Abstract
Background
To evaluate the efficacy and safety of latanoprost compared with timolol in the treatment of Asian patients with chronic angle-closure glaucoma (CACG).
Methods
Relevant trials were identified through systematic searches of Medline, EMBASE, PubMed, Cochrane Library, Google Scholar and several Chinese databases. The main outcome measures included absolute and relative reduction of intraocular pressure (IOP) at mean, peak and trough from baseline, ocular adverse effects and systemic adverse events.
Results
Seven randomized controlled trials with 685 patients were included. In comparison with timolol, latanoprost reduced absolute IOP in CACG patients by more than 2.3 mmHg (95%CI, 1.8∼2.9, P<0.01), 2.4 mmHg (95%CI, 1.9∼2.9, P<0.01) and 2.5 mmHg (95%CI, 1.6∼3.3, P<0.01) at mean, peak and trough, respectively. As for relative IOP, there is 9.0% (95%CI, 6.6∼11.4, P<0.01), 9.7% (95%CI, 7.6∼11.8, P<0.01), and 10.8% (95%CI, 7.4∼14.3, P<0.01) greater reduction among latanoprost users than among timolol users. The differences were statistically significant at all time points (1, 2, 4, 8, 12, and 24 weeks). More ocular adverse effects (OR = 1.49, 95% CI, 1.05∼2.10, P = 0.02) and less systemic adverse events (OR = 0.46, 95% CI, 0.25∼0.84, P = 0.01) were observed in latanoprost group in comparison with timolol group.
Conclusion
Compared with timolol, latanoprost was significantly more effective in lowering IOP of Asian patients with CACG, with higher risk of ocular adverse effects but lower risk of systemic adverse events, and might be a good substitute for CACG patients.
Introduction
Chronic angle-closure glaucoma (CACG), a disease characterized by elevated intraocular pressure (IOP) resulting from gradual angle closure and a decrease in aqueous humor outflow, is considered as a major form of glaucoma in Asia [1]–[6]. First-line therapy for CACG is peripheral iridotomy (PI) which is an invasive procedure [7], [8]. However, for many CACG patients, PI alone is insufficient to control IOP. Topical β-blockers such as timolol which reduces the aqueous humor generation is usually added to these patients to further lower their IOP.
Latanoprost, a representative of prostaglandin analogs which can significantly reduce IOP by increasing uveoscleral outflow, has been proved to be more effective in lowering IOP in patients with primary open-angle glaucoma (POAG) or ocular hypertension (OH) than timolol [9], [10]. As for CACG patients, however, the differences between these two eyedrops in efficacy and safety have not been systematically evaluated. Previous studies [11], [12] were designed differently, and were mainly performed in Asia-Pacific population. Whether there is difference in lowering IOP among different populations remains unclear. In addition, how long latanoprost can keep a stable level in lowering IOP than timolol is also of concern.
The purposes of this meta-analysis was to systematically evaluate the efficacy and safety of latanoprost compared with timolol in treating Asian patients with CACG, to compare the efficacy and safety difference between these two drugs in Chinese Mainland population and other Asia-Pacific population, and to evaluate how long could this difference last and the robustness of the available evidence.
Methods
Search strategy
Articles were identified through a computerized search up to March 2013 in the following data sources: MEDLINE, EMBASE, PubMed and Cochrane Library, Google Scholar and several Chinese databases including CBM (Chinese Biomedical Literature Database), CNKI (China National Knowledge Infrastructure), WANFANG DATA and VIP Database. The keywords were angle-closure glaucoma, latanoprost, xalatan, timolol and timoptol. Mesh terms were used if available. Otherwise the keywords were searched in full text. See Table S1 for search strategy in PubMed. References within the retrieved articles from the above search were used to search for additional trials. We also searched in Chinese Clinical Trial Registry, WHO International Clinical Trials Registry Platform and ClinicalTrials.gov for ongoing trials.
Inclusion criteria
Relevant clinical trials (RCT) were selected based on the protocol-determined selection criteria. (i) Study type: RCTs. (ii) Population: Patients with chronic angle-closure glaucoma, including primary chronic angle-closure glaucoma and residual chronic angle-closure glaucoma after laser or surgical treatment. (iii) Intervention: Latanoprost versus timolol in each group without combination of any other drugs. (iv) Primary outcome measures: Absolute and relative IOP reduction from baseline at mean, peak and trough were used for efficacy analysis; Occurrences of ocular and systemic adverse effects were used for safety analysis.
For latanoprost 0.005% once daily, the time points were as close to 12 hours for peak and 24 hours for trough after administration as possible. And for timolol 0.5% twice daily, the time points were as close to 2 hours for peak and 12 hours for trough after administration as possible [13]. Mean IOP was defined as the mean value of measurements at all time points throughout the 24-h cycle.
A broad focus on adverse effects was chosen to detect a variety of adverse effects, whether known or previously unrecognized. All cases reported in the studies with information falling under any of the terms ‘adverse effect’, ‘adverse drug reaction’, ‘side effect’, ‘toxic effect’, ‘adverse event’ and ‘complication’ were considered as adverse effects. Ocular adverse effects were defined as adverse effects related with eyes and the remaining was classified as systemic adverse effects in accordance with the term used in the trials.
Screening and data extraction
Trial eligibility was determined by two authors independently (C.R., D.Q.J.). Title, abstract, and medical subject heading words of the obtained publications were initially used for a rough judgment on eligibility of an article. Of the remaining identified publications, the full texts were downloaded for further judgment. Data extraction was performed according to a customized form by two authors (C.R., D.Q.J.) independently. The form covered information on article characteristics (authors, year of publication, location, language, center and funding), study design (type of study, control and intervention, length of wash-out period and study), participants (number, age, gender, race and surgical treatment), and outcomes (IOP, ocular and systemic adverse effects). Any disagreements in article selection and data extraction were resolved by discussion or a third authors (Y.Z.R).
Quality assessment
Methodological quality was evaluated (in duplicate by C.R. and D.Q.J.) using the Cochrane Collaboration tool for assessing risk of bias of clinical trials and figures were generated using RevMan (5.2) [14]. The tool included six individual domains: sequence generation, allocation concealment, blinding, incomplete outcome data, selective outcome report and other sources of bias. Each domain had one entry with the judgment of ‘Low risk’, ‘High risk’, or ‘Unclear risk’ of bias and a description of the design, conduct or observations that underlie the judgment. We contacted with the authors by email and waited for a response for at least 4 weeks when information reported in the trials was insufficient to make a judgment.
Statistical analysis
Outcome measures were assessed on an intention-to-treat (ITT) analysis. The ITT population was comprised of all randomized patients who received a minimum of 1 dose of active treatment and provided a valid baseline measurement. Otherwise, available case analysis was used.
Original data were obtained from the articles as much as possible; data that could not be obtained were calculated when necessary. When mean and standard deviation (SD) of IOP reduction (IOPR) were not available directly, they were calculated by the formulas as the following [15]–[17]:
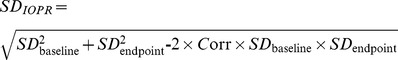 |
The correlation coefficient indicates correlation between baseline SD and endpoint SD of the measurement, and was calculated from trials with known SDchange. For studies that only reported standard errors (SEs), SD was calculated by the formula 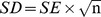 . The mean and SD of relative IOPR (IOPR%) were then estimated by the formulas [13], [18], [19]:
. The mean and SD of relative IOPR (IOPR%) were then estimated by the formulas [13], [18], [19]:
A random-effects model was used if trials were heterogeneous on the basis of the Q statistic for heterogeneity and the reasons for the heterogeneity could not be identified [20], [21]. Otherwise a fixed-effect model was used to estimate the pooled effects.
The mean difference (MD) was used to measure the absolute difference between the mean values of two groups in a clinical trial. The MD here referred to the subtraction of mean value in latanoprost group from the mean value in timolol group. The odds ratio (OR) were estimated for the adverse effects using ITT analysis. In the pooling of odds ratio, the Mantel-Haenszel method was used for the fixed-effect model. The method described by DerSimonian and Laird [22] was used for the random-effects model.
Subgroup analyses between Chinese Mainland population and other Asia-Pacific population were used to investigate heterogeneity of efficacy in different populations. Sensitivity analyses were undertaken to evaluate the effect of quality of randomized controlled trials in terms of the study design, withdrawal rate and pharmaceutical industry support. To detect publication biases, we explored asymmetry in funnel plots. Egger's measure of publication bias was calculated if more than 10 studies were included [23].
Results
Study eligibility
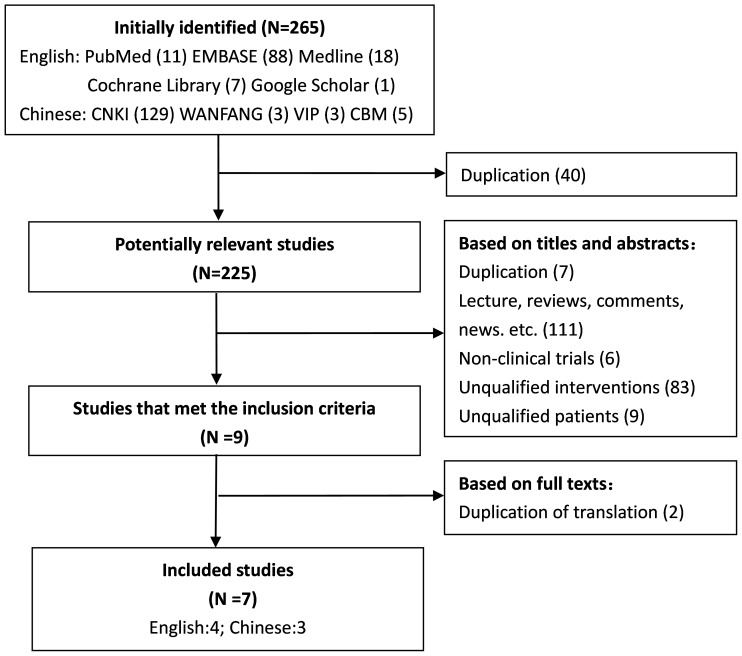
The selection flow of studies was summarized in Figure 1. In case more than one reason for exclusion was present, only the first reason encountered was listed. The initial search identified 125 studies in English and 140 studies in Chinese. Finally, 7 RCTs [24]–[30] were included in this meta-analysis.
Figure 1. Flow diagram of the results of the search strategy.
Trials characteristics
Characteristics of 7 RCTs were summarized in Table 1. These RCTs were conducted in various countries including China [26], [28]–[30], Indonesia [25], Malaysia [25], Philippines [25], Singapore [24], [25], Thailand [25], and India [27]. A total of 685 patients were included with 343 in latanoprost group and 342 in timolol group. Three trials [24], [27], [30] claimed that the authors had no relevant financial interest. Baseline and endpoint IOP were summarized in Table 2. Four trials [24], [25], [27], [28] reported the severity of the adverse effects and no treatment related serious adverse events were observed.
Table 1. Baseline Characteristics of Eligible Randomized Clinical Trial.
| Studies | Design | Latanoprost (%) | Timolol(%) | Center | Location | Funding | Total No. | Withdrawals (%) | Age (Mean±SD) | Sex (M/F) | Population | Types of glaucoma | Operations | Washout period | Length |
| Aung 2000 | DB, PG | 0.005 eve | 0.5bid | Multi | Singapore | Singapore Eye Research Institute (partly) | 32 | 9.4 | T:64±7 C:64±8 | 16/16 | Chinese, Malay, Indian | Primary chronic angle-closure glaucoma | After peripheral iridotomy | Yes | 2 w |
| Chew 2004 | DB, PG | 0.005 eve | 0.5bid | Multi | Hong Kong, Indonesia, Malaysia, Philippines, Singapore, Taiwan, Thailand | Pharmacia Corporation | 275 | 6.2 | T:63.3±9.4 C:63.1±9.5 | 70/205 | Asian or Pacific Islander | Chronic angle-closure glaucoma | After peripheral iridotomy | Yes | 12 w |
| Kong 2005 | SB, PG | 0.005 eve | 0.5bid | Single | China | NR | 49 | 16.3 | T:61.6±8.8 C:60.4±7.9 | 14/35 | Chinese | Residual angle-closure glaucoma | After out-filtrating surgery or iridotomy | Yes | 8 w |
| Liu 2006 | NR, PG | 0.005 eve | 0.5bid | Single | China | NR | 60 | 0 | - | 35/25 | Chinese | Chronic angle-closure glaucoma | NR | Yes | 2 w |
| Sihota 2004 | DB, CR | 0.005 eve | 0.5bid | Single | India | NR | 30 | 0 | 57.7±7.4 | 18/12 | Indian | Primary chronic angle-closure glaucoma | After laser iridotomy | Yes | 3 m |
| Wang 2002 | NR, PG | 0.005 eve | 0.5bid | Single | China | NR | 68 | 17.6 | - | - | Chinese | Residual angle-closure glaucoma | After peripheral iridotomy | Yes | 6 m |
| Zhao 2012 | OL, PG | 0.005 eve | 0.5bid | Multi | China | Pharmacia Upjohn, China (acquired by Pfizer Inc) | 142 | 0.7 | T:63±7.3 C:61.3±8.9 | 47/94 | Chinese | Chronic angle-closure glaucoma | After laser or surgical peripheral iridotomy | Yes | 8 w |
CR indicates crossover; DB, double blind; NR, not reported; OL, open label; PG, parallel group; SB, single blind.
eve = evening regimen, bid = twice per day, w = week, m = month.
Table 2. Outcome Measurements of Eligible Randomized Clinical Trial.
| Studies | Time Points | Baseline IOP (mmHg) (Mean(SD)) | Endpoint IOP (mmHg) (Mean(SD)) | Ocular adverse effects (Times) | Systemic adverse effects (Times) | ||||||||||||
| Mean | Peak | Trough | Mean | Peak | Trough | ||||||||||||
| Latanoprost | Timolol | Latanoprost | Timolol | Latanoprost | Timolol | Latanoprost | Timolol | Latanoprost | Timolol | Latanoprost | Timolol | Latanoprost | Timolol | Latanoprost | Timolol | ||
| Aung2000 | Mean,peak(9AM), trough (5PM) | 25.7(3.6) | 25.2(4.1) | 27.2(3.8) | 26.8(4.5) | 24.2(3.8) | 23.5(4.3) | 16.9(5.2) | 19.4(2.4) | 17.0(4.7) | 20.0(2.6) | 16.8(5.8) | 18.9(2.8) | 14 | 12 | 0 | 1 |
| Chew2004 | Mean,peak(9AM), trough(5 PM) | 25.0(5.5) | 25.9(6.3) | 25.2(5.5) | 25.9(6.5) | 24.8(6.0) | 25.9(7.1) | 17.5(5.0) | 20.7(6.9) | NR | NR | NR | NR | 61 | 57 | 17 | 28 |
| Kong2005 | Mean,peak(9AM), trough(4PM) | 24.8(3.3)* | 25.8(3.9)* | 24.7(3.9) | 26.0(4.4) | 24.8(3.5) | 25.5(4.2) | 15.7(3.2)* | 18.1(3.6)* | 16.1(3.9) | 17.5(4.0) | 15.3(3.2) | 18.8(4.1) | 0 | 3 | 0 | 2 |
| Liu2006 | Peak(9AM) | NR | NR | 25.3(4.1) | 24.2(3.5) | NR | NR | NR | NR | 19.1(3.4) | 20.3(2.5) | NR | NR | 2 | 0 | 0 | 0 |
| Sihota2004 | Mean,peak(10AM),trough(7PM) | 23.4(2.1) | 23.4(2.1) | 24.6(3.9) | 24.6(3.9) | 22.4(3.1) | 22.4(3.1) | 15.3(1.8) | 17.4(1.7) | 14.6(2.8) | 17.9(3.6) | 15.6(3.1) | 16.9(3.8) | 6 | 2 | 0 | 0 |
| Wang2002 | Peak(9AM) | NR | NR | 24.1(1.0) | 24.1(1.1) | NR | NR | NR | NR | 17.0(1.0) | 19.1(1.3) | NR | NR | 4 | 5 | 0 | 0 |
| Zhao2012 | Mean,peak(9AM),trough(4PM) | 24.3(3.0) | 24.2(3.0) | NR | NR | NR | NR | 17.6(3.8) | 19.3(3.9) | NR | NR | NR | NR | 42 | 24 | 1 | 5 |
*Pooled value of IOP measured at 10 AM. and 4 PM; NR, not reported.
Trials quality
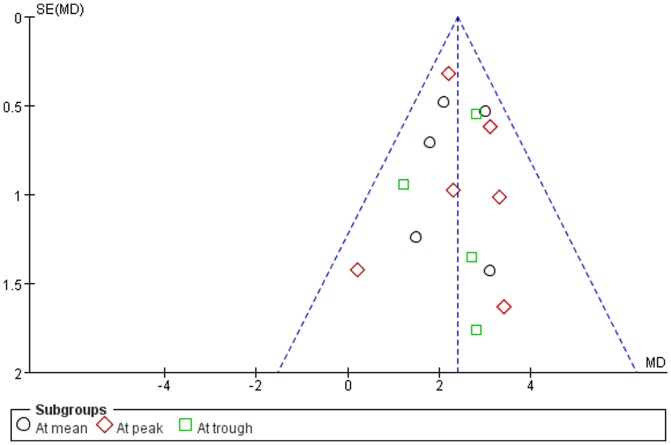
Five authors [24], [25], [27], [28], [30] replied our emails with detailed information. Figure 2 presented all judgments (‘Low risk’, ‘High risk’ and ‘Unclear risk’) in a cross-tabulation of study by entry. We were confident of blinding of participants and personnel in three trials [24], [25], [27], blinding of outcome assessment in four trials [24], [25], [27], [28], random sequence generation in four trials [24], [25], [27], [30], and allocation concealment in five trials [24], [25], [27], [28], [30]. There was no clear evidence of publication bias on the funnel plot, although the number of publications included was small (Figure 3).
Figure 2. Risk of bias summary.
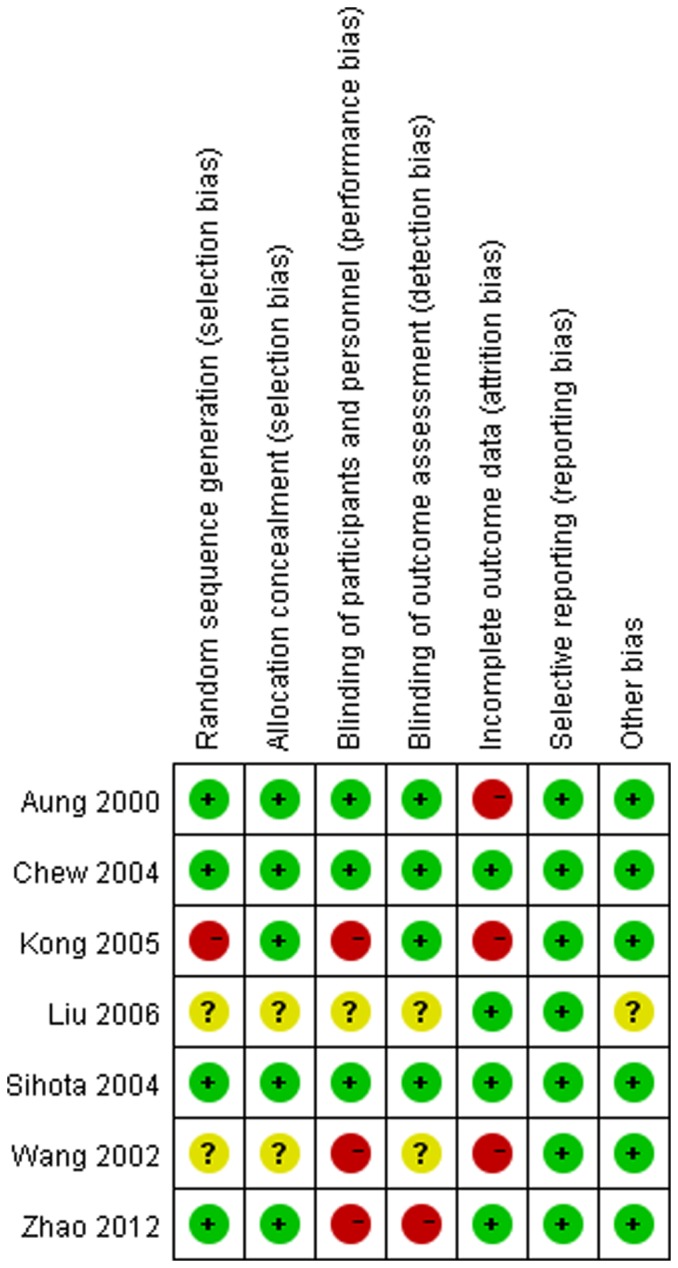
Red stands for high risk of bias, green stands for low risk of bias and yellow stands for unclear risk of bias.
Figure 3. Funnel plots of RCTs comparing lantanoprost with timolol in IOP reduction.
Efficacy
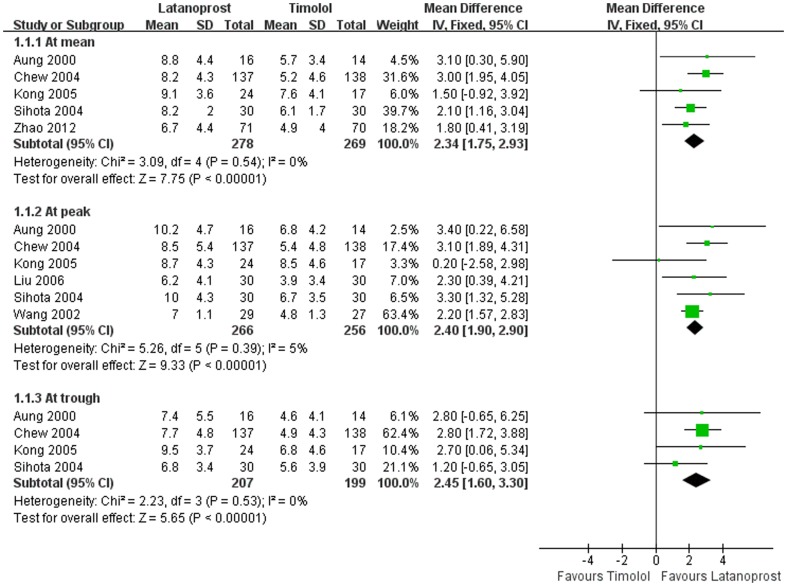
Absolute IOP reduction. Pooled absolute IOP reductions from baseline for latanoprost and timolol were shown in Figure 4. Mean differences in absolute IOP reduction between two groups were 2.3 mmHg (P<0.01) at mean, 2.4 mmHg (P<0.01) at peak and 2.5 mmHg (P<0.01) at trough, respectively.
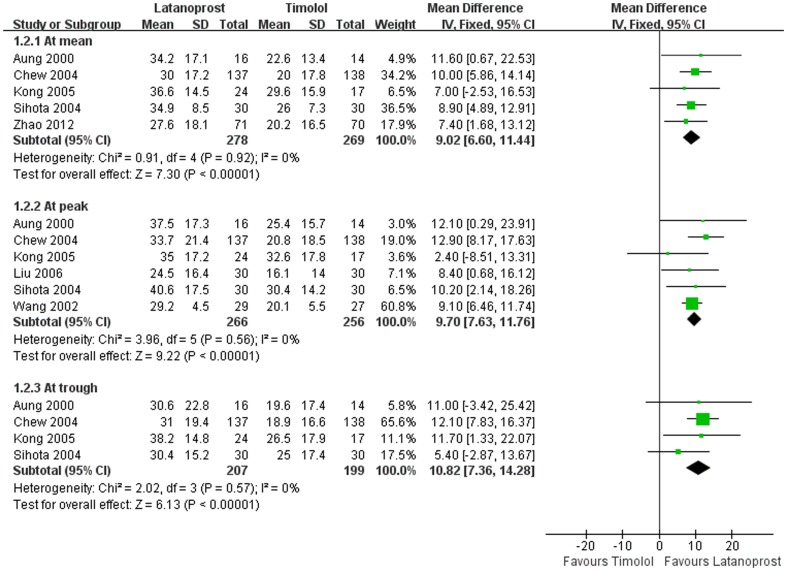
Relative IOP reduction. Relative IOP reductions from baseline of two drugs were shown in Figure 5. Mean differences in relative IOP reduction between latanoprost and timolol were 9.0% (P<0.01) at mean, 9.7% (P<0.01) at peak, and 10.8% (P<0.01) at trough, respectively.
Effects at different time points. Absolute reductions in mean and peak IOP between latanoprost and timolol at various time points were shown in Table 3. The differences were all statistically significant with greater reduction in IOP of latanoprost.
Sensitivity analysis. All trials were divided into subgroups by method of blinding and withdrawal, respectively. There were no statistically significant differences in absolute and relative IOP reduction between double blind/single blind group and open label/not reported group (P = 0.26, 0.36), and between groups with withdrawal rate less than 10% or 10% or more (P = 0.10, 0.22). (See Table S2)
Subgroup analysis. Mean differences in absolute IOP reductions at average, peak and trough between latanoprost and timolol were 1.7 mmHg, 2.1 mmHg and 2.7 mmHg in Chinese Mainland population, and 2.5 mmHg, 3.2 mmHg and 2.4 mmHg in other Asia-Pacific population, respectively. For relative IOP reduction at mean, peak and trough, the mean differences between latanoprost and timolol were 7.3%, 8.7% and 11.7% in Chinese mainland population, and 9.6%, 12.2% and 10.7% in other Asia-Pacific population, respectively. The differences between subgroups were neither statistically significant in absolute nor relative mean IOP reduction (P>0.05). There were no significant differences in absolute IOP reduction between studies with pharmaceutical industry funding and studies without funding (P = 0.38), though greater mean difference of IOP reduction was observed in funded studies (2.6 mmHg, 95%CI, 1.7∼3.4) than the studies that did not report any funding (2.1 mmHg, 95%CI, 1.3∼3.0). (See Table S3 and Figure S1)
Figure 4. Comparison of Absolute IOP reductions between latanoprost and timolol.
Figure 5. Comparison of Relative IOP reductions between latanoprost and timolol.
Table 3. Absolute IOP reduction from baseline at different time points.
| Time Points | No. of trials | No. of patients | Mean difference (mmHg)(95%CI) |
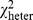 (p value) (p value) |
Zoverall (p value) | |
| Latanoprost | Timolol | |||||
| IOP reduction at mean | ||||||
| 1 week | 1 | 71 | 70 | 1.8(0.4,3.2) | - | 2.54(0.01) |
| 2 weeks | 2 | 87 | 84 | 2.1(0.9,3.3) | 0.58(0.45) | 3.48(<0.01) |
| 4 weeks | 1 | 71 | 70 | 1.6(0.3,3.0) | - | 2.32(0.02) |
| 8 weeks | 2 | 95 | 87 | 1.7(0.5,2.9) | 0.04(0.83) | 2.81(<0.01) |
| 12 weeks | 2 | 167 | 168 | 2.5(1.8, 3.2) | 1.56(0.21) | 6.99(<0.01) |
| IOP reduction at peak | ||||||
| 1 week | 3 | 69 | 58 | 2.0(1.3,2.6) | 0.99(0.61) | 6.15(<0.01) |
| 2 weeks | 4 | 99 | 88 | 2.0(1.4,2.6) | 1.79(0.62) | 6.42(<0.01) |
| 4 weeks | 2 | 53 | 44 | 1.9(1.3,2.6) | 1.00(0.32) | 5.90(<0.01) |
| 8 weeks | 2 | 53 | 44 | 1.9(1.4,2.5) | 1.54(0.21) | 6.64(<0.01) |
| 12 weeks | 2 | 196 | 195 | 2.3(1.8,2.9) | 3.45(0.18) | 8.25(<0.01) |
| 24 weeks | 1 | 29 | 27 | 2.2(1.5,2.9) | - | 6.29(<0.01) |
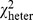 =
= 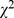 test for subgroup differences.
test for subgroup differences.
All pooling was undertaken using fixed effect model as no heterogeneity was detected by Q test.
Safety
Overall, 129 ocular adverse events (AEs) and 18 systemic AEs were recorded in latanoprost group and 103 ocular AEs and 36 systemic AEs were recorded in timolol group by the 7 clinical studies in our analysis. The frequencies of the most common ocular and systemic AEs in both groups are shown in Table 4.
Table 4. Risk of adverse effects with latanoprost and timolol.
| Adverse Effects | No. of trials | No. of events/No. of patients | Pooled OR (95%CI) |
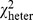
|
P value | |
| Latanoprost | Timolol | |||||
| Ocular | ||||||
| Discomfort* | 5 | 30/284 | 16/284 | 1.97(1.05,3.69) | 0.82 | 0.03 |
| Blurred vision | 3 | 29/224 | 23/224 | 1.33(0.72,2.46) | 3.70 | 0.37 |
| Conjunctival hyperemia | 4 | 29/254 | 12/254 | 2.72(1.33,5.59) | 1.33 | <0.01 |
| Keratitis | 1 | 9/137 | 8/138 | 1.14(0.43,3.05) | - | 0.79 |
| Uncontrolled IOP | 2 | 4/59 | 8/58 | 0.48(0.14,1.62) | 1.24 | 0.24 |
| Total | 7 | 129/343 | 103/342 | 1.49(1.05,2.10) | 9.75 | 0.02 |
| Systemic | ||||||
| Headache | 2 | 2/153 | 4/154 | 0.55(0.11,2.63) | 0.16 | 0.45 |
| Cardiac disorder** | 2 | 0/96 | 6/94 | 0.13(0.02,1.08) | 0.06 | 0.06 |
| Dizziness | 1 | 1/137 | 4/138 | 0.25(0.03,2.23) | - | 0.21 |
| Total | 4 | 18/249 | 36/248 | 0.46(0.25,0.84) | 1.41 | 0.01 |
* Discomfort include: eye discomfort, foreign body sensation, eye irritation.
**Cardiac disorder include: palpitation, cardiac arrhythmia.
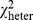 =
= 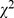 test for subgroup differences.
test for subgroup differences.
All pooling was undertaken using fixed effect model as no heterogeneity was detected by Q test.
Ocular Adverse Effects. Latanoprost caused more ocular adverse effects (OR = 1.49, 95% CI, 1.05∼2.10, P = 0.02) than timolol. The risks for discomfort and conjunctival hyperemia were significantly higher in latanoprost than timolol (P = 0.03 and P<0.01) (Table 4).
Systemic Adverse Events. More systemic adverse effects were observed in patients treated with timolol than latanoprost (OR = 0.46, 95% CI, 0.25∼0.84, P = 0.01). The risk for cardiac disorder was higher in timolol with a borderline statistical value (P = 0.06) (Table 4).
Discussion
In this meta-analysis, the findings from 7 RCTs showed that latanoprost once daily could achieve lower IOP in Asian patients with CACG than timolol twice daily, with differences in absolute IOP reduction for mean, peak and trough of 2.3 mmHg, 2.4 mmHg and 2.5 mmHg, and differences in relative IOP reductions of 9.0%, 9.7% and 10.8%, respectively. These differences could last from 1 week up to 6 months with magnitudes of 2.0 mmHg, 2.0 mmHg, 1.9 mmHg, 1.9 mmHg, 2.3 mmHg, and 2.2 mmHg at 1 week, 2 weeks, 4 weeks, 8 weeks, 12 weeks and 24 weeks, respectively. Sensitivity analysis based on study design and withdrawal rate didn't change the results. There were no statistically significant differences in lowering IOP between Chinese Mainland population and Asia-Pacific population (non-Chinese Mainland). As for safety, latanoprost caused more ocular adverse effects and less systemic adverse events than timolol. Our findings confirmed the results of previous trials that latanoprost might be a substitute for timolol in the treatment of patients with CACG.
The difference of mean IOP reduction between these two drugs (2.3 mmHg and 9.0%) in patients with CACG was similar to that in patients with POAG or OH [11], [12], for whom the mechanism of latanoprost in reducing IOP was mainly due to increase uveoscleral outflow. One important inclusion criteria for the 7 trials was that there should be at least one quadrant without peripheral anterior synechiae. This criterion was a consideration of the traditional mechanism of latanoprost which was thought to be dependent on the area of visible ciliary body face. However, Ritch et al. [31] found that the efficacy of latanoprost in lowering IOP was independent of the height of ciliary body face. The trial by Kook et al. [32] demonstrated that latanoprost could still significantly reduce the IOP of CACG patients with no visible ciliary body face by percentages of 25.5%∼36.1%. Therefore, latanoprost might produce IOP-lowering effect via passage other than ciliary body face, for example, trabecular meshwork. If so, latanoprost might be a treatment choice for CACG patients at different stages, which deserved further study.
In this meta-analysis, it was found that both latanoprost and timolol were well tolerated by CACG patients. However, latanoprost produced more cases of ocular discomfort (P = 0.03) and conjunctival hyperemia (P<0.01) than timolol, but less systemic advents especially cardiac disorder (P = 0.06). As a whole, latanoprost produced higher risk of ocular adverse effects (P = 0.02) and lower risk of systemic adverse events than timolol (P = 0.01). More ocular adverse effects observed in patients treated by latanoprost might be related to its proinflammatory effect [33]. In addition, higher percentage of benzalkonium chloride, a known irritant of conjunctiva and cornea [34], was involved in latanoprost eyedrop (0.2 mg/ml) than that in timolol eyedrop (0.1 mg/ml), which might be another reason of ocular discomfort [25]. On the contrary, it should be noted that systemic adverse events were more serious than ocular adverse effects. One trial [30] included in the meta-analysis reported that some cases from the timolol group had to stop using timolol because of its systemic adverse events. In view of this, latanoprost may be more suitable for those CACG patients who had concurrently systemic diseases.
The seven trials included in the present study had relatively high quality (Figure 2). Most trials suffered no clear bias in randomization, allocation concealment and outcome reporting. Although only three of these seven trials were free of bias in terms of blinding, sensitivity analysis based on blinding found no significant difference. Considering that the effect of eyedrops is usually dependent on the compliance of patients, we also did sensitivity analysis based on withdraw rate less than 10% or not. There was no significant difference between the two subgroups. Although only seven trials were included, we did funnel plots and found no publication bias. From the above, we believe that the results in this meta-analysis are robust.
Besides the discriminating among mean, peak, and trough moments, the analysis included both absolute and relative IOP reduction. The included studies did not vary in concentration of the drug, moment of applying, and frequency of dosing for the different medicines. Latanoprost 0.005% eye drops were directly compared with timolol 0.5% eye drops in all of the trials. We undertook subgroup analysis and sensitivity analyses to explore the heterogeneity between population, study design and withdrawals and the results were all robust. However, it should be noted that the differences between these two drugs might be only due to the different frequency of use daily. That is, latanoprost once daily might produce better compliance than timolol twice daily [35], [36]. This issue needs to be addressed in future studies.
Some limitations remain in the present study. Firstly, only 7 studies met our inclusion criteria and were included in this meta-analysis, and the numbers of participants in the trials were also limited (range, 30∼275). Secondly, several trials lacked adequate randomization, allocation concealment, masking, and complete outcome data, which may leave them vulnerable to bias. The imputation of missing values also introduces bias. However, the studies that contributed the most weight to this meta-analysis were also the most methodologically stringent, and according to the result of sensitivity analysis, it is unlikely that poorer quality trials significantly biased the pooled estimates. Thirdly, timolol is commonly used in Europe as 0.25% slow release gel, once or twice daily, so the applicability of the present study may not be as relevant in other populations. Fourthly, not all included studies have all the data for all time points. This might cause bias to the results. Finally, publication bias is inevitable; our research was restricted to studies published in journals or in certain trial registers.
In summary, the present meta-analysis showed that latanoprost once daily could achieve better effect in lowering IOP of CACG patients than timolol twice daily, with higher risk of ocular adverse effects but lower risk of systemic adverse events. Thus, latanoprost may be a good substitute for timolol to lower IOP of CACG patients.
Supporting Information
Subgroup analysis of IOP reduction between latanoprost and timolol in studies with and without pharmaceutical industry funding.
(TIF)
Search strategy and results.
(DOCX)
Sensitivity analysis of absolute IOP reduction at peak between latanoprost and timolol.
(DOCX)
Subgroup analysis of IOP reduction between latanoprost and timolol in Chinese Mainland population and Other Asia Pacific population.
(DOCX)
PRISMA Checklist.
(DOC)
Funding Statement
This study was supported by Pfizer China, Beijing. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Bourne RR, Sukudom P, Foster PJ, Tantisevi V, Jitapunkul S, et al. (2003) Prevalence of glaucoma in Thailand: a population based survey in Rom Klao District, Bangkok. Br J Ophthalmol 87: 1069–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Foster PJ, Baasanhu J, Alsbirk PH, Munkhbayar D, Uranchimeg D, et al. (1996) Glaucoma in Mongolia. A population-based survey in Hovsgol province, northern Mongolia. Arch Ophthalmol 114: 1235–1241. [DOI] [PubMed] [Google Scholar]
- 3. Foster PJ, Oen FT, Machin D, Ng TP, Devereux JG, et al. (2000) The prevalence of glaucoma in Chinese residents of Singapore: a cross-sectional population survey of the Tanjong Pagar district. Arch Ophthalmol 118: 1105–1111. [DOI] [PubMed] [Google Scholar]
- 4. He M, Foster PJ, Ge J, Huang W, Zheng Y, et al. (2006) Prevalence and clinical characteristics of glaucoma in adult Chinese: a population-based study in Liwan District, Guangzhou. Invest Ophthalmol Vis Sci 47: 2782–2788. [DOI] [PubMed] [Google Scholar]
- 5. Vijaya L, George R, Arvind H, Baskaran M, Paul PG, et al. (2006) Prevalence of angle-closure disease in a rural southern Indian population. Arch Ophthalmol 124: 403–409. [DOI] [PubMed] [Google Scholar]
- 6. Yamamoto T, Iwase A, Araie M, Suzuki Y, Abe H, et al. (2005) The Tajimi Study report 2: prevalence of primary angle closure and secondary glaucoma in a Japanese population. Ophthalmology 112: 1661–1669. [DOI] [PubMed] [Google Scholar]
- 7. McGalliard JN, Wishart PK (1990) The effect of Nd:YAG iridotomy on intraocular pressure in hypertensive eyes with shallow anterior chambers. Eye (Lond) 4 (Pt 6): 823–829. [DOI] [PubMed] [Google Scholar]
- 8. Robin AL, Pollack IP (1982) Argon laser peripheral iridotomies in the treatment of primary angle closure glaucoma. Long-term follow-up. Arch Ophthalmol 100: 919–923. [DOI] [PubMed] [Google Scholar]
- 9. Camras CB (1996) Comparison of latanoprost and timolol in patients with ocular hypertension and glaucoma: a six-month masked, multicenter trial in the United States. The United States Latanoprost Study Group. Ophthalmology 103: 138–147. [DOI] [PubMed] [Google Scholar]
- 10. Watson P, Stjernschantz J (1996) A six-month, randomized, double-masked study comparing latanoprost with timolol in open-angle glaucoma and ocular hypertension. The Latanoprost Study Group. Ophthalmology 103: 126–137. [DOI] [PubMed] [Google Scholar]
- 11. Alsagoff Z, Aung T, Ang LP, Chew PT (2000) Long-term clinical course of primary angle-closure glaucoma in an Asian population. Ophthalmology 107: 2300–2304. [DOI] [PubMed] [Google Scholar]
- 12. Rosman M, Aung T, Ang LP, Chew PT, Liebmann JM, et al. (2002) Chronic angle-closure with glaucomatous damage: long-term clinical course in a North American population and comparison with an Asian population. Ophthalmology 109: 2227–2231. [DOI] [PubMed] [Google Scholar]
- 13. van der Valk R, Webers CA, Schouten JS, Zeegers MP, Hendrikse F, et al. (2005) Intraocular pressure-lowering effects of all commonly used glaucoma drugs: a meta-analysis of randomized clinical trials. Ophthalmology 112: 1177–1185. [DOI] [PubMed] [Google Scholar]
- 14.Higgins JP, Green S (2011) Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 Chapter 8: Assessing risk of bias in included studies: The Cochrane Collaboration.
- 15.Higgins JP, Green S (2011) Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 Chapter 16: Special topics in statistics: The Cochrane Collaboration.
- 16. Abrams KR, Gillies CL, Lambert PC (2005) Meta-analysis of heterogeneously reported trials assessing change from baseline. Stat Med 24: 3823–3844. [DOI] [PubMed] [Google Scholar]
- 17. Follmann D, Elliott P, Suh I, Cutler J (1992) Variance imputation for overviews of clinical trials with continuous response. J Clin Epidemiol 45: 769–773. [DOI] [PubMed] [Google Scholar]
- 18. Cheng JW, Cai JP, Li Y, Wei RL (2009) A meta-analysis of topical prostaglandin analogs in the treatment of chronic angle-closure glaucoma. J Glaucoma 18: 652–657. [DOI] [PubMed] [Google Scholar]
- 19. Zhang WY, Po AL, Dua HS, Azuara-Blanco A (2001) Meta-analysis of randomised controlled trials comparing latanoprost with timolol in the treatment of patients with open angle glaucoma or ocular hypertension. Br J Ophthalmol 85: 983–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Higgins JP, Thompson SG (2002) Quantifying heterogeneity in a meta-analysis. Stat Med 21: 1539–1558. [DOI] [PubMed] [Google Scholar]
- 21. Higgins JP, Thompson SG, Deeks JJ, Altman DG (2003) Measuring inconsistency in meta-analyses. BMJ 327: 557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. DerSimonian R, Kacker R (2007) Random-effects model for meta-analysis of clinical trials: an update. Contemp Clin Trials 28: 105–114. [DOI] [PubMed] [Google Scholar]
- 23. Egger M, Davey SG, Schneider M, Minder C (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ 315: 629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Aung T, Wong HT, Yip CC, Leong JY, Chan YH, et al. (2000) Comparison of the intraocular pressure-lowering effect of latanoprost and timolol in patients with chronic angle closure glaucoma: a preliminary study. Ophthalmology 107: 1178–1183. [DOI] [PubMed] [Google Scholar]
- 25. Chew PTK, Aung T, Aquino MV, Rojanapongpun P (2004) Intraocular pressure–reducing effects and safety of latanoprost versus timolol in patients with chronic angle-closure glaucoma. Ophthalmology 111: 427–434. [DOI] [PubMed] [Google Scholar]
- 26. Wang NL, Fan ZG, Wu HP, Li DZ, Huang Y, et al. (2002) Drug therapy for residual angle-closure glaucoma after lsscr iridectomy[in Chinese]. Chin J Ophthalmol 38: 712–716. [PubMed] [Google Scholar]
- 27. Sihota R, Saxena R, Agarwal HC, Gulati V (2004) Crossover Comparison of Timolol and Latanoprost in Chronic Primary Angle-closure Glaucoma. Arch Ophthalmol 122: 185–189. [DOI] [PubMed] [Google Scholar]
- 28. Kong XM, Sun XH, GE L, Meng FR, Guo WY, et al. (2005) The clinical study of latanoprost in treating with residual angle-closure glaucoma[in Chinese]. Chin J Pract Ophthalmo 23: 475–478. [Google Scholar]
- 29. Liu YL, Liu Z, Gao N, Yu DZ (2006) Effect of indometacin in the treatment of glaucoma with latanoprost and timolol[in Chinese]. Shandong Med J 46: 52–53. [Google Scholar]
- 30. Zhao J, Ge J, Sun X, Wang N (2013) Intraocular Pressure-reducing Effects of Latanoprost Versus Timolol in Chinese Patients With Chronic Angle-closure Glaucoma. J Glaucoma 22: 591–596. [DOI] [PubMed] [Google Scholar]
- 31. Ritch R, Ishikawa H, Rothman R, Yu G, Liebmann JM (2002) The efficacy of latanoprost is independent of the width of the ciliary body face. J Glaucoma 11: 239–243. [DOI] [PubMed] [Google Scholar]
- 32. Kook MS, Cho HS, Yang SJ, Kim S, Chung J (2005) Efficacy of latanoprost in patients with chronic angle-closure glaucoma and no visible ciliary-body face: a preliminary study. J Ocul Pharmacol Ther 21: 75–84. [DOI] [PubMed] [Google Scholar]
- 33. Astin M, Stjernschantz J (1997) Mediation of prostaglandin f2 alpha-induced ocular surface hyperemia by sensory nerves in rabbits. Curr Eye Res 16: 886–890. [DOI] [PubMed] [Google Scholar]
- 34. Lazarus HM, Imperia PS, Botti RE, Mack RJ, Lass JH (1989) An in vitro method which assesses corneal epithelial toxicity due to antineoplastic, preservative and antimicrobial agents. Lens Eye Toxic Res 6: 59–85. [PubMed] [Google Scholar]
- 35. Patel SC, Spaeth GL (1995) Compliance in patients prescribed eyedrops for glaucoma. Ophthalmic Surg 26: 233–236. [PubMed] [Google Scholar]
- 36.Perfetti S, Varotto A, Massagrandi S, Pagliani F, Bonomi L (1998) Glaucoma and quality of the life. Acta Ophthalmol Scand Suppl 227: 52. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Subgroup analysis of IOP reduction between latanoprost and timolol in studies with and without pharmaceutical industry funding.
(TIF)
Search strategy and results.
(DOCX)
Sensitivity analysis of absolute IOP reduction at peak between latanoprost and timolol.
(DOCX)
Subgroup analysis of IOP reduction between latanoprost and timolol in Chinese Mainland population and Other Asia Pacific population.
(DOCX)
PRISMA Checklist.
(DOC)