Abstract
Importance:
Patients leaving treatment for alcohol-use disorders (AUDs) are not typically offered evidence-based continuing care, although research suggests that continuing care is associated with better outcomes. A smartphone-based application could provide effective continuing care.
Objective:
To determine whether patients leaving residential treatment for AUDs with a smartphone application to support recovery have fewer risky drinking days than control-group patients.
Design:
An un-blinded randomized controlled trial. Patients were randomized to treatment as usual or treatment as usual plus a smartphone with A-CHESS, an application designed to improve continuing care for AUDs. “A-CHESS” stands for Addiction – Comprehensive Health Enhancement Support System.
Setting:
Three residential programs operated by one treatment organization in the Midwestern US and 2 residential programs operated by one organization in the Northeastern US.
Participants:
349 patients who met the criteria for DSM-IV alcohol dependence when they entered residential treatment. 179 were randomized to the control group and 170 to the treatment group.
Intervention:
Treatment as usual varied across programs; none offered patients coordinated continuing care after discharge. A-CHESS provides monitoring, information, communication, and support services to patients, including ways for patients and counselors to stay in contact. The intervention lasted 8 months and the follow-up period lasted 4 months.
Main Outcome Measure:
Risky drinking days—the number of days during which a patient’s drinking in a 2-hour period exceeded, for men, 4 standard drinks and for women, 3 standard drinks. Patients were asked to report their risky drinking days in the previous 30 days on surveys taken 4, 8, and 12 months after discharge from residential treatment.
Results:
For the 8 months of the intervention and 4 months of follow-up, patients in the A-CHESS group reported significantly fewer risky drinking days than patients in the control group (M = 1.39 vs. 2.75, respectively; P = .003; 95% CI [.46, 2.27]).
Conclusions and Relevance:
The findings suggest that a multi-featured smartphone application may have significant benefit to patients in continuing care for AUDs.
Trial registration:
clinicaltrials.gov Identifier: NCT01003119
Keywords: alcohol dependence, eHealth, mobile devices, continuing care, risky drinking days
Alcohol dependence is a lifetime psychiatric diagnosis.1,2 Like other chronic illnesses (e.g., diabetes, hypertension), alcohol use disorders (AUDs) have both physiological and behavioral components. AUDs also have relapse rates similar to those of other chronic illnesses.3 It has been estimated that about 1 in 4 AUDs patients remain continuously abstinent in the first year after treatment.4
Although evidence shows that continuing care for alcohol and drug use disorders is associated with better outcomes,5 patients leaving treatment for AUDs are not typically offered aftercare with ongoing monitoring.3,6 Regular or severity-adjusted check-ups to assess a patient’s status and modify treatment goals, if indicated, are common for other chronic conditions but rare for addiction.3 This scarcity arises in part from the strained addiction treatment system, which is financially overburdened, labor intensive, and unstable.7 Insufficient continuing care persists despite the cost of alcohol abuse and dependence in the US, estimated to be about $184.6 billion per year.8
Technology offers a way of providing continuing care for AUDs. A smartphone app could make recovery support, information, and monitoring available almost constantly. Compared to traditional care, technology can give personalized care while using less counselor time and be available at the moment of greatest need.
This paper describes a randomized trial of a mobile technology application called A-CHESS (Alcohol – Comprehensive Health Enhancement Support System). A-CHESS was designed to improve continuing care for AUDs by offering emotional and instrumental support at almost any time and place.9 The theoretical basis of A-CHESS is self-determination theory (SDT), which posits that meeting 3 needs contributes to an individual’s adaptive functioning: being perceived as competent, feeling related to others, and feeling internally motivated and not coerced in one’s actions.10,11 SDT was chosen because evidence suggested that SDT’s 3 constructs could be causal mechanisms that would affect CHESS targets, and because SDT is broad and fundamental enough to cover a complex, multifaceted eHealth intervention such as CHESS.12 This paper reports the primary outcome from a trial that hypothesized that patients leaving residential care for AUDs who received treatment as usual plus a multi-featured smartphone application would have fewer risky drinking days over 12 months than patients receiving only treatment as usual. We also report on 2 secondary outcomes: abstinence and negative consequences of drinking.
METHODS
Study Design and Participants
The A-CHESS study was an un-blinded randomized trial with 349 patients who met the criteria for DSM-IV alcohol dependence upon entering treatment at 3 residential programs operated by one nonprofit in the Midwestern US and 2 programs operated by another nonprofit in the Northeastern US. Patients had to be at least 18 years old, willing to be randomized, and able to identify 2 backup contacts—people who could provide information about how to reach the patient for one year. Patients were not approached to be recruited if their patient records showed a psychiatric or medical condition that would have precluded participating in the study (a history of suicidality, a significant developmental or cognitive impairment that would limit the ability to use A-CHESS, or vision problems).
Study Procedures
In the 3 Midwestern programs, residential treatment consisted of cognitive behavioral therapy, motivational interviewing, and psychoeducation, conducted almost entirely in group therapy. In the 2 Northeastern programs, residential treatment consisted of group therapy (based on cognitive behavioral therapy and psychoeducation), case management services, supportive individual counseling (based on motivational interviewing and cognitive behavioral therapy), and 3 community AA meetings per week.
An onsite project coordinator employed by each program identified eligible patients from the program’s administrative database. About 2 weeks before an eligible patient left residential treatment, the coordinator discussed the study with the patient, including procedures, data to be collected, and risks and benefits. Willing patients gave written informed consent and were enrolled. The coordinator then collected pretest data and contacted the project director to get a group assignment. Within each program, patients were randomized to control or A-CHESS in a 1:1 ratio using a computer-generated random allocation sequence with blocks of 8. Randomization was implemented using sequentially numbered containers. The study was approved by the Institutional Review Board at the University of Wisconsin – Madison and registered at clinicaltrials.gov (NCT01003119).
The control group received treatment as usual for 12 months; the A-CHESS group received treatment as usual plus a smartphone with A-CHESS for the 8-month intervention period and treatment as usual only during the 4-month follow-up. Recruitment took place from February 2010 through June 2011 and the intervention from February 2010 through May 2012. Recruitment ended 2 months early because it took less time than planned; accordingly, the intervention period started and stopped 2 months earlier than planned.
Description of the Interventions
At all residential treatment locations, counselors encouraged patients to receive continuing care, but such care was not required or monitored.
Patients in the A-CHESS group received a smartphone with the A-CHESS application, phone service, and a data plan. A-CHESS had both static content (e.g., audio-guided relaxation) and interactive features. For example, if a patient neared a high-risk location (a bar she used to frequent), GPS initiated an alert asking the patient if she wanted to be there. eTable 1 shows A-CHESS services; screen shots of A-CHESS are available at http://chess.wisc.edu/achess-archive/. Each patient using A-CHESS had a unique account. A-CHESS use data were automatically collected in time-stamped server log files, including when a patient accessed A-CHESS, the service(s) selected, duration of service use, pages viewed, and messages sent or received. Counselors could, with patient permission, access information about the patient’s A-CHESS use. Before leaving residential treatment, patients were required to demonstrate a minimal understanding of A-CHESS (i.e., the ability to set up their profile and use the discussion board and texting features) and to have entered at least 2 people (who could be the same as or different from the 2 backup contacts) to be contacted if they pressed the app’s panic button. Patients were free to use the phones for personal purposes throughout the intervention. Only the use of A-CHESS services was monitored.
Implementation
Counselors were asked to treat study participants as they would normally treat a patient who had left residential treatment—i.e., respond to patient-initiated requests for referrals or information, but not offer counseling per se—though counselors of A-CHESS patients received updates about patients through the app. Patients in the A-CHESS group were asked each week to complete a reduced version of the Brief Alcohol Monitoring (BAM) Index,13 which included protective and risky items related to drinking (such as lifestyle balance, quality of sleep, negative affect, and recent substance use) and displayed a patient’s results graphically over time. With a patient’s permission, A-CHESS automatically sent notifications to counselors if a BAM score exceeded a preset threshold or the BAM was not completed. During the intervention, patients completed 3,751 weekly BAMs and shared 97.0% (3,637) of these with counselors. Patients were much more willing to share their BAM results (93.5% allowed this) than that they had had a lapse (41.9% allowed this). The time counselors spent responding to patients in the study was not tracked, though informal feedback suggested the time was minimal. One counselor estimated she spent 2 hours/week to follow 120 patients.
Researchers called patients to administer the outcome survey at 4, 8, and 12 months after discharge from treatment. The survey asked about risky drinking days, quality of life, treatment services received, and coping behavior, and took 15 to 25 minutes to complete. If researchers’ calls and messages went unanswered, researchers called backup contacts. On average, 20 calls were required per patient to complete 3 phone surveys.
Outcomes and Measures
It was hypothesized that patients with A-CHESS would have fewer risky drinking days (the primary outcome) as well as greater abstinence and fewer negative consequences of drinking (secondary outcomes) than control-group patients. Data for all 3 outcomes came from the telephone survey conducted 4, 8, and 12 months after discharge from residential treatment. We considered adding biomarkers, but they have been found not to add sufficiently to the accuracy of self-reported measures to warrant being used.14
Risky drinking days were defined as days on which a patient’s drinking in a 2-hour period exceeded, for men, 4 standard drinks and for women, 3 standard drinks. Patients reported the number of risky drinking days they had in the previous 30 days. For abstinence, patients reported whether they had any drinks in the previous 30 days. Negative consequences of drinking came from The Short Inventory of Problems – Revised (SIP-R).15,16 This instrument has items rated on a 5-point Likert-type scale from “hardly ever” to “very likely.” We retained 4 of these items (not eating properly, hurting someone, having one’s status damaged, having money problems), made 3 items dichotomous (lost job, being arrested, having an accident), and added 1 dichotomous item (involvement with the Department of Children and Family Services). Because of these departures from the established instrument, the 8 items were examined individually rather than as a single scale.
Statistical Analysis
Planned recruitment of 175 patients per group was estimated from the effect size (h = 0.34) observed in a telephone-based intervention,17 α = .05, .80 power, and 20% attrition.
The primary outcome, risky drinking days, was analyzed with mixed-effects models. These models account for correlated measurements within patients, use all available data (allowing for intention-to-treat rather than only complete-case analysis), and provide unbiased estimates when data are missing at random.18 Each model included a random effect for patient and fixed effects for treatment program (a design variable), intervention arm (A-CHESS vs. control), month (4, 8, and 12), and arm-by-month interaction, using a first-order autoregressive covariance structure for the repeated measure of month. Secondary outcomes consisted of rating scales and dichotomous variables. Rating scales measuring negative consequences of drinking were analyzed with the mixed-effects approach used for the primary outcome. Abstinence and dichotomous negative consequences of drinking were analyzed using Fisher’s exact test. All analyses were conducted with IBM SPSS (v.21) using a 2-sided α of .05.
RESULTS
Baseline Characteristics and A-CHESS Use Data
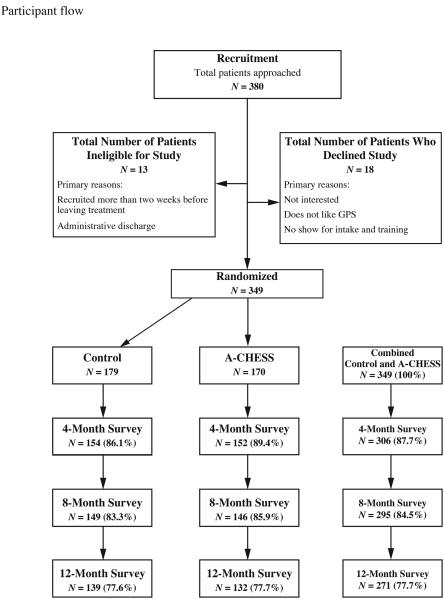
The Figure shows the flow of patients from initial screening through the end of the follow-up period, and Table 1 shows baseline characteristics of enrolled patients. Most patients were white (80%), male (61%), and unemployed (79%); most used or abused drugs in addition to alcohol (63%). Mean patient age was 38 years (SD = 10; median = 39).
Figure.
Participant flow.
Table 1.
Baseline Demographics Characteristics by Treatment Groupa
| Characteristic | Control (n=179) No., % |
A-CHESS (n=170) No., % |
|---|---|---|
| Age, mean (SD), y | 38.4 (11.2) | 38.3 (9.5) |
| Male | 109 (60.9) | 103 (60.6) |
| Started drinking before age 15 | 121 (67.6) | 115 (67.6) |
| Race | ||
| Caucasian | 142 (79.3) | 138 (81.2) |
| African American | 24 (13.4) | 21 (12.4) |
| Other | 13 (7.3) | 11 (6.5) |
| Highest level of education | ||
| < HS | 28 (15.6) | 42 (24.7) |
| HS diploma or GED | 136 (76) | 115 (67.6) |
| 4-year degree or above | 15 (8.4) | 13 (7.6) |
| Reasons for beginning treatment: Own initiativeb |
91 (50.8) | 83 (48.8) |
| Post-treatment living arrangement | ||
| Alone | 22 (12.3) | 21 (12.4) |
| With family | 83 (46.4) | 77 (45.3) |
| With roommates | 7 (3.9) | 11 (6.5) |
| Shelter | 3 (1.7) | 3 (1.8) |
| Halfway house | 59 (33) | 55 (32.4) |
| Unknown | 5 (2.8) | 3 (1.8) |
| Use/abuse drugs besides alcohol | 113 (63.1) | 105 (61.8) |
| Other drugs used/abusedcd | ||
| Cocaine | 43 (38.4) | 50 (47.6) |
| Stimulants (not including cocaine) | 22 (19.6) | 25 (23.8) |
| Opiates | 51 (45.5) | 45 (42.9) |
| Have other mental health problems/issues | 81 (45.3) | 83 (48.8) |
| Drinking or other drug use has led to:c | ||
| Loss of job or legal issues | 165 (92.2) | 159 (93.5) |
| Loss of significant relationship | 160 (89.4) | 147 (86.5) |
| Continues to be affected by history of emotional or physical trauma |
100 (55.9) | 86 (50.6) |
| Not currently employed or self-employed | 138 (77.1) | 136 (80) |
| Completed residential treatment | 160 (89.1) | 159 (93.7) |
| Median length of stay in residential treatment, days |
50 | 58.5 |
Abbreviations: SD, standard deviation; HS, high school; GED, General Educational Development
Data presented as percentage of patients unless otherwise indicated
Patients who indicated they began treatment on their own initiative, without also endorsing any other options (i.e., family pressure, employer pressure, court referral, state agency)
Percentages do not sum to 100 because patients could endorse multiple items
One control-group patient did not respond to this item.
Although 179 patients were randomized to the A-CHESS group, 286 phones were given to patients during the study because 113 phones were replaced: 56 did not work properly, 19 were stolen, 20 were damaged by patients, and 22 were lost. No patients withdrew from the study, although 21 patients in the control group and 14 patients in the A-CHESS group did not provide data for any of the 3 surveys. The rate of survey completion was not significantly different between groups (Figure). Patients were included in the analysis if they provided any outcome data according to the intention-to-treat principle.
During the 8-month intervention period, patients randomized to the A-CHESS group used the system, on average, 40% of days (mean number of days of use: 97.36; median: 103) and viewed a mean number of 1,967 pages (median: 1,745). Of the 170 patients who received A-CHESS, 122 (71.7%) pressed the panic button at least once. Because patients could press the button in error, intended use was defined as going beyond the panic button main page to at least one other page; 98 did this. Other information about patient use of A-CHESS has been published elsewhere.19
Risky Drinking Days
Patients in the A-CHESS group reported significantly fewer risky drinking days (Table 2) than patients in the control group for the intervention and follow-up period (P = .003) and at months 4 (P = .020) and 12 (P =.032), but not month 8 (P = .096). The effects of program, month, and the group-by-month interaction were not significant (Ps = .536, .649, and .865, respectively). The results were consistent when all 2- and 3-way interactions were included in the model, with significant effects of A-CHESS overall (main effect; P = .003) and at months 4 and 12 (simple effects; Ps = .002 and .044), but not at month 8 (P = .259) or for any other factor or interaction (all Ps > .05). Examining only cases with complete risky-drinking-day data produced similar results (Table 2). Sensitivity analyses were conducted using 6 sets of values. The pattern of results changed only when missing risky-drinking-day data were replaced with the maximum possible value (eTable2).
Table 2.
Group Differencesa on Risky Drinking Days Overall and by Month
| Effect | Control M (SE) |
A-CHESS M (SE) |
Mean difference (95% CI) |
t (df) | P | db | hc |
|---|---|---|---|---|---|---|---|
| Analysis of All Available Datad | |||||||
| Overall | 2.75 (.34) | 1.39 (.34) | 1.37 (.46, 2.27) | 2.98 (287.69) | .003 | .23 | .18 |
| By month: | |||||||
| 4 months | 3.01 (0.48) | 1.50 (0.47) | 1.52 (0.24, 2.80) | 2.32 (802.26) | .020 | .25 | .19 |
| 8 months | 2.65 (0.48) | 1.54 (0.49) | 1.11 (−0.20, 2.42) | 1.67 (809.01) | .096 | .18 | .15 |
| 12 months | 2.60 (0.49) | 1.13 (0.50) | 1.47 (0.13, 2.81) | 2.15 (819.05) | .032 | .24 | .21 |
| Analysis of Complete Cases Onlye | |||||||
| Overall | 2.75 (0.35) | 1.23 (0.35) | 1.53 (.61, 2.44) | 3.28 (275.79) | .001 | .25 | .16 |
| By month: | |||||||
| 4 months | 3.22 (0.49) | 1.02 (0.49) | 2.20 (0.88, 3.52) | 3.27 (757.44) | 0.001 | .36 | .12 |
| 8 months | 2.43 (0.49) | 1.59 (0.49) | 0.84 (−0.48, 2.16) | 1.25 (757.44) | 0.210 | .14 | .24 |
| 12 months | 2.61 (0.49) | 1.07 (0.49) | 1.53 (0.21, 2.85) | 2.28 (757.44) | 0.023 | .25 | .14 |
The data were skewed because most patients reported no risky drinking days at each time point.To examine whether results were robust to the distribution of the data,the analysis was re-run after separately applying various transformations (, , , , and ) to the outcome variable. Because the pattern of results across the transformations was consistent with the untransformed data, only results using the untransformed values are reported. Results also were robust when analyzed using negative binomial regression.
Cohen’s d is calculated as the mean difference divided by the pooled standard deviation (in all cases, spooled=6.05, the pooled standard deviation at 4 months).
Cohen’s h is calculated as , where P1 and P2 are the proportion of days with risky drinking (mean RDD days divided by 30) for the control group and A-CHESS, respectively.
Model estimated means based on 314 patients (158 Control; 156 A-CHESS) because 35 patients provided no survey data (21 Control; 14 A-CHESS)
Model estimated means based on 279 patients (143 Control; 136 A-CHESS) because 70 patients (36 Control; 34 A-CHESS) had missing risky drinking day data on at least one survey.
Abstinence
The odds of reporting abstinence in the previous 30 days (Table 3) were greater for A-CHESS than control-group patients, with significant differences at months 8 and 12 (Ps = .038, .014, respectively) but not at month 4 (P = .132). A-CHESS patients were also more likely than control-group patients to report abstinence at all 3 time points (P = .032).
Table 3.
Prevalence and Odds of Abstinencea by Month
| Prevalence of Abstinenceb, n (%) |
Odds of Abstinencec |
|||||
|---|---|---|---|---|---|---|
| Control | A-CHESS | Control | A-CHESS | OR (95% CI) | Pd | |
| Month 4 | 105 (68%) | 118 (76%) | 2.10 | 3.11 | 1.48 (.90-2.43) | .132 |
| Month 8 | 101 (67%) | 114 (78%) | 2.02 | 3.56 | 1.76 (1.05-2.96) | .038 |
| Month 12 | 95 (66%) | 107 (79%) | 1.90 | 3.69 | 1.94 (1.14-3.31) | .017 |
| Months 4, 8, & 12e |
63 (40%) | 81 (52%) | 0.66 | 1.08 | 1.65 (1.05-2.57) | .032 |
Abbreviations: OR, Odds ratio; CI, Confidence interval.
Abstinence is defined as a patient reporting no drinking in the past 30 days.
% reporting abstinence of relapse = nreporting abstinence ÷ ntotal reports; % reporting relapse = 100 − % reporting abstinence
Odds of abstinence = nreporting abstinence ÷ nreporting relapse; OR = oddsA-CHESS ÷ oddsControl
P values calculated using Fisher’s exact test
Reported abstinence at all three time points (months 4, 8, and 12)
Negative Consequences of Drinking
No significant differences were found between groups overall or by month for any of the negative consequences (not eating properly, hurting someone, having one’s status damaged, having money problems, losing a job, being arrested, having an accident, or involvement with the DCFS).
Patients reported so few of the dichotomous consequences that monthly comparisons between groups could not be made. Instead, Fisher’s exact test was used to compare the proportion of patients in each group reporting the consequence at any time point. Patients in jail at the time of a survey were counted as having an arrest.
Post-Hoc Analyses
A post-hoc analysis examined the relationship between cumulative system use and number of risky drinking days. Cumulative system use was defined in 3 ways: number of pages viewed, number of days used, and number of services used. Each type of use was separately added to the primary analysis as a time-varying covariate. The number of risky drinking days was significantly predicted by the number of pages viewed (per 100 pages: B = 0.036, P = .007, 95% CI [−0.069, −0.002) and days used (B = −0.01, P = .007, 95% CI [−0.02, −0.00], but not the number of services used (B = −0.06, P = .465, 95% CI [−0.22, 0.10]).
The effect of A-CHESS on post-treatment symptoms and consequences of alcohol dependence was further explored by replacing risky-drinking-day data from the primary analysis with the 10 individual BAM items and the composite BAM risk, protection, and overall scores as outcomes in separate models. Significant effects were found for the individual items of keeping busy and abstinence confidence, and for the BAM protection score (eTable 2).
AUD severity was not collected. The closest collected measure was patients’ baseline self-report of the number of previous times in treatment (mean = 4.67; SD = 9.15; range = 0 to 50), which was not significant when included as a covariate in the primary analysis.
Mediation Analysis
We tested whether the relationship between intervention and number of risky drinking days was mediated by the three constructs of self-determination theory. Perceived competence, relatedness, and autonomous motivation were measured using modified versions of the Drug-Taking Confidence Questionnaire, the Important People Survey, and the Treatment Self-Regulation Questionnaire, respectively. Mediation was examined using the Test for Joint Significance,20 which requires a significant relationship between (1) the intervention and potential mediator (path a) and (2) the outcome and the potential mediator, controlling for intervention (path b). Of the 3 constructs, only perceived competence at 4 months was found to mediate the relationship between intervention and number of risky drinking days at 8 months (path: B [95% CI], P-value) (a: 0.219 [.004, .434], P = .046; b: −.969 [1.650, −.289], P = .005)
COMMENT
In this randomized trial, patients reported their drinking-related behavior for the past 30 days at 4, 8, and 12 months post-residential treatment. Patients who received treatment as usual plus A-CHESS reported a lower average number of risky drinking days (1.39 vs. 2.75; P = .003) and higher likelihood of consistent abstinence (51.9% vs. 39.6%; P = .032) than patients who received only treatment as usual, but no difference in negative consequences of drinking.
One of self-determination theory’s 3 constructs (competence) was a significant mediator. We have observed in past work that context seems to influence which constructs are most salient. For example, relatedness was the only construct that significantly mediated the relationship between a CHESS intervention for parents of pediatric asthma patients and asthma control, perhaps because children’s medication adherence requires that parents receive considerable social support.21 It may be that recovery from addiction is such a complex process that building competence is more important than in asthma control.
The literature supports the effectiveness of continuing care in improving outcomes for AUDs,22 as well as for computer-based interventions for AUDs.23-27 Although high-quality studies have been published about computer-based interventions for continuing care of other chronic illnesses (e.g., diabetes and heart disease), they are rare for AUDs.28 To our knowledge, no other large randomized trial has been reported about the effectiveness of mobile technology for the continuing care of AUDs.
Rates of patient participation in A-CHESS were high compared to usual rates of participation in aftercare for AUDs.17,29 More than 90% of patients in the A-CHESS group used the system at least once during months 1-4, and 58% of patients used the application in the last week of the 8-month intervention period. In contrast, two studies found participation in aftercare for substance use disorders to be 59%29 and 60%30 at the end of 3 months. A study of Hazelden’s MORE program—consisting of 7 sequential, web-based modules, along with periodic contact with a personal recovery coach—showed that only 40% of patients accessed any module.28
The study has limitations. Patients in the treatment group received a smartphone while those in the control group did not, and the app included a weekly self-assessment, possibly producing an assessment effect31 and more counselor contact than for those in the control group. The study involved only patient self-report, without urine testing, and each survey asked about drinking only in the past 30 days, which does not capture a complete picture of each patient’s drinking and can under- or over-estimate drinking behavior. Using the standard SIP-R or a similar assessment would have allowed comparisons to other trials. The study involved only 2 treatment organizations and 5 programs, and most patients were white males in their 30s and 40s. A test involving more programs, greater control over the types of continuing care patients received, and more diverse populations is needed to confirm our results and increase their generalizability. Furthermore, a longer intervention period may be merited, given that patients have a chronic disease.
Whether smartphones will be practical as continuing care of AUDs depends in part on the cost and whether it will be reimbursed. In this study, 8 months of A-CHESS cost about $597/patient, based on 1 hour/month of counselor time at $90/hour divided by 50 patients, 1 hour/month for system administrator time at $50/hour divided by 170 patients, $60/month for each data plan, and $100 to buy each phone. The cost of interventions such as A-CHESS will decrease dramatically as more people have smartphones and data plans of their own, though low-income patients may be less likely to have them.
If other studies confirm our results, such applications could provide the type of care identified as most effective—that is, care that continues at least 12 months and involves proactive efforts to change patient behaviors.32 The Affordable Care Act emphasizes (via accountable care organizations) a single payment for a defined population, with a reward for good outcomes. An A-CHESS-like system may be economically viable under these rules, especially if reductions in other healthcare costs offset the cost of phones and data plans.
Thousands of healthcare apps for smartphones are available, with more appearing every day, but very few have been rigorously tested. The under-treatment of AUDs and the severity of problems associated with AUDs make it critical to develop applications that work. The promising results of this trial in continuing care for AUDs point to the possible value of a smartphone intervention for treating AUDs and perhaps other chronic illnesses.
Supplementary Material
ACKNOWLEDGMENTS
Funding: This work was supported by the National Institute on Alcohol Abuse and Alcoholism (grant R01 AA017192)
Role of the Sponsor: The funding body approved the design of the study but had no role in the conduct of the study; collection, management, analysis, and interpretation of the data; or preparation, review, or approval of the manuscript.
Additional contributions: We are grateful to Timothy B. Baker, Ph.D., at the University of Wisconsin School of Medicine and Public Health, for his conceptual and design advice. We also thank Adam Maus, M.S., from the Center for Health Enhancement Systems Studies, for his development work on A-CHESS.
Footnotes
Author’s contributions: Dr. Gustafson had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. All authors have had full access to all the data in the study.
Study concept and design: Gustafson, McTavish, Isham
Acquisition of data: McTavish, Chih, Atwood, Boyle, Levy, Driscoll, Chisholm, Dillenburg, Isham
Analysis and interpretation of data: Gustafson, McTavish, Chih, Atwood, Johnson, Boyle, Driscoll, Dillenburg, Isham, Shah
Drafting of manuscript: Gustafson, McTavish, Atwood, Johnson
Critical revision of the manuscript for important intellectual content: Gustafson, McTavish, Chih, Atwood, Johnson, Boyle, Levy, Driscoll, Chisholm, Dillenburg, Isham, Shah
Statistical analysis: Chih, Atwood
Obtained funding: Gustafson
Administrative, technical, or material support: Gustafson, McTavish, Chih, Atwood, Johnson, Boyle, Levy, Driscoll, Chisholm, Dillenburg, Isham, Shah
Study supervision: Gustafson, McTavish
Conflict-of-interest disclosures: The authors report no conflicts of interest.
Financial Disclosures: None reported.
Disclaimer: The opinions, results, and conclusions reported in this article are those of the authors and are independent from the funding source.
REFERENCES
- 1.Culverhouse R, Bucholz KK, Crowe RR, et al. Long-term stability of alcohol and other substance dependence diagnoses and habitual smoking. JAMA Psychiatry. 2005;62:753–760. doi: 10.1001/archpsyc.62.7.753. [DOI] [PubMed] [Google Scholar]
- 2.Lapham SC, Stout R, Laxton G, Skipper BJ. Persistence of addictive disorders in a first-offender driving while impaired population. JAMA Psychiatry. 2011;68(11):1151–1157. doi: 10.1001/archgenpsychiatry.2011.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McLellan AT, Lewis DC, O’Brien CP, Kleber HD. Drug dependence, a chronic medical illness: Implications for treatment, insurance, and outcomes evaluation. JAMA. 2000;284:1689–1695. doi: 10.1001/jama.284.13.1689. [DOI] [PubMed] [Google Scholar]
- 4.Miller WR, Walters ST, Bennett ME. How effective is alcoholism treatment in the United States? J Stud Alcohol. 2001;62(2):211–20. doi: 10.15288/jsa.2001.62.211. [DOI] [PubMed] [Google Scholar]
- 5.McLellan AT, McKay JR, Forman R, Cacciola J, Kemp J. Reconsidering the evaluation of addiction treatment: From retrospective follow-up to concurrent recovery monitoring. Addiction. 2005;100:447–458. doi: 10.1111/j.1360-0443.2005.01012.x. [DOI] [PubMed] [Google Scholar]
- 6.White WL, Boyle M, Loveland D. Alcoholism/addiction as chronic disease: From rhetoric to programal reality. Alcohol Treat Q. 2002;20:107–129. [Google Scholar]
- 7.McLellan AT, Carise D, Kleber HD. Can the national addiction treatment infrastructure support the public’s demand for quality care? J Subst Abuse Treat. 2003;25(2):117–121. [PubMed] [Google Scholar]
- 8.Harwood HJ. Updating estimates of the economic costs of alcohol abuse in the United States: Estimates, update methods, and data. National Institute on Alcohol Abuse and Alcoholism; Bethesda, MD: 2000. [Google Scholar]
- 9.Gustafson DH, Shaw BR, Isham A, Baker T, Boyle MG, Levy M. Explicating an evidence-based, theoretically informed, mobile technology-based system to improve outcomes for people in recovery for alcohol dependence. Subst Use Misuse. 2011;46:96–111. doi: 10.3109/10826084.2011.521413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ryan RM, Deci EL. Self-regulation and the problem of human autonomy: Does psychology need choice, self-determination, and will? J Pers. 2006;74(6):1557–1586. doi: 10.1111/j.1467-6494.2006.00420.x. [DOI] [PubMed] [Google Scholar]
- 11.Ryan RM, Deci EL. Self-determination theory and the facilitation of intrinsic motivation, social development, and well-being. Am Psychol. 2000;55(1):68–78. doi: 10.1037//0003-066x.55.1.68. [DOI] [PubMed] [Google Scholar]
- 12.Pingree S, Hawkins R, Baker T, DuBenske L, Roberts LJ, Gustafson DH. The value of theory for enhancing and understanding e-Health interventions. Am J Prev Med. 2010;38(1):103–109. doi: 10.1016/j.amepre.2009.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cacciola JS, Alterman AI, Dephilippis D, et al. Development and initial evaluation of Brief Addiction Monitor (BAM) J Subst Abuse Treat. 2013;44(3):256–263. doi: 10.1016/j.jsat.2012.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Babor TF, Steinberg K, Anton R, Del Boca F. Talk is cheap: Measuring drinking outcomes in clinical trials. J Stud Alcohol. 2000;61(1):55–63. doi: 10.15288/jsa.2000.61.55. [DOI] [PubMed] [Google Scholar]
- 15.Miller WR, Tonigan JS, Longabaugh R. The Drinker Inventory of Consequences (DrinC): An instrument for assessing adverse consequences of alcohol abuse: Test manual. US Department of Health and Human Services, Public Health Service, National Institutes of Health, National Institute on Alcohol Abuse and Alcoholism; 1995. (No. 95) [Google Scholar]
- 16.Kiluk BD, Dreifuss JA, Weiss RD, Morgenstern J, Carroll KM. The Short Inventory of Problems – Revised (SIP-R): Psychometric properties within a large, diverse sample of substance use disorder treatment seekers. Psychol Addict Behav. 2013;27(1):307–314. doi: 10.1037/a0028445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McKay JR, Lynch KG, Shepard DS, et al. The effectiveness of telephone-based continuing care in the programal management of alcohol and cocaine use disorders: 12-month outcomes. J Consult Clin Psychol. 2004;72:967–979. doi: 10.1037/0022-006X.72.6.967. [DOI] [PubMed] [Google Scholar]
- 18.White IR, Horton NJ, Carpenter J, Pocock SJ. Strategy for intention to treat analysis in randomised trials with missing outcome data. BMJ. 2011;342:d40. doi: 10.1136/bmj.d40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McTavish FM, Chih M-Y, Shah D, Gustafson DH. How patients recovering from alcoholism use a smartphone intervention. J Dual Diagn. 2012;8(4):294–304. doi: 10.1080/15504263.2012.723312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cohen J, Cohen P. Applied Multiple Regression/Correlation Analysis for the Behavioral Sciences. Erlbaum; Hillsdale, NJ: 1983. [Google Scholar]
- 21.Gustafson DH, Wise M, Bhattacharya A. The effects of combining web-based eHealth with telephone nurse case management for pediatric asthma control: A randomized controlled trial. J Med Internet Res. 2012;14(4):e101. doi: 10.2196/jmir.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McKay JR. Is there a case for extended interventions for alcohol and drug use disorders? Addiction. 2005;100:1594–1610. doi: 10.1111/j.1360-0443.2005.01208.x. [DOI] [PubMed] [Google Scholar]
- 23.Hester RK, Squires DD, Delaney HD. The Drinker’s Checkup: 12-month outcomes of a controlled programal trial of a stand-alone software program for problem drinkers. J Subst Abuse Treat. 2005;28:159–169. doi: 10.1016/j.jsat.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 24.Hester RK, Delaney HD, Campbell W, Handmaker N. A web application for moderation training: Initial results of a randomized programal trial. J Subst Abuse Treat. 2009;37:266–276. doi: 10.1016/j.jsat.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kypri K, Saunders JB, Williams SM, et al. Web-based screening and brief intervention for hazardous drinking: A double-blind randomized controlled trial. Addiction. 2004;99:1410–1417. doi: 10.1111/j.1360-0443.2004.00847.x. [DOI] [PubMed] [Google Scholar]
- 26.Kypri K, Langley JD, Saunders JB, Cashell-Smith ML, Herbison P. Randomized controlled trial of web-based alcohol screening and brief intervention in primary care. JAMA Intern Med. 2008;168:530–536. doi: 10.1001/archinternmed.2007.109. [DOI] [PubMed] [Google Scholar]
- 27.Carroll K, Ball SA, Martino S, Nich C, Babuscio TA, Rounsaville BJ. Enduring effects of a computer-assisted training program for cognitive behavioral therapy: A 6-month follow-up of CBT4CBT. Drug Alcohol Depend. 2009;100:178–181. doi: 10.1016/j.drugalcdep.2008.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Klein AA, Slaymaker VJ, Dugosh KL, McKay JR. Computerized continuing care support for alcohol and drug dependence: A preliminary analysis of usage and outcomes. J Subst Abuse Treat. 2012;42:25–34. doi: 10.1016/j.jsat.2011.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McKay JR, McLellan AT, Alterman AI, Cacciola JS, Rutherford MJ, O’Brien CP. Predictors of participation in aftercare sessions and self-help groups following completion of intensive outpatient treatment for substance abuse. J Studies Alcohol Drugs. 1998;59:152–162. doi: 10.15288/jsa.1998.59.152. [DOI] [PubMed] [Google Scholar]
- 30.Lash SJ, Stephens RS, Burden JL, et al. Contracting, prompting, and reinforcing substance use disorder continuing care: A randomized programal trial. Psychol Addict Behav. 2007;21:387–397. doi: 10.1037/0893-164X.21.3.387. [DOI] [PubMed] [Google Scholar]
- 31.McCambridge J, Kypri K. Can simply answering research questions change behaviour? Systematic review and meta analysis of brief alcohol intervention trials. PLoS ONE. 2011;6(10):e23748. doi: 10.1371/journal.pone.0023748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McKay JR. Continuing care research: What we have learned and where we are going. J Subst Abuse Treat. 2009;36:131–145. doi: 10.1016/j.jsat.2008.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.