Abstract
Adolescence is a peak period for the onset of depression, and it is also a time marked by substantial stress as well as neural development within the brain reward circuitry. In the current review, we provide a selective overview of current animal and human research investigating the relationship among reward processes, stress, and depression. Three separate, but related, etiological models examine the differential roles that stress may play with regard to reward dysfunction and adolescent depression. First, the reward mediation model suggests that acute and chronic stress contribute to reward deficits, which in turn, potentiate depressive symptoms and/or increase the risk for depression. Second, in line with the stress generation perspective, it is plausible that premorbid reward-related dysfunction generates stress, in particular interpersonal stress, which then leads to the manifestation of depressive symptoms. Last, consistent with a diathesis-stress model, the interaction between stress and premorbid reward dysfunction may contribute to the onset of depression. Given the equifinal nature of depression, these models could shed important light on different etiological pathways during adolescence, particularly as they may relate to understanding the heterogeneity of depression. To highlight the translational potential of these insights, a hypothetical case study is provided as means of demonstrating the importance of targeting reward dysfunction in both assessment and treatment of adolescent depression.
Keywords: Major Depressive Disorder, Reward Circuitry, Stress Exposure, Stress Generation, Mesolimbic Pathway, Anterior Cingulate Cortex, Prefrontal Cortex
Adolescent depression is a major public health concern and is associated with significant emotional and socioeconomic burden.1,2 The point prevalence of major depressive disorder (MDD) among adolescents is estimated between 3–8%.3 Moreover, 40% of depressed adolescents experience a recurrent episode within 2 years of their initial diagnosis, and 70% will have a recurrence within 5 years.3,4 Despite these alarming epidemiological data, the etiological and pathophysiological mechanisms contributing to adolescent MDD remain unclear.
A preponderance of research has found that stress is a robust predictor of the onset, maintenance, and severity of MDD.5 For example, in community samples of adolescent, adult, and elderly populations, approximately 80% of depressive episodes were preceded by stressful life events.6–8 In addition, antecedent chronic stressors have been linked to poorer prognosis, more frequent relapse, and higher depressive symptoms.9–12 Notably, for adolescents in particular, life stress plays a central role in MDD onset.13,14 Adolescents experience a greater frequency of interpersonal stressors relative to younger and older individuals,15 which may stem from a greater investment in peer relationships coupled with increased autonomy from parents. At the same time, deficient peer and parental relationships have been shown to generate relational stressors and subsequent depressive symptoms among adolescents, underscoring the need to examine interpersonal stressors as a pathway to adolescent depression.16
In addition to being a time of substantial interpersonal stress, adolescence is also a critical period of neurobiological growth within the brain reward circuitry (i.e., mesocorticolimbic regions).17–20 During adolescence, the brain undergoes structural changes in gray and white matter subcomponents as well as subcortical regions, particularly in the basal ganglia.21,22 Consistent with this notion, imaging studies have implicated key developmental differences in neurobiological regions critical for reward processing, namely the prefrontal and the mesolimbic cortex.23,24 In this context, Casey and colleagues (2008) posit that prefrontal regions are less developed in comparison to the limbic systems in adolescent, and furthermore, that such discordant development may explain the critical role of reward circuitry in adolescent MDD, as the adolescent brain is learning and engaging reward with “more developed equipment” (i.e., mesolimbic system), while the “tools” to modulate reward responsiveness are not mature (i.e., prefrontal cortex).25 Alternatively, Davey and colleagues suggest that prefrontal cortex development during adolescence increases the pursuit of reward (e.g., romantic relationships, status); however, failure to obtain these more complex goals ultimately suppresses the reward system and increases the likelihood of MDD.18 Irrespective of the specific anatomical mechanisms, these theories suggest that the development of the reward circuitry plays a prominent role in the occurrence of depressive disorders, which is consistent with promising preliminary data indicating dysfunctional neural response to reward feedback as a predictor of adolescent depression.26
As a whole, research suggests that stress and reward deficits (as well as the associated neural dysfunction) each contribute to the occurrence of adolescent MDD. However, given the temporal overlap of stress and neural development during adolescence, the interplay of these processes may confer the greatest vulnerability for the onset of MDD. Indeed, researchers have begun to disentangle the relationship between stress and reward processing as a means of better understanding adolescent MDD. In the current review, we provide a selective overview of current animal and human research, which was obtained by conducting a PubMed search using keywords including adolescent depression, reward processing, adolescent stress, brain reward circuitry. Three separate, but related, etiological models examine the differential roles that stress may play with regard to reward dysfunction and adolescent MDD. While the models are presented separately for the purpose of more clearly delineating the extant research, it bears mentioning that that the models are, in fact, interrelated. First, a large corpus of research has found that both acute and chronic stress contributes to reward dysfunction, and these deficits, may then lead to the onset of MDD (see Figure 1A). Support for this reward mediation model has been found in animal models,27 adults,28,29 and to a lesser extent, adolescents.30 Second, transactional models of MDD posit that individuals possess certain characteristics or engage in a specific pattern of behaviors that lead to the occurrence of stressors.31 Specifically, adolescents characterized by pre-existing reward dysfunction may have a tendency to withdraw from peers or not attend to important social cues, and these deficits may in turn generate interpersonal or relational stressors (stress generation model). Over time, these accumulated stressors may lead to the onset of MDD (see Figure 1B). To date, research has explored cognitive32 and interpersonal16,33 predictors of adolescent stress generation; however, research has not examined the role that reward dysfunction exerts on the stress generation process. In this context, interesting findings have emerged examining the role of peer evaluation, which may shed important light on the role of reward dysfunction in the context of stress generation. Last, stress exposure models suggest that the interaction between premorbid vulnerability factors and stress leads to the development of MDD. Such a perspective is consistent with diathesis-stress models of MDD such as Beck’s cognitive theory of depression.34 Consistent with these etiological perspectives, we propose a titration model whereby the degree of vulnerability may be contingent on the magnitude of the stress as well as degree of reward dysfunction. Vulnerability may be operationalized in such a way that, for example, the greater reward dysfunction an individual possesses, the fewer stressful life events may be needed for depression to emerge. Conversely, less reward dysfunction may necessitate greater stress for depressive symptoms to arise. Within this diathesis-stress perspective, reward dysfunction alone may not predict depression, but rather, it is the interaction between reward deficits and stress, which may contribute to the occurrence of depression (see Figure 1C). Given the equifinal nature of MDD, these models may shed important light on different etiological pathways culminating in depression during adolescence, particularly as they may relate to understanding the heterogeneity of MDD. Over time, such insight may be used to develop more effective prevention, intervention, and treatment programs. Thus, in the final section of the current review paper, a hypothetical case study is provided as means of demonstrating the importance of targeting reward dysfunction in both assessment and treatment of adolescent depression.
Figure 1.
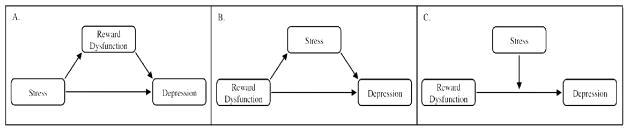
Examining the Relationship among Stress, Reward Dysfunction, and Depression
Note. (A) Reward Mediation Model; (B) Stress Generation Model, (C) Titration Model
Reward Dysfunction Model: The Impact of Stress on Reward Processes
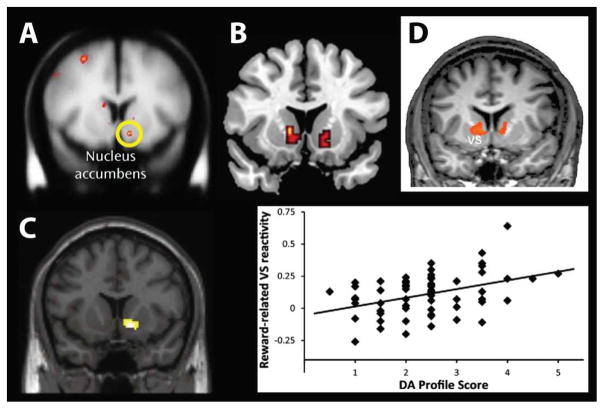
In comparison to stress reactivity (i.e., stress generation) and stress exposure (i.e., diathesis-stress perspective) models, the impact of stress on reward dysfunction and subsequent depression has provided the most consistent findings across animal, adolescent, and adult research (see Figure 1A). These studies have utilized a variety of approaches to examine the impact of acute and chronic stress on reward-related neurotransmitters – particularly dopamine (DA) – especially as it relates to the associated impact of DA signaling within the reward brain circuitry (e.g., nucleus accumbens (Nacc), orbitofrontal cortex (OFC), ventral tegmental area (VTA)). Research with animals has provided an ideal setting to examine the impact of stress on reward functioning, and these studies have clearly demonstrated that stress negatively impacts reward processes. For example, chronically stressed animals show diminished appetitive behaviors, reduced DA release in the Nacc in response to palatable food, and reduced DA transporter binding (a possible compensatory down-regulation stemming from reduced DA signaling) in the Nacc.35–40 Further, stress-induced reduction in dopaminergic output from the Nacc has been associated with coping failures and maintenance of depression-like behaviors such as helplessness.27 Importantly, pretreatment DA agonists are found to prevent the stress-induced reward processing deficits,41 while antidepressant medication reverse these deficits.39 These animal findings are intriguing in light of human neuroimaging evidence highlighting blunted Nacc activation to rewards in both adolescents42 and adults43 with MDD (see Figure 2A).
Figure 2.
Exemplary findings implicating ventral striatal regions (particularly the nucleus accumbens) in the pathophysiology of major depression. (A) Relative to healthy controls, unmedicated MDD subjects show reduced ventral striatal activation to reward feedback (Pizzagalli et al., 2009; Reprinted with permission from The American Journal of Psychiatry, (Copyright ©2009). American Psychiatric Association.); (B) Among Israeli soldiers, combat exposure is associated with reduced ventral striatal activation to reward relative to before combat exposure (Admon et al., 2013); (C) Among healthy controls, a genetic score for DA signaling based on five different polymorphic loci predicted 10.9% of the variance in ventral striatal reactivity during a gambling task (Nikolova et al., 2011); and (D) Among individuals with MDD, neurofeedback targeting the ventromedial PFC was associated with secondary increases in ventral striatal activity to positive stimuli (Linden et al., 2012). All figures reproduced with permission from each publisher and corresponding author.
Animal studies have also demonstrated the deleterious effects of stress on reward function during developmentally sensitive periods. Specifically, chronic stress occurring early in development has been found to lead to long term dysfunction in the mesolimbic dopaminergic pathway, contributing to depressive-like behavior including reduced motivation to obtain rewards, reduced social motivation, and blunted acquisition and expression of Pavlovian appetitive conditioning.44–50 Such early stress, however, does not affect other developmental processes such as eye opening and body weight, suggesting that reward processing might be especially sensitive to early adversity.45 As a whole, animal studies have demonstrated behavioral and physiological markers of stress-induced reward dysfunction that mirror abnormalities seen in depressed individuals, which are especially pronounced in response to early stress. Thus, when stress occurs during developmentally sensitive periods, it may greatly impair reward processing and increase susceptibility to MDD.
The first evidence in humans linking stress exposure with reduction in reward responsivity was derived from samples of U.S. Army cadets and college students as they reported experiencing less pleasure following stressful events (field training exercises and final examinations, respectively) compared to a control situation (i.e., a non-stress period).51 Notably, the same study also found that the deleterious effect of stress on hedonic capacity was particularly strong for subjects with family histories of MDD.51 Expanding on these earlier findings, healthy adults exposed to both acute (e.g., threat of shock) and more prolonged (e.g., final examinations in high-school students) stress demonstrated a reduced ability to modulate behavior as a function of past reward, and such deficits correlated to impaired functioning in the prefrontal cortex.28,52,53 Similar to the animal literature, the harmful impact of stress seems specific to reward processing, as acute stress in the form of threat of shock did not impair responses to aversive fearful faces54 or the ability to learn from punishments.55
While comparatively fewer studies have examined the effect of stress on reward dysfunction in adolescence, child maltreatment and experimental manipulations in healthy youth may provide an interesting lens through which to examine this relationship. Echoing preclinical findings showing that exposure to early adversities can have deleterious long-term consequences on adult’s reward responsiveness, Pechtel and Pizzagalli recently reported that women with a history of childhood sexual abuse were characterized by deficits in their ability to use previously rewarded – but not punished – information to guide decision making.56 Along similar lines, relative to healthy individuals, adults with a history of childhood maltreatment rated rewarding cues as less positive and displayed diminished Nacc response during anticipation of rewards but not punishments.57,58 Finally, in healthy adolescents, a computer-based ball-tossing game was utilized to probe the impact of social exclusion as a stressor.59 The results indicated that greater activity in the subgenual anterior cingulate cortex (ACC) was correlated with greater distress during social exclusion. Moreover, activity within the Nacc was associated with less distress, and in fact, Nacc activation was found to modulate subgenual ACC activity as well as individuals’ level of social rejection sensitivity.59
Although these findings provide evidence that ongoing or early life stress is associated with decreased reward responsiveness, poor ecological validity (i.e., threat of shock manipulations within a lab setting versus early life adversity), and retrospective assessments of early life stress represent important limitations of these initial studies. To address these limitations, Admon and colleagues recently assessed healthy 18 year-old soldiers prior to deployment and prospectively followed them for 18 months following active duty in high conflict areas. Results indicated that following military stress there is a reduction in Nacc response to reward (see Figure 2B), and furthermore, such stress-induced reward responsiveness deficits are associated with higher levels of depressive symptoms following stress.29 Similar to animal research, these data suggest that stress potentiates reward dysfunction and may increase susceptibility to depressive episodes, especially if stress occurs during developmentally sensitive periods. At the same time, while these findings have shed important light on the abnormal developmental trajectory, further research is warranted to better understand the pathways in which stress can induce reward dysfunction among children and adolescents.
A Stress Generation Perspective: The Impact of Reward Dysfunction on Stress Onset
A large corpus of research has examined the stress generation effect, which seeks to disentangle the reciprocal relationship between stress and depression.31 Such research stems from Hammen’s seminal work, which shows that previous depressive episodes in adult women predict subsequent MDD as a result of generating a greater number of interpersonal, but not non-interpersonal, stressors.60 Inherent to the stress generation framework is the belief that an individual possesses certain characteristics or behaviors that lead to the occurrence of dependent interpersonal stressors (i.e., stressors in which an individual is at least partly responsible for the occurrence). To date, research has demonstrated the role of stress generation in predicting diagnoses of depression in children and adolescents,61 adult men,62 and adult women63. More recent research has explored cognitive32 and interpersonal16,33 predictors of stress generation, but the direct link between reward dysfunction and stress generation, as a potential etiological pathway of MDD, has not to be examined. Therefore, direct evidence for the stress generation is scarce. Nevertheless, as reward dysfunction negatively affects both approach and avoidance tendencies, there is reason to believe that reward deficits may generate interpersonal or relational stressors. In part, individuals possessing avoidance-related deficits may withdraw and/or isolate from social situations to the frustration of partners, family and friends. Alternatively, youth exhibiting approach-related dysfunction may not respond to salient social cues, and despite proactive efforts, may paradoxically “push people away.” Over time these accumulated interpersonal stressors may lead to the onset of MDD (see Figure 1B).
Recent peer evaluation studies provide an interesting medium to delineate the relationship between reward dysfunction and stress generation. To do so, researchers have employed a chat room task, which simulates the transient online peer evaluations occurring on various social media sites (e.g., Facebook). While the administrations of the tasks vary, participants typically create a user profile detailing personal interests. Then, participants are made to believe that this profile will be shared with other adolescents at participating sites, and these youth will thus have an opportunity to determine whether they will accept or reject a participant’s invitation to chat online. After completing the offline portion of the chat room paradigm, which includes participants generating their own bank of peer acceptances and rejections, participants return to the lab 1–2 weeks later, and fMRI data are collected while participants are shown the same pictures of adolescents from the previous session. After each picture is displayed, teens are alerted as to whether they were “accepted” or “rejected” by their peers. Following each acceptance or rejection trial, participants are also asked to indicate the level of distress the trial elicits. To date, the chat room task has generated a number of interesting findings. While “rejected” trials are consistent with the reward mediation model (i.e., Figure 1A) in which stress triggers reward dysfunction, acceptance trials that elicit a blunted reward response may simulate a stress generation effect. For example, when examining healthy adolescents ages 9–17, Guyer and colleagues (2012) reported greater caudate and putamen activity following acceptance feedback, which is consistent with greater striatal responses to positive emotion.64 Future peer evaluation neuroimaging studies would benefit from examining adolescents exhibiting a blunted acceptance response or hypoactivation within the striatum, as this may indicate a preexisting reward deficit. A potential consequence of not “experiencing” acceptance in social situations is that such adolescents may act in ways that disrupt relationships thereby potentiating interpersonal stressors. The accumulation of this stress over time may contribute to the occurrence of depressive symptoms. In line with this approach, peer evaluation neuroimaging studies could, ultimately, highlight potential neurobiological mechanisms that lead to greater relational stressors, which may increase vulnerability to MDD.
In addition to promising neuroimaging data, there is also a growing empirical literature linking genes and reward dysfunction, which suggests that there may be an additional pathway contributing to the stress generation effect. For example, the DA D2 receptor gene (DRD2) has been called the “reward gene” by some researchers, as it is believed that A1 allele carriers on this gene have a greater likelihood of developing disorders characterized by reward deficiencies including depression.65–67 Critically, children carrying A1 allele exhibit more social problems and are more withdrawn, suggesting that a genetic predisposition may account for reward-related deficits.68 In line with this assumption, adults carrying the A1 allele on the DA D2 receptor gene (DRD2) exhibit reduced striatal responsivity to reward and impaired reward learning.69–72 Interestingly, other genetic polymorphisms on the D3 and D4 DA receptors were also associated with MDD, acting as a “phenotypic modifier for MDD”.73,74 In a related study, Nikolova and colleagues (2011) recently demonstrated that a genetic profile for DA signaling based on five different polymorphic loci better explained variability in reward-related Nacc reactivity than single genes, suggesting that a better understanding of DA deficiency may be mediated by the integrative effect of several genes (see Figure 2C).75 As a whole, these findings strongly suggest that the inheritance of alleles that encode neuronal receptors and transporters of DA may impact reward processing. Such genetic variability may thus contribute to reward dysfunction even prior to stress exposure, and reward deficits may in turn lead to the development of interpersonal stressors, and ultimately increase susceptibility for future MDD.
Altogether, the pioneering neurobiological and genetics research described above has provided kindling for a “new chapter” in stress generation research, and in doing so, has developed an important bridge between clinical psychology and neuroscience. At the same time, future research is needed to ascertain the specific neurobiological mechanisms and genetic vulnerabilities that underlie reward deficits as this may, ultimately, provide key information about the etiology of stress generation.
A Titration Perspective: The Relative Effect of Reward Dysfunction and Stress
Diathesis-stress perspectives of MDD have often followed a titration model whereby the interaction of the relative magnitude of stress and a premorbid vulnerability are accounted for in determining one’s likelihood of developing MDD (i.e., moderation framework; see Figure 1C). For example, the hopelessness theory of depression asserts that individuals possessing less negative inferential styles (i.e., internal, stable, and global) require more stressful life events to confer vulnerability for hopelessness depression.76 Monozygotic twin studies provide an interesting platform to examine the titration perspective given the shared genetic makeup and at times, environmental backgrounds. Such overlap has allowed researchers to disentangle the interrelationships among genes, stress, and psychopathology. For example, Kendler and Halberstadt (2012) completed extensive interviews with 14 pairs of monozygotic twins presenting with a discordant history of MDD.77 Interestingly, relative to the unaffected twins, the co-twins reported that depressive episodes were triggered by traumatic life events, romantic upsets, and diminished intimacy. These findings suggest that, despite shared genetic risk, stressful life events significantly increased one’s susceptibility to MDD. Conversely, in a twin study of adolescents between the ages of 11–17 (n = 780 pairs), the presence of Val66Met genotype (a polymorphism in the brain-derived neurotrophic factor (BDNF) gene associated with the onset of MDD) did not predict depressive symptoms.78 However, the interaction between Val66Met and stress was significantly associated with higher levels of depressive symptoms, suggesting that genes moderate the relationship between life events and subsequent depressive episodes.78 Taken together, these studies suggest that vulnerability to MDD may be the results of both stress and genetic factors; however, these studies do not adequately examine the role of reward dysfunction.
As mentioned previously, individuals possessing the A1 allele on the DRD2 gene are more likely to develop reward dysfunction. However, it is also important to note that following exposure to stress, A1 allele carriers report a higher prevalence rate of psychopathology and greater comorbidity.79,80 Furthermore, while stress exposure models suggest that stress triggers reward dysfunction leading to the development of mental illness,81,82 it is clear that stress severity alone does not determine the onset of psychiatric illness, which leaves room for additional factors such as premorbid vulnerability. In support of this notion, Bogdan et al. (2011) found that genetic variation of the corticotropin-releasing hormone type 1 receptor gene (CRHR1), a key component of HPA axis activity that regulates stress response, modulates the ability to learn from rewarding signals in an acute stress – but not no-stress – condition.83 In other words, stress-induced reward responsiveness deficits were greatest in healthy carriers of a genetic variants previously associated with increased MDD risk.83 These findings may suggest that genetic predispositions underlying reward dysfunction and stress response may increase susceptibility to future depression during times of stress exposure, which is consistent with the titration perspective in that the interaction between stress and premorbid reward dysfunction is believed to contribute to the onset of depression.
Treatment Implications: Designing a Clinic for the Future
Within our selective review of the literature, we presented three separate, but related, models delineating the relationship among stress, reward dysfunction, and adolescent MDD. Given the equifinal nature of MDD, we believe that insights from these models may shed important light on different etiological pathways culminating in depression during adolescence, particularly as they may relate to understanding the heterogeneity of MDD. Over time, such insights may be used to develop more effective prevention, intervention, and treatment programs. Therefore, in the proceeding section we describe a hypothetical case study as means of demonstrating the importance of targeting reward dysfunction in both assessment and treatment of adolescent depression. Although there is a preponderance of caveats to the vision outlined below for the Clinic for the Future (including economic and empirical obstacles), it is important to evaluate how translational research may one day shape our approach to understanding psychopathology and designing intervention.
Hypothetical Case Study
D.D. is an 18 year-old male reporting recurrent MDD. Specifically, D.D. experienced his first depressive episode at age 13, and to date, has experienced 3 other episodes, each persisting for approximately 4 months. In the current episode, D.D.’s symptoms include depressed mood, anhedonia, fatigue, insomnia, feelings of worthlessness, and inattention. Prior to the depressive episode, D.D. was an accomplished runner, and it was not uncommon for him to run 5–6 miles daily. However, since the onset of this most recent episode, D.D. struggles to get out of bed in the morning for school. While reserved, D.D. has a close-knit group of friends. In recent months, however, he has begun to withdraw socially and has exhibited a heightened sensitivity to peer criticism. Such withdrawal and peer conflict has contributed to the recurrence of his depressive symptoms and is consistent with a stress generation model of MDD.31
During the initial clinical assessment, D.D. was asked to undergo a brief neuroimaging session in which he completed a social evaluation task (i.e., peer acceptance versus rejection) while fMRI data were collected. This task was selected given D.D.’s diminished motivation and avoidance-based behavior, especially around peers. Consistent with research examining blunted reward response to positive social stimuli,64 D.D. demonstrated decreased Nacc activity in response to peer acceptance. These findings suggest that D.D.’s positive social experiences may not be reinforced, decreasing his likelihood of pursuing these opportunities, and thus, potentially contributing to the recurrence and maintenance of his depressive symptomology. Given D.D.’s symptom and neural profile, he received a combination of cognitive behavior therapy coupled with a novel reward retraining task relying on neurofeedback. During cognitive behavior therapy, D.D. worked collaboratively with his therapist: (a) creating and adhering to behavioral schedules – with a particular emphasis on being active during the weeks following the initial sessions, (b) completing thought records to challenge negative automatic thoughts and underlying schemas, and (c) developing mastery in resolving interpersonal discord. During his biweekly fMRI and neurofeedback sessions, D.D. was instructed to focus on raising a visual bar presented on a computer screen. In fact, through real-time fMRI analysis, the bar represented D.D.’s level of Nacc activation, and the reward retraining was intended to “recondition” reward dysfunction by targeting hypoactivation within the Nacc, a critical factor in the etiology of D.D.’s depressive episode. Over the ensuing weeks, CBT and reward retraining neurofeedback were found to be effective in reducing D.D.’s depressive symptoms, and given the improvements across symptoms, behavior, and neural functioning, it is believed that the likelihood of MDD recurrence has also been reduced.
To be very clear, the assessment and treatment described above are not presently in practice, and significant advancements are needed before such approaches could conceivably be utilized on an individual basis. Notably, although neurofeedback is a relatively new field, previous studies have shown that it improves self-regulation of emotion networks in healthy and MDD individuals,84–86 highlighting substantial promise. Of particular relevance here, these improvements were achieved by successfully targeting regions prominently implicated in the pathophysiology of MDD, including the subgenual PFC,84 amygdala,85 and ventromedial PFC.86 For example, in a recent proof-of-concept study that included four neurofeedback sessions, eight individuals with MDD learned to up-regulate the ventrolateral PFC, an area critically implicated in positive affective experience.86 Even more promising, neurofeedback targeting the ventromedial PFC has also been associated with increased ventral striatal activation to positive stimuli (see Figure 2D), highlighting improvements in a larger network within the brain reward pathway.86
In sum, at present, integrating neural assessments into everyday clinical practice is not empirically supported. However, as the National Institute of Mental Health Strategic Plan has emphasized the need to develop “new, brain-behavior-environmental targets for intervention research” and to “broaden the focus of what is meant by outcome measures in treatment research,” forward progress is anticipated. In the end, bridging the divide between clinical psychology and neuroscience may lead to more effective prevention, intervention, and treatment programs.
Future Directions
Despite an improved understanding of adolescent MDD, there are key empirical gaps, particularly with respect to understanding age- and gender-related differences in MDD. Specifically, adolescence is the peak period for the onset of MDD, and gender differences that arise in mid-adolescence perpetuate throughout adulthood. Additionally, a diagnosis of MDD is characterized by large heterogeneity, which directly impacts the course for the disorder and the approach to treatment. Therefore, future research is warranted to better address these issues.
Age and Gender
Between the ages of 12 and 18, there is a near fivefold increase in the prevalence of MDD with approximately 20% of adolescents experiencing a depressive episode.87 Further, after the age of 14, girls report twice as many depressive episodes compared to boys, and this 2:1 ratio persists throughout adulthood.88 Despite these alarming data, little is known about factors that increase vulnerability in youth and, particularly, females. Structural and functional neuroimaging studies, however, may provide key insight about why adolescence is a critical developmental window to examine the onset of MDD and gender differences that arise therein. Specifically, whereas the females’ total brain volume peaks at 10.5 years, the males’ brain does so at 14.5 years.89–91 While the brain volumes converge in the early twenties, there remain important differences pertaining to structure and function that persist throughout the life course.91–95 These sexually dimorphic changes may have implications for processing and regulating emotional stimuli, and therefore, puberty may provide a unique opportunity to examine how differential neurobiological activity increases vulnerability to MDD. For example, in a recent study examining puberty, gender-specific neural response to reward, and adolescent depressive symptoms, Forbes and colleagues (2010) indicated that advanced pubertal maturation was associated with less striatal and more medial PFC activity during rewarding trials, which is the same pattern of results she found in adults.42 In addition, the study highlighted important hormonal differences as testosterone was positively correlated with reward anticipation in boys, but negatively associated with striatal reactivity in both girls and boys during rewarding outcomes.42 Despite these promising results, additional research is warranted to better understand the integrative effect pertaining to puberty, neural development, reward functioning, and MDD.
MDD Heterogeneity
Given the heterogeneity of MDD, researchers have sought to identify behavioral indicators and biomarkers in order to improve diagnostic and treatment efforts. One promising area of research has been the study of anhedonia, which is considered to be a trait marker (i.e., a characteristic that is not state dependent)96,97 and endophenotype (i.e., an intermediate marker more closely associated with neurobiological and environmental risk factors than the syndrome itself)98 of MDD. The study of anhedonia has led researchers to examine reward processing deficits, especially as it relates to characterizing neural abnormalities that may potentiate reward dysfunction. Such efforts have contributed to fuel the development of the National Institute of Mental Health’s Research Domain Criteria initiative to underscore the importance of examining broad dimensions of abnormal functioning across symptoms, behaviors, genes, and neurobiology as a means of identifying core underpinnings of psychopathology.99,100 It is expected that the identification of underlying mechanisms that cut across disorders may enable researchers and clinicians to develop more effective prevention and intervention programs.
Of note, anhedonia itself encompasses a broad array of reward processes and motivational components. Treadway and Zald (2011) suggest that consummatory (i.e., goal-directed behavior) and motivational (i.e., force driving one’s actions toward a desired goal) processes associated with anhedonia significantly differ with respect to neurobiological processes and may result in the manifestation of different types of symptoms.101 Despite these important differences, within the current review, reward dysfunction is utilized ubiquitously without highlighting these important, fine-grain differences. Nevertheless, these distinct reward processes may have important implications for the proposed models. Namely, it may be that different reward processes are more closely associated with stress dysfunction, stress reactivity, or stress exposure. For example, early adversities have been shown to negatively affect anticipatory as opposed to consummatory processes,57 suggesting that stress can impact specific reward components. At present, further research is needed to better delineate the relationship between specific reward processes, susceptibility to stress, and vulnerability to depression as this may have important etiological and treatment consequences.
Summary
Recent research has made significant advancements in unpacking etiological mechanisms critically implicated in adolescent MDD. Each of the three models outlined above provide a distinct, but related, starting point to better integrate clinical psychology and neuroscience research. Building a bridge between these two fundamental sciences will only serve to improve our understanding of the onset, maintenance, recurrence of adolescent MDD. In time, advancements in our understanding will fuel more effective prevention and treatment programs, which will ease the untold economic and emotional cost associated with adolescent MDD.
Acknowledgments
Randy P. Auerbach was partially supported through generous funding from: Tommy Fuss Fund, the Adam Corneel Young Investigator Award awarded by McLean Hospital, the Kaplen Fellowship on Depression awarded by Harvard Medical School, NIMH K23MH097786, and the Klingenstein Third Generation Foundation Adolescent Depression Fellowship. Roee Admon was supported by an anonymous donation to McLean Hospital and the Adam Corneel Young Investigator Award awarded by McLean Hospital. Diego A. Pizzagalli was partially supported through the NIMH R01MH68376 and the 1R01MH095809. Dr. Pizzagalli has received consulting fees from ANT North America Inc. (Advanced Neuro Technology), AstraZeneca, Shire, Servier, and Ono Pharma USA for projects unrelated to the current research.
References
- 1.Greden JF. The burden of recurrent depression: causes, consequences, and future prospects. J Clin Psychiatry. 2001;62 (Suppl 22):5–9. [PubMed] [Google Scholar]
- 2.Sartorius N. The economic and social burden of depression. J Clin Psychiatry. 2001;62 (Suppl 15):8–11. [PubMed] [Google Scholar]
- 3.Costello EJ, Mustillo S, Erkanli A, Keeler G, Angold A. Prevalence and development of psychiatric disorders in childhood and adolescence. Arch Gen Psychiatry. 2003;60:837–44. doi: 10.1001/archpsyc.60.8.837. [DOI] [PubMed] [Google Scholar]
- 4.Birmaher B, Arbelaez C, Brent D. Course and outcome of child and adolescent major depressive disorder. Child Adolesc Psychiatr Clin N Am. 2002;11:619–37. doi: 10.1016/s1056-4993(02)00011-1. [DOI] [PubMed] [Google Scholar]
- 5.Grant KE, Compas BE, Thurm AE, McMahon SD, Gipson PY. Stressors and child and adolescent psychopathology: measurement issues and prospective effects. JJ Clin Child Adolesc Psychol. 2004;33:412–25. doi: 10.1207/s15374424jccp3302_23. [DOI] [PubMed] [Google Scholar]
- 6.Brilman EI, Ormel J. Life events, difficulties and onset of depressive episodes in later life. Psychol Med. 2001;31:859–69. doi: 10.1017/s0033291701004019. [DOI] [PubMed] [Google Scholar]
- 7.Fava GA, Munari F, Pavan L, Kellner R. Life events and depression. A replication J Affect Disord. 1981;3:159–65. doi: 10.1016/0165-0327(81)90040-9. [DOI] [PubMed] [Google Scholar]
- 8.Paykel ES, Myers JK, Dienelt MN, Klerman GL, Lindenthal JJ, Pepper MP. Life events and depression. A controlled study. Arch Gen Psychiatry. 1969;21:753–60. doi: 10.1001/archpsyc.1969.01740240113014. [DOI] [PubMed] [Google Scholar]
- 9.Lloyd C. Life events and depressive disorder reviewed. II. Events as precipitating factors. Arch Gen Psychiatry. 1980;37:541–8. doi: 10.1001/archpsyc.1980.01780180055005. [DOI] [PubMed] [Google Scholar]
- 10.Paykel ES. Life events and affective disorders. Acta psychiatrica Scandinavica Supplementum. 2003:61–6. doi: 10.1034/j.1600-0447.108.s418.13.x. [DOI] [PubMed] [Google Scholar]
- 11.Tennant C. Life events, stress and depression: a review of recent findings. Aust N Z J Psychiatry. 2002;36:173–82. doi: 10.1046/j.1440-1614.2002.01007.x. [DOI] [PubMed] [Google Scholar]
- 12.Leskela U, Rytsala H, Komulainen E, et al. The influence of adversity and perceived social support on the outcome of major depressive disorder in subjects with different levels of depressive symptoms. Psychol Med. 2006;36:779–88. doi: 10.1017/S0033291706007276. [DOI] [PubMed] [Google Scholar]
- 13.Lewinsohn PM, Allen NB, Seeley JR, Gotlib IH. First onset versus recurrence of depression: differential processes of psychosocial risk. J Abnorm Psychol. 1999;108:483–9. doi: 10.1037//0021-843x.108.3.483. [DOI] [PubMed] [Google Scholar]
- 14.Monroe SM, Harkness KL. Life stress, the “kindling” hypothesis, and the recurrence of depression: considerations from a life stress perspective. Psychol Rev. 2005;112:417–45. doi: 10.1037/0033-295X.112.2.417. [DOI] [PubMed] [Google Scholar]
- 15.Rudolph KD. Developmental influences on interpersonal stress generation in depressed youth. J Abnorm Psychol. 2008;117:673–9. doi: 10.1037/0021-843X.117.3.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Auerbach RP, Bigda-Peyton JS, Eberhart NK, Webb CA, Ho MH. Conceptualizing the prospective relationship between social support, stress, and depressive symptoms among adolescents. J Abnorm Child Psychol. 2011;39:475–87. doi: 10.1007/s10802-010-9479-x. [DOI] [PubMed] [Google Scholar]
- 17.Morgan JK, Olino TM, McMakin DL, Ryan ND, Forbes EE. Neural response to reward as a predictor of increases in depressive symptoms in adolescence. Neurobiol Dis. 2012 doi: 10.1016/j.nbd.2012.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Davey CG, Yucel M, Allen NB. The emergence of depression in adolescence: development of the prefrontal cortex and the representation of reward. Neurosci Biobehav Rev. 2008;32:1–19. doi: 10.1016/j.neubiorev.2007.04.016. [DOI] [PubMed] [Google Scholar]
- 19.Auerbach RP, Ho MH. A cognitive-interpersonal model of adolescent depression: the impact of family conflict and depressogenic cognitive styles. J Clin Child Adolesc Psychol. 2012;41:792–802. doi: 10.1080/15374416.2012.727760. [DOI] [PubMed] [Google Scholar]
- 20.Conley CS, Rudolph KD. The emerging sex difference in adolescent depression: interacting contributions of puberty and peer stress. Dev Psychopathol. 2009;21:593–620. doi: 10.1017/S0954579409000327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sowell ER, Thompson PM, Holmes CJ, Jernigan TL, Toga AW. In vivo evidence for post-adolescent brain maturation in frontal and striatal regions. Nat Neurosci. 1999;2:859–61. doi: 10.1038/13154. [DOI] [PubMed] [Google Scholar]
- 22.Sowell ER, Peterson BS, Thompson PM, Welcome SE, Henkenius AL, Toga AW. Mapping cortical change across the human life span. Nat Neurosci. 2003;6:309–15. doi: 10.1038/nn1008. [DOI] [PubMed] [Google Scholar]
- 23.Giedd JN, Blumenthal J, Jeffries NO, et al. Brain development during childhood and adolescence: a longitudinal MRI study. Nat Neurosci. 1999;2:861–3. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- 24.Gogtay N, Giedd JN, Lusk L, et al. Dynamic mapping of human cortical development during childhood through early adulthood. Proc Natl Acad Sci U S A. 2004;101:8174–9. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Casey BJ, Jones RM, Hare TA. The adolescent brain. Ann N Y Acad Sci. 2008;1124:111–26. doi: 10.1196/annals.1440.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bress JN, Foti D, Kotov R, Klein DN, Hajcak G. Blunted neural response to rewards prospectively predicts depression in adolescent girls. Psychophysiology. 2013;50:74–81. doi: 10.1111/j.1469-8986.2012.01485.x. [DOI] [PubMed] [Google Scholar]
- 27.Mangiavacchi S, Masi F, Scheggi S, Leggio B, De Montis MG, Gambarana C. Long-term behavioral and neurochemical effects of chronic stress exposure in rats. J Neurochem. 2001;79:1113–21. doi: 10.1046/j.1471-4159.2001.00665.x. [DOI] [PubMed] [Google Scholar]
- 28.Bogdan R, Pizzagalli DA. Acute stress reduces reward responsiveness: implications for depression. Biol Psychiatry. 2006;60:1147–54. doi: 10.1016/j.biopsych.2006.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Admon R, Lubin G, Rosenblatt JD, et al. Imbalanced neural responsivity to risk and reward indicates stress vulnerability in humans. Cereb Cortex. 2013;23:28–35. doi: 10.1093/cercor/bhr369. [DOI] [PubMed] [Google Scholar]
- 30.Masten CL, Eisenberger NI, Borofsky LA, et al. Neural correlates of social exclusion during adolescence: understanding the distress of peer rejection. Soc Cogn Affect Neurosci. 2009;4:143–57. doi: 10.1093/scan/nsp007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hammen C. Stress and depression. Annu Rev Clin Psychol. 2005;1:293–319. doi: 10.1146/annurev.clinpsy.1.102803.143938. [DOI] [PubMed] [Google Scholar]
- 32.Auerbach RP, Eberhart NK, Abela JR. Cognitive vulnerability to depression in Canadian and Chinese adolescents. J Abnorm Child Psychol. 2010;38:57–68. doi: 10.1007/s10802-009-9344-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Auerbach R, Ho M. An integrated cognitive-interpersonal model of depression: The impact of family conflict and depressogenic cognitive styles. J Clin Child Adolesc Psychol. 2012;41:792–802. doi: 10.1080/15374416.2012.727760. [DOI] [PubMed] [Google Scholar]
- 34.Beck AT. The current state of cognitive therapy: A 40-year retrospective. Arch Gen Psychiatry. 2005;62:953–9. doi: 10.1001/archpsyc.62.9.953. [DOI] [PubMed] [Google Scholar]
- 35.Katz RJ, Roth KA, Carroll BJ. Acute and chronic stress effects on open field activity in the rat: implications for a model of depression. Neurosci Biobehav Rev. 1981;5:247–51. doi: 10.1016/0149-7634(81)90005-1. [DOI] [PubMed] [Google Scholar]
- 36.Isovich E, Engelmann M, Landgraf R, Fuchs E. Social isolation after a single defeat reduces striatal dopamine transporter binding in rats. Eur J Neurosci. 2001;13:1254–6. doi: 10.1046/j.0953-816x.2001.01492.x. [DOI] [PubMed] [Google Scholar]
- 37.Isovich E, Mijnster MJ, Flugge G, Fuchs E. Chronic psychosocial stress reduces the density of dopamine transporters. Eur J Neurosci. 2000;12:1071–8. doi: 10.1046/j.1460-9568.2000.00969.x. [DOI] [PubMed] [Google Scholar]
- 38.Lucas LR, Celen Z, Tamashiro KL, et al. Repeated exposure to social stress has long-term effects on indirect markers of dopaminergic activity in brain regions associated with motivated behavior. Neuroscience. 2004;124:449–57. doi: 10.1016/j.neuroscience.2003.12.009. [DOI] [PubMed] [Google Scholar]
- 39.Bekris S, Antoniou K, Daskas S, Papadopoulou-Daifoti Z. Behavioural and neurochemical effects induced by chronic mild stress applied to two different rat strains. Behav Brain Res. 2005;161:45–59. doi: 10.1016/j.bbr.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 40.Di Chiara G, Tanda G. Blunting of reactivity of dopamine transmission to palatable food: a biochemical marker of anhedonia in the CMS model? Psychopharmacology. 1997;134:351–3. doi: 10.1007/s002130050465. discussion 71–7. [DOI] [PubMed] [Google Scholar]
- 41.Anisman H, Sklar LS. Catecholamine depletion in mice upon reexposure to stress: mediation of the escape deficits produced by inescapable shock. J Comp Physiol Psychol. 1979;93:610–25. doi: 10.1037/h0077603. [DOI] [PubMed] [Google Scholar]
- 42.Forbes EE, Ryan ND, Phillips ML, et al. Healthy adolescents’ neural response to reward: associations with puberty, positive affect, and depressive symptoms. J Am Acad Child Adolesc Psychiatry. 2010;49:162–72. e1–5. doi: 10.1097/00004583-201002000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pizzagalli DA, Holmes AJ, Dillon DG, et al. Reduced caudate and nucleus accumbens response to rewards in unmedicated individuals with major depressive disorder. Am J Psychiatry. 2009;166:702–10. doi: 10.1176/appi.ajp.2008.08081201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Anisman H, Matheson K. Stress, depression, and anhedonia: caveats concerning animal models. Neurosci Biobehav Rev. 2005;29:525–46. doi: 10.1016/j.neubiorev.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 45.Matthews K, Robbins TW. Early experience as a determinant of adult behavioural responses to reward: the effects of repeated maternal separation in the rat. Neurosci Biobehav Rev. 2003;27:45–55. doi: 10.1016/s0149-7634(03)00008-3. [DOI] [PubMed] [Google Scholar]
- 46.Pryce CR, Dettling AC, Spengler M, Schnell CR, Feldon J. Deprivation of parenting disrupts development of homeostatic and reward systems in marmoset monkey offspring. Biol Psychiatry. 2004;56:72–9. doi: 10.1016/j.biopsych.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 47.Strekalova T, Spanagel R, Bartsch D, Henn FA, Gass P. Stress-induced anhedonia in mice is associated with deficits in forced swimming and exploration. Neuropsychopharmacology. 2004;29:2007–17. doi: 10.1038/sj.npp.1300532. [DOI] [PubMed] [Google Scholar]
- 48.Pryce CR, Dettling A, Spengler M, Spaete C, Feldon J. Evidence for altered monoamine activity and emotional and cognitive disturbance in marmoset monkeys exposed to early life stress. Ann N Y Acad Sci. 2004;1032:245–9. doi: 10.1196/annals.1314.030. [DOI] [PubMed] [Google Scholar]
- 49.Ruedi-Bettschen D, Pedersen EM, Feldon J, Pryce CR. Early deprivation under specific conditions leads to reduced interest in reward in adulthood in Wistar rats. Behav Brain Res. 2005;156:297–310. doi: 10.1016/j.bbr.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 50.Mintz M, Ruedi-Bettschen D, Feldon J, Pryce CR. Early social and physical deprivation leads to reduced social motivation in adulthood in Wistar rats. Behav Brain Res. 2005;156:311–20. doi: 10.1016/j.bbr.2004.08.017. [DOI] [PubMed] [Google Scholar]
- 51.Berenbaum H, Connelly J. The effect of stress on hedonic capacity. J Abnorm Psychol. 1993;102:474–81. doi: 10.1037//0021-843x.102.3.474. [DOI] [PubMed] [Google Scholar]
- 52.Ossewaarde L, Qin S, Van Marle HJ, van Wingen GA, Fernandez G, Hermans EJ. Stress-induced reduction in reward-related prefrontal cortex function. Neuroimage. 2011;55:345–52. doi: 10.1016/j.neuroimage.2010.11.068. [DOI] [PubMed] [Google Scholar]
- 53.Nikolova Y, Bogdan R, Pizzagalli DA. Perception of a naturalistic stressor interacts with 5-HTTLPR/rs25531 genotype and gender to impact reward responsiveness. Neuropsychobiology. 2012;65:45–54. doi: 10.1159/000329105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Robinson OJ, Letkiewicz AM, Overstreet C, Ernst M, Grillon C. The effect of induced anxiety on cognition: threat of shock enhances aversive processing in healthy individuals. Cogn Affect Behav Neurosci. 2011;11:217–27. doi: 10.3758/s13415-011-0030-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Berghorst LH, Bogdan R, Frank MJ, Pizzagalli DA. Acute stress selectively reduces reward sensitivity. Front Hum Neurosci. 2013;7:133. doi: 10.3389/fnhum.2013.00133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pechtel P, Pizzagalli DA. Disrupted reinforcement learning and maladaptive behavior in women with a history of childhood sexual abuse: a high-density event-related potential study. JAMA Psychiatry. 2013;70:499–507. doi: 10.1001/jamapsychiatry.2013.728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dillon DG, Holmes AJ, Birk JL, Brooks N, Lyons-Ruth K, Pizzagalli DA. Childhood adversity is associated with left basal ganglia dysfunction during reward anticipation in adulthood. Biol Psychiatry. 2009;66:206–13. doi: 10.1016/j.biopsych.2009.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mehta MA, Gore-Langton E, Golembo N, Colvert E, Williams SC, Sonuga-Barke E. Hyporesponsive reward anticipation in the basal ganglia following severe institutional deprivation early in life. J Cogn Neurosci. 2010;22:2316–25. doi: 10.1162/jocn.2009.21394. [DOI] [PubMed] [Google Scholar]
- 59.Masten CL, Eisenberger NI, Borofsky LA, et al. Neural correlates of social exclusion during adolescence: understanding the distress of peer rejection. Soc Cogn Affect Neurosci. 2009;4:143–57. doi: 10.1093/scan/nsp007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hammen C. Generation of stress in the course of unipolar depression. J Abnorm Psychol. 1991;100:555–61. doi: 10.1037//0021-843x.100.4.555. [DOI] [PubMed] [Google Scholar]
- 61.Rudolph KD. Developmental influences on interpersonal stress generation in depressed youth. J Abnorm Psychol. 2008;117:673–9. doi: 10.1037/0021-843X.117.3.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cui X-J, Vaillant GE. Does depression generate negative life events? Journal of Nervous and Mental Disease. 1997;185:145–50. doi: 10.1097/00005053-199703000-00003. [DOI] [PubMed] [Google Scholar]
- 63.Hammen C, Shih JH, Brennan PA. Intergenerational transmission of depression: test of an interpersonal stress model in a community sample. J Consult Clin Psychol. 2004;72:511–22. doi: 10.1037/0022-006X.72.3.511. [DOI] [PubMed] [Google Scholar]
- 64.Guyer AE, Choate VR, Pine DS, Nelson EE. Neural circuitry underlying affective response to peer feedback in adolescence. Soc Cogn Affect Neurosci. 2012;7:81–92. doi: 10.1093/scan/nsr043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Noble EP. D2 dopamine receptor gene in psychiatric and neurologic disorders and its phenotypes. Am J Med Genet B Neuropsychiatr Genet. 2003;116B:103–25. doi: 10.1002/ajmg.b.10005. [DOI] [PubMed] [Google Scholar]
- 66.Blum K, Sheridan PJ, Wood RC, et al. The D2 dopamine receptor gene as a determinant of reward deficiency syndrome. J R Soc Med. 1996;89:396–400. doi: 10.1177/014107689608900711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kestler LP, Malhotra AK, Finch C, Adler C, Breier A. The relation between dopamine D2 receptor density and personality: preliminary evidence from the NEO personality inventory-revised. Neuropsychiatry Neuropsychol Behav Neurol. 2000;13:48–52. [PubMed] [Google Scholar]
- 68.Marino C, Vanzin L, Giorda R, et al. An assessment of transmission disequilibrium between quantitative measures of childhood problem behaviors and DRD2/Taql and DRD4/48bp-repeat polymorphisms. Behav Genet. 2004;34:495–502. doi: 10.1023/B:BEGE.0000038487.80597.7e. [DOI] [PubMed] [Google Scholar]
- 69.Jocham G, Klein TA, Neumann J, von Cramon DY, Reuter M, Ullsperger M. Dopamine DRD2 polymorphism alters reversal learning and associated neural activity. J Neurosci. 2009;29:3695–704. doi: 10.1523/JNEUROSCI.5195-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Klein TA, Neumann J, Reuter M, Hennig J, von Cramon DY, Ullsperger M. Genetically determined differences in learning from errors. Science. 2007;318:1642–5. doi: 10.1126/science.1145044. [DOI] [PubMed] [Google Scholar]
- 71.Cohen MX, Young J, Baek JM, Kessler C, Ranganath C. Individual differences in extraversion and dopamine genetics predict neural reward responses. Brain Res Cogn Brain Res. 2005;25:851–61. doi: 10.1016/j.cogbrainres.2005.09.018. [DOI] [PubMed] [Google Scholar]
- 72.Volkow ND, Fowler JS, Wang GJ, Goldstein RZ. Role of dopamine, the frontal cortex and memory circuits in drug addiction: insight from imaging studies. Neurobiol Learn Mem. 2002;78:610–24. doi: 10.1006/nlme.2002.4099. [DOI] [PubMed] [Google Scholar]
- 73.Lopez Leon S, Croes EA, Sayed-Tabatabaei FA, Claes S, Van Broeckhoven C, van Duijn CM. The dopamine D4 receptor gene 48-base-pair-repeat polymorphism and mood disorders: a meta-analysis. Biol psychiatry. 2005;57:999–1003. doi: 10.1016/j.biopsych.2005.01.030. [DOI] [PubMed] [Google Scholar]
- 74.Dikeos DG, Papadimitriou GN, Avramopoulos D, et al. Association between the dopamine D3 receptor gene locus (DRD3) and unipolar affective disorder. Psychiatric genetics. 1999;9:189–95. doi: 10.1097/00041444-199912000-00005. [DOI] [PubMed] [Google Scholar]
- 75.Nikolova YS, Ferrell RE, Manuck SB, Hariri AR. Multilocus genetic profile for dopamine signaling predicts ventral striatum reactivity. Neuropsychopharmacology. 2011;36:1940–7. doi: 10.1038/npp.2011.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Abramson LY, Metalsky GI, Alloy LB. Hopelessness depression: A theory-based subtype of depression. Psychol Rev. 1989;96:358–72. [Google Scholar]
- 77.Kendler KS, Halberstadt LJ. The road not taken: life experiences in monozygotic twin pairs discordant for major depression. Mol Psychiatry. 2012 doi: 10.1038/mp.2012.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chen J, Li X, McGue M. Interacting effect of BDNF Val66Met polymorphism and stressful life events on adolescent depression. Genes Brain Behav. 2012 doi: 10.1111/j.1601-183X.2012.00843.x. [DOI] [PubMed] [Google Scholar]
- 79.Lawford BR, Young R, Noble EP, Kann B, Ritchie T. The D2 dopamine receptor (DRD2) gene is associated with co-morbid depression, anxiety and social dysfunction in untreated veterans with post-traumatic stress disorder. Eur Psychiatry. 2006;21:180–5. doi: 10.1016/j.eurpsy.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 80.Comings DE, Muhleman D, Gysin R. Dopamine D2 receptor (DRD2) gene and susceptibility to posttraumatic stress disorder: a study and replication. Biol Psychiatry. 1996;40:368–72. doi: 10.1016/0006-3223(95)00519-6. [DOI] [PubMed] [Google Scholar]
- 81.Blum K, Sheridan PJ, Wood RC, et al. The D2 dopamine receptor gene as a determinant of reward deficiency syndrome. J R Soc Med. 1996;89:396–400. doi: 10.1177/014107689608900711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lawford BR, Young R, Noble EP, Kann B, Ritchie T. The D2 dopamine receptor (DRD2) gene is associated with co-morbid depression, anxiety and social dysfunction in untreated veterans with post-traumatic stress disorder. Eur Psychiatry. 2006;21:180–5. doi: 10.1016/j.eurpsy.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 83.Bogdan R, Santesso DL, Fagerness J, Perlis RH, Pizzagalli DA. Corticotropin-releasing hormone receptor type 1 (CRHR1) genetic variation and stress interact to influence reward learning. J Neurosci. 2011;31:13246–54. doi: 10.1523/JNEUROSCI.2661-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hamilton JP, Glover GH, Hsu JJ, Johnson RF, Gotlib IH. Modulation of subgenual anterior cingulate cortex activity with real-time neurofeedback. Hum Brain Mapp. 2011;32:22–31. doi: 10.1002/hbm.20997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Johnston SJ, Boehm SG, Healy D, Goebel R, Linden DE. Neurofeedback: A promising tool for the self-regulation of emotion networks. Neuroimage. 2010;49:1066–72. doi: 10.1016/j.neuroimage.2009.07.056. [DOI] [PubMed] [Google Scholar]
- 86.Linden DE, Habes I, Johnston SJ, et al. Real-time self-regulation of emotion networks in patients with depression. PloS one. 2012;7:e38115. doi: 10.1371/journal.pone.0038115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hankin BL, Abramson LY. Development of gender differences in depression: description and possible explanations. Ann Med. 1999;31:372–9. doi: 10.3109/07853899908998794. [DOI] [PubMed] [Google Scholar]
- 88.Hankin BL, Mermelstein R, Roesch L. Sex differences in adolescent depression: stress exposure and reactivity models. Child Dev. 2007;78:279–95. doi: 10.1111/j.1467-8624.2007.00997.x. [DOI] [PubMed] [Google Scholar]
- 89.Lenroot RK, Gogtay N, Greenstein DK, et al. Sexual dimorphism of brain developmental trajectories during childhood and adolescence. Neuroimage. 2007;36:1065–73. doi: 10.1016/j.neuroimage.2007.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lenroot RK, Giedd JN. Sex differences in the adolescent brain. Brain Cogn. 2010;72:46–55. doi: 10.1016/j.bandc.2009.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sowell ER, Peterson BS, Kan E, et al. Sex differences in cortical thickness mapped in 176 healthy individuals between 7 and 87 years of age. Cereb Cortex. 2007;17:1550–60. doi: 10.1093/cercor/bhl066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Christakou A, Halari R, Smith AB, Ifkovits E, Brammer M, Rubia K. Sex-dependent age modulation of frontostriatal and temporo-parietal activation during cognitive control. Neuroimage. 2009;48:223–36. doi: 10.1016/j.neuroimage.2009.06.070. [DOI] [PubMed] [Google Scholar]
- 93.Giedd JN, Snell JW, Lange N, et al. Quantitative magnetic resonance imaging of human brain development: ages 4–18. Cereb Cortex. 1996;6:551–60. doi: 10.1093/cercor/6.4.551. [DOI] [PubMed] [Google Scholar]
- 94.Goldstein JM, Seidman LJ, Horton NJ, et al. Normal sexual dimorphism of the adult human brain assessed by in vivo magnetic resonance imaging. Cereb Cortex. 2001;11:490–7. doi: 10.1093/cercor/11.6.490. [DOI] [PubMed] [Google Scholar]
- 95.Knickmeyer RC, Styner M, Short SJ, et al. Maturational trajectories of cortical brain development through the pubertal transition: unique species and sex differences in the monkey revealed through structural magnetic resonance imaging. Cereb Cortex. 2010;20:1053–63. doi: 10.1093/cercor/bhp166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Loas G. Vulnerability to depression: a model centered on anhedonia. J Affect Disord. 1996;41:39–53. doi: 10.1016/0165-0327(96)00065-1. [DOI] [PubMed] [Google Scholar]
- 97.Meehl PE. Hedonic capacity: some conjectures. Bull Menninger Clin. 1975;39:295–307. [PubMed] [Google Scholar]
- 98.Hasler G, Drevets WC, Manji HK, Charney DS. Discovering endophenotypes for major depression. Neuropsychopharmacology. 2004;29:1765–81. doi: 10.1038/sj.npp.1300506. [DOI] [PubMed] [Google Scholar]
- 99.Insel T, Cuthbert B, Garvey M, et al. Research domain criteria (RDoC): toward a new classification framework for research on mental disorders. Am J Psychiatry. 2010;167:748–51. doi: 10.1176/appi.ajp.2010.09091379. [DOI] [PubMed] [Google Scholar]
- 100.Sanislow CA, Pine DS, Quinn KJ, et al. Developing constructs for psychopathology research: research domain criteria. J Abnorm Psychol. 2010;119:631–9. doi: 10.1037/a0020909. [DOI] [PubMed] [Google Scholar]
- 101.Treadway MT, Zald DH. Reconsidering anhedonia in depression: lessons from translational neuroscience. Neurosci Biobehav Rev. 2011;35:537–55. doi: 10.1016/j.neubiorev.2010.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]