Abstract
Purpose
Survival benefit from adjuvant chemotherapy is established for stage III colon cancer; however, uncertainty exists for stage II patients. Tumor heterogeneity, specifically microsatellite instability (MSI) which is more common in right-sided cancers, may be the reason for this observation. We examined the relationship between adjuvant chemotherapy and overall 5-year mortality for stage II colon cancer by location (right- versus left-side) as a surrogate for MSI.
Methods
Using Surveillance, Epidemiology, and End Results (SEER)-Medicare data, we identified Medicare beneficiaries from 1992 to 2005 with AJCC stage II (n=23,578) and III (n=17,148) primary adenocarcinoma of the colon who underwent surgery for curative intent. Overall 5-year mortality was examined with Kaplan-Meier survival analysis and Cox proportional hazards regression with propensity score weighting.
Results
Eighteen percent (n=2,941) of stage II patients with right-sided cancer and 22% (n=1,693) with left-sided cancer received adjuvant chemotherapy. After adjustment, overall 5-year survival benefit from chemotherapy was observed only for stage III patients (right-sided: HR 0.64; 95% CI, 0.59–0.68, p<0.001 and left-sided: HR 0.61; 95% CI, 0.56–0.68, p<0.001). No survival benefit was observed for stage II patients with either right-sided (HR 0.97; 95% CI, 0.87–1.09, p=0.64) or left-sided cancer (HR 0.97; 95% CI, 0.84–1.12, p=0.68).
Conclusions
Among Medicare patients with stage II colon cancer, a substantial number receive adjuvant chemotherapy. Adjuvant chemotherapy did not improve overall 5-year survival for either right- or left-sided colon cancers. Our results reinforce existing guidelines and should be considered in treatment algorithms for older adults with stage II colon cancer.
Introduction
Adjuvant chemotherapy for patients with completely resected stage III colon cancer is considered standard clinical practice.1 However, controversy exists surrounding the use of adjuvant chemotherapy for patients with stage II colon cancer. Despite evidence that adjuvant chemotherapy for stage II patients may not be beneficial and guidelines that do not routinely recommend its use for patients with stage II colon cancer,2 a substantial number of patients are still receiving this treatment. Utilizing the Surveillance, Epidemiology, and End Results (SEER)-Medicare linked database, Schrag et al.3 found that between 1991 and 1996, 27% of patients over age 65, with surgically resected stage II colon cancer received chemotherapy. This pattern was recently confirmed by O’Connor et al.4 utilizing an updated SEER-Medicare database from 1992 to 2005.
With recent attention on tumor biology and the recognition that there is a great deal of heterogeneity,5–10 should we be asking whether there is a subset of patients with stage II colon cancer who would be more likely to benefit from adjuvant chemotherapy? Heterogeneity in microsatellite instability (MSI) status is common in colon cancers, and is an independent predictor of survival: MSI-high tumors have a better overall prognosis11–15 and significantly decreased risk of metastases.14 Ribic et al.11 investigated the effectiveness of adjuvant chemotherapy with fluorouracil for both stage II and stage III colon cancer patients by MSI status. They found a survival benefit from fluorouracil-based adjuvant chemotherapy for microsatellite-stable and MSI-low stage II and stage III colon cancers, but not for those with MSI-high tumors.
MSI is seen predominantly in right-sided colon cancers with an estimated 20–25% of right-sided stage II cancers being MSI-high; MSI-high tumors of the left colon rarely exist, across all stages.10,12,16–19 This raises the possibility for a differential benefit from chemotherapy based on tumor location. We used the linked SEER-Medicare dataset to examine the relationship between adjuvant chemotherapy and overall 5-year mortality for right- versus left-sided stage II colon cancer. We hypothesized that left-sided stage II cancers would demonstrate a greater benefit from receipt of adjuvant chemotherapy compared to right-sided cancers.
METHODS
This study was reviewed by the University of Wisconsin-Madison Health Sciences Institutional Review Board and determined to be exempt.
Data Sources
We utilized the linked SEER registry and Medicare claims databases to identify patients diagnosed with colon cancer between 1992 and 2005. SEER cancer registry information includes patient demographics, tumor characteristics, first course of treatment, and survival for persons newly diagnosed with cancer. The SEER-Medicare dataset has successfully linked 93% of individuals aged 65 or older at diagnosis to their Medicare record.20,21
Patient Selection
All Medicare-enrolled patients aged 66 years and older diagnosed in a SEER area from 1992 to 2005 were eligible for our study if they had a diagnosis of colon (International Classification of Diseases for Oncology ICD-O-3 site codes 18.0-18.9, and 19.9) adenocarcinoma (ICD-O-3 morphology codes 8140-47, 8210-11, 8220-21, 8260-63, 8480-81, and 8490) that were diagnosed at either American Joint Committee on Cancer (AJCC) stage II or III. Patients with rectal cancer (site code 20.9) were excluded, as well as patients with mucinous cystadenocarcinoma (morphology code 8470).22 We further selected for patients who underwent surgery for likely curative intent within six months of diagnosis by using International Classification of Diseases, ninth revision, Clinical Modification (ICD-9-CM) procedure codes: 45.7x (partial excision of large intestine) and 45.8x (total intra-abdominal colectomy). Patients were also required to have continuous enrollment in Medicare Part A and B from 12 months prior to the date of their diagnosis to five years following date of discharge, death, or December 31, 2005 to facilitate ascertainment of overall health status, post-operative chemotherapy administration, and survival. Patients were excluded who were also enrolled in a Health Maintenance Organization during the same time period, diagnosed with another malignancy one year before or after the date of colon cancer diagnosis, or if their first diagnosis of colon cancer was made after death (i.e., on autopsy).
From an initial cohort of 46,627 patients, sequential exclusions were carried out for tumors with an unknown location or from the appendix (site codes 18.8, 18.9 and 18.1), unknown tumor grade, initial cancer treatment other than surgery (pre-operative radiation or chemotherapy), missing nodal assessment, and death within 30 days of surgery or prior to discharge from the surgical hospitalization; yielding a final sample size was 40,726 patients.
Outcome Variable
The primary outcome measure was all-cause five-year mortality, defined as death within 5 years of primary surgery for colon cancer from the SEER Patient Entitlement and Diagnosis Summary File (PEDSF).
Explanatory Variables
The primary explanatory variable was receipt of adjuvant chemotherapy. We defined postoperative chemotherapy based on prior work by Bradley et al,23 Dobie et al,24 and our recently published paper on adjuvant chemotherapy for stage II colon cancer patients with poor prognostic features.4 Claims were included within the following treatment window: beginning with the date of primary surgery and ending with (a) claim date after which there were 3 months free of colon cancer treatment; (b) evidence of distant cancer recurrence; or (c) 9 months after primary surgery, whichever came first.23,24 Patients with at least one claim in this treatment window were classified as having received any chemotherapy, without differentiation between type of chemotherapy received. Capecitabine administration was captured using Healthcare Common Procedure Coding System (HCPCS) codes J8520 and J8521. Patients without any claims in this treatment window were considered to have received no chemotherapy.
Analyses were stratified by tumor location and AJCC stage. Dividing tumor location into right- and left-side follows a validated approach described by Meguid et al.25 and Benedix et al.26 and was used in our previously published paper examining the relationship between colon cancer location (right- versus left-side) and five-year mortality by stage.27 Right-sided colon cancers were identified by ICD-O-3 site codes: 18.0–Cecum, 18.2–Ascending colon, 18.3–Hepatic flexure of colon, and 18.4–Transverse colon. Left-sided colon cancers were defined with codes 18.5–Splenic flexure of colon, 18.6–Descending colon, 18.7–Sigmoid colon, and 19.9–Rectosigmoid. AJCC stage II–III was identified from the SEER data.
Control Variables
Patient sociodemographic variables included age, gender, race/ethnicity, marital status, SEER registry region, and rural/urban county of residence. Census tract median level of household income and median level of education were used as proxies for patient socioeconomic status. Patient risk adjustment and treatment variables included hospitalization in the year prior to diagnosis, the presence of any in-hospital complication,28 and a score for overall health status. Each patient was assigned a Centers for Medicare and Medicaid Services Hierarchical Condition Categories (HCC)29 score. HCC score is a measure of healthcare utilization and was used as a marker of overall health status. Data on oncologist visit within 30 days of surgical discharge was also extracted. We also incorporated several cancer-specific variables, including year of diagnosis (divided into periods of colonoscopy coverage by Medicare)30 and poor prognostic features (diagnosis in the setting of intestinal obstruction or perforation, emergent admission for surgery, T4 stage, poor/undifferentiated tumor histology, and fewer than 12 lymph nodes examined at surgery).2
Statistical Analyses
We compared the frequency of all patient sociodemographic, risk adjustment, treatment, and cancer-specific variables by receipt of chemotherapy (none vs. any) for right- and left-sided colon cancers within each stage (II–III) using χ2 tests for categorical variables and two-way analysis of variance tests for continuous variables. Cox proportional hazards regression was conducted to obtain adjusted hazards ratios (HRs) and 95% confidence intervals (CIs) for all-cause five-year mortality for patients who did or did not receive chemotherapy by tumor location within each stage group, controlling for all other variables.
In clinical practice, decisions to treat patients with chemotherapy are based on a number of factors including age, comorbidities, tumor stage and grade, lymphovascular invasion, and patient/provider preferences. We further adjusted the Cox proportional hazard ratios with propensity score weighting to address potential bias in estimates of the true treatment effect given the observational nature of the dataset.31–34 Propensity scores were calculated for each patient using models to estimate each patient’s probability of receiving adjuvant chemotherapy conditional on sociodemographic and clinical characteristics (Table 1). Propensity score weights based on the inverse of the propensity score were included in the fully adjusted models.35 Kaplan-Meier survival analysis was adjusted for propensity scores. Overall five-year survival rates were compared for patients receiving any versus no adjuvant chemotherapy within each stage (II–III) and stratified by location (right- vs left-sided colon cancer).
Table 1.
Propensity score models
| Stage II Right-Sided Cancer | Stage II Left-Sided Cancer | Stage III (Right- and Left-Sided Cancer) | |
|---|---|---|---|
| Main Effect Variables | Age | Age | Age |
| Race | Race | Race | |
| In-hospital complications | Marital Status | Census-tract education | |
| Visit to oncologist within 30 days | In-hospital complications | In-hospital complications | |
| Tumor grade | Visit to oncologist within 30 days | Diagnosis in the setting of obstruction | |
| Tumor grade | Visit to oncologist within 30 days Tumor grade |
||
| Interactions | Age*Complication | Age*Complication | Age*Complication |
| Age*Oncologist visit | Age*Oncologist visit | Age*Oncologist visit |
Analyses were conducted with Stata 12.0 software (StataCorp, College Station, Texas). All tests of significance used two-sided p-values at the p<0.05 level; 95% CIs were calculated using robust estimates of the standard error.
RESULTS
Sample Characteristics
We included 40,726 patients in our study using the patient selection algorithm described in the Methods section and stratified groups by AJCC stage and tumor location. Eighteen percent (n=2,941) of stage II patients with right-sided cancer and 22% (n=1,693) with left-sided cancer received adjuvant chemotherapy compared to 56% (n=6,496) of stage III right-sided cancer and 60% (n=3,284) with stage III left-sided cancer.
Patients who received adjuvant chemotherapy were more likely to be younger, white, male, and married; have a higher household income; be diagnosed with a more advanced tumor; have a better HCC score; have less hospitalizations in the previous year; and be seen by an oncologist within 30 days of surgery. Similar patterns were seen within tumor location groups for stage II (Table 2) and stage III patients (Table 3).
Table 2.
Characteristics of Medicare beneficiaries undergoing resection for Stage II colon cancer stratified by location and chemotherapy (N=23,578)
| Right-Sided Cancer (n=15,992) | Left-Sided Cancer (n=7,586) | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Characteristic | No Chemotherapy (n=13,051) | Any Chemotherapy (n=2,941) | p-value | No Chemotherapy (n=5,893) | Any Chemotherapy (n=1,693) | p-value |
| Patient Characteristics | ||||||
| Age, % | <0.001 | <0.001 | ||||
| 65–69 | 7.7 | 21.7 | 10.3 | 25.5 | ||
| 70–74 | 15.9 | 31.0 | 19.3 | 33.0 | ||
| 75–79 | 23.2 | 28.8 | 23.9 | 26.2 | ||
| 80–84 | 26.0 | 12.7 | 24.2 | 12.2 | ||
| 85+ | 27.2 | 5.8 | 22.4 | 3.1 | ||
| Male gender, % | 36.1 | 44.5 | <0.001 | 44.8 | 50.6 | <0.001 |
| Race/ethnicity, % | 0.016 | 0.024 | ||||
| White | 87.7 | 87.2 | 83.9 | 85.9 | ||
| Black | 5.8 | 4.8 | 6.9 | 4.7 | ||
| Asian or Pacific Islander | 3.0 | 3.6 | 5.0 | 5.2 | ||
| Hispanic or Other | 3.5 | 4.3 | 4.2 | 4.2 | ||
| Marital status, % | <0.001 | <0.001 | ||||
| Married | 44.6 | 60.2 | 48.0 | 62.4 | ||
| Widowed | 40.7 | 25.6 | 36.7 | 24.0 | ||
| Single, Separated, or Divorced | 11.6 | 11.1 | 12.1 | 11.0 | ||
| Unknown | 3.1 | 3.1 | 3.2 | 2.6 | ||
| SEER registry, % | <0.001 | 0.023 | ||||
| Connecticut | 11.3 | 7.8 | 11.3 | 9.1 | ||
| Detroit | 9.5 | 12.6 | 10.3 | 11.3 | ||
| Hawaii | 1.4 | 1.2 | 2.1 | 1.4 | ||
| Iowa | 14.0 | 13.3 | 12.3 | 13.2 | ||
| New Mexico | 2.3 | 2.0 | 3.1 | 2.8 | ||
| Seattle | 7.7 | 7.5 | 7.6 | 6.5 | ||
| Utah | 2.8 | 2.2 | 3.1 | 3.1 | ||
| Atlanta & Rural Georgia | 3.7 | 4.0 | 4.2 | 4.3 | ||
| Kentucky | 4.4 | 5.5 | 4.4 | 5.1 | ||
| Louisiana | 3.7 | 3.9 | 4.4 | 5.4 | ||
| New Jersey | 9.5 | 10.5 | 8.7 | 10.3 | ||
| California | 29.8 | 29.5 | 28.8 | 27.6 | ||
| Residence location, % | 0.003 | 0.121 | ||||
| Major metropolitan | 55.2 | 58.7 | 56.0 | 56.7 | ||
| Metropolitan or urban | 34.5 | 31.4 | 34.0 | 32.0 | ||
| Less urban or rural | 10.3 | 10.0 | 10.0 | 11.3 | ||
| Median household income (census tract), $ in thousands, mean (SD) | 45.0 (21.9) | 46.1 (22.7) | 0.016 | 43.8 (22.3) | 45.2 (22.1) | 0.030 |
| Less than 12 yrs education (census tract), %, mean (SD) | 18.7 (12.2) | 19.0 (12.6) | 0.247 | 19.8 (12.9) | 19.5 (13.0) | 0.511 |
| Year of diagnosis by Medicare colonoscopy coverage, % | <0.001 | <0.001 | ||||
| 1992–1997 | 33.0 | 31.4 | 38.2 | 34.9 | ||
| 1998-June 2001 | 24.2 | 28.0 | 23.3 | 27.9 | ||
| July 2001–2005 | 42.9 | 40.6 | 38.5 | 37.2 | ||
| Tumor Characteristics | ||||||
| Poor Prognostic Features | ||||||
| Emergent admission, % | 18.9 | 15.8 | <0.001 | 20.4 | 18.6 | 0.098 |
| Intestinal obstruction on admission, % | 3.3 | 3.3 | 0.957 | 7.2 | 8.8 | 0.026 |
| Intestinal perforation on admission, % | 1.4 | 2.3 | <0.001 | 2.7 | 5.5 | <0.001 |
| T stage, % | <0.001 | <0.001 | ||||
| T3 | 87.5 | 79.9 | 86.7 | 80.0 | ||
| T4 | 12.6 | 20.1 | 13.3 | 20.0 | ||
| Number of nodes resected, % | 0.007 | 0.19 | ||||
| 0–11 nodes | 49.3 | 46.6 | 66.2 | 64.5 | ||
| 12 or more nodes | 50.7 | 53.4 | 33.8 | 35.5 | ||
| Tumor grade, % | <0.001 | 0.415 | ||||
| Well-differentiated | 7.3 | 6.4 | 8.7 | 8.2 | ||
| Moderately-differentiated | 69.9 | 65.2 | 79.6 | 79.0 | ||
| Poorly or undifferentiated | 22.8 | 28.4 | 11.7 | 12.8 | ||
| Comorbidity & Treatment Measures | ||||||
| HCC risk score, mean (SD) | 2.00 (1.39) | 1.74 (1.19) | <0.001 | 2.06 (1.39) | 1.77 (1.16) | <0.001 |
| Any hospitalization in the previous year, % | 29.8 | 20.7 | <0.001 | 26.3 | 18.7 | <0.001 |
| Rehospitalization within 30 days of discharge, % | 11.6 | 8.7 | <0.001 | 12.7 | 7.9 | <0.001 |
| Any in-hospital surgical complication, % | 3.0 | 2.2 | 0.013 | 5.2 | 5.9 | 0.287 |
| Oncologist visit within 30 days of surgery, % | 21.9 | 55.3 | <0.001 | 22.6 | 53.3 | <0.001 |
AJCC = American Joint Committee on Cancer; SD = standard deviation; HCC = Hierarchical Condition Categories
Table 3.
Characteristics of Medicare beneficiaries undergoing resection for Stage III colon cancer stratified by location and chemotherapy (N=17,148)
| Right-Sided Cancer (n=11,655) | Left-Sided Cancer (n=5,493) | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Characteristic | No Chemotherapy (n=5,159) | Any Chemotherapy (n=6,496) | p-value | No Chemotherapy (n=2,209) | Any Chemotherapy (n=3,284) | p-value |
| Patient Characteristics | ||||||
| Age, % | <0.001 | <0.001 | ||||
| 65–69 | 4.7 | 18.5 | 6.9 | 22.0 | ||
| 70–74 | 10.3 | 28.5 | 14.3 | 32.0 | ||
| 75–79 | 19.4 | 29.6 | 22.9 | 27.8 | ||
| 80–84 | 28.2 | 17.4 | 28.4 | 14.4 | ||
| 85+ | 37.3 | 6.0 | 27.5 | 3.7 | ||
| Male gender, % | 32.7 | 41.3 | <0.001 | 44.5 | 52.2 | <0.001 |
| Race/ethnicity, % | 0.001 | 0.012 | ||||
| White | 85.3 | 85.6 | 79.9 | 81.9 | ||
| Black | 7.3 | 6.0 | 9.2 | 6.9 | ||
| Asian or Pacific Islander | 3.8 | 4.3 | 6.6 | 7.3 | ||
| Hispanic or Other | 3.6 | 4.2 | 4.4 | 3.9 | ||
| Marital status, % | <0.001 | <0.001 | ||||
| Married | 38.4 | 58.2 | 43.9 | 61.9 | ||
| Widowed | 46.6 | 27.1 | 40.5 | 23.6 | ||
| Single, Separated, or Divorced | 12.0 | 11.8 | 12.5 | 11.9 | ||
| Unknown | 3.1 | 2.9 | 3.2 | 2.6 | ||
| SEER registry, % | 0.003 | 0.565 | ||||
| Connecticut | 9.9 | 10.2 | 11.5 | 10.2 | ||
| Detroit | 10.1 | 11.3 | 10.5 | 10.1 | ||
| Hawaii | 1.8 | 1.6 | 2.7 | 2.9 | ||
| Iowa | 13.1 | 12.2 | 11.0 | 11.2 | ||
| New Mexico | 2.7 | 2.7 | 3.3 | 2.7 | ||
| Seattle | 7.7 | 7.2 | 6.2 | 6.4 | ||
| Utah | 3.4 | 3.2 | 3.8 | 3.4 | ||
| Atlanta & Rural Georgia | 3.9 | 4.0 | 4.0 | 3.9 | ||
| Kentucky | 4.7 | 5.0 | 4.9 | 4.7 | ||
| Louisiana | 3.6 | 4.4 | 3.9 | 5.1 | ||
| New Jersey | 9.9 | 11.6 | 8.9 | 9.9 | ||
| California | 29.4 | 26.5 | 29.2 | 29.3 | ||
| Residence location, % | 0.330 | 0.627 | ||||
| Major metropolitan | 57.5 | 56.2 | 56.5 | 55.2 | ||
| Metropolitan or urban | 32.3 | 33.5 | 33.5 | 34.7 | ||
| Less urban or rural | 10.3 | 10.3 | 10.0 | 10.0 | ||
| Median household income (census tract), $ in thousands, mean (SD) | 43.8 (21.0) | 46.4 (22.6) | <0.001 | 42.4 (20.2) | 45.2 (22.3) | <0.001 |
| Less than 12 yrs education (census tract), %, mean (SD) | 19.3 (12.7) | 18.5 (12.4) | 0.0014 | 20.5 (13.1) | 19.4 (12.7) | 0.003 |
| Year of diagnosis by Medicare colonoscopy coverage, % | <0.001 | <0.001 | ||||
| 1992–1997 | 33.1 | 28.2 | 36.4 | 31.4 | ||
| 1998-June 2001 | 23.5 | 25.7 | 21.5 | 25.0 | ||
| July 2001–2005 | 43.4 | 46.1 | 42.1 | 43.6 | ||
| Tumor Characteristics | ||||||
| Poor Prognostic Features | ||||||
| Emergent admission, % | 25.9 | 17.1 | <0.001 | 26.8 | 16.0 | <0.001 |
| Intestinal obstruction on admission, % | 4.9 | 3.9 | 0.013 | 8.7 | 6.4 | 0.001 |
| Intestinal perforation on admission, % | 2.4 | 1.3 | <0.001 | 3.1 | 1.7 | 0.001 |
| T stage, % | <0.001 | <0.001 | ||||
| T1, or T2 | 8.8 | 10.9 | 9.8 | 13.1 | ||
| T3 | 70.4 | 70.3 | 71.3 | 70.7 | ||
| T4 | 20.8 | 18.8 | 18.9 | 16.2 | ||
| Number of nodes resected, % | <0.001 | 0.029 | ||||
| 0–11 nodes | 46.0 | 42.5 | 62.4 | 59.5 | ||
| 12 or more nodes | 54.0 | 57.5 | 37.6 | 40.5 | ||
| Tumor grade, % | 0.158 | 0.944 | ||||
| Well-differentiated | 4.3 | 4.7 | 5.8 | 5.6 | ||
| Moderately-differentiated | 58.9 | 60.1 | 73.2 | 73.5 | ||
| Poorly or undifferentiated | 36.8 | 35.2 | 21.1 | 20.9 | ||
| Comorbidity & Treatment Measures | ||||||
| HCC risk score, mean (SD) | 2.9 (1.6) | 2.3 (1.2) | 0.0014 | 2.9 (1.6) | 2.3 (1.3) | <0.001 |
| Any hospitalization in the previous year, % | 33.1 | 22.9 | <0.001 | 30.9 | 18.5 | <0.001 |
| Rehospitalization within 30days of discharge, % | 16.0 | 8.6 | <0.001 | 15.5 | 8.7 | <0.001 |
| Any in-hospital surgical complication, % | 3.8 | 2.6 | <0.001 | 6.5 | 3.6 | <0.001 |
| Oncologist visit within 30 days of surgery, % | 27.5 | 59.2 | <0.001 | 25.6 | 55.9 | <0.001 |
AJCC = American Joint Committee on Cancer; SD = standard deviation; HCC = Hierarchical Condition Categories
Survival Benefit of Chemotherapy by Tumor Location
Unadjusted models showed a significant survival benefit for all groups from adjuvant chemotherapy for all groups: stage II right-sided (HR 0.66; 95% CI, 0.61 to 0.72), stage II left-sided (HR 0.61; 95% CI, 0.55 to 0.68), stage III right-sided (HR 0.50; 95% CI, 0.47 to 0.53), and stage III left-sided (HR 0.44; 95% CI, 0.40 to 0.47) (Table 4). However, after adjustment for all patient sociodemographic, risk adjustment, treatment, and cancer-specific variables, patients with stage II right-sided cancer no longer showed a benefit from adjuvant chemotherapy (HR 0.92; 95% CI, 0.85 to 1.01). Finally, when propensity score weighting was included to address selection bias owing to nonrandom treatment assignment, a survival benefit was no longer found for either group of stage II colon cancer patients (right-side: HR 0.97; 95% CI, 0.87 to 1.09; left-side: HR 0.97; 95% CI, 0.84 to 1.12).
Table 4.
Adjusted hazard ratios and 95% confidence intervals for 5 year mortality, by stage and location
| Stage II Right-Sided (n=15,992) | Stage II Left-Sided (n=7,586) | Stage III Right-Sided (n=11,655) | Stage III Left-Sided (n=5,493) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||
| HR | 95% CI | p-value | HR | 95% CI | p-value | HR | 95% CI | p-value | HR | 95% CI | p-value | |
| Unadjusted | ||||||||||||
| No Chemotherapy | 1.00 | 1.00 | 1.00 | 1.00 | ||||||||
| Any Chemotherapy | 0.66 | 0.61, 0.72 | <0.001 | 0.61 | 0.55, 0.68 | <0.001 | 0.50 | 0.47, 0.53 | <0.001 | 0.44 | 0.40, 0.47 | <0.001 |
| Adjusted for all covariates a | ||||||||||||
| No Chemotherapy | 1.00 | 1.00 | 1.00 | 1.00 | ||||||||
| Any Chemotherapy | 0.92 | 0.85,1.01 | 0.07 | 0.83 | 0.74, 0.93 | 0.001 | 0.64 | 0.60 0.68 | <0.001 | 0.59 | 0.54, 0.65 | <0.001 |
| Adjusted for all covariates with propensity score weightingb | ||||||||||||
| No Chemotherapy | 1.00 | 1.00 | 1.00 | 1.00 | ||||||||
| Any Chemotherapy | 0.97 | 0.87, 1.09 | 0.64 | 0.97 | 0.84, 1.12 | 0.68 | 0.64 | 0.59, 0.68 | <0.001 | 0.61 | 0.56, 0.68 | <0.001 |
| HR, hazard ratio; CI, confidence interval | ||||||||||||
Cox regression model controlling for age, gender, ethnicity, marital status, residence, SEER registry, year of diagnosis by Medicare colonoscopy coverage, census-tract income and education, hospitalizations in prior year, any in-hospital complication, HCC risk score, and poor prognostic features
See Table 1 for variables included in the propensity score models
Kaplan-Meier survival analysis with propensity score weighting compared 5-year survival between patients who received any versus no adjuvant chemotherapy by tumor location (right- versus left-side) within each stage group (Fig 1a through d). No difference was observed between treated and untreated patients with either stage II right-sided (Wald χ2=0.27, p=0.60) or stage II left-sided cancers (Wald χ2=0.28, p=0.60) by Cox regression-based test for equality of survival curves. In contrast, all groups of stage III patients derived a significant survival benefit from chemotherapy (right-side: Wald χ2=243.91; p<0.001; left-side: Wald χ2=133.37; p<0.001).
Figure 1.

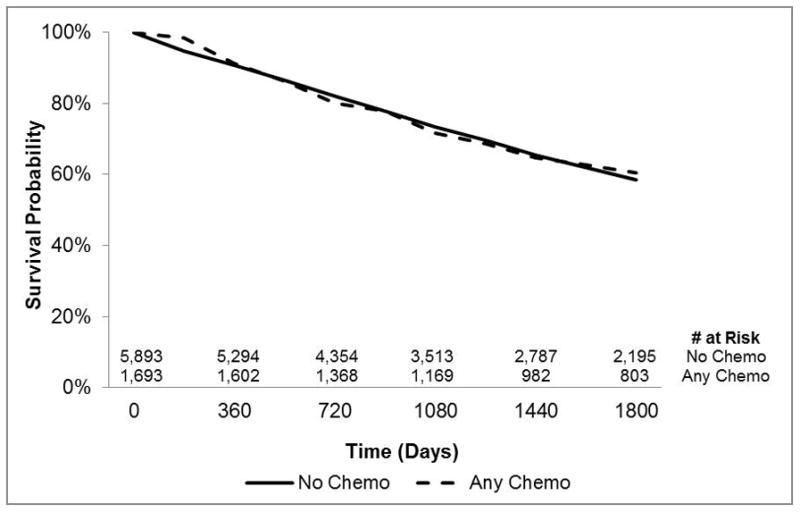


Kaplan-Meier survival curves showing overall survival by chemotherapy administration, tumor location (right- vs left-side), and stage group with propensity score weightin(Panel 1a: Stage II right-sided disease, Panel 1b: Stage II left-sided disease, Panel 1c: Stage III right-sided disease, Panel 1d: Stage III left-sided disease
DISCUSSION
Adjuvant chemotherapy is standard of care for patients with resected stage III colon cancer,1 while the benefit for stage II patients remains unclear. Some studies have found no significant survival benefit for stage II patients who receive this treatment,3,4 while others report a definite benefit of adjuvant chemotherapy over surgery alone for stage II patients.36,37 However, the majority of evidence shows only a small absolute survival benefit, in the range of 2–4%.38–40 This small benefit needs to be compared to the high likelihood of side effects from treatment. Given the previously identified relationship between MSI and response to chemotherapy, our primary aim was to determine if a subset of patients with stage II colon cancer, defined by tumor location as a surrogate for MSI status, could be identified that received a survival benefit from adjuvant chemotherapy. We found that, after controlling for multiple patient, risk adjustment, treatment, and cancer-specific variables in addition to propensity score weighting, there was no survival benefit for either stage II right-sided or stage II left-sided colon cancer patients who received any adjuvant chemotherapy compared to those who did not. Consistent with previous studies,4,37,39,41–46 we do show a significant survival benefit for stage III patients who receive adjuvant chemotherapy, regardless of tumor location.
Colon tumor heterogeneity has been described by many groups.5–7 More specifically, different gene expressions have been described between tumors in the right and left colon, such as higher MSI in right-sided cancers and more chromosomal instability in left-sided cancers.8–10 In a previous study, Ribic et al.11 found a differential benefit from fluorouracil-based adjuvant chemotherapy for both stage II and stage III colon cancers with microsatellite-stable and MSI-low tumors compared to those with MSI-high tumors. And further analysis of patients included in the MOSAIC trial37 points to a potential benefit of adding oxaliplatin to adjuvant 5-fluorouracil/leucovorin in patients with MSI-high stage III colon cancer.47 MSI-high tumors are more prevalent on the right side of the colon10,16–19 and even more so in stage II right-sided cancers.48 MSI status of a tumor is not always readily available. In this study, we used tumor location (right- versus left-side) as a proxy for MSI status and did not find any significant benefit for traditional fluorouracil-based adjuvant chemotherapy for either stage II right-sided or stage II left-sided colon cancers.
There are limitations to this study. First, we examine only Medicare beneficiaries age 66 years and older at the time of diagnosis, which limits the applicability. Molecular etiologies of MSI in colon cancers are associated with age. MSI-high tumors in patients less than 50 years old are four times more likely to be due to a germline mutation in a DNA mismatch repair gene than in patients older than 50, who are four times more likely to have MSI due to MLH1 methylation.16 This study should be repeated in a broader population before applying our results to younger patients. Second, unmeasured factors such as patient preferences or provider practice patterns may play a role in patient outcome. We attempted to limit the impact of these unmeasured factors by adjusting our models with propensity score weighting for receipt of chemotherapy and including a clinically relevant patient, risk-adjustment, and treatment variables in our final models. The major limitation of this study is the fact that MSI status was not available in this data set, which prohibits direct testing of our hypothesis that MSI status impacts benefit from adjuvant chemotherapy in this population of patients. A related limitation is that due to the administrative claim data from Medicare, we were unable to specifically look at survival benefit by type of adjuvant chemotherapy. This limitation is especially relevant in light of the recent findings from further analysis of patients in the MOSAIC trial.37,47 Further studies are needed to examine the effect of different types of adjuvant chemotherapy by colon cancer location and MSI status.
Despite these limitations, our findings add to the existing literature surrounding the controversy of adjuvant chemotherapy in stage II colon cancers. We know that substantial numbers of patients with stage II colon cancer continue to receive adjuvant chemotherapy despite current guidelines that do not recommend routine use.2 Our results reinforce these guidelines and show that a simple division of stage II colon cancers as right- or left-sided does not identify a subgroup of patients that might receive benefit from adjuvant chemotherapy. When added to the existing literature, our results highlight the fact that the relationship between tumor biology, tumor location, and response to adjuvant chemotherapy is not straightforward. Future efforts to personalize treatment of colon cancers will likely involve direct gene expression testing for each individual. This practice will also continue to play an important role in shaping future clinical trials that examine adjuvant chemotherapy regimens for colon cancer.
SYNOPSIS.
Among Medicare patients with stage II colon cancer, adjuvant chemotherapy does not improve overall 5-year survival for either right- or left-sided colon cancers. This lack of benefit should be considered in treatment algorithms for older adults with colon cancer.
Acknowledgments
The authors thank Research Specialist Lauren Weeth-Feinstein, MPH, for her assistance in formatting and proofing this manuscript.
Footnotes
Disclosures and Research Support: None of the authors have conflicts to disclose. This manuscript represents an original work of the authors, and has not been previously published. The results were presented as a poster at Digestive Disease Week, May 18–21, 2013, Orlando, FL. Dr. Weiss was supported by the Agency for Healthcare Research and Quality (AHRQ)/National Research Service Award (NRSA) T-32 Institutional Training Program Grant Number: 5-T32-HS00083. This project also received funding from the University of Wisconsin Carbone Cancer Center (UWCCC) Support Grant from the National Cancer Institute, grant number P30 CA014520. This project was also supported by the Health Innovation Program, the UW School of Medicine and Public Health from The Wisconsin Partnership Program, and the-Community Academic Partnerships core of the University of Wisconsin Institute for Clinical and Translational Research (UW ICTR) through the National Center for Advancing Translational Sciences (NCATS), grant UL1TR000427. The ideas and opinions expressed herein are those of the author(s) and endorsement by the State of California, Department of Public Health the National Cancer Institute, the National Institutes of Health, and the Centers for Disease Control and Prevention or their Contractors and Subcontractors is not intended nor should be inferred. This study used the linked SEER-Medicare database. The interpretation and reporting of these data are the sole responsibility of the authors. The collection of the California cancer incidence data used in this study was supported by the California Department of Public Health as part of the statewide cancer reporting program mandated by California Health and Safety Code Section 103885; the National Cancer Institute’s Surveillance, Epidemiology and End Results Program under contract N01-PC-35136 awarded to the Northern California Cancer Center, contract N01-PC-35139 awarded to the University of Southern California, and contract N02-PC-15105 awarded to the Public Health Institute; and the Centers for Disease Control and Prevention’s National Program of Cancer Registries, under agreement #U55/CCR921930-02 awarded to the Public Health Institute. The authors acknowledge the efforts of the Applied Research Program, NCI; the Office of Research, Development and Information, CMS; Information Management Services (IMS), Inc.; and the Surveillance, Epidemiology, and End Results (SEER) Program tumor registries in the creation of the SEER-Medicare database.
References
- 1.NIH consensus conference. Adjuvant therapy for patients with colon and rectal cancer. JAMA. 1990;264:1444–50. [PubMed] [Google Scholar]
- 2.Benson AB, 3rd, Schrag D, Somerfield MR, et al. American Society of Clinical Oncology recommendations on adjuvant chemotherapy for stage II colon cancer. J Clin Oncol. 2004;22:3408–19. doi: 10.1200/JCO.2004.05.063. [DOI] [PubMed] [Google Scholar]
- 3.Schrag D, Rifas-Shiman S, Saltz L, Bach PB, Begg CB. Adjuvant chemotherapy use for Medicare beneficiaries with stage II colon cancer. J Clin Oncol. 2002;20:3999–4005. doi: 10.1200/JCO.2002.11.084. [DOI] [PubMed] [Google Scholar]
- 4.O’Connor ES, Greenblatt DY, LoConte NK, Gangnon RE, Liou JL, Heise CP, Smith MA. Adjuvant chemotherapy for stage II colon cancer with poor prognostic features. J Clin Oncol. 2011;29:3381–8. doi: 10.1200/JCO.2010.34.3426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lanza G, Jr, Maestri I, Ballotta MR, Dubini A, Cavazzini L. Relationship of nuclear DNA content to clinicopathologic features in colorectal cancer. Mod Pathol. 1994;7:161–5. [PubMed] [Google Scholar]
- 6.Bufill JA. Colorectal cancer: evidence for distinct genetic categories based on proximal or distal tumor location. Ann Intern Med. 1990;113:779–88. doi: 10.7326/0003-4819-113-10-779. [DOI] [PubMed] [Google Scholar]
- 7.Iacopetta B. Are there two sides to colorectal cancer? Int J Cancer. 2002;101:403–8. doi: 10.1002/ijc.10635. [DOI] [PubMed] [Google Scholar]
- 8.Glebov OK, Rodriguez LM, Nakahara K, et al. Distinguishing right from left colon by the pattern of gene expression. Cancer Epidemiol Biomarkers Prev. 2003;12:755–62. [PubMed] [Google Scholar]
- 9.Birkenkamp-Demtroder K, Olesen SH, Sorensen FB, Laurberg S, Laiho P, Aaltonen LA, Orntoft TF. Differential gene expression in colon cancer of the caecum versus the sigmoid and rectosigmoid. Gut. 2005;54:374–84. doi: 10.1136/gut.2003.036848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gervaz P, Bucher P, Morel P. Two colons-two cancers: paradigm shift and clinical implications. J Surg Oncol. 2004;88:261–6. doi: 10.1002/jso.20156. [DOI] [PubMed] [Google Scholar]
- 11.Ribic CM, Sargent DJ, Moore MJ, et al. Tumor microsatellite-instability status as a predictor of benefit from fluorouracil-based adjuvant chemotherapy for colon cancer. N Engl J Med. 2003;349:247–57. doi: 10.1056/NEJMoa022289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Popat S, Hubner R, Houlston RS. Systematic review of microsatellite instability and colorectal cancer prognosis. J Clin Oncol. 2005;23:609–18. doi: 10.1200/JCO.2005.01.086. [DOI] [PubMed] [Google Scholar]
- 13.Hemminki A, Mecklin JP, Jarvinen H, Aaltonen LA, Joensuu H. Microsatellite instability is a favorable prognostic indicator in patients with colorectal cancer receiving chemotherapy. Gastroenterology. 2000;119:921–8. doi: 10.1053/gast.2000.18161. [DOI] [PubMed] [Google Scholar]
- 14.Malesci A, Laghi L, Bianchi P, et al. Reduced likelihood of metastases in patients with microsatellite-unstable colorectal cancer. Clinical Cancer Res. 2007;13:3831–9. doi: 10.1158/1078-0432.CCR-07-0366. [DOI] [PubMed] [Google Scholar]
- 15.Samowitz WS, Curtin K, Ma KN, Schaffer D, Coleman LW, Leppert M, Slattery ML. Microsatellite instability in sporadic colon cancer is associated with an improved prognosis at the population level. Cancer Epidemiol Biomarkers Prev. 2001;10:917–23. [PubMed] [Google Scholar]
- 16.Poynter JN, Siegmund KD, Weisenberger DJ, et al. Molecular characterization of MSI-H colorectal cancer by MLHI promoter methylation, immunohistochemistry, and mismatch repair germline mutation screening. Cancer Epidemiol Biomarkers Prev. 2008;17:3208–15. doi: 10.1158/1055-9965.EPI-08-0512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thibodeau SN, Bren G, Schaid D. Microsatellite instability in cancer of the proximal colon. Science. 1993;260:816–9. doi: 10.1126/science.8484122. [DOI] [PubMed] [Google Scholar]
- 18.Kim H, Jen J, Vogelstein B, Hamilton SR. Clinical and pathological characteristics of sporadic colorectal carcinomas with DNA replication errors in microsatellite sequences. Am J Pathol. 1994;145:148–56. [PMC free article] [PubMed] [Google Scholar]
- 19.Thibodeau SN, French AJ, Cunningham JM, et al. Microsatellite instability in colorectal cancer: different mutator phenotypes and the principal involvement of hMLH1. Cancer Res. 1998;58:1713–8. [PubMed] [Google Scholar]
- 20.Potosky AL, Riley GF, Lubitz JD, Mentnech RM, Kessler LG. Potential for cancer related health services research using a linked Medicare-tumor registry database. Med Care. 1993;31:732–48. [PubMed] [Google Scholar]
- 21.Warren JL, Klabunde CN, Schrag D, Bach PB, Riley GF. Overview of the SEER-Medicare data: content, research applications, and generalizability to the United States elderly population. Med Care. 2002;40:IV-3-18. doi: 10.1097/01.MLR.0000020942.47004.03. [DOI] [PubMed] [Google Scholar]
- 22.Lo NS, Sarr MG. Mucinous cystadenocarcinoma of the appendix. The controversy persists: a review. Hepato-gastroenterology. 2003;50:432–7. [PubMed] [Google Scholar]
- 23.Bradley CJ, Given CW, Dahman B, Fitzgerald TL. Adjuvant chemotherapy after resection in elderly Medicare and Medicaid patients with colon cancer. Arch Intern Med. 2008;168:521–9. doi: 10.1001/archinternmed.2007.82. [DOI] [PubMed] [Google Scholar]
- 24.Dobie SA, Baldwin LM, Dominitz JA, Matthews B, Billingsley K, Barlow W. Completion of therapy by Medicare patients with stage III colon cancer. J Natl Cancer Inst. 2006;98:610–9. doi: 10.1093/jnci/djj159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meguid RA, Slidell MB, Wolfgang CL, Chang DC, Ahuja N. Is there a difference in survival between right- versus left-sided colon cancers? Ann Surg Oncol. 2008;15:2388–94. doi: 10.1245/s10434-008-0015-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Benedix F, Kube R, Meyer F, Schmidt U, Gastinger I, Lippert H. Comparison of 17,641 patients with right- and left-sided colon cancer: differences in epidemiology, perioperative course, histology, and survival. Dis Colon Rectum. 2010;53:57–64. doi: 10.1007/DCR.0b013e3181c703a4. [DOI] [PubMed] [Google Scholar]
- 27.Weiss JM, Pfau PR, O’Connor ES, King J, LoConte N, Kennedy G, Smith MA. Mortality by stage for right- vs. left-sided colon cancer: Analysis of SEER-Medicare data. J Clin Oncol. 2011;29:4401–4409. doi: 10.1200/JCO.2011.36.4414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morris AM, Baldwin LM, Matthews B, Dominitz JA, Barlow WE, Dobie SA, Billingsley KG. Reoperation as a quality indicator in colorectal surgery: a population-based analysis. Ann Surg. 2007;245:73–9. doi: 10.1097/01.sla.0000231797.37743.9f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ash AS, Ellis RP, Pope GC, et al. Using diagnoses to describe populations and predict costs. Health Care Financ Rev. 2000;21:7–28. [PMC free article] [PubMed] [Google Scholar]
- 30.Fenton JJ, Cai Y, Green P, Beckett LA, Franks P, Baldwin LM. Trends in colorectal cancer testing among Medicare subpopulations. Am J Prev Med. 2008;35:194–202. doi: 10.1016/j.amepre.2008.05.029. [DOI] [PubMed] [Google Scholar]
- 31.Rosenbaum PR, Rubin DB. The Central Role of the Propensity Score in Observational Studies for Causal Effects. Biometrika. 1983;70:41–55. [Google Scholar]
- 32.Rubin DB. Estimating causal effects from large data sets using propensity scores. Ann Intern Med. 1997;127:757–63. doi: 10.7326/0003-4819-127-8_part_2-199710151-00064. [DOI] [PubMed] [Google Scholar]
- 33.Rubin DB. The design versus the analysis of observational studies for causal effects: parallels with the design of randomized trials. Stat Med. 2007;26:20–36. doi: 10.1002/sim.2739. [DOI] [PubMed] [Google Scholar]
- 34.D’Agostino RB., Jr Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat Med. 1998;17:2265–81. doi: 10.1002/(sici)1097-0258(19981015)17:19<2265::aid-sim918>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 35.Imbens GW. The role of the propensity score in estimating dose-response functions. Biometrika. 2000;87:706–710. [Google Scholar]
- 36.Wilkinson NW, Yothers G, Lopa S, Costantino JP, Petrelli NJ, Wolmark N. Long-term survival results of surgery alone versus surgery plus 5-fluorouracil and leucovorin for stage II and stage III colon cancer: pooled analysis of NSABP C-01 through C-05. A baseline from which to compare modern adjuvant trials. Ann Surg Oncol. 2010;17:959–66. doi: 10.1245/s10434-009-0881-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Andre T, Boni C, Navarro M, et al. Improved overall survival with oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment in stage II or III colon cancer in the MOSAIC trial. J Clin Oncol. 2009;27:3109–16. doi: 10.1200/JCO.2008.20.6771. [DOI] [PubMed] [Google Scholar]
- 38.Gray R, Barnwell J, McConkey C, Hills RK, Williams NS, Kerr DJ. Adjuvant chemotherapy versus observation in patients with colorectal cancer: a randomised study. Lancet. 2007;370:2020–9. doi: 10.1016/S0140-6736(07)61866-2. [DOI] [PubMed] [Google Scholar]
- 39.Gill S, Loprinzi CL, Sargent DJ, et al. Pooled analysis of fluorouracil-based adjuvant therapy for stage II and III colon cancer: who benefits and by how much? J Clin Oncol. 2004;22:1797–806. doi: 10.1200/JCO.2004.09.059. [DOI] [PubMed] [Google Scholar]
- 40.Figueredo A, Charette ML, Maroun J, Brouwers MC, Zuraw L. Adjuvant therapy for stage II colon cancer: a systematic review from the Cancer Care Ontario Program in evidence-based care’s gastrointestinal cancer disease site group. J Clin Oncol. 2004;22:3395–407. doi: 10.1200/JCO.2004.03.087. [DOI] [PubMed] [Google Scholar]
- 41.Sargent D, Sobrero A, Grothey A, et al. Evidence for cure by adjuvant therapy in colon cancer: observations based on individual patient data from 20,898 patients on 18 randomized trials. J Clin Oncol. 2009;27:872–7. doi: 10.1200/JCO.2008.19.5362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mamounas E, Wieand S, Wolmark N, et al. Comparative efficacy of adjuvant chemotherapy in patients with Dukes’ B versus Dukes’ C colon cancer: results from four National Surgical Adjuvant Breast and Bowel Project adjuvant studies (C-01, C-02, C-03, and C-04) J Clin Oncol. 1999;17:1349–55. doi: 10.1200/JCO.1999.17.5.1349. [DOI] [PubMed] [Google Scholar]
- 43.Sharif S, O’Connell MJ, Yothers G, Lopa S, Wolmark N. FOLFOX and FLOX regimens for the adjuvant treatment of resected stage II and III colon cancer. Cancer Invest. 2008;26:956–63. doi: 10.1080/07357900802132550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wolmark N, Rockette H, Mamounas E, et al. Clinical trial to assess the relative efficacy of fluorouracil and leucovorin, fluorouracil and levamisole, and fluorouracil, leucovorin, and levamisole in patients with Dukes’ B and C carcinoma of the colon: results from National Surgical Adjuvant Breast and Bowel Project C-04. J Clin Oncol. 1999;17:3553–9. doi: 10.1200/JCO.1999.17.11.3553. [DOI] [PubMed] [Google Scholar]
- 45.Wolmark N, Rockette H, Fisher B, et al. The benefit of leucovorin-modulated fluorouracil as postoperative adjuvant therapy for primary colon cancer: results from National Surgical Adjuvant Breast and Bowel Project protocol C-03. J Clin Oncol. 1993;11:1879–87. doi: 10.1200/JCO.1993.11.10.1879. [DOI] [PubMed] [Google Scholar]
- 46.Efficacy of adjuvant fluorouracil and folinic acid in B2 colon cancer. International Multicentre Pooled Analysis of B2 Colon Cancer Trials (IMPACT B2) Investigators. J Clin Oncol. 1999;17:1356–63. [PubMed] [Google Scholar]
- 47.Flejou J-F, Andre T, Chibaudel B, et al. Effect of adding oxaliplatin to adjuvant 5-fluorouracil/leucovorin (5FU/LV) in patients with defective mismatch repair (dMMR) colon cancer stage II and III included in the MOSIAC study. J Clin Oncol. 2013;31 (suppl; abstr 3524) [Google Scholar]
- 48.Jernvall P, Makinen MJ, Karttunen TJ, Makela J, Vihko P. Microsatellite instability: impact on cancer progression in proximal and distal colorectal cancers. Eur J Cancer. 1999;35:197–201. doi: 10.1016/s0959-8049(98)00306-2. [DOI] [PubMed] [Google Scholar]


