Abstract
Purpose
Cardiovascular disease is the leading non-cancer cause of death among survivors of childhood cancer. Ejection fraction (EF) and fractional shortening (FS) are common echocardiographic measures of cardiac function but newer imaging modalities may provide additional information about pre-clinical disease. This study aimed to evaluate these modalities in detection of anthracycline induced cardiac toxicity.
Methods
We compared mean radial displacement, EF, and FS among 17 adult survivors of childhood cancer exposed to ≥ 300 mg/m2 of anthracyclines to 17 age, sex-matched healthy controls. Survivors with a history of cardiac directed radiation, diabetes, or heart disease were excluded.
Results
Survivors (35% male), mostly with history of treatment for a solid tumor, had a median age at diagnosis of 15 years (1–20) and 27 years (18–50) at evaluation. Median anthracycline exposure was 440 (range 300–645) mg/m2. FS (35.5% vs. 39.6%, p < 0.01) and radial displacement (5.6 mm vs. 6.7 mm, p = 0.02) were significantly lower in survivors compared to controls, respectively. Although the mean EF was lower in survivors versus controls (55.4% vs. 59.7%) it was not statistically significant (p = 0.057). All echocardiographic measures were inversely associated with anthracycline dose, though radial displacement was no longer significantly correlated with anthracycline dose after controlling for survival time (p = 0.07), while EF remained correlated (p = 0.003).
Implications for Cancer Survivors
Radial displacement, EF, and FS are lower in childhood cancer survivors compared to controls. In this study radial displacement added no new information beyond the traditional measures, but clinical utility remains undetermined and requires further longitudinal study.
Keywords: Cancer Survivorship, Cardiotoxicity, Echocardiography
Introduction
Until the latter half of the 20th century most childhood cancer diagnoses resulted in death. Currently, multiple studies report upwards of 80% survival for all patients presenting with a pediatric malignancy largely due to the efforts of the large cooperative groups, advanced therapeutic protocols, newer treatment regimens, and improved supportive care.[1–5] With decreasing mortality and a concomitant rise in new diagnoses, [6] thousands of children and young adults join hundreds of thousands of survivors of childhood cancer in the United States each year.[4, 5] There is a growing recognition of the adverse effects resulting from diagnosis of and treatment for a pediatric malignancy with nearly two thirds of survivors reporting a chronic medical condition and over a quarter with severe or life-threatening disorders.[7]
The leading non-cancer cause of death among childhood cancer survivors is cardiovascular disease (CVD).[5] Cardiac directed radiation therapy and/or exposure to anthracycline containing regimens have been the most strongly associated with late cardiac toxicity and contribute to a 5–10 fold increased risk of cardiovascular mortality compared to sibling controls or the general population.[1, 8–10] Despite limitations due to cardiac toxicity, the anthracyclines remain one of the most potent anti-neoplastic classes and are used in nearly half to two thirds of all pediatric oncology patients.[2, 11] While many reviews have addressed cardiac toxicity following cancer therapy, many questions regarding the pathophysiology, appropriate screening, and management remain.[2, 12–15]
There has been an evolution in cardiac functional assessment over time. Of the most traditional and widely available techniques are fractional shortening (FS) and ejection fraction (EF) obtained by two-dimensional (2D) echocardiography.[16] Changes in EF and FS are late markers of anthracycline-induced cardiotoxicity, reflecting advanced myocardial dysfunction that is less likely amenable to therapeutic intervention.[17] Serum biomarkers and novel imaging modalities have been a recent focus of investigation with hopes of identifying subclinical cardiac dysfunction at a potentially more treatable stage. Newer echocardiographic techniques including Tissue Doppler Imaging (TDI) and 3D and 4D echocardiographic imaging with strain and strain rate [18, 19] have been employed in a variety of clincial applications in both pediatric and adult patients. TDI imaging assesses velocity signals from tissues of high amplitude and low frequency, such as the myocardium rather than the fluid and pressure dynamics measured by traditional Doppler echocardiocgraphy.[20] Strain and strain rate measurements including radial displacement have been used to differentiate between active and passive movement of myocardial segments and valvular rings and more objectively evaluate regional components of myocardial function. While not yet widely adopted, these modalities are currently most often used to diagnose and track acute changes in ischemic cardiomyopathy, monitoring of cardiac resynchronization therapy, or assessment of graft rejection following heart transplant.[21]
The aim of our study was to evaluate the use of newer echocardiographic measures in the detection of cardiac toxicity among cancer survivors previously exposed to anthracycline containing therapies.
Methods
Participants
Cancer survivors were recruited from the Long-Term Follow-Up Clinic at the University of Minnesota, Minneapolis, MN. Inclusion criteria included: age < 21 years at the time of cancer diagnosis, ≥ 18 years at study entry, in remission and surviving ≥ 5 years from diagnosis, and a cumulative anthracycline exposure ≥ 300 mg/m2. Cumulative anthracycline exposure was defined as doxorubicin dose + 0.833 * daunorubicin dose, according to the Children’s Oncology Group Long-Term Follow-Up Guidelines.[22] Survivors with ongoing myelosuppressive therapy, a history of cardiac directed radiation therapy, known cardiovascular disease, or diabetes mellitus were excluded. An age and gender matched comparison group was recruited from healthy community volunteers. The study was approved by the University of Minnesota Institutional Review Board.
Echocardiography
All studies were performed in the University of Minnesota Medical Center, Fairview Echocardiography Laboratory by a single echosonographer and cardiologist (S.S.), both blinded to control or survivor status. Participants underwent 2-dimensional, pulse wave, continuous wave, and color flow Doppler transthoracic echo, as well as tissue Doppler imaging (TDI) and strain imaging using a Philips iE33 Ultrasound Machine (Philips Healthcare, Andover, MA, USA) with a variable transducer.
Left ventricular end-diastolic volume (EDV) and left ventricular end-systolic volume (ESV) were obtained from 4 chamber view to estimate the EF equal to EDV-ESV/EDV, multiplied by 100. Left ventricular end-diastolic dimension (EDD) and left ventricular end-systolic dimensions (ESD) from M-mode guided by a parasternal short axis image were obtained to estimate the FS equal to EDD-ESD/EDD, multiplied by 100. The peak velocity of early (E) and late (A) ventricular filling and the E/A ratio were measured by transmitral pulsed wave Doppler. Deceleration time was measured as the interval from the peak of the E velocity extrapolated to baseline. Early and late diastolic tissue motions (E′ and A′) were measured with spectral TDI. Mitral E′/A′ and E/E′ ratios were calculated for each subject. Left ventricular myocardial performance index (MPI), also known as Tei index, was measured by using the isovolumic contraction time (IVCT) + isovolumic relaxation time (IVRT) divided by ejection time (ET).[23]
Two-dimensional gray-scale, parasternal, short-axis images were obtained at the level of the papillary muscle to evaluate radial function of the left ventricle. Cine-loops of 3 cardiac cycles triggered by the R wave of the QRS complex were saved digitally. Myocardial velocity data were analyzed off-line using dedicated QLAB quantification software (Philips Healthcare, Andover, MA, USA). To evaluate left ventricular radial function peak systolic strain, early and late peak diastolic strain rate, and radial displacement, images were obtained using a standard 6-segment regional analysis from the short-axis view. Due to variability in strain and strain rate, the more consistent radial displacement measurement is reported. All strain imaging was assessed with measures generated by a single investigator (A.C.D.). The same investigator repeated a smaller subset of 20 studies (10 survivors and 10 controls) to obtain intra-observer reliability and a second investigator (R.M.G.) independently processed this same smaller subset to obtain inter-observer reliability.
Statistical Analysis
The study was powered at 80% to detect a 1.0 standard deviation difference in radial displacement between cases and controls at the 0.05 level of significance. A standard deviation of ± 2.2 mm in healthy adult subjects was estimated from the literature.[24] Descriptive statistics were calculated for demographic and echocardiographic variables. Means were compared between survivors and controls using unpaired two-sample t-tests and linear regression was performed to compare correlation between continuous variables with and without adjustments. Adjustments were limited to one additional variable to maintain adequate statistical power. Results were considered significant at an alpha level of < 0.05. Analyses were performed with SAS software version 9.2 (SAS Institute Inc., Cary, NC, USA).
Results
Participant Characteristics
Characteristics of survivors and controls are shown in Table I. Consistent with the study design, there were no significant differences in gender or age. The study population was predominantly young adult female sarcoma survivors, on average over a decade from diagnosis with moderate to high dose anthracycline exposures (cumulative dose ≥ 300 mg/m2). None had experienced acute or early-onset cardiac toxicity. One survivor was being treated for hypertension with lisinopril and incidentally noted to have a bicuspid aortic valve. A previously undiagnosed and asymptomatic dilated aortic root was identified in a control subject.
Table I.
Characteristics of Study Population
| Survivors (n = 17) | Controls (n = 17) | |
|---|---|---|
| Gender (male) | 6 (35 %) | 6 (35 %) |
| Median age in years (range) | ||
| At diagnosis | 15 (1–20) | -- |
| At survey | 27 (18–50) | 25 (18–50) |
| Survival time | 17 (5–30) | -- |
| Diagnosis | -- | |
| Osteosarcoma | 10 (58.8%) | |
| Rhabdomyosarcoma | 3 (17.6%) | |
| Ewing Sarcoma | 1 (5.9%) | |
| Synovial Sarcoma | 1 (5.9%) | |
| Lymphoma | 1 (5.9%) | |
| Wilms Tumor | 1 (5.9%) | |
| Median anthracycline dose and range (mg/m2) | 440 (300–645) | -- |
Fractional Shortening and Ejection Fraction
Survivors had statistically lower average FS and a trend toward lower average EF compared to controls (Table II), while, in aggregate, still remaining in a normal range (FS ≥ 28% and EF ≥ 55%). Two controls and 4 survivors fell below the normal EF threshold and one survivor was below the normal threshold for FS. FS and EF were moderately well correlated with each other (r = 0.33, p = 0.056).
Table II.
Echocardiographic Measures
| Survivors | Controls | p-value | |
|---|---|---|---|
| Ejection Fraction (%) | 55.4 ± 6.7 | 59.7 ± 6.2 | 0.057 |
| Fractional Shortening (%) | 35.5 ± 3.8 | 39.6 ± 4.7 | 0.009 |
| E/A Ratio | 1.65 ± 0.53 | 1.78 ± 0.53 | 0.49 |
| Medial E/E′ Ratio | 7.33 ± 2.09 | 6.6 ± 2.02 | 0.32 |
| Lateral E/E′ Ratio | 5.35 ± 1.73 | 4.84 ± 1.47 | 0.37 |
| MPI (Tei Index) | 0.4 ± 0.14 | 0.39 ± 0.09 | 0.26 |
| Radial Displacement (mm) | 5.61 ± 1.16 | 6.73 ± 1.52 | 0.025 |
All values expressed as mean ± standard deviation
MPI – myocardial performance index
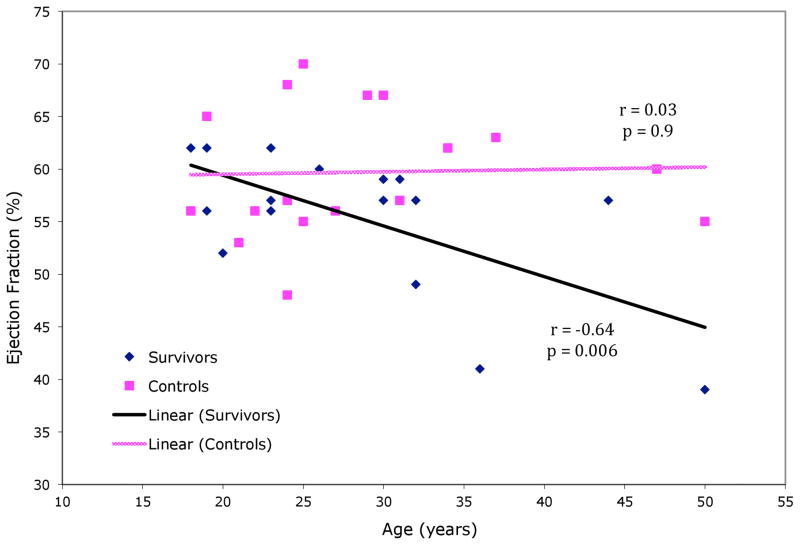
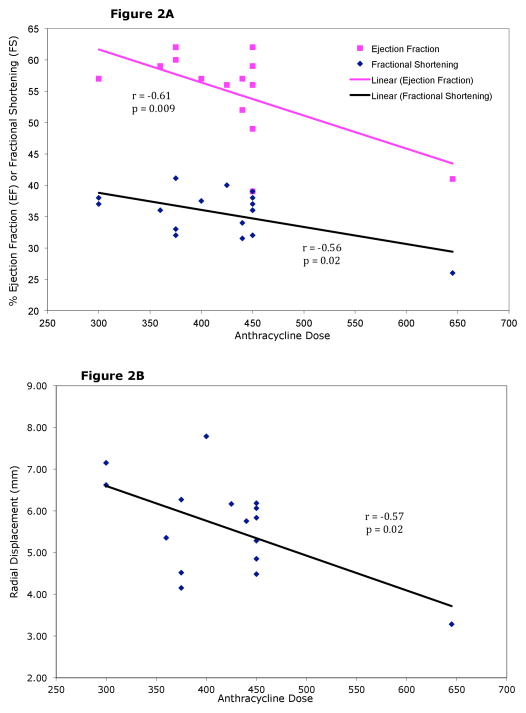
Among controls there was no correlation between age and FS (r = 0.28, p = 0.27) or EF (r = 0.03, p = 0.9). In the survivor group there was no correlation between age and FS (r = −0.02, p=0.95). There was a statistically significant decline in EF associated with advancing age (r = −0.64, p = 0.006) (Figure 1), increasing survival time (r = −0.48, p = 0.05), and increasing anthracycline dose (r = −0.61, p = 0.009), but not with earlier age at diagnosis (r = −0.32, p = 0.22). EF did, however, decline with age at diagnosis after controlling for survival time (r = −0.64, p = 0.03). FS in survivors declined with increasing anthracycline doses (r = −0.56, p = 0.02) but not with earlier age at diagnosis (r = 0.12, p = 0.66) or increased survival time (r = −0.11, p = 0.69). FS in survivors did not decline with age at diagnosis after controlling for survival time either (r = 0.14, p = 0.86). Figure 2A shows the effect of anthracycline dose on EF and FS respectively. Ventricular filling (E/A ratio) was not statistically different between survivors and controls (Table II).
Figure 1.
Correlation of Ejection Fraction versus Age
Figure 2.
Figure 2A. Anthracycline Dose versus EF and FS
Figure 2B. Anthracycline Dose versus Radial Displacement
Radial Displacement
Radial displacement was statistically significantly lower in survivors compared to controls (Table II). While normal ranges are not yet well established, decline in radial displacement is associated with decreased cardiac function. Radial displacement increased with advancing age in the control population (r = 0.56, p = 0.02), but not in survivors (r = 0.06, p = 0.82). Radial displacement declined with increasing anthracycline dose (r = −0.57, p = 0.02) (Figure 2B) but was not correlated with age at diagnosis (r = 0.2, p = 0.46) or survival time (r = −0.07, p = 0.8). Radial displacement also did not correlate with age at diagnosis after controlling for survival time (r = 0.2, p = 0.77). Inter-observer reliability for radial displacment showed an average difference of 16% between 2 readers with a correlation of 0.65 (p = 0.002) while intra-observer reliability showed an average difference of 12% with a correlation of 0.74 (p = 0.0002). TDI measures including medial and lateral mitral E/E′ ratios and the left ventricular MPI (Tei index) revealed no differences between the survivor and control groups.
Traditional Versus Novel Echocardiographic Measures
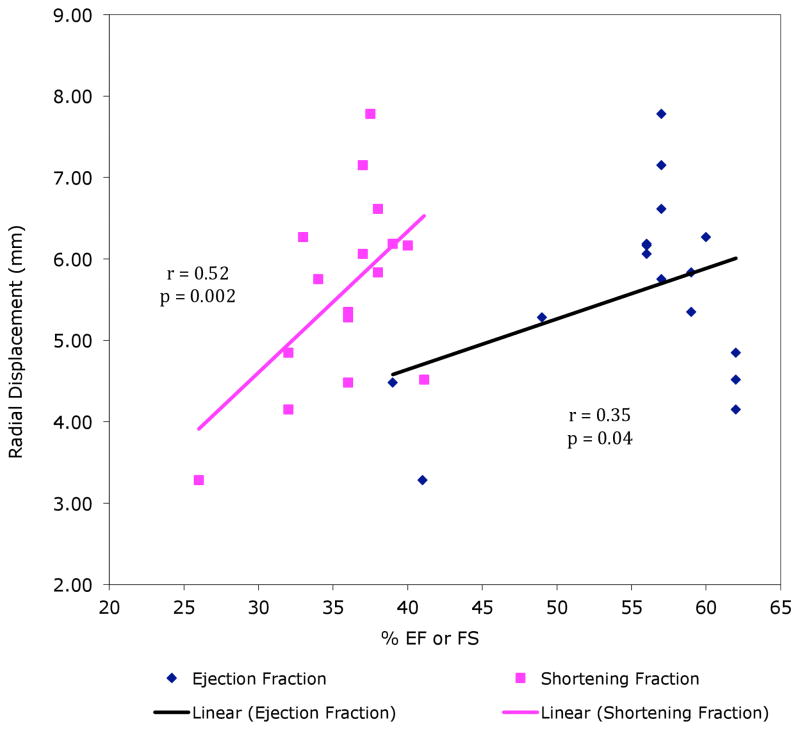
Radial displacement was significantly correlated with both FS (r = 0.52, p = 0.002) and EF (r = 0.35, p = 0.04), as shown in Figure 3. Despite all three measures being associated with each other, the significant difference in radial displacement between survivors and controls remained even after adjustment for FS (p = 0.004) or EF (p = 0.03).
Figure 3.
Correlation of Radial Displacement versus EF & FS
After adjusting for survival time, there was still a significant association between EF and anthracycline dose (p = 0.003) but the association between radial displacement and anthracycline dose was attenuated (p = 0.07). The association between radial displacement and anthracycline dose remained significant after controlling for FS (p=0.03), but not after contolling for EF (p = 0.07).
Discussion
While the natural progression of anthracycline-induced cardiomyopathy remains unclear, the cardiovascular morbidity and mortality among survivors of childhood cancer has been well documented, suggesting benefit to early detection with the goal of improving therapeutic interventions. Using traditional and novel echocardiographic measures, we assessed a high-risk population of childhood cancer survivors and found significant reductions in cardiac function compared to age- and gender-matched healthy controls. All measures significantly decreased with increasing anthracyline dose, though radial displacement appeared to attenuate over time.
Only a few studies have investigated TDI technologies among cancer patients exposed to cardiotoxic therapies, primarily in medical oncology patients or in children during anthracycline infusion or short-term follow-up. Changes have been demonstrated in both early and late ventricular filling ratios (E/A) as well as tissue velocities (E/E′), MPI, and global or longitudinal strain.[25–32] Many of these studies also demonstrated lower cardiac function among cancer patients compared to controls, albeit with measurements remaining within a normal range. This is the one of the first studies to assess these parameters late after therapy (median 17 years following exposure) among adult (median age 27 years) surviors of childhood cancer. Similar to adult studies, we also found some TDI measurements decreased in survivors compared to healthy controls. We found strong correlation between EF, FS and radial displacement, raising the question as to whether any additional prognostic information can be generated by this novel measurement. Both EF and radial displacement were significantly correlated with anthracycline dose, but after controlling for survival time, EF remained significantly correlated and radial displacement lost this association. As cardiotoxicity takes time to develop, survival time can impact the detection of myocardial dysfunction. While the specific pathophysiology has yet to be fully elucidated, the loss of association with survival time may suggest a role for radial displacement in early preclinical detection of myocardial injury. However, it remains unclear if this finding predicts ongoing or future dysfunction.
Of the novel measurements investigated in this study, we found radial displacement to be the most accurate. Examination of the data showed that over 11% of all strain and strain rate values measured in both survivors and controls fell out of normal physiologic ranges, whereas less than 1% of all radial displacement data fell out of normal physiologic ranges. Radial displacement, which is related through a mathematical relationship of temporal integration of velocity and spatial integration of strain, is much less variable and can act as an adequate surrogate for radial strain.[21, 24, 33]
The inter-observer reliability of 16% and correlation of 0.65 found in this study for radial displacement is similar to previous strain and strain rate imaging estimates of 6.9–17% reliability and 0.75–0.79 correlation.[34] The intra-observer reliability of 12% and correlation of 0.74 is also similar to previous estimates of 5–10.1% reliability and 0.78–0.87 correlation.[34] In this study, inter-observer reliability for EF and FS was not determined as a single cardiologist provided those data, though it has previously been reported at 6–15%.[35–37] Intra-observer reliability for EF and FS was not calculated since they were not repeatedly measured, though it has been reported at 6–11%.[35–37] Overall the technologies appear to be similar with respect to both inter- and intra-observer reliability based on these estimates.
This initial assessment of novel echocardiographic technologies among childhood cancer survivors has a number of limitations. Our study sample was recruited from patients attending our local survivor clinic who might be more likely to participate in research.[38] However, this should not particularly effect comparisons between the various echocardiocraphic measures. While the study was adequately powered to detect a 1.0 standard deviation difference in radial displacement, the small sample limited our ability to perform more detailed multiple variable regression and the cross-sectional nature of the study inhibits conclusions concerning long-term changes in the myocardium. Finally, more advanced TDI imaging remains a relatively new modality not readily available in most clinical settings and requires unique hardware and software to run and interpret, [39] limiting more generalized application.
Using traditional and novel echocardiograpy, we measured cardiac function among adult survivors of childhood cancer with significant historical exposures to anthracyclines and identified significant decreases in myocardial function compared to healthy controls. Detection of cardiac toxicity prior to changes in FS and/or EF will help guide care for these patients. However, it remains unclear if TDI imaging can provide sufficient additional clinical information beyond traditional echocardiography to become an adjuct to current screening practices. Potentially a useful monitoring technique, the clinical advantage of radial displacement, strain and strain rate over traditional measurements of EF and FS remains to be elucidated. Longitudinal study of pediatric cancer patients is necessary to more thoroughly evaluate this modality.
Acknowledgments
Supported by a Minnesota Medical Foundation Research Grant (D. A. Mulrooney, PI), Cancer Center Support (CORE) Grant No. CA21765 from the National Cancer Institute, the American Lebanese Syrian Associate Charities (ALSAC), Memphis, TN, and the Children’s Cancer Research Fund, Minneapolis, MN.
Ms. Ashley Schemp, coordinator of the Long-Term Follow-Up Clinic at the University of Minnesota, Ms. Christine Jacox, research coordinator for this project, and the Biostatistical Design and Analysis Center at the University of Minnesota.
Footnotes
Conflict of Interest Statement: No authors have relevant interests or affiliations to disclose.
References
- 1.Shankar S, Marina N, Hudson M, Hodgson D, Adams M, Landier W, et al. Monitoring for cardiovascular disease in survivors of childhood cancer: report from the Cardiovascular Disease Task Force of the Children’s Oncology Group. Pediatrics. 2008;121(2):e387–96. doi: 10.1542/peds.2007-0575. [DOI] [PubMed] [Google Scholar]
- 2.Lipshultz SE, Adams MJ. Cardiotoxicity after childhood cancer: beginning with the end in mind. J Clin Oncol. 2010;28(8):1276–81. doi: 10.1200/JCO.2009.26.5751. JCO.2009.26.5751 [pii] [DOI] [PubMed] [Google Scholar]
- 3.Armstrong GT, Liu Q, Yasui Y, Neglia JP, Leisenring W, Robison LL, et al. Late mortality among 5-year survivors of childhood cancer: a summary from the Childhood Cancer Survivor Study. J Clin Oncol. 2009;27(14):2328–38. doi: 10.1200/JCO.2008.21.1425. JCO.2008.21.1425 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mulrooney DA, Neglia JP, Hudson MM. Caring for adult survivors of childhood cancer. Curr Treat Options Oncol. 2008;9(1):51–66. doi: 10.1007/s11864-008-0054-4. [DOI] [PubMed] [Google Scholar]
- 5.Mertens AC, Liu Q, Neglia JP, Wasilewski K, Leisenring W, Armstrong GT, et al. Cause-specific late mortality among 5-year survivors of childhood cancer: the Childhood Cancer Survivor Study. J Natl Cancer Inst. 2008;100(19):1368–79. doi: 10.1093/jnci/djn310. djn310 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.LAG R, MAS, JGG, ML, TT, JLY, et al. Cancer Incidence and Survival among Children and Adolescents: United States SEER Program 1975–1995. Bethesda: National Cancer Institute, SEER Program, National Institutes of Health; 1999. [Google Scholar]
- 7.Oeffinger KC, Mertens AC, Sklar CA, Kawashima T, Hudson MM, Meadows AT, et al. Chronic health conditions in adult survivors of childhood cancer. N Engl J Med. 2006;355(15):1572–82. doi: 10.1056/NEJMsa060185. 355/15/1572 [pii] [DOI] [PubMed] [Google Scholar]
- 8.Mulrooney DA, Yeazel MW, Kawashima T, Mertens AC, Mitby P, Stovall M, et al. Cardiac outcomes in a cohort of adult survivors of childhood and adolescent cancer: retrospective analysis of the Childhood Cancer Survivor Study cohort. BMJ. 2009;339:b4606. doi: 10.1136/bmj.b4606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tukenova M, Guibout C, Oberlin O, Doyon F, Mousannif A, Haddy N, et al. Role of cancer treatment in long-term overall and cardiovascular mortality after childhood cancer. J Clin Oncol. 2010;28(8):1308–15. doi: 10.1200/JCO.2008.20.2267. JCO.2008.20.2267 [pii] [DOI] [PubMed] [Google Scholar]
- 10.Hawkins MM, Kingston JE, Kinnier Wilson LM. Late deaths after treatment for childhood cancer. Arch Dis Child. 1990;65(12):1356–63. doi: 10.1136/adc.65.12.1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Krischer J, Epstein S, Cuthbertson D, Goorin A, Epstein M, Lipshultz S. Clinical cardiotoxicity following anthracycline treatment for childhood cancer: the Pediatric Oncology Group experience. J Clin Oncol. 1997;15(4):1544–52. doi: 10.1200/JCO.1997.15.4.1544. [DOI] [PubMed] [Google Scholar]
- 12.Lipshultz SE, Alvarez JA, Scully RE. Anthracycline associated cardiotoxicity in survivors of childhood cancer. Heart. 2008;94(4):525–33. doi: 10.1136/hrt.2007.136093. 94/4/525 [pii] [DOI] [PubMed] [Google Scholar]
- 13.Barry E, Alvarez JA, Scully RE, Miller TL, Lipshultz SE. Anthracycline-induced cardiotoxicity: course, pathophysiology, prevention and management. Expert Opin Pharmacother. 2007;8(8):1039–58. doi: 10.1517/14656566.8.8.1039. [DOI] [PubMed] [Google Scholar]
- 14.Franco VI, Henkel JM, Miller TL, Lipshultz SE. Cardiovascular effects in childhood cancer survivors treated with anthracyclines. Cardiol Res Pract. 2011;2011:134679. doi: 10.4061/2011/134679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Trachtenberg BH, Landy DC, Franco VI, Henkel JM, Pearson EJ, Miller TL, et al. Anthracycline-associated cardiotoxicity in survivors of childhood cancer. Pediatr Cardiol. 2011;32(3):342–53. doi: 10.1007/s00246-010-9878-3. [DOI] [PubMed] [Google Scholar]
- 16.Armstrong GT, Plana JC, Zhang N, Srivastava D, Green DM, Ness KK, et al. Screening adult survivors of childhood cancer for cardiomyopathy: comparison of echocardiography and cardiac magnetic resonance imaging. J Clin Oncol. 2012;30(23):2876–84. doi: 10.1200/JCO.2011.40.3584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ewer MS, Lenihan DJ. Left ventricular ejection fraction and cardiotoxicity: is our ear really to the ground? J Clin Oncol. 2008;26(8):1201–3. doi: 10.1200/JCO.2007.14.8742. [DOI] [PubMed] [Google Scholar]
- 18.Po MJ, Lorsakul A, Duan Q, Yeroushalmi KJ, Hyodo E, Oe Y, et al. In-vivo clinical validation of cardiac deformation and strain measurements from 4D ultrasound. Conf Proc IEEE Eng Med Biol Soc. 2010;2010:41–4. doi: 10.1109/IEMBS.2010.5626332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hor KN, Baumann R, Pedrizzetti G, Tonti G, Gottliebson WM, Taylor M, et al. Magnetic resonance derived myocardial strain assessment using feature tracking. J Vis Exp. 2011;(48) doi: 10.3791/2356. 2356 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Citro R, Bossone E, Kuersten B, Gregorio G, Salustri A. Tissue Doppler and strain imaging: anything left in the echo-lab? Cardiovascular ultrasound. 2008;6:54. doi: 10.1186/1476-7120-6-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sutherland GR, Di Salvo G, Claus P, D’hooge J, Bijnens B. Strain and strain rate imaging: a new clinical approach to quantifying regional myocardial function. J Am Soc Echocardiogr. 2004;17(7):788–802. doi: 10.1016/j.echo.2004.03.027. S0894731704003074 [pii] [DOI] [PubMed] [Google Scholar]
- 22.Group AAoPSoHOCsO. Long-term follow-up care for pediatric cancer survivors. Pediatrics. 2009;123(3):906–15. doi: 10.1542/peds.2008-3688. 123/3/906 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tei C, Nishimura RA, Seward JB, Tajik AJ. Noninvasive Doppler-derived myocardial performance index: correlation with simultaneous measurements of cardiac catheterization measurements. J Am Soc Echocardiogr. 1997;10(2):169–78. doi: 10.1016/s0894-7317(97)70090-7. S0894731797000230 [pii] [DOI] [PubMed] [Google Scholar]
- 24.Ganame J, Claus P, Uyttebroeck A, Renard M, D’hooge J, Bijnens B, et al. Myocardial dysfunction late after low-dose anthracycline treatment in asymptomatic pediatric patients. J Am Soc Echocardiogr. 2007;20(12):1351–8. doi: 10.1016/j.echo.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 25.Kapusta L, Thijssen JM, Groot-Loonen J, Antonius T, Mulder J, Daniëls O. Tissue Doppler imaging in detection of myocardial dysfunction in survivors of childhood cancer treated with anthracyclines. Ultrasound Med Biol. 2000;26(7):1099–108. doi: 10.1016/s0301-5629(00)00252-0. S0301-5629(00)00252-0 [pii] [DOI] [PubMed] [Google Scholar]
- 26.Kapusta L, Thijssen JM, Groot-Loonen J, van Druten JA, Daniëls O. Discriminative ability of conventional echocardiography and tissue Doppler imaging techniques for the detection of subclinical cardiotoxic effects of treatment with anthracyclines. Ultrasound Med Biol. 2001;27(12):1605–14. doi: 10.1016/s0301-5629(01)00470-7. S0301562901004707 [pii] [DOI] [PubMed] [Google Scholar]
- 27.Kapusta L, Groot-Loonen J, Thijssen JM, DeGraaf R, Daniëls O. Regional cardiac wall motion abnormalities during and shortly after anthracyclines therapy. Med Pediatr Oncol. 2003;41(5):426–35. doi: 10.1002/mpo.10383. [DOI] [PubMed] [Google Scholar]
- 28.Stapleton G, Stapleton S, Martinez A, Ayres N, Kovalchin J, Bezold L, et al. Evaluation of longitudinal ventricular function with tissue Doppler echocardiography in children treated with anthracyclines. J Am Soc Echocardiogr. 2007;20(5):492–7. doi: 10.1016/j.echo.2006.10.011. [DOI] [PubMed] [Google Scholar]
- 29.Senju N, Ikeda S, Koga S, Miyahara Y, Tsukasaki K, Tomonaga M, et al. The echocardiographic Tei-index reflects early myocardial damage induced by anthracyclines in patients with hematological malignancies. Heart Vessels. 2007;22(6):393–7. doi: 10.1007/s00380-007-0985-x. [DOI] [PubMed] [Google Scholar]
- 30.Karakurt C, Koçak G, Ozgen U. Evaluation of the left ventricular function with tissue tracking and tissue Doppler echocardiography in pediatric malignancy survivors after anthracycline therapy. Echocardiography. 2008;25(8):880–7. doi: 10.1111/j.1540-8175.2008.00695.x. ECHO695 [pii] [DOI] [PubMed] [Google Scholar]
- 31.Poterucha JT, Kutty S, Lindquist RK, Li L, Eidem BW. Changes in left ventricular longitudinal strain with anthracycline chemotherapy in adolescents precede subsequent decreased left ventricular ejection fraction. J Am Soc Echocardiogr. 2012;25(7):733–40. doi: 10.1016/j.echo.2012.04.007. [DOI] [PubMed] [Google Scholar]
- 32.Tsai HR, Gjesdal O, Wethal T, Haugaa KH, Fosså A, Fosså SD, et al. Left ventricular function assessed by two-dimensional speckle tracking echocardiography in long-term survivors of Hodgkin’s lymphoma treated by mediastinal radiotherapy with or without anthracycline therapy. Am J Cardiol. 2011;107(3):472–7. doi: 10.1016/j.amjcard.2010.09.048. [DOI] [PubMed] [Google Scholar]
- 33.Dandel M, Hetzer R. Echocardiographic strain and strain rate imaging--clinical applications. Int J Cardiol. 2009;132(1):11–24. doi: 10.1016/j.ijcard.2008.06.091. S0167-5273(08)00863-2 [pii] [DOI] [PubMed] [Google Scholar]
- 34.Zhang L, Gao J, Xie M, Yin P, Liu W, Li Y, et al. Left ventricular three-dimensional global systolic strain by real-time three-dimensional speckle-tracking in children: feasibility, reproducibility, maturational changes, and normal ranges. J Am Soc Echocardiogr. 2013;26(8):853–9. doi: 10.1016/j.echo.2013.05.002. [DOI] [PubMed] [Google Scholar]
- 35.Krenning BJ, Kirschbaum SW, Soliman OI, Nemes A, van Geuns RJ, Vletter WB, et al. Comparison of contrast agent-enhanced versus non-contrast agent-enhanced real-time three-dimensional echocardiography for analysis of left ventricular systolic function. Am J Cardiol. 2007;100(9):1485–9. doi: 10.1016/j.amjcard.2007.06.042. [DOI] [PubMed] [Google Scholar]
- 36.Malm S, Frigstad S, Sagberg E, Larsson H, Skjaerpe T. Accurate and reproducible measurement of left ventricular volume and ejection fraction by contrast echocardiography: a comparison with magnetic resonance imaging. J Am Coll Cardiol. 2004;44(5):1030–5. doi: 10.1016/j.jacc.2004.05.068. [DOI] [PubMed] [Google Scholar]
- 37.Malm S, Sagberg E, Larsson H, Skjaerpe T. Choosing apical long-axis instead of two-chamber view gives more accurate biplane echocardiographic measurements of left ventricular ejection fraction: a comparison with magnetic resonance imaging. J Am Soc Echocardiogr. 2005;18(10):1044–50. doi: 10.1016/j.echo.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 38.Ness KK, Leisenring W, Goodman P, Kawashima T, Mertens AC, Oeffinger KC, et al. Assessment of selection bias in clinic-based populations of childhood cancer survivors: a report from the childhood cancer survivor study. Pediatr Blood Cancer. 2009;52(3):379–86. doi: 10.1002/pbc.21829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dandel M, Hetzer R. Echocardiographic strain and strain rate imaging--clinical applications. Int J Cardiol. 2009;132(1):11–24. doi: 10.1016/j.ijcard.2008.06.091. [DOI] [PubMed] [Google Scholar]