Abstract
Warburg’s metabolic hypothesis is based on the assumption that a cancer cell’s respiration must be under attack, leading to its damage, in order to obtain increased glycolysis. Although this may not apply to all cancers, there is some evidence proving that primarily abnormally functioning mitochondrial complexes are indeed related to cancer development. Thus, mutations in complex II (succinate dehydrogenase (SDH)) lead to the formation of pheochromocytoma/paraganglioma.
Mutations in one of the SDH genes (SDHx mutations) lead to succinate accumulation associated with very low fumarate levels, increased glutaminolysis, the generation of reactive oxygen species (ROS), and pseudohypoxia. This results in significant changes in signaling pathways (many of them dependent on the stabilization of hypoxia-inducible factor (HIF)) including oxidative phosphorylation, glycolysis, specific expression profiles, as well as genomic instability and increased mutability resulting in tumor development.
Although there is currently no very effective therapy for SDHx-related metastatic pheochromocytomas/paragangliomas, targeting their fundamental metabolic abnormalities may provide a unique opportunity for the development of novel and more effective forms of therapy for these tumors.
Key Terms: SDHx, glycolysis, Warburg effect, reactive oxygen species, succinate dehydrogenase, pheochromocytoma, paraganglioma, renal cell carcinoma, gastrointestinal stromal tumor, hypoxia, pseudohypoxia
Introduction
In previous innovative work, Hanahan and Weinberg determined unique hallmarks of cancer that together constitute a fundamental principle that provides a logical framework for understanding the remarkable diversity, yet nevertheless similarity, of various cancers. Six hallmarks of cancer, namely sustaining proliferative signaling, evading growth suppressors, activating invasion and metastasis, enabling replicative immortality, promoting angiogenesis, and resisting cell death, are the driving forces that ultimately cause cancer cell development and spread, leading to patient death (Hanahan and Weinberg 2000). Recently, Hanahan and Weinberg have added two new emerging hallmarks, evading immune destruction and reprogramming energy metabolism (Hanahan and Weinberg 2011).
Additional scientific studies have also shown that altered energy metabolism is as widespread in cancer cells as many of the other cancer-associated traits that have been well accepted as hallmarks of cancer (Levine and Puzio-Kuter 2010). This raises the question of whether deregulating cellular energy metabolism could be a core hallmark of cancer cells. In fact, redirection of energy metabolism is largely orchestrated by proteins that are involved in one way or another in programming the core hallmarks of cancer. When viewed in this way, abnormal oxidative phosphorylation (OXPHOS) is simply another phenotype that is caused by altered oncogenes or tumor suppressor genes (Levine and Puzio-Kuter 2010). Multiple lines of evidence indicate that the process of tumorigenesis is often associated with altered metabolism. In 1926, Otto Warburg reported that cancer cells produce most of their adenosine triphosphate (ATP) via “aerobic glycolysis” (Warburg 1926). A significant glycolytic production of ATP despite aerobic conditions, referred to as the Warburg effect, was found to be characteristic of most cancer cells (Warburg 1956). Warburg reasoned that respiration must be damaged in cancers because high levels of O2 are unable to suppress the production of lactic acid by cancer cells (known as the Pasteur effect). However, new studies have demonstrated that tumor mitochondria are fairly functional with regards to respiration and ATP synthesis, exhibiting almost normal respiratory control ratios and capabilities for the oxidation of respiratory substrates (Bensinger and Christofk 2012; Eakin, et al. 1972; Krejci 2012; Nakajima and Van Houten 2012).
Although mitochondria are fairly functional in the majority of cancers, some cancers were found with mutations in genes linked to paramout mitochondrial processes, the Krebs cycle (tricarboxylic acid cycle (TCA)) (Linehan and Rouault 2013; Zhang, et al. 2013) and OXPHOS. Mitochondrial complex II, also known as succinate dehydrogenase (SDH), is one such protein involved in both TCA and OXPHOS. This membrane complex catalyzes the oxidation of succinate to fumarate in TCA and serves as an electron donor to complex III via CoQ (Eng, et al. 2003; Gottlieb and Tomlinson 2005). Succinate oxidation results in the reduction of ubiquinone (CoQ) to ubiquinol at the mitochondrial inner membrane as one part of the respiration electron transfer chain (Sun, et al. 2005b). SDH is composed of four subunits (SDHA-D), all encoded by nuclear genes (Baysal 2003, 2008; Cascon, et al. 2008; Sun, et al. 2005a; Yankovskaya, et al. 2003). The large SDHA subunit is catalytic. The conversion of succinate to fumarate is accomplished by SDHA through reduction of a flavin dinucleotide (FAD), a molecule bound to its protein moiety. This reaction is measured as SDH activity. Electrons are then passed to three Fe-S centers bound to SDHB, which eventually transfers them to ubiquinone (coenzyme Q). The smaller subunits, SDHC and SDHD, bind ubiquinone and anchor the entire complex to the inner membrane of the mitochondria (Rustin, et al. 2002).
Deleterious mutations in any of the SDH genes invariably result in decreased SDH activity or a significant reduction or complete absence of the protein (Gill, et al. 2011; Korpershoek, et al. 2011; Rustin et al. 2002; van Nederveen, et al. 2009; Yang, et al. 2012). Inherited defects in particular SDH subunits in humans are associated with variable clinical presentations ranging from early-onset devastating encephalomyopathy to tumor susceptibility or optic atrophy. Homozygous or compound heterozygous mutations in SDHA cause metabolic neurodegenerative disorders like congenital Leigh syndrome and late-onset optic atrophy, ataxia, and myopathy (Birch-Machin, et al. 2000; Burnichon, et al. 2010; Horvath, et al. 2006; Levitas, et al. 2010; Parfait, et al. 2000). Recently, Alston et al. (Alston, et al. 2012) presented the first patient with hypotonia and leukodystrophy due to a novel homozygous SDHB mutation. Heterozygous mutations in SDHA-D predispose to tumorigenesis (Figure 1) (Astuti, et al. 2003; Astuti, et al. 2004; Bayley, et al. 2006; Benn, et al. 2006; Cascon et al. 2008; Eng et al. 2003; Maher and Eng 2002; Schiavi, et al. 2005). The detailed molecular and cellular mechanisms linking these latter SDH mutations and tumorigenesis have not been fully elucidated. Thus, consistent with Knudson’s two-hit hypothesis for tumorigenesis, a heterozygous germline mutation in an SDH gene is associated with a loss of the wild-type allele, or other silencing mechanisms (e.g. methylation) of the wild-type allele are present in a tumor (Astuti et al. 2003; Astuti et al. 2004; Bardella, et al. 2011; Baysal 2003, 2004, 2008; Baysal, et al. 2000; Eng et al. 2003; Gimenez-Roqueplo, et al. 2003; Killian, et al. 2013; Letouze, et al. 2013; Ni, et al. 2012; Ni, et al. 2008; Sandgren, et al. 2010) as the starting point for tumor development. Moreover, the pathophysiology of distinct clinical phenotypes associated with abnormalities in SDH subunits remains to be determined (Timmers, et al. 2009b). Detailed knowledge about SDH mutations is available in a database (LOVD v.2.0 - Leiden Open Variation Database, http://www.lovd.nl/2.0) (Bayley, et al. 2005).
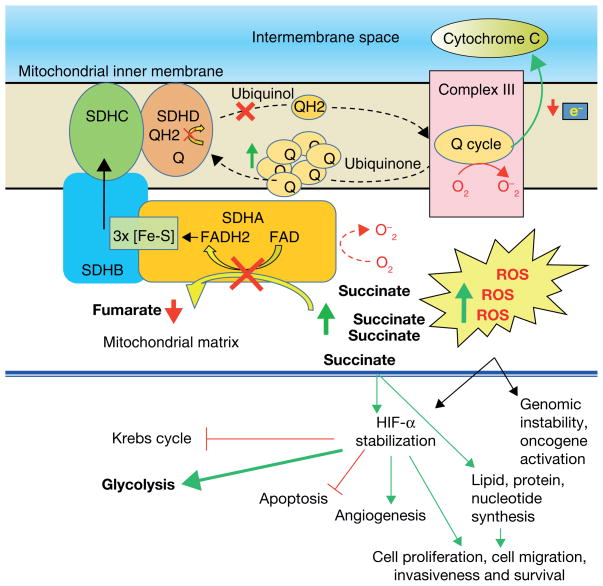
Figure 1.
The succinate dehydrogenase complex (SDH), as a member of the tricarboxylic acid cycle (TCA), catalyzes the oxidation of succinate to fumarate. In this reaction, two hydrogen atoms are removed from succinate by flavine adenine dinucleotide (FAD). These electrons from the reduced SDH-FADH2 complex are then transferred to ubiquinol-ubiquinone (coenzyme Q), a soluble component of the electron transport system of complex II. In the Q cycle, the sequential oxidation and reduction of the lipophilic electron carrier, coenzyme Q, generates protons that are transferred to complex III, with the ultimate generation of ATP (complex V). Coenzyme Q, beside its function in the respiratory chain as an electron carrier mediating electron transfer between the various dehydrogenases and the cytochrome pathway, also works as a powerful antioxidant in biological membranes. Dysfunction of SDH inactivates the electron transport chain and the Krebs cycle. A lack or suboptimal level of SDH activity will not only cause decreased ATP production, but will also result in increased reactive oxygen species (ROS) with succinate accumulation. An increase in ROS, like the accumulation of succinate, leads to stabilization of HIF-α. HIF-α stabilization subsequently activates glycolysis, cell proliferation, cell migration, invasiveness, and angiogenesis and inhibits apoptosis. The overexpression of ROS triggers genomic instability, oncogene activation, and tumor suppressor inactivation.
Abbreviations: e−- electron; FAD- flavin adenine dinucleotide; FADH2- flavin adenine dinucleotide hydroquinone; ROS- reactive oxygen species; Q- ubiquinone; QH2- ubiquinol; SDHA- The succinate dehydrogenase complex subunit A; SDHB- succinate dehydrogenase complex subunit B; SDHC- succinate dehydrogenase complex subunit C; SDHD- succinate dehydrogenase complex subunit D.
Although these findings led to a renewed interest in cancer metabolism, our knowledge on the specifics of tumor metabolism is still fragmented. Nevertheless, multiple lines of evidence indicate that the process of tumorigenesis is often associated with altered metabolism. In this review we show and discuss how mutations in SDH subunits can lead to reprogramming of cancer-related metabolism. Also, this paper reviews recent findings related to key metabolites, transcription factors, and enzymes that play an important role in the regulation of cancer metabolism, and that blocking these metabolic pathways or restoring altered pathways can lead to new approaches in cancer treatment.
Pheochromocytoma and Paraganglioma
PHEOs/PGLs are rare neuroendocrine tumors that produce catecholamines (Lenders, et al. 2005). PHEOs/PGLs arise from three distinct parts of the neural crest: the adrenal medulla (PHEOs) and the sympathetic and parasympathetic paraganglia (extradrenal PGLs) (Papaspyrou, et al. 2011).
One third or more of PHEO/PGL cases have a familial etiology (Erlic, et al. 2009; Gimenez-Roqueplo, et al. 2012; Neumann, et al. 2002). This group is heterogeneous with diverse hereditary backgrounds due to germline mutations in 16 susceptibility genes to date. Some of these include neurofibromatosis type 1 (NF1) (Viskochil, et al. 1990), the ret proto-oncogene (RET) (Mulligan, et al. 1993), the von Hippel-Lindau (VHL) (Latif, et al. 1993) tumor suppressor, the succinate dehydrogenase (SDH) subunits (SDHA/B/C/D) (Astuti, et al. 2001; Baysal et al. 2000; Burnichon et al. 2010; Niemann and Muller 2000), succinate dehydrogenase complex assembly factor 2 (SDHAF2) (Hao, et al. 2009), transmembrane protein 127 (TMEM127) (Jiang and Dahia 2011; Qin, et al. 2010; Yao, et al. 2010), the MAX protein (MAX) (Comino-Mendez, et al. 2011), kinesin family member 1B (KIF1B) (Schlisio, et al. 2008; Yeh, et al. 2008), the 2-oxoglutarate-dependent prolyl hydroxylase enzymes (PHD2) (Eltzschig, et al. 2009; Ladroue, et al. 2008), isocitrate dehydrogenase 1 (IDH1) (Gaal, et al. 2010), and most recently hypoxia-inducible transcription factor 2 alpha (HIF2A) (Toledo, et al. 2013; Zhuang, et al. 2012), fumarate hydratase (FH) (Castro-Vega, et al. 2013), and H-RAS protein (H-RAS) (Crona, et al. 2013). Somatic mutations of these genes are also involved in PHEO/PGL tumors (Burnichon, et al. 2012a; Crona et al. 2013; Dahia 2013; Weber, et al. 2012). Hereditary and sporadic PHEOs/PGLs can be divided into two groups based on their transcription profile revealed by genome-wide expression microarray analysis (Burnichon, et al. 2011; Galan and Kann 2013; Lopez-Jimenez, et al. 2010; Vicha, et al. 2013). The first group (cluster 1) includes tumors carrying VHL and SDHx (SDHD, SDHB, SDHC, SDHA, SDHAF2) mutations and also accounts for about 30% of sporadic tumors (Burnichon et al. 2011; Dahia, et al. 2005; Lopez-Jimenez et al. 2010). The second group (cluster 2) represents tumors carrying NF1, RET, and KIF1Bβ mutations, and also includes about 70% of sporadic tumors (Burnichon et al. 2011; Galan and Kann 2013; Gimenez-Roqueplo et al. 2012; Shah, et al. 2012). The newly discovered TMEM127 and MAX genes are most likely associated with cluster 2, and HIF-2α with cluster 1 (Burnichon, et al. 2012b; Burnichon et al. 2011; Lorenzo, et al. 2012; Zhuang et al. 2012). However, a subset of MAX-related tumors may have impaired SDH activity, and metabolomics in these tumors could uncover new data that could be very useful clinically for their diagnosis (Rapizzi, et al. 2012).
In cluster 1, VHL/SDHx mutations lead to impaired degradation and accumulation of HIF-1/2α and display signatures of pseudohypoxia, angiogenesis, increased ROS, and reduced oxidative response resulting in changes in cell metabolism (energy metabolism regulation). VHL and SDH subunit mutations distribute tumors to separate subclusters within cluster 1 (Burnichon, et al. 2009; Dahia et al. 2005; Eisenhofer, et al. 2004; Lopez-Jimenez et al. 2010). Cluster 2-related PHEOs/PGLs are linked together by the activation of kinase signaling pathways driven by oncogenes that are involved in kinase signaling, translation, initiation, protein synthesis, and genes involved in neural/neuroendocrine identity (Burnichon et al. 2011; Dahia et al. 2005; Jiang and Dahia 2011; Powers, et al. 2007; Shah et al. 2012; Yeh et al. 2008). Cluster 1 is characterized by immature catecholamine phenotypic features of associated tumors (Eisenhofer et al. 2004). The immature phenotype involves reduced or absent expression of numerous catecholamine biosynthetic and secretory pathway components, mainly phenylethanolamine N-methyltransferase (PNMT), the enzyme that converts norepinephrine to epinephrine (Eisenhofer, et al. 2012; Eisenhofer, et al. 2011b). Also, SDH-related tumors often produce dopamine. Thus, cluster 1 tumors can be distinguished from cluster 2 tumors by the absence of epinephrine production (Burnichon et al. 2012b; Eisenhofer et al. 2004; Eisenhofer et al. 2012; Eisenhofer, et al. 2011a; Eisenhofer et al. 2011b). Most recently, Imperiale et al. (Imperiale, et al. 2013) evaluated metabolic characteristics of PHEOs/PGLs tumors using 1H high-resolution magic angle spinning (HRMAS) NMR spectroscopy. SDHx-related tumors were characterized by an increase in succinate levels, significantly lower values of glutamate and lower values of ATP/ADP/AMP in SDHx-related tumors compared to other subtypes. VHL tumors were found to have the highest values of glutathione (GSH) compared to other PHEOs/PGLs. This study showed that HRMAS NMR spectroscopy is a future promising method for investigating the metabolomic profile of various PHEOs/PGLs.
SDH dysfunction and metabolic changes
Succinate accumulation
It is well documented that abnormal SDH function induces an accumulation of succinate (Hobert, et al. 2012; King, et al. 2006; Rao, et al. 2013; Selak, et al. 2005). Very recently, Lendvai et al. (Lendvai, et al. 2013) showed that tissue levels of succinate in PGLs due to SDHB/D mutations were several-fold higher. Their results showed that the mean fumarate concentration in SDHB-related PGLs is significantly lower than in the apparently sporadic PHEO/PGL group. Lendvai et al. (Lendvai et al. 2013) also demonstrated a significantly increased succinate to fumarate ratio in SDHB-related PGLs and suggested this ratio may be used as a new metabolic marker for the detection of SDHB-related PHEOs/PGLs. Thus, mass spectrometric-based measurements of succinate:fumarate ratios in PHEO/PGL tumor tissue may provide a novel method to identify patients to be tested for SDHB/C/D mutations. The measurements could also be useful for assessing metabolic factors responsible for variable clinical presentations of tumors resulting from mutations of different SDHx genes. Also, plasma organic acid analysis may provide an effective and inexpensive screening method to determine the presence of SDHx mutations in the near future (Hobert et al. 2012).
The accumulation of specific metabolites has been illustrated in different tumor models with inherited and acquired alterations of enzymes of the TCA cycle, such as fumarate in cases of FH gene mutations (Isaacs, et al. 2005) and 2-hydroxyglutarate (2HG) in mutations in one of the 2 isocitrate dehydrogenase genes (IDH1/2) (Dang, et al. 2009). These findings have important implications for our understanding of tumorigenesis because these metabolites convey oncogenic signals (oncometabolites) (Kaelin and McKnight 2013).
Succinate that accumulates in the mitochondrial matrix due to SDH dysfunction leaks out into the cytosol, where it inhibits the activity of HIF-1/2α prolyl hydroxylase enzymes (PHDs - PHD1, 2, and 3, also known as Egln2, 1, and 3, respectively) that hydroxylate two prolyl residues (Dann and Bruick 2005). PHDs are members of a large superfamily of α-ketoglutarate-dependent dioxygenases. PHD action normally requires oxygen and α-ketoglutarate as cosubstrates and ferrous iron and ascorbate as cofactors. (Hewitson, et al. 2003; Kaelin and Ratcliffe 2008). Succinate competes with α-ketoglutarate in binding to the PHD enzyme. Therefore, increasing succinate levels offset the effect of PHD activity. A lack of SDH activity inhibits succinate-ubiquinone activity; thus, electrons that would normally transfer through the SDHB subunit to the ubiquinone pool are instead donated to molecular oxygen to give a superoxide anion with a subsequent increase of ROS production and oxidative stress. ROS exposure also inhibits the interaction of HIF-α and PHDs, similar to the accumulation of succinate, but it is proposed that such an inhibition of this interaction by ROS may be more important for tumorigenesis (Guzy, et al. 2008; Majmundar, et al. 2010; Yankovskaya et al. 2003). The inhibition of the HIF-α-PHD interaction leads to the stabilization of HIF-α and activation of the HIF complex (Lee, et al. 2005). HIF-α regulates the transcription of a number of genes that are known to be involved in tumorigenesis and angiogenesis, extracellular matrix elements, and coordinated suppression of oxidoreductase enzymes, all processes that would be directly or indirectly regulated by the activation of HIF-1α and/or HIF-2α (Dahia et al. 2005; Favier and Gimenez-Roqueplo 2010; Keith, et al. 2012; Mole, et al. 2009; Selak et al. 2005; Semenza 2010, 2011, 2012). HIF-1α and HIF-2α regulate both shared and unique target genes and pathways. The common shared targets are VEGF, GLUT-1, GLUT-3, and HK2. HIF-1α exclusively stimulates the expression of several glycolytic enzymes, whereas the embryonic transcription factors Oct-4, cyclin D1, platelet-derived growth factor (PDGF), and erythropoietin (EPO) are activated in a HIF-2α-dependent manner (Figure 2) (Florczyk, et al. 2011; Franke, et al. 2013; Furlow, et al. 2009; Koh, et al. 2011; Patel and Simon 2008; Rankin, et al. 2007; Singh, et al. 2013). The differential effects of these two transcription factors in numerous cellular systems are now well established and reviewed, including their link to the pathogenesis of PHEO and PGL (Branco-Price, et al. 2012; Chiavarina, et al. 2012; Holmquist-Mengelbier, et al. 2006; Jochmanova, et al. 2013; Keith et al. 2012; Koh et al. 2011; Semenza 2012). Despite the fact that Pollard et al. (Pollard, et al. 2005; Pollard, et al. 2006) found relatively more common HIF-2α overexpression in VHL PHEOs and PGLs, whereas in SDHx-related tumors nuclear HIF-1α staining was more prominent, Gimenez-Roqueplo et al. (Gimenez-Roqueplo, et al. 2001; Gimenez-Roqueplo, et al. 2002) described overexpression of HIF-2α and VEGF in patients with PHEOs and PGLs carrying SDHB and SDHD mutations compared with sporadic PHEOs and PGLs, and Favier et al. (Favier, et al. 2009) found overexpression of HIF-2α mRNA in both VHL and SDH-related PHEO and PGL. Also, Eisenhofer et al. (Eisenhofer et al. 2004) and Koh et al. (Koh et al. 2011) support the leading role of HIF-2α in tumor development and progression in cluster 1 tumors as well as their unique noradrenergic phenotype (Jochmanova et al. 2013). The important role of HIF-2α in various developmental issues is also supported by previous observations performed in fetal paraganglia and neuroblastoma (Favier, et al. 1999; Jochmanova et al. 2013; Nilsson, et al. 2005; Tian, et al. 1998).
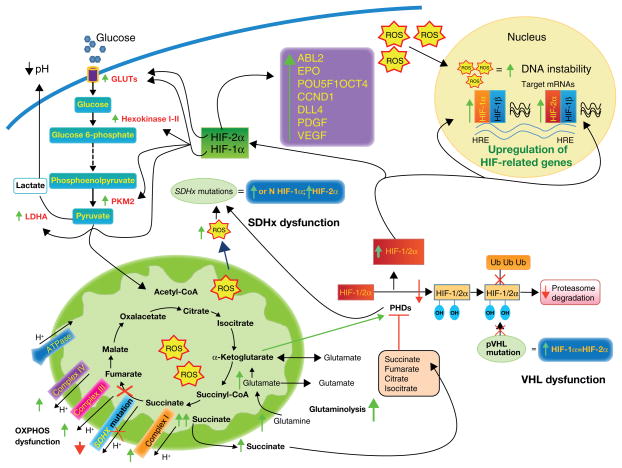
Figure 2.
Different mechanisms have been proposed to explain the link between SDHx mutations and tumorigenesis. First, the loss of function of SDH causes an accumulation of succinate and the overproduction of reactive oxygen species (ROS). Second, inactivation or dysfunction of SDH inhibits the activity of HIF-α prolyl hydroxylase enzymes (PHDs). Inhibition of PHDs results in an insufficient hydroxylation of HIF-1/2α. The unhydroxylated HIF-1/2α protein cannot be degraded by the proteasome, and HIF-1/2α is stabilized. This stabilization can be overcome by α-ketoglutarate. VHL mutations result in similar inadequate HIF-1/2α proteasome degradation and HIF-1/2α stabilization. The stabilization of HIF-1α rather than HIF-2α increases glycolysis (due to overexpression of some glycolytic enzymes) and can regulate glutaminolysis. HIF-2α stabilization is involved in the direct or indirect activation of a number of genes that are known to be involved in the inhibition of apoptosis, tumorigenesis, and angiogenesis. The stabilization of HIF-1/2α leads to an upregulation of HIF-related genes due to binding to hypoxia-responsive elements (HRE) and to an overexpression of hypoxia-related genes. Increased ROS accumulation results in oxidative DNA damage and genomic instability and inhibits PHDs, similarly to the accumulation of succinate. Increasing activity of complex I, III, and IV may be a compensatory reaction to a lack of or decreased complex II activity in SDHx- related tumors.
Abbreviations: ABL2- ABL2 protein tyrosine-protein dinase; CCDN1- cyclin D1; DLL4- delta-like protein 4; EPO- erythropoietin; GLUTs- glucose transporters; HIF- hypoxia-inducible factor; HRE- hypoxia-responsive elements; LDH-A- lactate dehydrogenase A; OXPHOS- oxidative phosphorylation; PDGF- platelet-derived growth factor; PHDs- prolyl hydroxylases; PKM2- pyruvate kinase muscle isozyme 2; POU5F1 OCT4- POU domain, class 5, tarnscription factor 1 isoform; pVHL- protein of the von Hippel-Lindau tumor suppressor gene; ROS- reactive oxygen species; SDH- succinate dehydrogenase; Ub- ubiquitin; VEGF- vascular endothelial growth factor.
Similary, the mechanism of PHD inhibition by succinate is likely to extend to other numbers of a large superfamily of α-ketoglutarate-dependent dioxygenases. One of them is the factor inhibiting HIF (FIH), which normally hydroxylates HIF-1α on the asparagine 803 residue. This blocks its interaction with the coactivators histone acetyltransferase p300 (p300) and cAMP-response element-binding protein (CBP) under normoxic conditions (Lando, et al. 2002; Mahon, et al. 2001) and thus inhibits the transactivation of HIF target genes (Cascon and Tennant 2012; Khan, et al. 2011). Also, SDHx mutations inhibit activity of the jumonji-domain (JmjC) histone demethylases (Cervera, et al. 2009; Xiao, et al. 2012). These enzymes use α-ketoglutarate to remove the methyl groups found on arginines and lysines of histones H3 and 4 (Agger, et al. 2008). SDHx mutations decrease histone demethylase activity (specifically the JMJD3 demethylase) and lead to increased methylation of histone H3 (H3K27me3) (Cervera et al. 2009). Similary, very recently Letouze et al. (Letouze et al. 2013) showed that increased tumor levels of succinate lead to DNA hypermethylation, a process causing global changes in gene expression as a critical tumorigenic mechanism. These modulations in the pseudohypoxic signature observed in SDHx-related tumors can distinguish the gene expression phenotypes observed in the two subgroups of tumors in cluster 1.
Reactive oxygen species (ROS)
A lack of SDH activity results in increases in steady-state levels of O2− to H2O2 that could then form more powerful oxidants, such as hydroxyl radicals through Haber-Weiss–driven Fenton chemistry as well as organic hydroperoxides capable of causing chronic metabolic oxidative stress (Owens, et al. 2012; Slane, et al. 2006; Spitz 2011). Chronic ROS exposure can result in oxidative damage to mitochondrial and cellular proteins, lipids, and nucleic acids. These normoxic ROS accelerate the DNA-damaging processes as a “mutator phenotype,” causing genomic instability, as well as an increase in glucose consumption and sensitivity to glucose deprivation-induced toxicity and slower growth rates. ROS are also involved in Ras-Raf-MEK signaling. Ras-Raf-MEK signaling activation causes the mediation of protection against apoptotic cell death induced by increased oxidative stress (Jiang, et al. 2005). The activity of the ROS-generating enzyme Nox1 is required for vascular endothelial growth factor (VEGF), a potent stimulator of tumor angiogenesis (Dudkina, et al. 2005; Pan, et al. 2009; Rustin et al. 2002; Slane et al. 2006). Fliedner et al. (Fliedner, et al. 2012) detected elevated superoxide dismutase 2 expression in SDHB-derived PHEOs/PGLs that is indirect evidence for increased ROS production and may reflect elevated oxidative stress.
Warburg effect
A lack of SDH activity and consequent other changes lead to the Warburg effect in SDHx-related tumors. Because metabolic control over the glycolytic rate can be applied at many steps in the glycolytic pathway (Dang, et al. 1997; Gatenby and Gillies 2004), most studies in cancer support the hypothesis that control over glycolytic flux primarily resides at the transport and phosphorylation steps (upregulation of glucose transporters (notably GLUT-1 and GLUT-3) and hexokinase 2 (HK2)) (Choi, et al. 2013; Gatenby and Gillies 2004; Mathupala, et al. 2009). HIF-α enhances the glycolytic pathway by increasing target gene expression from GLUT-1, GLUT-3, through HK2 and pyruvate kinase variant M2 (PKM2) to lactate dehydrogenase-A (LDH-A) and other glycolytic and anabolic enzymes and metabolites (Osthus, et al. 2000; Soga 2013). Some expression studies have not found over-expression of GLUT-1 in SDHx-related tumors (Favier et al. 2009; Fliedner et al. 2012; Lopez-Jimenez et al. 2010). Moreover, increased expression of GLUT-3 and HK2 mRNAs observed in SDHx-related tumors (Favier et al. 2009; Fliedner et al. 2012) can explain the high sensitivity of [18F]-FDG PET for SDHx-related tumors, mainly observed in SDHB-related PHEOs/PGLs (Taieb, et al. 2009; Timmers, et al. 2012; Timmers, et al. 2009a; Timmers, et al. 2007; Zelinka, et al. 2008). Fliedner et al. (Fliedner et al. 2012) detected the M2 isoform of pyruvate kinase (PKM2) mRNA, which appeared to be possibly elevated in SDHB-mutant tumors. PKM2 is generated by increased transcription and alternative splicing of the PKM2 gene through a HIF-1α and c-Myc-mediated process. PKM2 catalyzes the final and also rate-limiting reaction in the glycolytic pathway and promotes tumorigenesis by regulating the Warburg effect. PKM2 also possesses a positive feedback regulation towards HIF-1α. PKM2 interacts with HIF-1α in the nucleus and functions as a transcriptional coactivator to enhance the expression of HIF-1α target genes that promote the shift from OXPHOS to glycolytic metabolism (Luo, et al. 2011; Luo and Semenza 2011, 2012). Also, overexpression of LDH-A has been found in SDHx-related tumors (Favier et al. 2009; Fliedner et al. 2012). In proliferating cancer cells, the majority of the pyruvate generated from glucose (>90%) is converted to lactate by LDH-A, where it is readily secreted into the extracellular environment. By converting pyruvate to lactate, LDH-A recovers the NAD+ needed to maintain glycolysis and ATP production. This step is critical for the maintenance of tumor proliferation in vivo (Fantin, et al. 2006; Jones and Thompson 2009). LDH-A may be upregulated by a high glycolytic flux through the carbohydrate-response elements (ChoREs) by binding HIF or myc products (Semenza 2002a; Semenza 2002b; Walenta and Mueller-Klieser 2004). Moreover, both LDH-A and mitochondria activity are mutually regulated at the level of metabolites. They depend on the availability of pyruvate and on the NADH/NAD+ ratio. The generation of lactate and the export of intracellular acid leads to an acidic tumor microenvironment, which is correlated with a poor prognosis and may facilitate tumor invasion and metastasis leading to the stimulation of cell migration and angiogenesis (Chiche, et al. 2010; Vegran, et al. 2011). Thus, activation of HIF-α, c-myc, and other proteins stimulate many processes that result in the Warburg effect in these tumors (Cairns, et al. 2011; Deberardinis, et al. 2008; Gogvadze, et al. 2010; Jones and Thompson 2009; Koppenol, et al. 2011; Levine and Puzio-Kuter 2010; Vogelstein and Kinzler 2004; Yuneva 2008).
Glutamine metabolism
Tannahill et al. (Tannahill, et al. 2013) showed that a dysfunctional TCA cycle pointed towards an alternative source of succinate. The microarray study showed a significantly higher concentration of glutamine transporter SLC3A2 mRNA. Thus, substantial increases in succinate accumulation have been demonstrated through processes involving increased import and metabolism of glutamine (Tannahill et al. 2013). Therefore, we suggest that glutamine metabolism can be involved in SDHx-related tumors. Succinate can be derived from glutamine through anaplerosis by α-ketoglutarate. Recently, Imperiale et al. (Imperiale et al. 2013) found significantly lower values of glutamate in SDHx-related tumors compared to other subtypes. These catabolic pathways are reversible and involve the removal of nitrogen as part of the mechanism that regulates nitrogen homeostasis; the carbon skeleton from glutaminolysis may eventually enter anabolic or anaplerotic processes (including the formation of nucleotides, lipids, and proteins) (Dang 2010; Eng and Abraham 2010; Yuneva 2008).
Meng et al. (Meng, et al. 2010) observed that nitrogen source restriction repressed carbon metabolic pathways, including glucose utilization. Therefore, the interconversion between glutamine and α-ketoglutarate serves as the bridge connecting nitrogen and carbon metabolism. Thus, glutaminolysis and the Warburg effect become two integral parts of the cellular machinery to balance the carbon and nitrogen metabolism. Glutaminolysis also supports the production of molecules, such as glutathione (GSH) and NADPH, which protect cells from oxidative stress.
OXPHOS proteins
OXPHOS proteins include the electron transport chain components, ATP synthase, and the adenine nucleotide translocator (ANT). Information about other OXPHOS proteins besides complex II in SDHx-related tumors is limited. Favier et al. (Favier et al. 2009) suggested a lower expression of OXPHOS protein complexes I to IV in SDHx- and VHL-related tumors than in PHEOs/PGLs harboring NF1 and RET mutations, but complex V expression was relatively similar in all patients. Also, the activity of complexes II, III, or IV was found to be decreased in SDHx- and VHL-related PHEOs/PGLs, but the differences were smaller for complexes III and IV. In contrast, other groups showed that the activity of SDH or respiratory chain enzyme complex II is low in SDHx-related tumors and associated with increased activities of respiratory chain complexes I, III, and IV and citrate synthase. All these factors suggest a compensatory response to the lack of SDH activity (Fliedner et al. 2012; Rao et al. 2013). However, as shown by Rao et al., the apparently increased activity of complex I, III, IV, and citrate synthase in the SDHx-related tumors does not lead to a full restoration of ATP/ADP/AMP, because the concentration of ATP/ADP/AMP was consistently very low in all SDHx-related tumors (Rao et al. 2013). Rao et al. found positive relationships between mitochondrial complex II function, tumor ATP/ADP/AMP content, and tumor catecholamine contents, and suggested the possibility that differences in energy metabolism might also contribute to the lower tumor tissue catecholamine contents in cluster 1 than in cluster 2 tumors. Thus, increased activity of complex I, III, and IV may be a compensatory reaction to a lack of or decreased complex II activity in these tumors.
Thus, the generation of ROS as well as pseudohypoxia and succinate accumulation results in significant changes in key pathways: HIF, glycolysis, angiogenesis, genomic instability, increased cell cycle, and increased mutability.
In summary, increased ROS production has been suggested to contribute to tumorigenesis in SDHB-related tumors (Goffrini, et al. 2009; Guzy et al. 2008; Huang and Lemire 2009). SDHx mutation-induced increases in ROS have recently been shown to cause genomic instability that may contribute to tumorigenesis (Owens et al. 2012; Slane et al. 2006). Second, accumulation of succinate leads to widespread changes, from stabilization of HIF-α through inhibition of PHD to DNA hypermethylation, a process causing global changes in gene expression. This accumulation of succinate accompanies low fumarate in malignant SDHB tumors. This high succinate:fumarate ratio can be used as a predictor of malignancy in the future. Third, the specific catecholamine phenotype in SDHx-related tumors may be due to down-regulation of HIF-1α and up-regulation of HIF-2α. Fourth, not only glycolysis, but also glutaminolysis may be involved in SDHx-related tumors.
Future treatment options to attack metabolic alterations in SDHx-related tumors
Understanding specific metabolic alterations characteristic and unique to SDHx-related PHEOs/PGLs and increasing availability and implementation of molecular profiling and metabolomics in clinical medicine opens new promising options for the use of multiple and personalized metabolic-specific molecular targeted therapies in the near future, as originally suggested by Eng (Eng et al. 2003). Several key events involved in the pathogenesis of SDHx-related PHEOs/PGLs have been described such as 1) an increase in ROS production resulting in oxidative stress and stabilization of HIF-1/2α; 2) accumulation of succinate which inhibits 2-oxoglutarate (2OG)-dependent dioxygenases and causes hypermethylated and pseudohypoxic phenotypes. Identification of subgroups of specific molecular-metabolic phenotypes may be especially useful in personalized medicine. Futhermore, targeted therapies hold promise for the treatment of metastatic SDHx-related tumors. Thus, although outlined below separately, these approaches are viewed as tightly interconnected and should be combined when appropriate treatments or knowledge are available.
Restoration of SDH activity
An increase in the expression and stabilization of SDH proteins is crucial to prevent various metabolic dysregulations resulting from the absent SDHB protein and therefore dysfunctional mitochondrial complex II in SDHx-related tumors. Recently, Yang et al. demonstrated that the loss of SDHB function was due to a reduction in the mutant protein half-life by rapid proteasome degradation. The authors found that histone deacetylase inibitors (HDACi) stabilized the half-life of SDHB mutated proteins and their activity (Yang et al. 2012). However, this approach, although of interest in the near future, cannot be applied to patients with nonsense SDHx mutations or SDHx gene deletions, since no protein is generated and the second allele is missing by the mechanism of loss of heterozygosity. Furthermore, it is expected that this approach will only partially increase the availability of mutated (and still dysfunctional) protein that will only improve mitochondrial function to a certain degree leading to persistent metabolic dysregulations (although to a lesser degree). Therefore, this therapy most likely will need to be combined with other therapeutic approaches.
Restoration of PHD activity
As we described, both succinate and ROS contribute to inactivation of PHD and subsequent stabilization of the HIF-1/2α pathway. PHD action normally requires oxygen and α-ketoglutarate as co-substrates. PHD inactivation by succinate is competitive with α-ketoglutarate. Therefore, increasing the α-ketoglutarate to succinate ratio levels by treatment with α-ketoglutarate derivatives could critically affect PHD activity and decrease the stabilization of HIF-1/2α (Jokilehto and Jaakkola 2010; MacKenzie, et al. 2007; Tennant, et al. 2009). Furthermore, esterified α-ketoglutarate induces apoptosis and inhibits tumor growth. These effects are independent of HIF-α but dependent on the presence of PHD3 (Tennant and Gottlieb 2010).
Direct activation of PHD by the activator KRH102053 increases HIF-1/2α hydroxylation and promotes its degradation (Choi, et al. 2008; Nepal, et al. 2011). Targeting HIF-1/2α with their specific inhibitors (e.g., currently by either the direct inhibitor PX-478 or the indirect inhibitor PX-12; both targeted to HIF-1α) has shown antitumoral activity in human tumor xenografts in mice and also seems to be promising for malignant PHEO/PGL (Semenza 2007; Welsh, et al. 2004; Welsh, et al. 2003). However, currently there are no HIF-2α inhibitors that would be preferable in the treatment of SDHx-related PHEO/PGL. Neverthless, it is expected that these compounds will be introduced in the near future (Rogers, et al. 2013).
Prevent ROS damaging
A rationale for using antioxidants in HIF-1/2α-driven tumors was recently provided by Gao et al., who examined the antitumorigenic effect of the antioxidant NAC (Gao, et al. 2007). They reported that NAC treatment resulted in reduced HIF-1α expression and in inhibition of in vivo tumor formation in a HIF-driven model of tumorigenesis. Ni and Eng (Ni and Eng 2012) concluded that the lipid soluble antioxidant α-tocopherol can selectively protect SDHxvar+ cells from oxidative damage and apoptosis resistance and rebalance the redox metabolites NAD/NADH, which is a promising opportunity to prevent the development of tumors in patients with SDHx mutations. This concept is very unique, introducing prevention for the first time in treatment of SDHx carriers. Thus, α-tocopherol, ascorbic acid, and HDACi could be administered over a long period and could serve as a novel therapeutic paradigm for preventing the development of SDHx-related PHEOs/PGLs (Ni and Eng 2012; Yang et al. 2012).
HSP90 inhibitors
Malignant SDHx-related PHEO/PGL overexpresses heat shock protein 90 (HSP90), a molecular chaperone that assists in binding to HIF-1/2α and promotes its stability by preventing ubiquitination and proteasomal degradation of HIF-1/2α (Liu, et al. 2007; Mahalingam, et al. 2009; Semenza 2010). Thus, inhibitors of HSP90, such as geldanamycin and analogues like 17-allylaminogeldanamycin (17-AAG; tanespimycin), 17-dimethylaminoethylamino-17-demethoxygeldanamycin (17-DMAG; alespimicin), or other new analogues, are promising therapeutic agents (Isaacs, et al. 2002; Northcott, et al. 2012). Giubelino et al. (Giubellino, et al. 2013) demonstrated potent inhibition of proliferation and migration of PHEO cell lines and induced degradation of key Hsp90 clients by 17-AAG and ganetespib. They also showed the efficacy of 17-AAG and ganetespib in reducing metastatic burden and increasing survival in metastatic model of PHEO (Giubellino et al. 2013).
Glycolysis inhibition
In addition, when the TCA cycle is genetically compromised, as is the case in SDHx-related PHEO/PGL, glycolytic addiction of the tumor cells is ensured. These tumors are “glucose addicts” as revealed by their almost 100% of positivity on [18F]-FDG PET. This may prove to be an Achilles’ heel of these tumors. Thus, strategies disrupting glycolytic mechanisms can be used in the future.
A small-molecule inhibitor of GLUT1, such as WZB117 or STF-31, downregulates glycolysis and inhibits cancer cell growth in vitro and in vivo (Chan, et al. 2011; Liu, et al. 2012). In addition, dichloroacetate (DCA) and 3-bromopyruvate (3BP) reverse cancer-specific aerobic glycolysis (Cardaci, et al. 2012; El Sayed, et al. 2012; Kluza, et al. 2012; Kumar, et al. 2012; Matsushita, et al. 2012; Michelakis, et al. 2008; Sutendra, et al. 2013; Sutendra and Michelakis 2013). DCA down-regulates pyruvate dehydrogenase kinase (PDK), which, under normal conditions, up-regulates the glycolysis enzyme pyruvate dehydrogenase (Kluza et al. 2012; Kumar et al. 2012; Michelakis et al. 2008; Sutendra et al. 2013; Sutendra and Michelakis 2013), shifting metabolism from glycolysis to glucose oxidation and selectively inducing apoptosis in cancer cells (Xie, et al. 2011). Furthermore, inhibitors of HK2 (Pedersen 2012; Yu, et al. 2012) may also represent a novel therapeutic approach to malignant SDHx-related PHEO/PGL.
Disruption of pH regulators
In addition, activation of the HIF-1α pathway enhances glycolytic metabolism and generates increased amounts of lactic and carbonic acids. This poses considerable cellular stress and requires a continous regulation by several pH-regulating systems. It has been shown that disruption of these proteins may provide an effective avenue for future targeted therapies in different cancer models (Parks, et al. 2013). However, several studies have shown a predominant expression of HIF-2α over HIF-1α in SDH-related tumors (Eisenhofer et al. 2004; Favier et al. 2009; Jochmanova et al. 2013), suggesting a leading role for HIF-2α in SDHx-related tumorigenesis. Thus, the identification of subgroups of patients with preferential or combined activation of HIF-1α or HIF-1/2α, respectively, would help in the development of “personalized” approaches in this type of therapy. In these patients, disrupting pH-regulating capacities and the export of lactic acid from tumor cells (by disrupting MCTs) could reduce glycolysis and growth rates. Additional strategies could be developed by disrupting glycolytic mechanisms.
Disruption of alternative signaling pathways
Additional treatment strategies could target abnormally functioning pathways, possibly in conjunction with targeting metabolic pathways. For example, the pseudohypoxic response and abnormal energy status of tumor cells activate kinase signaling pathways such as PI3kinase/AKT, RAS/RAF/ERK, and mTOR1/p70s6K, which leads to abnormal cell growth and a lack of apoptotic capacity (Abraham and Eng 2010; Choo, et al. 2010; Nolting and Grossman 2012). Favier et al. showed that the mTOR pathway was potentially activated in half of PHEO/PGLs (Favier, et al. 2012). Nölting et al. showed that combination treatment with dual NVP-BEZ235 (a PI3K/mTORC1 inhibitor) and lovastatin (an inhibitor of ERK signaling) had a significant additive effect in mice PHEO MPC and MTT cells and resulted in the inhibition of both AKT and mTORC1/p70S6K signaling without ERK upregulation (Nolting, et al. 2012). However, recently, Ghayee et al. (Ghayee, et al. 2013) suggested that the use of mTOR inhibitors alone for metastatic SDHB PHEOs/PGLs may not achieve good therapeutic efficacy in these patients.
In summary, recent advances and insights into SDHx-related PHEOs/PGLs as tumors with significant changes in their metabolism may lead to major advances in the treatment of these tumors.
Acknowledgments
Funding
This work was supported by the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development and the National Institute of Neurological Disorders and Stroke at the National Institutes of Health, and by MH CZ – DRO, University Hospital Motol, Prague, Czech Republic 00064203.
This work was supported by the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development at the National Institutes of Health. We thank Victoria Martucci and Ankita Reddy for their technical assistance.
Footnotes
DISCLOSURE STATEMENT: The authors have nothing to disclose.
Declaration of interest
The authors declare no conflict of interest.
Author contributions
AV, DT, and KP contributed to the manuscript conception and design, data collection and interpretation, writing, editing, and final proof. KP provided administrative support.
References
- Abraham RT, Eng CH. A metabolic (re-)balancing act. Mol Cell. 2010;38:481–482. doi: 10.1016/j.molcel.2010.05.008. [DOI] [PubMed] [Google Scholar]
- Agger K, Christensen J, Cloos PA, Helin K. The emerging functions of histone demethylases. Curr Opin Genet Dev. 2008;18:159–168. doi: 10.1016/j.gde.2007.12.003. [DOI] [PubMed] [Google Scholar]
- Alston CL, Davison JE, Meloni F, van der Westhuizen FH, He L, Hornig-Do HT, Peet AC, Gissen P, Goffrini P, Ferrero I, et al. Recessive germline SDHA and SDHB mutations causing leukodystrophy and isolated mitochondrial complex II deficiency. J Med Genet. 2012;49:569–577. doi: 10.1136/jmedgenet-2012-101146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astuti D, Hart-Holden N, Latif F, Lalloo F, Black GC, Lim C, Moran A, Grossman AB, Hodgson SV, Freemont A, et al. Genetic analysis of mitochondrial complex II subunits SDHD, SDHB and SDHC in paraganglioma and phaeochromocytoma susceptibility. Clin Endocrinol (Oxf) 2003;59:728–733. doi: 10.1046/j.1365-2265.2003.01914.x. [DOI] [PubMed] [Google Scholar]
- Astuti D, Latif F, Dallol A, Dahia PL, Douglas F, George E, Skoldberg F, Husebye ES, Eng C, Maher ER. Gene mutations in the succinate dehydrogenase subunit SDHB cause susceptibility to familial pheochromocytoma and to familial paraganglioma. Am J Hum Genet. 2001;69:49–54. doi: 10.1086/321282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astuti D, Morris M, Krona C, Abel F, Gentle D, Martinsson T, Kogner P, Neumann HP, Voutilainen R, Eng C, et al. Investigation of the role of SDHB inactivation in sporadic phaeochromocytoma and neuroblastoma. Br J Cancer. 2004;91:1835–1841. doi: 10.1038/sj.bjc.6602202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardella C, Pollard PJ, Tomlinson I. SDH mutations in cancer. Biochim Biophys Acta. 2011 doi: 10.1016/j.bbabio.2011.07.003. [DOI] [PubMed] [Google Scholar]
- Bayley JP, Devilee P, Taschner PE. The SDH mutation database: an online resource for succinate dehydrogenase sequence variants involved in pheochromocytoma, paraganglioma and mitochondrial complex II deficiency. BMC Med Genet. 2005;6:39. doi: 10.1186/1471-2350-6-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayley JP, van Minderhout I, Weiss MM, Jansen JC, Oomen PH, Menko FH, Pasini B, Ferrando B, Wong N, Alpert LC, et al. Mutation analysis of SDHB and SDHC: novel germline mutations in sporadic head and neck paraganglioma and familial paraganglioma and/or pheochromocytoma. BMC Med Genet. 2006;7:1. doi: 10.1186/1471-2350-7-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baysal BE. On the association of succinate dehydrogenase mutations with hereditary paraganglioma. Trends Endocrinol Metab. 2003;14:453–459. doi: 10.1016/j.tem.2003.08.004. [DOI] [PubMed] [Google Scholar]
- Baysal BE. Genomic imprinting and environment in hereditary paraganglioma. Am J Med Genet C Semin Med Genet. 2004;129C:85–90. doi: 10.1002/ajmg.c.30018. [DOI] [PubMed] [Google Scholar]
- Baysal BE. Clinical and molecular progress in hereditary paraganglioma. J Med Genet. 2008;45:689–694. doi: 10.1136/jmg.2008.058560. [DOI] [PubMed] [Google Scholar]
- Baysal BE, Ferrell RE, Willett-Brozick JE, Lawrence EC, Myssiorek D, Bosch A, van der Mey A, Taschner PE, Rubinstein WS, Myers EN, et al. Mutations in SDHD, a mitochondrial complex II gene, in hereditary paraganglioma. Science. 2000;287:848–851. doi: 10.1126/science.287.5454.848. [DOI] [PubMed] [Google Scholar]
- Benn DE, Gimenez-Roqueplo AP, Reilly JR, Bertherat J, Burgess J, Byth K, Croxson M, Dahia PL, Elston M, Gimm O, et al. Clinical presentation and penetrance of pheochromocytoma/paraganglioma syndromes. J Clin Endocrinol Metab. 2006;91:827–836. doi: 10.1210/jc.2005-1862. [DOI] [PubMed] [Google Scholar]
- Bensinger SJ, Christofk HR. New aspects of the Warburg effect in cancer cell biology. Semin Cell Dev Biol. 2012;23:352–361. doi: 10.1016/j.semcdb.2012.02.003. [DOI] [PubMed] [Google Scholar]
- Birch-Machin MA, Taylor RW, Cochran B, Ackrell BA, Turnbull DM. Late-onset optic atrophy, ataxia, and myopathy associated with a mutation of a complex II gene. Ann Neurol. 2000;48:330–335. [PubMed] [Google Scholar]
- Branco-Price C, Zhang N, Schnelle M, Evans C, Katschinski DM, Liao D, Ellies L, Johnson RS. Endothelial cell HIF-1alpha and HIF-2alpha differentially regulate metastatic success. Cancer Cell. 2012;21:52–65. doi: 10.1016/j.ccr.2011.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnichon N, Briere JJ, Libe R, Vescovo L, Riviere J, Tissier F, Jouanno E, Jeunemaitre X, Benit P, Tzagoloff A, et al. SDHA is a tumor suppressor gene causing paraganglioma. Hum Mol Genet. 2010;19:3011–3020. doi: 10.1093/hmg/ddq206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnichon N, Buffet A, Parfait B, Letouze E, Laurendeau I, Loriot C, Pasmant E, Abermil N, Valeyrie-Allanore L, Bertherat J, et al. Somatic NF1 inactivation is a frequent event in sporadic pheochromocytoma. Hum Mol Genet. 2012a;21:5397–5405. doi: 10.1093/hmg/dds374. [DOI] [PubMed] [Google Scholar]
- Burnichon N, Cascon A, Schiavi F, Morales NP, Comino-Mendez I, Abermil N, Inglada-Perez L, de Cubas AA, Amar L, Barontini M, et al. MAX mutations cause hereditary and sporadic pheochromocytoma and paraganglioma. Clin Cancer Res. 2012b;18:2828–2837. doi: 10.1158/1078-0432.CCR-12-0160. [DOI] [PubMed] [Google Scholar]
- Burnichon N, Rohmer V, Amar L, Herman P, Leboulleux S, Darrouzet V, Niccoli P, Gaillard D, Chabrier G, Chabolle F, et al. The succinate dehydrogenase genetic testing in a large prospective series of patients with paragangliomas. J Clin Endocrinol Metab. 2009;94:2817–2827. doi: 10.1210/jc.2008-2504. [DOI] [PubMed] [Google Scholar]
- Burnichon N, Vescovo L, Amar L, Libe R, de Reynies A, Venisse A, Jouanno E, Laurendeau I, Parfait B, Bertherat J, et al. Integrative genomic analysis reveals somatic mutations in pheochromocytoma and paraganglioma. Hum Mol Genet. 2011;20:3974–3985. doi: 10.1093/hmg/ddr324. [DOI] [PubMed] [Google Scholar]
- Cairns RA, Harris IS, Mak TW. Regulation of cancer cell metabolism. Nat Rev Cancer. 2011;11:85–95. doi: 10.1038/nrc2981. [DOI] [PubMed] [Google Scholar]
- Cardaci S, Desideri E, Ciriolo MR. Targeting aerobic glycolysis: 3-bromopyruvate as a promising anticancer drug. J Bioenerg Biomembr. 2012;44:17–29. doi: 10.1007/s10863-012-9422-7. [DOI] [PubMed] [Google Scholar]
- Cascon A, Landa I, Lopez-Jimenez E, Diez-Hernandez A, Buchta M, Montero-Conde C, Leskela S, Leandro-Garcia LJ, Leton R, Rodriguez-Antona C, et al. Molecular characterisation of a common SDHB deletion in paraganglioma patients. J Med Genet. 2008;45:233–238. doi: 10.1136/jmg.2007.054965. [DOI] [PubMed] [Google Scholar]
- Cascon A, Tennant DA. From transcriptional profiling to tumor biology in pheochromocytoma and paraganglioma. Endocr Pathol. 2012;23:15–20. doi: 10.1007/s12022-012-9195-x. [DOI] [PubMed] [Google Scholar]
- Castro-Vega LJ, Buffet A, de Cubas AA, Cascon A, Menara M, Khalifa E, Amar L, Azriel S, Bourdeau I, Chabre O, et al. Germline mutations in FH confer predisposition to malignant pheochromocytomas and paragangliomas. Hum Mol Genet. 2013 doi: 10.1093/hmg/ddt639. [DOI] [PubMed] [Google Scholar]
- Cervera AM, Bayley JP, Devilee P, McCreath KJ. Inhibition of succinate dehydrogenase dysregulates histone modification in mammalian cells. Mol Cancer. 2009;8:89. doi: 10.1186/1476-4598-8-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan DA, Sutphin PD, Nguyen P, Turcotte S, Lai EW, Banh A, Reynolds GE, Chi JT, Wu J, Solow-Cordero DE, et al. Targeting GLUT1 and the Warburg effect in renal cell carcinoma by chemical synthetic lethality. Sci Transl Med. 2011;3:94ra70. doi: 10.1126/scitranslmed.3002394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiavarina B, Martinez-Outschoorn UE, Whitaker-Menezes D, Howell A, Tanowitz HB, Pestell RG, Sotgia F, Lisanti MP. Metabolic reprogramming and two-compartment tumor metabolism: opposing role(s) of HIF1alpha and HIF2alpha in tumor-associated fibroblasts and human breast cancer cells. Cell Cycle. 2012;11:3280–3289. doi: 10.4161/cc.21643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiche J, Brahimi-Horn MC, Pouyssegur J. Tumour hypoxia induces a metabolic shift causing acidosis: a common feature in cancer. J Cell Mol Med. 2010;14:771–794. doi: 10.1111/j.1582-4934.2009.00994.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi HJ, Song BJ, Gong YD, Gwak WJ, Soh Y. Rapid degradation of hypoxia-inducible factor-1alpha by KRH102053, a new activator of prolyl hydroxylase 2. Br J Pharmacol. 2008;154:114–125. doi: 10.1038/bjp.2008.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi J, Jung WH, Koo JS. Metabolism-related proteins are differentially expressed according to the molecular subtype of invasive breast cancer defined by surrogate immunohistochemistry. Pathobiology. 2013;80:41–52. doi: 10.1159/000339513. [DOI] [PubMed] [Google Scholar]
- Choo AY, Kim SG, Vander Heiden MG, Mahoney SJ, Vu H, Yoon SO, Cantley LC, Blenis J. Glucose addiction of TSC null cells is caused by failed mTORC1-dependent balancing of metabolic demand with supply. Mol Cell. 2010;38:487–499. doi: 10.1016/j.molcel.2010.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comino-Mendez I, Gracia-Aznarez FJ, Schiavi F, Landa I, Leandro-Garcia LJ, Leton R, Honrado E, Ramos-Medina R, Caronia D, Pita G, et al. Exome sequencing identifies MAX mutations as a cause of hereditary pheochromocytoma. Nat Genet. 2011;43:663–667. doi: 10.1038/ng.861. [DOI] [PubMed] [Google Scholar]
- Crona J, Delgado Verdugo A, Maharjan R, Stalberg P, Granberg D, Hellman P, Bjorklund P. Somatic mutations in H-RAS in sporadic pheochromocytoma and paraganglioma identified by exome sequencing. J Clin Endocrinol Metab. 2013;98:E1266–1271. doi: 10.1210/jc.2012-4257. [DOI] [PubMed] [Google Scholar]
- Dahia PL. The genetic landscape of pheochromocytomas and paragangliomas: somatic mutations take center stage. J Clin Endocrinol Metab. 2013;98:2679–2681. doi: 10.1210/jc.2013-2191. [DOI] [PubMed] [Google Scholar]
- Dahia PL, Ross KN, Wright ME, Hayashida CY, Santagata S, Barontini M, Kung AL, Sanso G, Powers JF, Tischler AS, et al. A HIF1alpha regulatory loop links hypoxia and mitochondrial signals in pheochromocytomas. PLoS Genet. 2005;1:72–80. doi: 10.1371/journal.pgen.0010008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang CV. Glutaminolysis: supplying carbon or nitrogen or both for cancer cells? Cell Cycle. 2010;9:3884–3886. doi: 10.4161/cc.9.19.13302. [DOI] [PubMed] [Google Scholar]
- Dang CV, Lewis BC, Dolde C, Dang G, Shim H. Oncogenes in tumor metabolism, tumorigenesis, and apoptosis. J Bioenerg Biomembr. 1997;29:345–354. doi: 10.1023/a:1022446730452. [DOI] [PubMed] [Google Scholar]
- Dang L, White DW, Gross S, Bennett BD, Bittinger MA, Driggers EM, Fantin VR, Jang HG, Jin S, Keenan MC, et al. Cancer-associated IDH1 mutations produce 2-hydroxyglutarate. Nature. 2009;462:739–744. doi: 10.1038/nature08617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dann CE, 3rd, Bruick RK. Dioxygenases as O2-dependent regulators of the hypoxic response pathway. Biochem Biophys Res Commun. 2005;338:639–647. doi: 10.1016/j.bbrc.2005.08.140. [DOI] [PubMed] [Google Scholar]
- Deberardinis RJ, Sayed N, Ditsworth D, Thompson CB. Brick by brick: metabolism and tumor cell growth. Curr Opin Genet Dev. 2008;18:54–61. doi: 10.1016/j.gde.2008.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudkina NV, Eubel H, Keegstra W, Boekema EJ, Braun HP. Structure of a mitochondrial supercomplex formed by respiratory-chain complexes I and III. Proc Natl Acad Sci U S A. 2005;102:3225–3229. doi: 10.1073/pnas.0408870102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eakin RT, Morgan LO, Gregg CT, Matwiyoff NA. Carbon-13 nuclear magnetic resonance spectroscopy of living cells and their metabolism of a specifically labeled 13C substrate. FEBS Lett. 1972;28:259–264. doi: 10.1016/0014-5793(72)80726-9. [DOI] [PubMed] [Google Scholar]
- Eisenhofer G, Huynh TT, Pacak K, Brouwers FM, Walther MM, Linehan WM, Munson PJ, Mannelli M, Goldstein DS, Elkahloun AG. Distinct gene expression profiles in norepinephrine- and epinephrine-producing hereditary and sporadic pheochromocytomas: activation of hypoxia-driven angiogenic pathways in von Hippel-Lindau syndrome. Endocr Relat Cancer. 2004;11:897–911. doi: 10.1677/erc.1.00838. [DOI] [PubMed] [Google Scholar]
- Eisenhofer G, Lenders JW, Siegert G, Bornstein SR, Friberg P, Milosevic D, Mannelli M, Linehan WM, Adams K, Timmers HJ, et al. Plasma methoxytyramine: a novel biomarker of metastatic pheochromocytoma and paraganglioma in relation to established risk factors of tumour size, location and SDHB mutation status. Eur J Cancer. 2012;48:1739–1749. doi: 10.1016/j.ejca.2011.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenhofer G, Lenders JW, Timmers H, Mannelli M, Grebe SK, Hofbauer LC, Bornstein SR, Tiebel O, Adams K, Bratslavsky G, et al. Measurements of plasma methoxytyramine, normetanephrine, and metanephrine as discriminators of different hereditary forms of pheochromocytoma. Clin Chem. 2011a;57:411–420. doi: 10.1373/clinchem.2010.153320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenhofer G, Pacak K, Huynh TT, Qin N, Bratslavsky G, Linehan WM, Mannelli M, Friberg P, Grebe SK, Timmers HJ, et al. Catecholamine metabolomic and secretory phenotypes in phaeochromocytoma. Endocr Relat Cancer. 2011b;18:97–111. doi: 10.1677/ERC-10-0211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Sayed SM, El-Magd RM, Shishido Y, Chung SP, Diem TH, Sakai T, Watanabe H, Kagami S, Fukui K. 3-Bromopyruvate antagonizes effects of lactate and pyruvate, synergizes with citrate and exerts novel anti-glioma effects. J Bioenerg Biomembr. 2012;44:61–79. doi: 10.1007/s10863-012-9409-4. [DOI] [PubMed] [Google Scholar]
- Eltzschig HK, Eckle T, Grenz A. PHD2 mutation and congenital erythrocytosis with paraganglioma. N Engl J Med. 2009;360:1361–1362. doi: 10.1056/NEJMc090088. author reply 1362. [DOI] [PubMed] [Google Scholar]
- Eng C, Kiuru M, Fernandez MJ, Aaltonen LA. A role for mitochondrial enzymes in inherited neoplasia and beyond. Nat Rev Cancer. 2003;3:193–202. doi: 10.1038/nrc1013. [DOI] [PubMed] [Google Scholar]
- Eng CH, Abraham RT. Glutaminolysis yields a metabolic by-product that stimulates autophagy. Autophagy. 2010;6:968–970. doi: 10.4161/auto.6.7.13082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erlic Z, Rybicki L, Peczkowska M, Golcher H, Kann PH, Brauckhoff M, Mussig K, Muresan M, Schaffler A, Reisch N, et al. Clinical predictors and algorithm for the genetic diagnosis of pheochromocytoma patients. Clin Cancer Res. 2009;15:6378–6385. doi: 10.1158/1078-0432.CCR-09-1237. [DOI] [PubMed] [Google Scholar]
- Fantin VR, St-Pierre J, Leder P. Attenuation of LDH-A expression uncovers a link between glycolysis, mitochondrial physiology, and tumor maintenance. Cancer Cell. 2006;9:425–434. doi: 10.1016/j.ccr.2006.04.023. [DOI] [PubMed] [Google Scholar]
- Favier J, Briere JJ, Burnichon N, Riviere J, Vescovo L, Benit P, Giscos-Douriez I, De Reynies A, Bertherat J, Badoual C, et al. The Warburg effect is genetically determined in inherited pheochromocytomas. PLoS One. 2009;4:e7094. doi: 10.1371/journal.pone.0007094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favier J, Gimenez-Roqueplo AP. Pheochromocytomas: the (pseudo)-hypoxia hypothesis. Best Pract Res Clin Endocrinol Metab. 2010;24:957–968. doi: 10.1016/j.beem.2010.10.004. [DOI] [PubMed] [Google Scholar]
- Favier J, Igaz P, Burnichon N, Amar L, Libe R, Badoual C, Tissier F, Bertherat J, Plouin PF, Jeunemaitre X, et al. Rationale for anti-angiogenic therapy in pheochromocytoma and paraganglioma. Endocr Pathol. 2012;23:34–42. doi: 10.1007/s12022-011-9189-0. [DOI] [PubMed] [Google Scholar]
- Favier J, Kempf H, Corvol P, Gasc JM. Cloning and expression pattern of EPAS1 in the chicken embryo. Colocalization with tyrosine hydroxylase. FEBS Lett. 1999;462:19–24. doi: 10.1016/s0014-5793(99)01476-3. [DOI] [PubMed] [Google Scholar]
- Fliedner SMJ, Kaludercic N, Jiang X, Hansikova H, Hajkova Z, Sladkova J, Limpuangthip A, Backlund PS, Wesley R, Martiniova L, et al. Warburg Effect’s Manifestation in Aggressive Pheochromocytomas and Paragangliomas: Insights from a Mouse Cell Model Applied to Human Tumor Tissue. PlosOne. 2012 doi: 10.1371/journal.pone.0040949. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florczyk U, Czauderna S, Stachurska A, Tertil M, Nowak W, Kozakowska M, Poellinger L, Jozkowicz A, Loboda A, Dulak J. Opposite effects of HIF-1alpha and HIF-2alpha on the regulation of IL-8 expression in endothelial cells. Free Radic Biol Med. 2011;51:1882–1892. doi: 10.1016/j.freeradbiomed.2011.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke K, Gassmann M, Wielockx B. Erythrocytosis: the HIF pathway in control. Blood. 2013;122:1122–1128. doi: 10.1182/blood-2013-01-478065. [DOI] [PubMed] [Google Scholar]
- Furlow PW, Percy MJ, Sutherland S, Bierl C, McMullin MF, Master SR, Lappin TR, Lee FS. Erythrocytosis-associated HIF-2alpha mutations demonstrate a critical role for residues C-terminal to the hydroxylacceptor proline. J Biol Chem. 2009;284:9050–9058. doi: 10.1074/jbc.M808737200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaal J, Burnichon N, Korpershoek E, Roncelin I, Bertherat J, Plouin PF, de Krijger RR, Gimenez-Roqueplo AP, Dinjens WN. Isocitrate dehydrogenase mutations are rare in pheochromocytomas and paragangliomas. J Clin Endocrinol Metab. 2010;95:1274–1278. doi: 10.1210/jc.2009-2170. [DOI] [PubMed] [Google Scholar]
- Galan SR, Kann PH. Genetics and molecular pathogenesis of pheochromocytoma and paraganglioma. Clin Endocrinol (Oxf) 2013;78:165–175. doi: 10.1111/cen.12071. [DOI] [PubMed] [Google Scholar]
- Gao P, Zhang H, Dinavahi R, Li F, Xiang Y, Raman V, Bhujwalla ZM, Felsher DW, Cheng L, Pevsner J, et al. HIF-dependent antitumorigenic effect of antioxidants in vivo. Cancer Cell. 2007;12:230–238. doi: 10.1016/j.ccr.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatenby RA, Gillies RJ. Why do cancers have high aerobic glycolysis? Nat Rev Cancer. 2004;4:891–899. doi: 10.1038/nrc1478. [DOI] [PubMed] [Google Scholar]
- Ghayee HK, Giubellino A, Click A, Kapur P, Christie A, Xie XJ, Martucci V, Shay JW, Souza RF, Pacak K. Phospho-mTOR is not upregulated in metastatic SDHB paragangliomas. Eur J Clin Invest. 2013;43:970–977. doi: 10.1111/eci.12127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill AJ, Pachter NS, Clarkson A, Tucker KM, Winship IM, Benn DE, Robinson BG, Clifton-Bligh RJ. Renal tumors and hereditary pheochromocytoma-paraganglioma syndrome type 4. N Engl J Med. 2011;364:885–886. doi: 10.1056/NEJMc1012357. [DOI] [PubMed] [Google Scholar]
- Gimenez-Roqueplo AP, Dahia PL, Robledo M. An update on the genetics of paraganglioma, pheochromocytoma, and associated hereditary syndromes. Horm Metab Res. 2012;44:328–333. doi: 10.1055/s-0031-1301302. [DOI] [PubMed] [Google Scholar]
- Gimenez-Roqueplo AP, Favier J, Rustin P, Mourad JJ, Plouin PF, Corvol P, Rotig A, Jeunemaitre X. The R22X mutation of the SDHD gene in hereditary paraganglioma abolishes the enzymatic activity of complex II in the mitochondrial respiratory chain and activates the hypoxia pathway. Am J Hum Genet. 2001;69:1186–1197. doi: 10.1086/324413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimenez-Roqueplo AP, Favier J, Rustin P, Rieubland C, Crespin M, Nau V, Khau Van Kien P, Corvol P, Plouin PF, Jeunemaitre X. Mutations in the SDHB gene are associated with extra-adrenal and/or malignant phaeochromocytomas. Cancer Res. 2003;63:5615–5621. [PubMed] [Google Scholar]
- Gimenez-Roqueplo AP, Favier J, Rustin P, Rieubland C, Kerlan V, Plouin PF, Rotig A, Jeunemaitre X. Functional consequences of a SDHB gene mutation in an apparently sporadic pheochromocytoma. J Clin Endocrinol Metab. 2002;87:4771–4774. doi: 10.1210/jc.2002-020525. [DOI] [PubMed] [Google Scholar]
- Giubellino A, Sourbier C, Lee MJ, Scroggins B, Bullova P, Landau M, Ying W, Neckers L, Trepel JB, Pacak K. Targeting heat shock protein 90 for the treatment of malignant pheochromocytoma. PLoS One. 2013;8:e56083. doi: 10.1371/journal.pone.0056083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goffrini P, Ercolino T, Panizza E, Giache V, Cavone L, Chiarugi A, Dima V, Ferrero I, Mannelli M. Functional study in a yeast model of a novel succinate dehydrogenase subunit B gene germline missense mutation (C191Y) diagnosed in a patient affected by a glomus tumor. Hum Mol Genet. 2009;18:1860–1868. doi: 10.1093/hmg/ddp102. [DOI] [PubMed] [Google Scholar]
- Gogvadze V, Zhivotovsky B, Orrenius S. The Warburg effect and mitochondrial stability in cancer cells. Mol Aspects Med. 2010;31:60–74. doi: 10.1016/j.mam.2009.12.004. [DOI] [PubMed] [Google Scholar]
- Gottlieb E, Tomlinson IP. Mitochondrial tumour suppressors: a genetic and biochemical update. Nat Rev Cancer. 2005;5:857–866. doi: 10.1038/nrc1737. [DOI] [PubMed] [Google Scholar]
- Guzy RD, Sharma B, Bell E, Chandel NS, Schumacker PT. Loss of the SdhB, but Not the SdhA, subunit of complex II triggers reactive oxygen species-dependent hypoxia-inducible factor activation and tumorigenesis. Mol Cell Biol. 2008;28:718–731. doi: 10.1128/MCB.01338-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- Hao HX, Khalimonchuk O, Schraders M, Dephoure N, Bayley JP, Kunst H, Devilee P, Cremers CW, Schiffman JD, Bentz BG, et al. SDH5, a gene required for flavination of succinate dehydrogenase, is mutated in paraganglioma. Science. 2009;325:1139–1142. doi: 10.1126/science.1175689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewitson KS, McNeill LA, Elkins JM, Schofield CJ. The role of iron and 2-oxoglutarate oxygenases in signalling. Biochem Soc Trans. 2003;31:510–515. doi: 10.1042/bst0310510. [DOI] [PubMed] [Google Scholar]
- Hobert JA, Mester JL, Moline J, Eng C. Elevated plasma succinate in PTEN, SDHB, and SDHD mutation-positive individuals. Genet Med. 2012;14:616–619. doi: 10.1038/gim.2011.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmquist-Mengelbier L, Fredlund E, Lofstedt T, Noguera R, Navarro S, Nilsson H, Pietras A, Vallon-Christersson J, Borg A, Gradin K, et al. Recruitment of HIF-1alpha and HIF-2alpha to common target genes is differentially regulated in neuroblastoma: HIF-2alpha promotes an aggressive phenotype. Cancer Cell. 2006;10:413–423. doi: 10.1016/j.ccr.2006.08.026. [DOI] [PubMed] [Google Scholar]
- Horvath R, Abicht A, Holinski-Feder E, Laner A, Gempel K, Prokisch H, Lochmuller H, Klopstock T, Jaksch M. Leigh syndrome caused by mutations in the flavoprotein (Fp) subunit of succinate dehydrogenase (SDHA) J Neurol Neurosurg Psychiatry. 2006;77:74–76. doi: 10.1136/jnnp.2005.067041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J, Lemire BD. Mutations in the C. elegans succinate dehydrogenase iron-sulfur subunit promote superoxide generation and premature aging. J Mol Biol. 2009;387:559–569. doi: 10.1016/j.jmb.2009.02.028. [DOI] [PubMed] [Google Scholar]
- Imperiale A, Moussallieh FM, Sebag F, Brunaud L, Barlier A, Elbayed K, Bachellier P, Goichot B, Pacak K, Namer IJ, et al. A new specific succinate-glutamate metabolomic hallmark in sdhx-related paragangliomas. PLoS One. 2013;8:e80539. doi: 10.1371/journal.pone.0080539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaacs JS, Jung YJ, Mimnaugh EG, Martinez A, Cuttitta F, Neckers LM. Hsp90 regulates a von Hippel Lindau-independent hypoxia-inducible factor-1 alpha-degradative pathway. J Biol Chem. 2002;277:29936–29944. doi: 10.1074/jbc.M204733200. [DOI] [PubMed] [Google Scholar]
- Isaacs JS, Jung YJ, Mole DR, Lee S, Torres-Cabala C, Chung YL, Merino M, Trepel J, Zbar B, Toro J, et al. HIF overexpression correlates with biallelic loss of fumarate hydratase in renal cancer: novel role of fumarate in regulation of HIF stability. Cancer Cell. 2005;8:143–153. doi: 10.1016/j.ccr.2005.06.017. [DOI] [PubMed] [Google Scholar]
- Jiang H, Zhang L, Koubi D, Kuo J, Groc L, Rodriguez AI, Hunter TJ, Tang S, Lazarovici P, Gautam SC, et al. Roles of Ras-Erk in apoptosis of PC12 cells induced by trophic factor withdrawal or oxidative stress. J Mol Neurosci. 2005;25:133–140. doi: 10.1385/JMN:25:2:133. [DOI] [PubMed] [Google Scholar]
- Jiang S, Dahia PL. Minireview: the busy road to pheochromocytomas and paragangliomas has a new member, TMEM127. Endocrinology. 2011;152:2133–2140. doi: 10.1210/en.2011-0052. [DOI] [PubMed] [Google Scholar]
- Jochmanova I, Yang C, Zhuang Z, Pacak K. Hypoxia-inducible factor signaling in pheochromocytoma: turning the rudder in the right direction. J Natl Cancer Inst. 2013;105:1270–1283. doi: 10.1093/jnci/djt201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jokilehto T, Jaakkola PM. The role of HIF prolyl hydroxylases in tumour growth. J Cell Mol Med. 2010;14:758–770. doi: 10.1111/j.1582-4934.2010.01030.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones RG, Thompson CB. Tumor suppressors and cell metabolism: a recipe for cancer growth. Genes Dev. 2009;23:537–548. doi: 10.1101/gad.1756509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaelin WG, Jr, McKnight SL. Influence of metabolism on epigenetics and disease. Cell. 2013;153:56–69. doi: 10.1016/j.cell.2013.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaelin WG, Jr, Ratcliffe PJ. Oxygen sensing by metazoans: the central role of the HIF hydroxylase pathway. Mol Cell. 2008;30:393–402. doi: 10.1016/j.molcel.2008.04.009. [DOI] [PubMed] [Google Scholar]
- Keith B, Johnson RS, Simon MC. HIF1alpha and HIF2alpha: sibling rivalry in hypoxic tumour growth and progression. Nat Rev Cancer. 2012;12:9–22. doi: 10.1038/nrc3183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan MN, Bhattacharyya T, Andrikopoulos P, Esteban MA, Barod R, Connor T, Ashcroft M, Maxwell PH, Kiriakidis S. Factor inhibiting HIF (FIH-1) promotes renal cancer cell survival by protecting cells from HIF-1alpha-mediated apoptosis. Br J Cancer. 2011;104:1151–1159. doi: 10.1038/bjc.2011.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killian JK, Kim SY, Miettinen M, Smith C, Merino M, Tsokos M, Quezado M, Smith WI, Jr, Jahromi MS, Xekouki P, et al. Succinate dehydrogenase mutation underlies global epigenomic divergence in gastrointestinal stromal tumor. Cancer Discov. 2013;3:648–657. doi: 10.1158/2159-8290.CD-13-0092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King A, Selak MA, Gottlieb E. Succinate dehydrogenase and fumarate hydratase: linking mitochondrial dysfunction and cancer. Oncogene. 2006;25:4675–4682. doi: 10.1038/sj.onc.1209594. [DOI] [PubMed] [Google Scholar]
- Kluza J, Corazao-Rozas P, Touil Y, Jendoubi M, Maire C, Guerreschi P, Jonneaux A, Ballot C, Balayssac S, Valable S, et al. Inactivation of the HIF-1alpha/PDK3 Signaling Axis Drives Melanoma toward Mitochondrial Oxidative Metabolism and Potentiates the Therapeutic Activity of Pro-Oxidants. Cancer Res. 2012;72:5035–5047. doi: 10.1158/0008-5472.CAN-12-0979. [DOI] [PubMed] [Google Scholar]
- Koh MY, Lemos R, Jr, Liu X, Powis G. The hypoxia-associated factor switches cells from HIF-1alpha- to HIF-2alpha-dependent signaling promoting stem cell characteristics, aggressive tumor growth and invasion. Cancer Res. 2011;71:4015–4027. doi: 10.1158/0008-5472.CAN-10-4142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koppenol WH, Bounds PL, Dang CV. Otto Warburg’s contributions to current concepts of cancer metabolism. Nat Rev Cancer. 2011;11:325–337. doi: 10.1038/nrc3038. [DOI] [PubMed] [Google Scholar]
- Korpershoek E, Favier J, Gaal J, Burnichon N, van Gessel B, Oudijk L, Badoual C, Gadessaud N, Venisse A, Bayley JP, et al. SDHA immunohistochemistry detects germline SDHA gene mutations in apparently sporadic paragangliomas and pheochromocytomas. J Clin Endocrinol Metab. 2011;96:E1472–1476. doi: 10.1210/jc.2011-1043. [DOI] [PubMed] [Google Scholar]
- Krejci A. Metabolic sensors and their interplay with cell signalling and transcription. Biochem Soc Trans. 2012;40:311–323. doi: 10.1042/BST20110767. [DOI] [PubMed] [Google Scholar]
- Kumar A, Kant S, Singh SM. Novel molecular mechanisms of antitumor action of dichloroacetate against T cell lymphoma: Implication of altered glucose metabolism, pH homeostasis and cell survival regulation. Chem Biol Interact. 2012;199:29–37. doi: 10.1016/j.cbi.2012.06.005. [DOI] [PubMed] [Google Scholar]
- Ladroue C, Carcenac R, Leporrier M, Gad S, Le Hello C, Galateau-Salle F, Feunteun J, Pouyssegur J, Richard S, Gardie B. PHD2 mutation and congenital erythrocytosis with paraganglioma. N Engl J Med. 2008;359:2685–2692. doi: 10.1056/NEJMoa0806277. [DOI] [PubMed] [Google Scholar]
- Lando D, Peet DJ, Gorman JJ, Whelan DA, Whitelaw ML, Bruick RK. FIH-1 is an asparaginyl hydroxylase enzyme that regulates the transcriptional activity of hypoxia-inducible factor. Genes Dev. 2002;16:1466–1471. doi: 10.1101/gad.991402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latif F, Duh FM, Gnarra J, Tory K, Kuzmin I, Yao M, Stackhouse T, Modi W, Geil L, Schmidt L, et al. von Hippel-Lindau syndrome: cloning and identification of the plasma membrane Ca(++)-transporting ATPase isoform 2 gene that resides in the von Hippel-Lindau gene region. Cancer Res. 1993;53:861–867. [PubMed] [Google Scholar]
- Lee S, Nakamura E, Yang H, Wei W, Linggi MS, Sajan MP, Farese RV, Freeman RS, Carter BD, Kaelin WG, Jr, et al. Neuronal apoptosis linked to EglN3 prolyl hydroxylase and familial pheochromocytoma genes: developmental culling and cancer. Cancer Cell. 2005;8:155–167. doi: 10.1016/j.ccr.2005.06.015. [DOI] [PubMed] [Google Scholar]
- Lenders JW, Eisenhofer G, Mannelli M, Pacak K. Phaeochromocytoma. Lancet. 2005;366:665–675. doi: 10.1016/S0140-6736(05)67139-5. [DOI] [PubMed] [Google Scholar]
- Lendvai N, Pawlosky R, Bullova P, Eisenhofer G, Patocs A, Veech RL, Pacak K. Succinate-to-Fumarate Ratio as a New Metabolic Marker to Detect the Presence of SDHB/D-related Paraganglioma: Initial Experimental and Ex Vivo Findings. Endocrinology. 2013 doi: 10.1210/en.2013-1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letouze E, Martinelli C, Loriot C, Burnichon N, Abermil N, Ottolenghi C, Janin M, Menara M, Nguyen AT, Benit P, et al. SDH Mutations Establish a Hypermethylator Phenotype in Paraganglioma. Cancer Cell. 2013;23:739–752. doi: 10.1016/j.ccr.2013.04.018. [DOI] [PubMed] [Google Scholar]
- Levine AJ, Puzio-Kuter AM. The control of the metabolic switch in cancers by oncogenes and tumor suppressor genes. Science. 2010;330:1340–1344. doi: 10.1126/science.1193494. [DOI] [PubMed] [Google Scholar]
- Levitas A, Muhammad E, Harel G, Saada A, Caspi VC, Manor E, Beck JC, Sheffield V, Parvari R. Familial neonatal isolated cardiomyopathy caused by a mutation in the flavoprotein subunit of succinate dehydrogenase. Eur J Hum Genet. 2010;18:1160–1165. doi: 10.1038/ejhg.2010.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linehan WM, Rouault TA. Molecular pathways: fumarate hydratase-deficient kidney cancer--targeting the warburg effect in cancer. Clin Cancer Res. 2013;19:3345–3352. doi: 10.1158/1078-0432.CCR-13-0304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Cao Y, Zhang W, Bergmeier S, Qian Y, Akbar H, Colvin R, Ding J, Tong L, Wu S, et al. A small-molecule inhibitor of glucose transporter 1 downregulates glycolysis, induces cell-cycle arrest, and inhibits cancer cell growth in vitro and in vivo. Mol Cancer Ther. 2012;11:1672–1682. doi: 10.1158/1535-7163.MCT-12-0131. [DOI] [PubMed] [Google Scholar]
- Liu YV, Baek JH, Zhang H, Diez R, Cole RN, Semenza GL. RACK1 competes with HSP90 for binding to HIF-1alpha and is required for O(2)-independent and HSP90 inhibitor-induced degradation of HIF-1alpha. Mol Cell. 2007;25:207–217. doi: 10.1016/j.molcel.2007.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Jimenez E, Gomez-Lopez G, Leandro-Garcia LJ, Munoz I, Schiavi F, Montero-Conde C, de Cubas AA, Ramires R, Landa I, Leskela S, et al. Research resource: Transcriptional profiling reveals different pseudohypoxic signatures in SDHB and VHL-related pheochromocytomas. Mol Endocrinol. 2010;24:2382–2391. doi: 10.1210/me.2010-0256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzo FR, Yang C, Ng Tang Fui M, Vankayalapati H, Zhuang Z, Huynh T, Grossmann M, Pacak K, Prchal JT. A novel EPAS1/HIF2A germline mutation in a congenital polycythemia with paraganglioma. J Mol Med (Berl) 2012 doi: 10.1007/s00109-012-0967-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo W, Hu H, Chang R, Zhong J, Knabel M, O’Meally R, Cole RN, Pandey A, Semenza GL. Pyruvate kinase M2 is a PHD3-stimulated coactivator for hypoxia-inducible factor 1. Cell. 2011;145:732–744. doi: 10.1016/j.cell.2011.03.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo W, Semenza GL. Pyruvate kinase M2 regulates glucose metabolism by functioning as a coactivator for hypoxia-inducible factor 1 in cancer cells. Oncotarget. 2011;2:551–556. doi: 10.18632/oncotarget.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo W, Semenza GL. Emerging roles of PKM2 in cell metabolism and cancer progression. Trends Endocrinol Metab. 2012;23:560–566. doi: 10.1016/j.tem.2012.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKenzie ED, Selak MA, Tennant DA, Payne LJ, Crosby S, Frederiksen CM, Watson DG, Gottlieb E. Cell-permeating alpha-ketoglutarate derivatives alleviate pseudohypoxia in succinate dehydrogenase-deficient cells. Mol Cell Biol. 2007;27:3282–3289. doi: 10.1128/MCB.01927-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahalingam D, Swords R, Carew JS, Nawrocki ST, Bhalla K, Giles FJ. Targeting HSP90 for cancer therapy. Br J Cancer. 2009;100:1523–1529. doi: 10.1038/sj.bjc.6605066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maher ER, Eng C. The pressure rises: update on the genetics of phaeochromocytoma. Hum Mol Genet. 2002;11:2347–2354. doi: 10.1093/hmg/11.20.2347. [DOI] [PubMed] [Google Scholar]
- Mahon PC, Hirota K, Semenza GL. FIH-1: a novel protein that interacts with HIF-1alpha and VHL to mediate repression of HIF-1 transcriptional activity. Genes Dev. 2001;15:2675–2686. doi: 10.1101/gad.924501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majmundar AJ, Wong WJ, Simon MC. Hypoxia-inducible factors and the response to hypoxic stress. Mol Cell. 2010;40:294–309. doi: 10.1016/j.molcel.2010.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathupala SP, Ko YH, Pedersen PL. Hexokinase-2 bound to mitochondria: cancer’s stygian link to the “Warburg Effect” and a pivotal target for effective therapy. Semin Cancer Biol. 2009;19:17–24. doi: 10.1016/j.semcancer.2008.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsushita K, Uchida K, Saigusa S, Ide S, Hashimoto K, Koike Y, Otake K, Inoue M, Tanaka K, Kusunoki M. Glycolysis inhibitors as a potential therapeutic option to treat aggressive neuroblastoma expressing GLUT1. J Pediatr Surg. 2012;47:1323–1330. doi: 10.1016/j.jpedsurg.2011.12.007. [DOI] [PubMed] [Google Scholar]
- Meng M, Chen S, Lao T, Liang D, Sang N. Nitrogen anabolism underlies the importance of glutaminolysis in proliferating cells. Cell Cycle. 2010;9:3921–3932. doi: 10.4161/cc.9.19.13139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michelakis ED, Webster L, Mackey JR. Dichloroacetate (DCA) as a potential metabolic-targeting therapy for cancer. Br J Cancer. 2008;99:989–994. doi: 10.1038/sj.bjc.6604554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mole DR, Blancher C, Copley RR, Pollard PJ, Gleadle JM, Ragoussis J, Ratcliffe PJ. Genome-wide association of hypoxia-inducible factor (HIF)-1alpha and HIF-2alpha DNA binding with expression profiling of hypoxia-inducible transcripts. J Biol Chem. 2009;284:16767–16775. doi: 10.1074/jbc.M901790200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulligan LM, Kwok JB, Healey CS, Elsdon MJ, Eng C, Gardner E, Love DR, Mole SE, Moore JK, Papi L, et al. Germ-line mutations of the RET proto-oncogene in multiple endocrine neoplasia type 2A. Nature. 1993;363:458–460. doi: 10.1038/363458a0. [DOI] [PubMed] [Google Scholar]
- Nakajima EC, Van Houten B. Metabolic symbiosis in cancer: Refocusing the Warburg lens. Mol Carcinog. 2012 doi: 10.1002/mc.21863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nepal M, Gong YD, Park YR, Soh Y. An activator of PHD2, KRH102140, decreases angiogenesis via inhibition of HIF-1alpha. Cell Biochem Funct. 2011;29:126–134. doi: 10.1002/cbf.1732. [DOI] [PubMed] [Google Scholar]
- Neumann HP, Bausch B, McWhinney SR, Bender BU, Gimm O, Franke G, Schipper J, Klisch J, Altehoefer C, Zerres K, et al. Germ-line mutations in nonsyndromic pheochromocytoma. N Engl J Med. 2002;346:1459–1466. doi: 10.1056/NEJMoa020152. [DOI] [PubMed] [Google Scholar]
- Ni Y, Eng C. Vitamin E Protects against Lipid Peroxidation and Rescues Tumorigenic Phenotypes in Cowden/Cowden-like Patient-derived Lymphoblast Cells with Germline SDHx Variants. Clin Cancer Res. 2012 doi: 10.1158/1078-0432.CCR-12-1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni Y, He X, Chen J, Moline J, Mester J, Orloff MS, Ringel MD, Eng C. Germline SDHx variants modify breast and thyroid cancer risks in Cowden and Cowden-like syndrome via FAD/NAD-dependant destabilization of p53. Hum Mol Genet. 2012;21:300–310. doi: 10.1093/hmg/ddr459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni Y, Zbuk KM, Sadler T, Patocs A, Lobo G, Edelman E, Platzer P, Orloff MS, Waite KA, Eng C. Germline mutations and variants in the succinate dehydrogenase genes in Cowden and Cowden-like syndromes. Am J Hum Genet. 2008;83:261–268. doi: 10.1016/j.ajhg.2008.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niemann S, Muller U. Mutations in SDHC cause autosomal dominant paraganglioma, type 3. Nat Genet. 2000;26:268–270. doi: 10.1038/81551. [DOI] [PubMed] [Google Scholar]
- Nilsson H, Jogi A, Beckman S, Harris AL, Poellinger L, Pahlman S. HIF-2alpha expression in human fetal paraganglia and neuroblastoma: relation to sympathetic differentiation, glucose deficiency, and hypoxia. Exp Cell Res. 2005;303:447–456. doi: 10.1016/j.yexcr.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Nolting S, Garcia E, Alusi G, Giubellino A, Pacak K, Korbonits M, Grossman AB. Combined blockade of signalling pathways shows marked anti-tumour potential in phaeochromocytoma cell lines. J Mol Endocrinol. 2012;49:79–96. doi: 10.1530/JME-12-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolting S, Grossman AB. Signaling pathways in pheochromocytomas and paragangliomas: prospects for future therapies. Endocr Pathol. 2012;23:21–33. doi: 10.1007/s12022-012-9199-6. [DOI] [PubMed] [Google Scholar]
- Northcott PA, Shih DJ, Remke M, Cho YJ, Kool M, Hawkins C, Eberhart CG, Dubuc A, Guettouche T, Cardentey Y, et al. Rapid, reliable, and reproducible molecular subgrouping of clinical medulloblastoma samples. Acta Neuropathol. 2012;123:615–626. doi: 10.1007/s00401-011-0899-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osthus RC, Shim H, Kim S, Li Q, Reddy R, Mukherjee M, Xu Y, Wonsey D, Lee LA, Dang CV. Deregulation of glucose transporter 1 and glycolytic gene expression by c-Myc. J Biol Chem. 2000;275:21797–21800. doi: 10.1074/jbc.C000023200. [DOI] [PubMed] [Google Scholar]
- Owens KM, Aykin-Burns N, Dayal D, Coleman MC, Domann FE, Spitz DR. Genomic instability induced by mutant succinate dehydrogenase subunit D (SDHD) is mediated by O2(−*) and H2O2. Free Radic Biol Med. 2012;52:160–166. doi: 10.1016/j.freeradbiomed.2011.10.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan JS, Hong MZ, Ren JL. Reactive oxygen species: a double-edged sword in oncogenesis. World J Gastroenterol. 2009;15:1702–1707. doi: 10.3748/wjg.15.1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papaspyrou K, Mewes T, Rossmann H, Fottner C, Schneider-Raetzke B, Bartsch O, Schreckenberger M, Lackner KJ, Amedee RG, Mann WJ. Head and neck paragangliomas: Report of 175 patients (1989–2010) Head Neck. 2011 doi: 10.1002/hed.21790. [DOI] [PubMed] [Google Scholar]
- Parfait B, Chretien D, Rotig A, Marsac C, Munnich A, Rustin P. Compound heterozygous mutations in the flavoprotein gene of the respiratory chain complex II in a patient with Leigh syndrome. Hum Genet. 2000;106:236–243. doi: 10.1007/s004390051033. [DOI] [PubMed] [Google Scholar]
- Parks SK, Chiche J, Pouyssegur J. Disrupting proton dynamics and energy metabolism for cancer therapy. Nat Rev Cancer. 2013;13:611–623. doi: 10.1038/nrc3579. [DOI] [PubMed] [Google Scholar]
- Patel SA, Simon MC. Biology of hypoxia-inducible factor-2alpha in development and disease. Cell Death Differ. 2008;15:628–634. doi: 10.1038/cdd.2008.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen PL. 3-Bromopyruvate (3BP) a fast acting, promising, powerful, specific, and effective “small molecule” anti-cancer agent taken from labside to bedside: introduction to a special issue. J Bioenerg Biomembr. 2012;44:1–6. doi: 10.1007/s10863-012-9425-4. [DOI] [PubMed] [Google Scholar]
- Pollard PJ, Briere JJ, Alam NA, Barwell J, Barclay E, Wortham NC, Hunt T, Mitchell M, Olpin S, Moat SJ, et al. Accumulation of Krebs cycle intermediates and over-expression of HIF1alpha in tumours which result from germline FH and SDH mutations. Hum Mol Genet. 2005;14:2231–2239. doi: 10.1093/hmg/ddi227. [DOI] [PubMed] [Google Scholar]
- Pollard PJ, El-Bahrawy M, Poulsom R, Elia G, Killick P, Kelly G, Hunt T, Jeffery R, Seedhar P, Barwell J, et al. Expression of HIF-1alpha, HIF-2alpha (EPAS1), and their target genes in paraganglioma and pheochromocytoma with VHL and SDH mutations. J Clin Endocrinol Metab. 2006;91:4593–4598. doi: 10.1210/jc.2006-0920. [DOI] [PubMed] [Google Scholar]
- Powers JF, Evinger MJ, Zhi J, Picard KL, Tischler AS. Pheochromocytomas in Nf1 knockout mice express a neural progenitor gene expression profile. Neuroscience. 2007;147:928–937. doi: 10.1016/j.neuroscience.2007.05.008. [DOI] [PubMed] [Google Scholar]
- Qin Y, Yao L, King EE, Buddavarapu K, Lenci RE, Chocron ES, Lechleiter JD, Sass M, Aronin N, Schiavi F, et al. Germline mutations in TMEM127 confer susceptibility to pheochromocytoma. Nat Genet. 2010;42:229–233. doi: 10.1038/ng.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rankin EB, Biju MP, Liu Q, Unger TL, Rha J, Johnson RS, Simon MC, Keith B, Haase VH. Hypoxia-inducible factor-2 (HIF-2) regulates hepatic erythropoietin in vivo. J Clin Invest. 2007;117:1068–1077. doi: 10.1172/JCI30117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao JU, Engelke UF, Rodenburg RJ, Wevers RA, Pacak K, Eisenhofer G, Qin N, Kusters B, Goudswaard AG, Lenders JW, et al. Genotype-Specific Abnormalities in Mitochondrial Function Associate with Distinct Profiles of Energy Metabolism and Catecholamine Content in Pheochromocytoma and Paraganglioma. Clin Cancer Res. 2013;19:3787–3795. doi: 10.1158/1078-0432.CCR-12-3922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapizzi E, Ercolino T, Canu L, Giache V, Francalanci M, Pratesi C, Valeri A, Mannelli M. Mitochondrial function and content in pheochromocytoma/paraganglioma of succinate dehydrogenase mutation carriers. Endocr Relat Cancer. 2012;19:261–269. doi: 10.1530/ERC-11-0263. [DOI] [PubMed] [Google Scholar]
- Rogers JL, Bayeh L, Scheuermann TH, Longgood J, Key J, Naidoo J, Melito L, Shokri C, Frantz DE, Bruick RK, et al. Development of inhibitors of the PAS-B domain of the HIF-2alpha transcription factor. J Med Chem. 2013;56:1739–1747. doi: 10.1021/jm301847z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rustin P, Munnich A, Rotig A. Succinate dehydrogenase and human diseases: new insights into a well-known enzyme. Eur J Hum Genet. 2002;10:289–291. doi: 10.1038/sj.ejhg.5200793. [DOI] [PubMed] [Google Scholar]
- Sandgren J, Diaz de Stahl T, Andersson R, Menzel U, Piotrowski A, Nord H, Backdahl M, Kiss NB, Brauckhoff M, Komorowski J, et al. Recurrent genomic alterations in benign and malignant pheochromocytomas and paragangliomas revealed by whole-genome array comparative genomic hybridization analysis. Endocr Relat Cancer. 2010;17:561–579. doi: 10.1677/ERC-09-0310. [DOI] [PubMed] [Google Scholar]
- Schiavi F, Boedeker CC, Bausch B, Peczkowska M, Gomez CF, Strassburg T, Pawlu C, Buchta M, Salzmann M, Hoffmann MM, et al. Predictors and prevalence of paraganglioma syndrome associated with mutations of the SDHC gene. JAMA. 2005;294:2057–2063. doi: 10.1001/jama.294.16.2057. [DOI] [PubMed] [Google Scholar]
- Schlisio S, Kenchappa RS, Vredeveld LC, George RE, Stewart R, Greulich H, Shahriari K, Nguyen NV, Pigny P, Dahia PL, et al. The kinesin KIF1Bbeta acts downstream from EglN3 to induce apoptosis and is a potential 1p36 tumor suppressor. Genes Dev. 2008;22:884–893. doi: 10.1101/gad.1648608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selak MA, Armour SM, MacKenzie ED, Boulahbel H, Watson DG, Mansfield KD, Pan Y, Simon MC, Thompson CB, Gottlieb E. Succinate links TCA cycle dysfunction to oncogenesis by inhibiting HIF-alpha prolyl hydroxylase. Cancer Cell. 2005;7:77–85. doi: 10.1016/j.ccr.2004.11.022. [DOI] [PubMed] [Google Scholar]
- Semenza G. Signal transduction to hypoxia-inducible factor 1. Biochem Pharmacol. 2002a;64:993–998. doi: 10.1016/s0006-2952(02)01168-1. [DOI] [PubMed] [Google Scholar]
- Semenza GL. HIF-1 and tumor progression: pathophysiology and therapeutics. Trends Mol Med. 2002b;8:S62–67. doi: 10.1016/s1471-4914(02)02317-1. [DOI] [PubMed] [Google Scholar]
- Semenza GL. Evaluation of HIF-1 inhibitors as anticancer agents. Drug Discov Today. 2007;12:853–859. doi: 10.1016/j.drudis.2007.08.006. [DOI] [PubMed] [Google Scholar]
- Semenza GL. Defining the role of hypoxia-inducible factor 1 in cancer biology and therapeutics. Oncogene. 2010;29:625–634. doi: 10.1038/onc.2009.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semenza GL. Oxygen sensing, homeostasis, and disease. N Engl J Med. 2011;365:537–547. doi: 10.1056/NEJMra1011165. [DOI] [PubMed] [Google Scholar]
- Semenza GL. Hypoxia-inducible factors: mediators of cancer progression and targets for cancer therapy. Trends Pharmacol Sci. 2012;33:207–214. doi: 10.1016/j.tips.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah U, Giubellino A, Pacak K. Pheochromocytoma: implications in tumorigenesis and the actual management. Minerva Endocrinol. 2012;37:141–156. [PMC free article] [PubMed] [Google Scholar]
- Singh RP, Franke K, Kalucka J, Mamlouk S, Muschter A, Gembarska A, Grinenko T, Willam C, Naumann R, Anastassiadis K, et al. HIF prolyl hydroxylase 2 (PHD2) is a critical regulator of hematopoietic stem cell maintenance during steady-state and stress. Blood. 2013;121:5158–5166. doi: 10.1182/blood-2012-12-471185. [DOI] [PubMed] [Google Scholar]
- Slane BG, Aykin-Burns N, Smith BJ, Kalen AL, Goswami PC, Domann FE, Spitz DR. Mutation of succinate dehydrogenase subunit C results in increased O2.-, oxidative stress, and genomic instability. Cancer Res. 2006;66:7615–7620. doi: 10.1158/0008-5472.CAN-06-0833. [DOI] [PubMed] [Google Scholar]
- Soga T. Cancer metabolism: key players in metabolic reprogramming. Cancer Sci. 2013;104:275–281. doi: 10.1111/cas.12085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitz DR. Metabolic oxidative stress and low dose radiation responses: are mitochondria involved. Health Phys. 2011;100:295. doi: 10.1097/hp.0b013e31820840aa. [DOI] [PubMed] [Google Scholar]
- Sun F, Huo X, Zhai Y, Wang A, Xu J, Su D, Bartlam M, Rao Z. Crystal structure of mitochondrial respiratory membrane protein complex II. Cell. 2005a;121:1043–1057. doi: 10.1016/j.cell.2005.05.025. [DOI] [PubMed] [Google Scholar]
- Sun L, Bartlam M, Liu Y, Pang H, Rao Z. Crystal structure of the pyridoxal-5′-phosphate-dependent serine dehydratase from human liver. Protein Sci. 2005b;14:791–798. doi: 10.1110/ps.041179105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutendra G, Dromparis P, Kinnaird A, Stenson TH, Haromy A, Parker JM, McMurtry MS, Michelakis ED. Mitochondrial activation by inhibition of PDKII suppresses HIF1a signaling and angiogenesis in cancer. Oncogene. 2013;32:1638–1650. doi: 10.1038/onc.2012.198. [DOI] [PubMed] [Google Scholar]
- Sutendra G, Michelakis ED. Pyruvate dehydrogenase kinase as a novel therapeutic target in oncology. Front Oncol. 2013;3:38. doi: 10.3389/fonc.2013.00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taieb D, Sebag F, Barlier A, Tessonnier L, Palazzo FF, Morange I, Niccoli-Sire P, Fakhry N, De Micco C, Cammilleri S, et al. 18F-FDG avidity of pheochromocytomas and paragangliomas: a new molecular imaging signature? J Nucl Med. 2009;50:711–717. doi: 10.2967/jnumed.108.060731. [DOI] [PubMed] [Google Scholar]
- Tannahill GM, Curtis AM, Adamik J, Palsson-McDermott EM, McGettrick AF, Goel G, Frezza C, Bernard NJ, Kelly B, Foley NH, et al. Succinate is an inflammatory signal that induces IL-1beta through HIF-1alpha. Nature. 2013;496:238–242. doi: 10.1038/nature11986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tennant DA, Frezza C, MacKenzie ED, Nguyen QD, Zheng L, Selak MA, Roberts DL, Dive C, Watson DG, Aboagye EO, et al. Reactivating HIF prolyl hydroxylases under hypoxia results in metabolic catastrophe and cell death. Oncogene. 2009;28:4009–4021. doi: 10.1038/onc.2009.250. [DOI] [PubMed] [Google Scholar]
- Tennant DA, Gottlieb E. HIF prolyl hydroxylase-3 mediates alpha-ketoglutarate-induced apoptosis and tumor suppression. J Mol Med (Berl) 2010;88:839–849. doi: 10.1007/s00109-010-0627-0. [DOI] [PubMed] [Google Scholar]
- Tian H, Hammer RE, Matsumoto AM, Russell DW, McKnight SL. The hypoxia-responsive transcription factor EPAS1 is essential for catecholamine homeostasis and protection against heart failure during embryonic development. Genes Dev. 1998;12:3320–3324. doi: 10.1101/gad.12.21.3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmers HJ, Chen CC, Carrasquillo JA, Whatley M, Ling A, Eisenhofer G, King KS, Rao JU, Wesley RA, Adams KT, et al. Staging and functional characterization of pheochromocytoma and paraganglioma by 18F-fluorodeoxyglucose (18F-FDG) positron emission tomography. J Natl Cancer Inst. 2012;104:700–708. doi: 10.1093/jnci/djs188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmers HJ, Chen CC, Carrasquillo JA, Whatley M, Ling A, Havekes B, Eisenhofer G, Martiniova L, Adams KT, Pacak K. Comparison of 18F-fluoro-L-DOPA, 18F-fluoro-deoxyglucose, and 18F-fluorodopamine PET and 123I-MIBG scintigraphy in the localization of pheochromocytoma and paraganglioma. J Clin Endocrinol Metab. 2009a;94:4757–4767. doi: 10.1210/jc.2009-1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmers HJ, Gimenez-Roqueplo AP, Mannelli M, Pacak K. Clinical aspects of SDHx-related pheochromocytoma and paraganglioma. Endocr Relat Cancer. 2009b;16:391–400. doi: 10.1677/ERC-08-0284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmers HJ, Kozupa A, Eisenhofer G, Raygada M, Adams KT, Solis D, Lenders JW, Pacak K. Clinical presentations, biochemical phenotypes, and genotype-phenotype correlations in patients with succinate dehydrogenase subunit B-associated pheochromocytomas and paragangliomas. J Clin Endocrinol Metab. 2007;92:779–786. doi: 10.1210/jc.2006-2315. [DOI] [PubMed] [Google Scholar]
- Toledo RA, Qin Y, Srikantan S, Morales NP, Li Q, Deng Y, Kim SW, Pereira MA, Toledo SP, Su X, et al. In vivo and in vitro oncogenic effects of HIF2A mutations in pheochromocytomas and paragangliomas. Endocr Relat Cancer. 2013;20:349–359. doi: 10.1530/ERC-13-0101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Nederveen FH, Gaal J, Favier J, Korpershoek E, Oldenburg RA, de Bruyn EM, Sleddens HF, Derkx P, Riviere J, Dannenberg H, et al. An immunohistochemical procedure to detect patients with paraganglioma and phaeochromocytoma with germline SDHB, SDHC, or SDHD gene mutations: a retrospective and prospective analysis. Lancet Oncol. 2009;10:764–771. doi: 10.1016/S1470-2045(09)70164-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vegran F, Boidot R, Michiels C, Sonveaux P, Feron O. Lactate influx through the endothelial cell monocarboxylate transporter MCT1 supports an NF-kappaB/IL-8 pathway that drives tumor angiogenesis. Cancer Res. 2011;71:2550–2560. doi: 10.1158/0008-5472.CAN-10-2828. [DOI] [PubMed] [Google Scholar]
- Vicha A, Musil Z, Pacak K. Genetics of pheochromocytoma and paraganglioma syndromes: new advances and future treatment options. Curr Opin Endocrinol Diabetes Obes. 2013;20:186–191. doi: 10.1097/MED.0b013e32835fcc45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viskochil D, Buchberg AM, Xu G, Cawthon RM, Stevens J, Wolff RK, Culver M, Carey JC, Copeland NG, Jenkins NA, et al. Deletions and a translocation interrupt a cloned gene at the neurofibromatosis type 1 locus. Cell. 1990;62:187–192. doi: 10.1016/0092-8674(90)90252-a. [DOI] [PubMed] [Google Scholar]
- Vogelstein B, Kinzler KW. Cancer genes and the pathways they control. Nat Med. 2004;10:789–799. doi: 10.1038/nm1087. [DOI] [PubMed] [Google Scholar]
- Walenta S, Mueller-Klieser WF. Lactate: mirror and motor of tumor malignancy. Semin Radiat Oncol. 2004;14:267–274. doi: 10.1016/j.semradonc.2004.04.004. [DOI] [PubMed] [Google Scholar]
- Warburg O. On the origin of cancer cells. Science. 1956;123:309–314. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- Warburg O, Wind F, Negelein E. Über den Stoffwechsel der Tumoren in Körper. Klinische Wochenschrift. 1926:829–832. [Google Scholar]
- Weber A, Hoffmann MM, Neumann HP, Erlic Z. Somatic Mutation Analysis of the SDHB, SDHC, SDHD, and RET Genes in the Clinical Assessment of Sporadic and Hereditary Pheochromocytoma. Horm Cancer. 2012;3:187–192. doi: 10.1007/s12672-012-0113-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh S, Williams R, Kirkpatrick L, Paine-Murrieta G, Powis G. Antitumor activity and pharmacodynamic properties of PX-478, an inhibitor of hypoxia-inducible factor-1alpha. Mol Cancer Ther. 2004;3:233–244. [PubMed] [Google Scholar]
- Welsh SJ, Williams RR, Birmingham A, Newman DJ, Kirkpatrick DL, Powis G. The thioredoxin redox inhibitors 1-methylpropyl 2-imidazolyl disulfide and pleurotin inhibit hypoxia-induced factor 1alpha and vascular endothelial growth factor formation. Mol Cancer Ther. 2003;2:235–243. [PubMed] [Google Scholar]
- Xiao M, Yang H, Xu W, Ma S, Lin H, Zhu H, Liu L, Liu Y, Yang C, Xu Y, et al. Inhibition of alpha-KG-dependent histone and DNA demethylases by fumarate and succinate that are accumulated in mutations of FH and SDH tumor suppressors. Genes Dev. 2012;26:1326–1338. doi: 10.1101/gad.191056.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie J, Wang BS, Yu DH, Lu Q, Ma J, Qi H, Fang C, Chen HZ. Dichloroacetate shifts the metabolism from glycolysis to glucose oxidation and exhibits synergistic growth inhibition with cisplatin in HeLa cells. Int J Oncol. 2011;38:409–417. doi: 10.3892/ijo.2010.851. [DOI] [PubMed] [Google Scholar]
- Yang C, Matro JC, Huntoon KM, Ye DY, Huynh TT, Fliedner SMJ, Breza J, Zhuang Z, Pacak K. Missense mutations in human SDHB gene increase protein degradation without altering intrinsic enzymatic function. FASEB J. 2012 doi: 10.1096/fj.12-210146. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yankovskaya V, Horsefield R, Tornroth S, Luna-Chavez C, Miyoshi H, Leger C, Byrne B, Cecchini G, Iwata S. Architecture of succinate dehydrogenase and reactive oxygen species generation. Science. 2003;299:700–704. doi: 10.1126/science.1079605. [DOI] [PubMed] [Google Scholar]
- Yao L, Schiavi F, Cascon A, Qin Y, Inglada-Perez L, King EE, Toledo RA, Ercolino T, Rapizzi E, Ricketts CJ, et al. Spectrum and prevalence of FP/TMEM127 gene mutations in pheochromocytomas and paragangliomas. JAMA. 2010;304:2611–2619. doi: 10.1001/jama.2010.1830. [DOI] [PubMed] [Google Scholar]
- Yeh IT, Lenci RE, Qin Y, Buddavarapu K, Ligon AH, Leteurtre E, Do Cao C, Cardot-Bauters C, Pigny P, Dahia PL. A germline mutation of the KIF1B beta gene on 1p36 in a family with neural and nonneural tumors. Hum Genet. 2008;124:279–285. doi: 10.1007/s00439-008-0553-1. [DOI] [PubMed] [Google Scholar]
- Yu SJ, Yoon JH, Yang JI, Cho EJ, Kwak MS, Jang ES, Lee JH, Kim YJ, Lee HS, Kim CY. Enhancement of hexokinase II inhibitor-induced apoptosis in hepatocellular carcinoma cells via augmenting ER stress and anti-angiogenesis by protein disulfide isomerase inhibition. J Bioenerg Biomembr. 2012;44:101–115. doi: 10.1007/s10863-012-9416-5. [DOI] [PubMed] [Google Scholar]
- Yuneva M. Finding an “Achilles’ heel” of cancer: the role of glucose and glutamine metabolism in the survival of transformed cells. Cell Cycle. 2008;7:2083–2089. doi: 10.4161/cc.7.14.6256. [DOI] [PubMed] [Google Scholar]
- Zelinka T, Timmers HJ, Kozupa A, Chen CC, Carrasquillo JA, Reynolds JC, Ling A, Eisenhofer G, Lazurova I, Adams KT, et al. Role of positron emission tomography and bone scintigraphy in the evaluation of bone involvement in metastatic pheochromocytoma and paraganglioma: specific implications for succinate dehydrogenase enzyme subunit B gene mutations. Endocr Relat Cancer. 2008;15:311–323. doi: 10.1677/ERC-07-0217. [DOI] [PubMed] [Google Scholar]
- Zhang C, Moore LM, Li X, Yung WK, Zhang W. IDH1/2 mutations target a key hallmark of cancer by deregulating cellular metabolism in glioma. Neuro Oncol. 2013 doi: 10.1093/neuonc/not087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang Z, Yang C, Lorenzo F, Merino M, Fojo T, Kebebew E, Popovic V, Stratakis CA, Prchal JT, Pacak K. Somatic HIF2A gain-of-function mutations in paraganglioma with polycythemia. N Engl J Med. 2012;367:922–930. doi: 10.1056/NEJMoa1205119. [DOI] [PMC free article] [PubMed] [Google Scholar]