetics of HDL cholesterol and paraoxonase activity in moderate alcohol consumers. Alcohol Clin Exp Res 26: ; 1430–1435. [DOI] [PubMed] [Google Scholar]
- 38.Jaouad L, de Guise C, Berrougui H, Cloutier M, Isabelle M, et al. (2006) Age-related decrease in high-density lipoproteins antioxidant activity is due to an alteration in the PON1's free sulfhydryl groups. Atherosclerosis 185: ; 91–200. [DOI] [PubMed] [Google Scholar]
- 39.Mackness B, Hunt R, Durrington PN, Mackness MI (1997) Increased immunolocalisation of paraoxonase, clusterin and apolipoprotein AI in the human artery wall with progression of atherosclerosis. Arterioscler Thromb Vasc Biol 17: ; 1233–1238. [DOI] [PubMed] [Google Scholar]
- 40.Reddy ST, Wadleigh DJ, Grijalva V, Ng C, Hama S, et al. (2001) Human paraoxonase-3 is an HDL-associated enzyme with biological activity similar to paraoxonase-1 protein but is not regulated by oxidised lipids. Arterioscler Thromb Vasc Biol 21: ; 542–547. [DOI] [PubMed] [Google Scholar]
- 41.Camps J, Marsillach J, Joven J (2009) The paraoxonases: role in human diseases and methodological difficulties in measurement. Crit Rev Clin Lab Sci 46: ; 83–106. [DOI] [PubMed] [Google Scholar]
- 42.Shih DM, Lusis AJ (2009) The roles of PON1 and PON2 in cardiovascular disease and innate immunity. Curr Opin Lipidol 20: ; 288–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shih DM, Xia YR, Wang XP, Wang SS, Bourquard N, et al. (2007) Decreased obesity and atherosclerosis in human paraoxonase 3 transgenic mice. Circ Res 100: ; 1200–1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shih DM, Gu L, Xia YR, Navab M, Li WF, et al. (1998) Mice lacking serum paraoxonase are susceptible to organophosphate toxicity and atherosclerosis. Nature 394: ; 284–287. [DOI] [PubMed] [Google Scholar]
- 45.Ng CJ, Bourquard N, Grijalva V, Hama S, Shih DM, et al. (2006) Paraoxonase-2 deficiency aggravates atherosclerosis in mice despite lower apolipoprotein-B-containing lipoproteins: anti-atherogenic role for paraoxonase-2. J Biol Chem 281: ; 29491–29500. [DOI] [PubMed] [Google Scholar]
- 46.Zhang K, Kaufman RJ (2006) The unfolded protein response: a stress signaling pathway critical for health and disease. Neurology 66 (2 Suppl 1); S102–S109. | [DOI] [PubMed] [Google Scholar]
- 47.Oyadomari S, Mori M (2004) Roles of CHOP/GADD153 in endoplasmic reticulum stress. Cell Death Differ 11: ; 381–389. [DOI] [PubMed] [Google Scholar]
- 48.Song B, Scheuner D, Ron D, Pennathur S, Kaufman RJ (2008) Chop deletion reduces oxidative stress, improves beta cell function, and promotes cell survival in multiple mouse models of diabetes. J Clin Invest 118: ; 378–3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ott, M Gogvadze V, Orrenius S, Zhivotovsky B (2007) Mitochondria, oxidative stress and cell death Apoptosis 5: ; 913–922. [DOI] [PubMed] [Google Scholar]
- 50.Horke S, Witte I, Wilgenbus P, Kruger M, Strand D, et al. (2007) Paraoxonase-2 reduces oxidative stress in vascular cells and decreases endoplasmic reticulum stress-induced caspase activation. Circ 115: ; 2055–2064. [DOI] [PubMed] [Google Scholar]
- 51.Horke S, Witte I, Altenhoffer S, Wilgenbus P, Goldeck M, et al. (2010) Paraoxonase–2 is down regulated by the Pseudomonas aeruginosa quorum-sensing signal N-(3-oxododecanyl)-L-homoserine lactone and attenuates oxidative stress induced by pyocyanin. Biochem J 42: ; 73–83. [DOI] [PubMed] [Google Scholar]
- 52.Rothem L, Hartman C, Dahan A, Lachter J, Eliakim R, et al. (2007). Paraoxonases are associated with intestinal inflammatory diseases and intracellularly localized to the endoplasmic reticulum. Free Radic Biol Med 43: ; 730–739. [DOI] [PubMed] [Google Scholar]
- 53.Aviram M, Rosenblat M (2004) Paraoxonases 1,2, and 3, oxidative stress, and macrophage foam cell formation during atherosclerosis development. Free Radic Biol Med 37: ; 1304–1316. [DOI] [PubMed] [Google Scholar]
- 54. Witte I, Altenhöfer S, Wilgenbus P, Amort J, Clement AM, et al. (2011) Beyond reduction of atherosclerosis: PON2 provides apoptosis resistance and stabilizes tumor cells. Cell Death and Disease 2: e112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Dwarakanath RS, Sahar S, Reddy MA, Castanotto D, Rossi JJ, et al. (2004) Regulation of monocyte chemoattractant protein-1 by the oxidized lipid, 13-hydroperoxyoctadecadienoic acid, in vascular smooth muscle cells via nuclear factor-κB (NF-κB). J Mol Cell Cardiol 36: 585–595. [DOI] [PubMed] [Google Scholar]
- 56.Fuhrman B, Khateeb J, Shiner M, Nitzan O, Karry R, et al. (2008) Urokinase plasminogen activator upregulates paraoxonase 2 expression in macrophages via an NADPH oxidase-dependent mechanism. Arterioscler Thromb Vasc Biol 28: ; 1361–1367. [DOI] [PubMed] [Google Scholar]
- 57. Shiner M, Fuhrman B, Aviram M (2004) Paraoxonase 2 (PON2) expression is upregulated via a reduced-nicotinamide-adenine-dinucleotide-phosphate (NADPH)-oxidase-dependent mechanism during monocytes differentiation into macrophages. Free Radic Biol Med 37: 2052–2063. [DOI] [PubMed] [Google Scholar]
- 58.Mackness B, Beltran-Debon R, Aragones G, Joven J, Camps J, et al. (2010) Human tissue distribution of paraoxonases 1 and 2 mRNA. IUBMB Life 62: ; 480–482. [DOI] [PubMed] [Google Scholar]
- 59.Yang Y, Zhang Y, Cuevas S, Villar VA, Escano C, et al. , (2012) Paraoxonase 2 decreases renal reactive oxygen species production, lowers blood pressure, and mediates dopamine D2 receptor-induced inhibition of NADPH oxidase. Free Radic Biol Med 53: ; 437–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Meilin E, Aviram M, Hayek T (2010) Paraoxonase 2 (PON2) decreases high glucose-induced macrophage triglycerides (TG) accumulation, via inhibition of NADPH-oxidase and DGATI activity: studies in PON2-deficient mice. Atherosclerosis. 208: ; 390–395. [DOI] [PubMed] [Google Scholar]
- 61.Devarajan A, Bourquard N, Hama S, Navab M, Grijalva VR, et al. (2011) Paraoxonase 2 deficiency alters mitochondrial function and exacerbates the development of atherosclerosis. Antioxid Redox Signal 14: ; 341–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Altenhöfer S, Witte I, Teiber JF, Wilgenbus P, Pautz A, et al. (2010) One enzyme, two functions: PON2 prevents mitochondrial superoxide formation and apoptosis independent from its lactonase activity. J Biol Chem 285: ; 24398–24403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chen D, Hu Y, Chen C, Yang F, Fang Z, et al. (2004) Polymorphisms of the paraoxonase gene and risk of preterm delivery. Epidemiology 215: ; 466–470 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. All data are included in the manuscript.