Abstract
Background
Data regarding the value of B-type natriuretic peptide measurements (BNP) in infants with single ventricle (SV) physiology are lacking. The objective of this analysis was to describe the changes in BNP in infants with SV physiology before and after superior cavopulmonary connection (SCPC) surgery.
Methods
BNP levels were measured by a core laboratory pre-SCPC (5.0 ± 1.6 months) and at age 14 months during a multicenter trial of angiotensin converting enzyme inhibition therapy in infants with SV. Multivariable longitudinal analysis was utilized to model the associations between BNP with three sets of grouped variables (echocardiographic, catheterization, growth). Multivariable analysis was performed to assess associations with patient characteristics at both visits. Associations between BNP and neurodevelopmental variables were investigated at the 14 month visit as neurodevelopmental assessment was performed only at this visit.
Results
BNP was significantly higher before SCPC (n=173) than at 14 months (n=134); median [IQR] 80.8 pg/ml [35–187] v. 34.5 pg/ml [17–67], p<0.01. BNP > 100 pg/ml was present in 72 (42%) subjects pre-SCPC and 21 (16%) at 14 months. In the 117 patients who had BNP at both visits, the median BNP decreased 32 pg/mL [1–79 pg/mL], p<0.01. In longitudinal multivariable analysis, higher BNP were associated with a higher end-systolic volume z-score (p=0.01), greater degree of atrioventricular (AV) valve regurgitation (p<0.01), lower weight z-score (p<0.01), and lower length z-score (p=0.02) In multivariable analyses, higher BNP at 14 months was associated with presence of arrhythmia post-SCPC surgery (p<0.01), prior Norwood procedure (p<0.01), longer length of hospital stay post-SCPC surgery (p=0.04), and lower Bayley Psychomotor Developmental Index (p=0.02).
Conclusion
BNP decreases in infants with SV from the pre- SCPC visit to 14 months. Higher BNP is associated with increased ventricular dilation in systole, increased AV valve regurgitation, impaired growth and poorer neurodevelopmental outcomes. Therefore, BNP may be a useful seromarker in identifying infants with SV at risk for worse outcomes.
Introduction
B-type natriuretic peptide (BNP) is produced by ventricular cardiac myocytes as a response to stretch.[1] BNP has been used to help differentiate between lung disease and cardiac disease [2,3], to help predict successful weaning from mechanical circulatory support [4], and to help predict successful medical treatment of a patent ductus arteriosus in neonates.[5] Elevated serum BNP has been associated with poor outcomes in adults and children with heart failure.[6,7]
Despite a growing knowledge of the utility of BNP in the management of heart disease, little is known about the changes that occur in BNP in patients with single ventricle (SV) physiology undergoing staged palliation. BNP has been shown to help differentiate between ventricular dysfunction and cavopulmonary connection failure in patients with SV disease after superior cavopulmonary connection (SCPC) or Fontan.[8] There are few data about BNP levels in patients who are between the first stage palliation and SCPC. This is the interval during which patients are at the highest risk for both mortality and significant morbidity.[9–11] Prior reports investigating BNP between stage I and SCPC surgery have been limited to single center, cross-sectional studies involving small numbers of patients.[12,13]
The objective of this study was to describe BNP trends before and after SCPC in a large cohort of infants with SV physiology. An additional objective was to look for associations between BNP and other variables known to be important in this patient population, including patient characteristics, echocardiographic and catheterization variables, growth parameters and neurodevelopmental outcomes.
Methods
This study is a secondary analysis of the NIH/NHLBI sponsored trial of angiotensin converting enzyme (ACE) inhibition in infants with SV trial conducted by the Pediatric Heart Network. The study design and main results of the trial have been previously published.[14,15] The study enrolled infants with SV physiology between 7 and 45 days of age with stable pulmonary and systemic blood flow that were predicted to undergo a SCPC (bidirectional Glenn, bilateral bidirectional Glenn, or hemi-Fontan procedure). Exclusion criteria included prematurity (gestational age <35weeks), small for gestational age (weight <10th percentile for gestational age), systemic oxygen saturation <65%, creatinine >1.0 mg/dL, absolute neutrophil count <1000 cells/ml, prior use of ACE inhibitor or other clinical situation which prevented the use of ACE inhibitor. Ten centers throughout North America enrolled patients between August, 2003 and May, 2007. Each participating center’s institutional review board or ethics board approved the study protocol and written informed consent was obtained from a parent or guardian.
Data Collection
Plasma BNP levels were obtained at the pre-SCPC visit and at the final study visit at 14 months of age. The sample for BNP was drawn after the subject had been in a sitting/supine position in a quiet room. One mililiter of whole blood was collected and placed into a pre-chilled lavender-topped EDTA tube.[16] The resulting plasma was dispensed into vials for storage and frozen immediately at −20° to −80° C. Samples were then sent to the serology core laboratory (Mayo Central Laboratory for Clinical Trials) on dry ice. BNP levels were determined using the Shionogi method.[17] Detailed anatomic diagnosis, gestational age, and birth weight were recorded. Height, weight, and head circumference were measured at seven different time points during the study (for this study the Pre-SCPC and 14month measurements were utilized for analysis) and adjusted for age using the WHO criteria. Echocardiograms were performed at the pre-SCPC visit and 14 month visit and analyzed in a core laboratory by a single reader. Measurements of ventricular volume, mass, end-systolic volume (ESV), end-diastolic volume (EDV) and ejection fraction were calculated using modified Simpson’s algorithm.[18] If cardiac catheterization was performed prior to SCPC surgery the data were also included. Neurodevelopmental testing with the Bayley Scale of Infant Development (BSID)-II was performed at the 14 month visit. Analysis was done for the following sections of the BSID-II: Mental Developmental Index (MDI) and Psychomotor Developmental Index (PDI).
Data Analysis
BNP values were log transformed (with natural base, logBNP) to attain an approximately normal distribution. All patients with a BNP measurement were included in the analysis, irrespective of type of surgery performed between the two time points. LogBNP was compared to other continuous variables using the Pearson correlation test. Mean logBNP was compared between categorical variables using the student t-test. Longitudinal multivariable analysis was utilized for the variables collected at both time points (echocardiographic, catheterization, and growth measures.) Multivariable analysis was performed for variables not assessed at multiple time points (patient characteristics, neurodevelopmental testing). Multivariable analysis was performed separately for each category of variables (echocardiographic, catheterization, growth, neurodevelopmental measures and patient characteristics), all models used logBNP as the outcome.
For longitudinal analysis of BNP, only the data of patients with BNP measured at both time points who also had a SCPC were included. Longitudinal multivariable modeling was performed for each set of grouped variables collected at both time points (echocardiographic, catheterization and growth measures) to identify associations with logBNP and included all available data. When applicable, the parameter estimate (PE) of longitudinal analysis is reported. Multivariable analyses of both neurodevelopmental measures and patient characteristics (Table 1) were performed to assess associations with logBNP. The multivariable analysis of neurodevelopmental measures had only logBNP at the 14 month visit as the outcome, as neurodevelopmental testing was only performed at that time. Multivariable modeling of logBNP at the pre-SCPC visit included only the patient characteristics present prior to the pre-SCPC visit. Multivariable modeling of logBNP at the 14 month visit included all relevant patient characteristics, regardless of time. Since some BNP measurements were missing, analysis was performed to determine potential bias in the patient sample. The characteristics of patients with and without BNP measurements at each time point were compared (t-test, Fisher exact test, Wilcoxon rank sum and Mantel-Haenszel test for linear trend, as appropriate). All analyses were performed using SAS statistical software version 9.3 (SAS Institute, Inc., Cary, NC).
Table 1.
Patient Characteristics Evaluated
| Patient Factors |
| Birthweight |
| Gestational Age |
| Race |
| Gender |
| Ethnicity |
| Anatomic Diagnosis (HLHS-yes/no) |
| Dominant Ventricular type (Left/Right/Mixed) |
| Age at the time of BNP test |
| Medical Factors during Neonatal Hospitalization |
| Age at Palliative Surgery |
| Length of Hospital Stay |
| Number of Discharge Medications |
| Discharge Oxygen Saturation |
| Number of Other Cardiac Surgical Procedures |
| Type of Palliative Surgery (Norwood v. Non-Norwood) |
| Cardiopulmonary Bypass Time |
| Aortic Cross Clamp Time |
| Use of Deep Hypothermic Circulatory Arrest |
| Medical Factors during SCPC Hospitalization |
| Age at SCPC Surgery |
| Length of Hospital Stay |
| Number of Discharge Medications |
| Discharge Oxygen Saturation |
| Number of Other Cardiac Surgical Procedures |
| Presence of Chylous Drainage |
| Presence of Post-Operative Arrhythmia |
BNP: brain natriuretic peptide, HLHS: hypoplastic left heart syndrome, SCPC: superior cavopulmonary connection.
Results
Participation
Among 230 subjects enrolled in the trial, 200 had a pre-SCPC visit at 5.0 ± 1.6 months; 28 patients withdrew before the pre-SCPC visit and 2 did not have a pre-SCPC visit but remained in the trial. Of the 202 patients who continued in the study after the pre-SCPC visit, 185 had a 14 month visit. Of the 185 patients who completed the 14 month visit, 175 had a SCPC procedure. The remaining 10 subjects had procedures physiologically different than a SCPC (Kawashima in 5, systemic to pulmonary shunt in 3 and no procedure in 2). A total of 117 subjects underwent a SCPC and had BNP measured at both the pre-SCPC and 14 month visits. Hypoplastic left heart syndrome was the most common diagnosis of the cohort representing 107 (61.8%) of the patients with a pre-SCPC BNP (n=173) and 80 (59.7%) of the patients with a 14-month BNP (n=134). The dominant ventricle in patients with a pre-SCPC BNP was the right ventricle in 120, left in 35 and mixed in 18, in patients with a 14-month BNP the dominant ventricle was the right ventricle in 94, left in 28, and mixed in 12. To explore potential participation bias, of the 200 patients who had a pre-SCPC visit, the characteristics of the 173 patients with BNP measurements were compared to 27 patients without BNP data. There were no differences with the single exception that patients with BNP measurements were more likely to have moderate or severe atrioventricular (AV) valve regurgitation versus mild or none (p=0.04 Fisher exact test). At the 14 month visit, the only significant difference between 134 patients with BNP measurements and 51 patients without BNP was race. Subjects with a BNP measurement were more likely to be African-American and less likely to be in ‘other’ category (the proportion of whites was similar for the two groups, and represented 80% of the total sample; p=0.04).
BNP measurements
BNP was higher in patients at the pre-SCPC visit, median 80.8 pg/mL (IQR, 35–187 pg/mL, n=173), versus the 14-month visit, median 34.5 pg/mL (IQR 17–67 pg/mL, n=134, p<0.01)). (Figure1) Of the 117 patients who underwent a SCPC surgery and had a BNP measurement at both visits, the median drop in BNP was 32 pg/mL (IQR 1–79 pg/mL, p<0.01). In the pre-SCPC sample (n=173), a weak correlation between greater age and lower logBNP was observed (r=−0.16, p=0.01), however, there was no association between age and logBNP after SCPC surgery (r=0.03, p=0.9, n=134)). In multivariable longitudinal analysis focusing on age and gender, there were no associations between age or race and logBNP. BNP > 100 pg/ml was present in 72 (42%) subjects pre-SCPC and 21 (16%) at months.
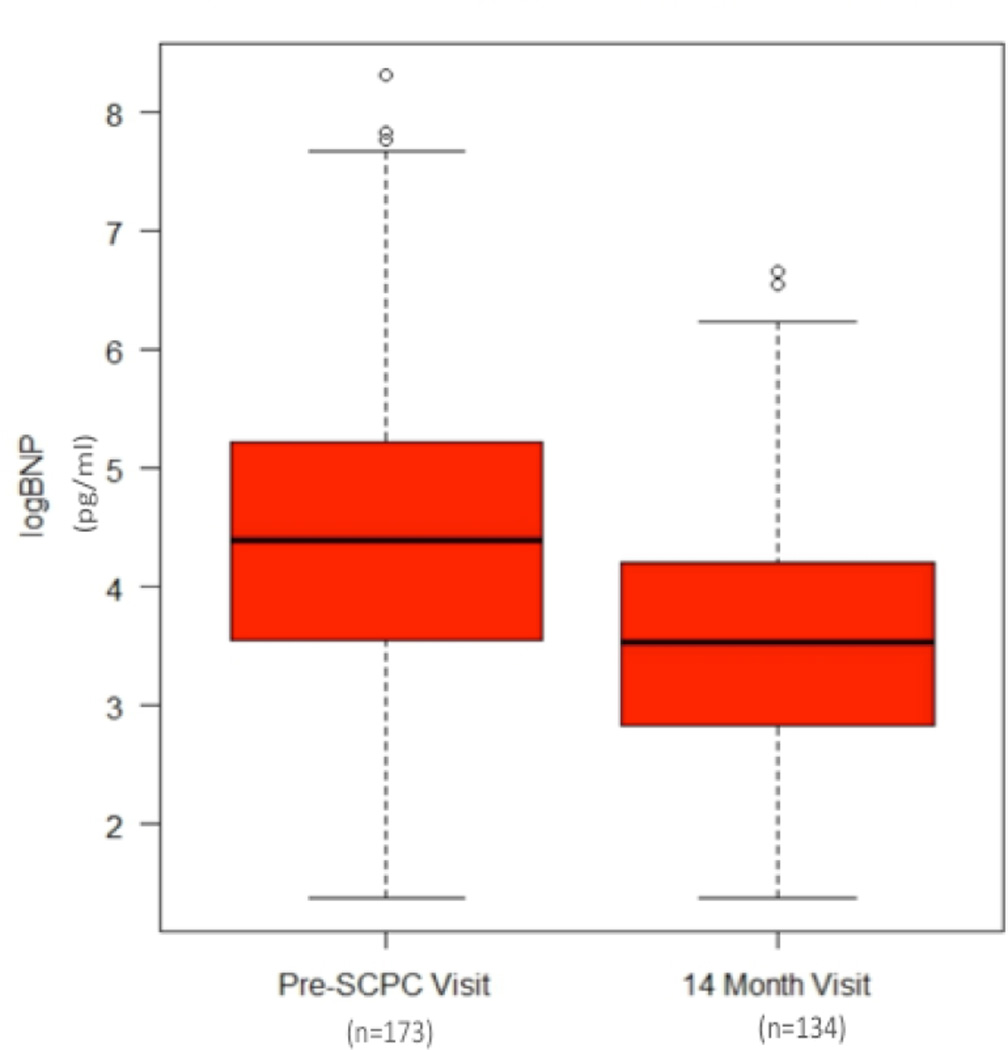
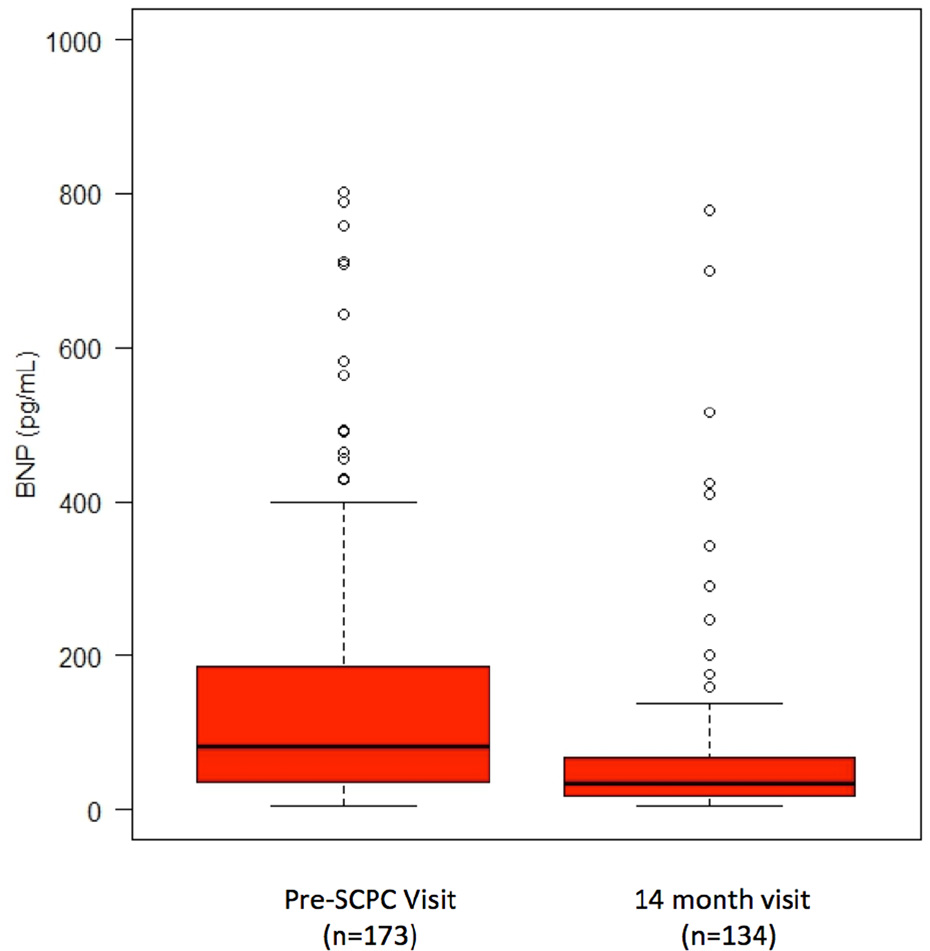
Figure 1.
Log-transformed brain natriuretic peptide (logBNP, Fig1A) was significantly higher in the pre-superior cavopulmonary connection (Pre-SCPC) time period (5.0 ± 1.6 months) compared to the 14 month visit (4.4 ± 1.3 vs. 3.5 ± 1.7, p<0.01). Raw median values of BNP were 80.8 pg/mL (IQR, 35–187 pg/mL, n=173) and 34.5pg/mL (IQR 17–67 pg/mL, n=134, p<0.01) at the pre-SCPC and 14-month time points, respectively.
Raw BNP values are presented at Fig 1B (6 Pre-SCPC outlying values, between 1100 and 4100 pg/mL, are excluded to improve resolution)
Associations between BNP and Echocardiography
In univariate analysis, higher logBNP at both time points was associated with increased end-systolic and end-diastolic volume z-scores, ventricular mass, lower ejection fraction z-score and moderate or severe AV valve regurgitation versus mild or none. Multivariable longitudinal analysis of logBNP (N-observations =290) that examined echocardiographic measures as correlates found associations between higher logBNP and greater end-systolic volume z-score and degree of AV valve regurgitation (Table 2).
Table 2.
Echocardiogram Measures and logBNP
| Echocardiogram Measure | Pre-SCPC Visit | 14 Month Visit | Multivariable Longitudinal Model |
|||
|---|---|---|---|---|---|---|
| End-Diastolic Vol z-score | r=0.39 | p<0.01 | r=0.41 | p<0.01 | ||
| End-Systolic Vol z-score | r=0.43 | p<0.01 | r=0.42 | p<0.01 | PE=0.13 | |
| Ejection Fraction z-score | r=−0.26 | p<0.01 | r=−0.24 | p<0.01 | ||
| Mass z-score | r=0.30 | p<0.01 | r=0.21 | p<0.01 | ||
| AV Valve Regurgitation* | ||||||
| none/mild | 77 pg/mL (30–180) | p<0.01 | 29 pg/mL (13–64), | p<0.01 | PE=0.54 | |
| moderate/severe | 107 pg/mL (60–270) | 52 pg/mL (32–127) | ||||
SCPC=superior cavopulmonary anastomosis, AV=atrioventricular, Echo=echocardiographic, PE= parameter estimate, Vol=volume
Displayed values for AV-valve regurgitation are median (IQR)
Associations between BNP and Catheterization
At the pre-SCPC visit, univariate analysis of catheterization measures found correlations between higher pre-SCPC logBNP and higher end-diastolic pressure (PE=0.1, p<0.01) as well as lower superior vena cava saturation (PE=−0.3, p=0.01). In multivariable analysis of logBNP that examined catheterization variables as correlates, the only association was a positive correlation between mean pulmonary artery pressure and logBNP levels at the pre-SCPC visit (R2=0.04, β=0.07 ±0.02, p=.01). LogBNP measured at the 14 month visit had no statistically significant associations with pre-SCPC catheterization measures.
Associations between BNP and Patient Characteristics
Higher logBNP at pre-SCPC was associated with a diagnosis of hypoplastic left heart syndrome (HLHS), Stage 1 Norwood procedure and right ventricle (RV) morphology in univariate analysis. Higher logBNP at the 14 month visit was associated with the diagnosis of HLHS, Stage 1 Norwood procedure, RV morphology, post-SCPC arrhythmia, lower birth weight and longer post-SCPC hospital stay in univariate analysis (Table 3). In the multivariable model of logBNP at the pre-SCPC visit, only the diagnosis of HLHS was associated with higher logBNP (R2=0.08, β=0.75 ± 0.2, p<0.01). In the multivariable model of logBNP at the 14 month visit, the presence of post-SCPC arrhythmia (β=0.92 ± 0.33, p<0.01), log transformed hospital length of stay after SCPC surgery (β=0.66 ± 0.22, p<0.01) and stage 1 Norwood procedure (β=0.26 ± 0.12, p=0.04) were associated with higher logBNP (R2=0.16, p<0.01). All associations remained statistically significant when degree of AV regurgitation was introduced into the model.
Table 3.
Significant univariate associations between Patient Variables and log-transformed brain natriuretic peptide (logBNP)
| Patient Variable | logBNP | p-value |
|---|---|---|
| Pre-SCPC BNP | ||
| Hypoplastic Left | ||
| Heart | ||
| yes | 4.7 | p<0.01 |
| no | 4.0 | |
| Norwood procedure | ||
| yes | 4.6 | p<0.01 |
| no | 3.8 | |
| Ventricle Type | ||
| Left | 3.8 | p<0.01 |
| Right | 4.6 | |
| Mixed | 4.2 | |
| 14 month BNP | ||
| Postoperative SCPC Arrhythmia | ||
| yes | 4.3 | p<0.01 |
| no | 3.4 | |
| Prior Norwood | ||
| yes | 3.6 | p<0.01 |
| no | 3.0 | |
| Ventricle Type | ||
| Left | 3.0 | p=0.04 |
| Right | 3.6 | |
| Mixed | 3.8 | |
| Length of Hospital Stay after SCPC, days | ||
| PE=0.2, p=0.02 | ||
SCPC = superior cavopulmonary connection
Associations between BNP and Growth
LogBNP had a negative correlation with weight and length z-scores in univariate analysis at both visits (Table 4). Head circumference z-score had a negative correlation with logBNP at the pre-SCPC visit; however, this association did not persist at the 14 month visit. Weight for length z-score was negatively correlated with logBNP at the month visit only. In longitudinal multivariable analysis of logBNP (N-observations=306) that examined growth variables as correlates, lower logBNP was associated with higher weight z-score, higher length z-score and the BNP sample being obtained at the 14-month visit versus pre-SCPC visit (table 4).
Table 4.
Growth Measures and LogBNP
| Growth Measure | Pre-SCPC Visit | 14 month Visit | Multivariable Longitudinal Model |
|||
|---|---|---|---|---|---|---|
| Weight for age z-score | r=−0.31 | p<0.01 | r=−0.42 | p<0.01 | PE=−0.23 | p<0.01 |
| Length for age z-score | r=−0.31 | p<0.01 | r=−0.38 | p<0.01 | ||
| Head Circumference for age z-score | r=−0.30 | p<0.01 | r=−0.09 | p=0.30 | ||
| Weight for Length z-score | r=−0.11 | p=0.15 | r=−0.33 | p<0.01 | PE=−0.19 | p=0.02 |
LogBNP= log-transformed brain natriuretic peptide, SCPC=superior cavopulmonary anastomosis
Associations between BNP and neurodevelopment
Neurodevelopmental testing was performed in 170 patients at the 14-month visit, of whom 149 had a BNP drawn at the pre-SCPC visit and 126 had a BNP drawn at the 14 month visit. LogBNP at the pre-SCPC visit negatively correlated with the Mental Developmental Index z-score (r=−0.21, p=0.01I) and Psychomotor Developmental Index (r=−0.22, p=0.01). LogBNP at the 14 month visit negatively correlated with the Psychomotor Developmental Index (r=−0.22, p=0.01) but not with the Mental Developmental Index (r=−0.12, p=0.20). In multivariable analysis of LogBNP, only the Psychomotor Developmental Index was weakly associated with logBNP measured at the 14-month visit (R2=0.05, p=0.04).
Discussion
This analysis is unique in that it represents the largest group of patients to date with SV heart disease who have had a systematic collection of BNP during the first 14 months of life. Describing BNP values and variation in this population is a valuable reference for the medical professionals taking care of children with SV. The large study population allowed for a robust evaluation of the association of BNP with many variables including echocardiographic, catheterization, neurodevelopmental and growth factors. It also allowed for longitudinal analysis at two distinct time points that represented two different physiologic conditions. The analysis shows that BNP declines after SCPC surgery and elevations in BNP are associated with echocardiographic measures of worse ventricular function as well as with poorer growth and developmental outcomes in infants with SV physiology.
We found that BNP values decreased significantly over time in this multicenter cohort of SV subjects to levels closer to that of normal pediatric patients. However, the median BNP at 14 months of age in this study remained slightly higher (4pg/mL to 10pg/mL) than previously published 95th centile of plasma BNP in pediatric controls.[19] The Pediatric Heart Network has previously reported on BNP values in 510 pediatric patients with a Fontan circulation.[20] In that study, involving subjects between the age of 6 and 18 years, BNP values were generally low (median 13.0pg/ml, IQR 7.1–25.9 pg/mL) and were associated with both age and gender.[20] Our study showed no association at pre-SCPC or at 14 months with gender. Previous reports in healthy children have shown that gender differences in BNP are not present until after 10 years of age.[19] There was a weak correlation with age, but this was only for the pre-SCPC samples. The results of our analyses combined with the results from PHN Fontan study demonstrate that serum BNP generally declines with each stage of surgical SV palliation. Therefore, when interpreting the serum BNP level in SV heart disease, stage of palliation is important.
The decrease in BNP levels following SCPC surgery seen in the present study confirms similar results reported in smaller studies.[21–25] The decrease in BNP levels following SCPC surgery supports previous findings that demonstrate a physiologic benefit of the SCPC compared to Stage 1 palliation on SV physiology.[26,27] In biventricular congenital heart disease, BNP levels increase in conjunction with ventricular dilation.[28–30] The decline in BNP after SCPC seen in this study is likely a result of the volume unloading of the SV. A decrease in ventricular volume results in a decrease in atrial and ventricular stretch and leads to decreased release of BNP. The association of higher BNP levels with moderate or severe systemic AV regurgitation at both the pre-SCPC and 14-month time points in this study also supports this theory and is consistent with similar findings reported in Fontan patients.[23]
Degree of ventricular hypertrophy and size has been associated with BNP in patients with and without congenital heart disease. [31–33] In our study, which included a heterogeneous group of SV’s, showed similar results to the previous reports. Ventricular mass, end-systolic and diastolic volumes were all associated with BNP in univariate analysis with end-systolic volume being a predictor of BNP in multivariate analysis. Therefore, SV with anatomy that leads to elevated ventricular mass or volume will likely have higher BNP.
Despite similar exercise capacity and echocardiographic measures of contractility, RV morphology in SV patients has been associated with worse outcomes.[27,34,35] Prior studies reported higher BNP levels in patients with RV morphology versus left after SCPC and total cavopulmonary connection surgery.[24] Our study found that RV morphology is associated with a higher BNP before but not following the SCPC. The association between RV dominance and higher BNP before SCPC persisted after controlling for degree of AV valve regurgitation. This indicates that the observed BNP elevation in RV dominant SV patients is not fully explained by worse AV valve function alone.
There were weak correlations between BNP and poorer growth and neurodevelopmental outcomes such as height z-score, weight z-score and the Bayley Psychomotor Developmental Index. Other patient characteristics that were associated with higher BNP at 14 months were arrhythmia after SCPC surgery and length of hospital stay after SCPC surgery.
BNP showed only weak associations with catheterization values in this population. Studies comparing catheterization measurements and BNP in pediatric heart disease are lacking. One study showed a correlation between BNP and right heart catheterization measurements in pediatric transplant recipients.[36] However, another study of 59 pediatric patients with an atrial septal defect or ventricular septal defect, there was no association between Qp:Qs and BNP.[37]
Limitations
Of the 230 patients enrolled, only 173 had BNP at the pre-SCPC visit and only 134 had BNP at the 14 month visit. The 173 patients with BNP at the pre-SCPC visit had a higher degree of AV valve regurgitation than the patients with missing data. Therefore, it is possible that if these 27 patients had a BNP measured then the median BNP measured pre-SCPC would have been lower. The results reported here reflect BNP values measured at only 2 time points (at 5 months and 14 months) and at times that the subjects were generally doing clinically well.
Conclusions
BNP has a predictable decline after SCPC surgery. Elevations in BNP are negatively correlated with important echocardiographic measures of ventricular function as well as growth and developmental outcomes in infants with SV physiology. Therefore, BNP may be a useful seromarker in helping determine overall cardiac health in this population and may aid in identifying infants with SV at high risk for poor outcomes.
Acknowledgments
Supported by U01 grants from the National Heart, Lung, and Blood Institute (HL068269, HL068270, HL068279, HL068281, HL068285, HL068292, HL068290, HL068288, HL085057) and the FDA Office of Orphan Products Development. Supported by T32 grant from the National Heart, Lung and Blood Institute (5T32 HL007710). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of NHLBI or NIH.
APPENDIX
National Heart, Lung, and Blood Institute: Gail Pearson, Victoria Pemberton, Mario Stylianou, Marsha Mathis*
Network Chair: Lynn Mahony, University of Texas Southwestern Medical Center
Data Coordinating Center: New England Research Institutes, Lynn Sleeper (PI), Steven Colan, Lisa Virzi*, Lisa Wruck*, Victor Zak, David F. Teitel
Core Clinical Site Investigators: Children’s Hospital Boston, Jane W. Newburger (PI), Roger Breitbart, Jami Levine, Ellen McGrath*, Carolyn Dunbar-Masterson; Children’s Hospital of New York, Daphne Hsu* (Study Chair), William Hellenbrand (PI)*, Ashwin Prakash*, Seema Mital*, Darlene Servedio*; Children’s Hospital of Philadelphia, Victoria L. Vetter (PI), Chitra Ravishankar, Sarah Tabbutt*, Meryl Cohen, Katherine Lee, Marisa Nolan, Stephanie Piacentino, Michelle Toms; Cincinnati Children’s Medical Center, D. Woodrow Benson (PI), Catherine Dent Krawczeski, Lois Bogenschutz, Teresa Barnard, Steven Schwartz*, David Nelson; North Carolina Consortium: Duke University, East Carolina University, Wake Forest University, Page A. W. Anderson (PI) – deceased, Jennifer Li (PI), Wesley Covitz, Kari Crawford*, Michael Hines*, James Jaggers*, Theodore Koutlas, Charlie Sang, Jr, Lori Jo Sutton, Mingfen Xu; Medical University of South Carolina, J. Philip Saul (PI), Andrew Atz, Girish Shirali*, Eric M. Graham, Teresa Atz; Primary Children’s Medical Center and the University of Utah, Salt Lake City, Utah, L. LuAnn Minich (PI), John A. Hawkins – deceased, Richard V. Williams, Linda M. Lambert, Marian E. Shearrow; Hospital for Sick Children, Toronto, Brian McCrindle (PI), Elizabeth Radojewski, Nancy Slater, Svetlana Khaikin, Susan McIntyre.
Auxiliary Sites: Children's Hospital of Wisconsin, Nancy Ghanayem, Kathy Mussatto, Michele Frommelt, Lisa Young-Borkowski; University of Michigan, Albert Rocchini, Laurie Rodgers-Augustyniak
Echocardiography Core Laboratory: Children’s Hospital Boston: Steven Colan, Renee Margossian
Genetics Core Laboratory: Children’s Hospital of New York: Wendy Chung, Liyong Deng, Patricia Lanzano
Protocol Review Committee: Michael Artman, Chair; Judith Massicot-Fisher, Executive Secretary; Timothy Feltes, Julie Johnson, Thomas Klitzner, Jeffrey Krischer, G. Paul Matherne
Data and Safety Monitoring Board: John Kugler, Chair; Rae-Ellen Kavey, Executive Secretary; David J. Driscoll, Mark Galantowicz, Sally A. Hunsberger, Thomas J. Knight, Holly Taylor, Catherine L. Webb
*no longer at the institution listed
Footnotes
The authors have no conflicts to disclose.
References
- 1.Mukoyama M, Nakao K, Hosoda K, Suga S, Saito Y, Ogawa Y, Shirakami G, Jougasaki M, Obata K, Yasue H, et al. Brain natriuretic peptide as a novel cardiac hormone in humans Evidence for an exquisite dual natriuretic peptide system, atrial natriuretic peptide and brain natriuretic peptide. J Clin Invest. 1991;87:1402–1412. doi: 10.1172/JCI115146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ko HK, Lee JH, Choi BM, Yoo KH, Son CS, Lee JW. Utility of the rapid B-type natriuretic peptide assay for detection of cardiovascular problems in newborn infants with respiratory difficulties. Neonatology. 2008;94:16–21. doi: 10.1159/000112584. [DOI] [PubMed] [Google Scholar]
- 3.Law YM, Hoyer AW, Reller MD, Silberbach M. Accuracy of plasma B-type natriuretic peptide to diagnose significant cardiovascular disease in children: the Better Not Pout Children! Study. J Am Coll Cardiol. 2009;54:1467–1475. doi: 10.1016/j.jacc.2009.06.020. [DOI] [PubMed] [Google Scholar]
- 4.Huang SC, Wu ET, Ko WJ, Lai LP, Hsu J, Chang CI, Chiu IS, Wang SS, Wu MH, Lin FY, Chen YS. Clinical implication of blood levels of B-type natriuretic peptide in pediatric patients on mechanical circulatory support. Ann Thorac Surg. 2006;81:2267–2272. doi: 10.1016/j.athoracsur.2005.12.061. [DOI] [PubMed] [Google Scholar]
- 5.Choi BM, Lee KH, Eun BL, Yoo KH, Hong YS, Son CS, Lee JW. Utility of rapid B-type natriuretic peptide assay for diagnosis of symptomatic patent ductus arteriosus in preterm infants. Pediatrics. 2005;115:e255–e261. doi: 10.1542/peds.2004-1837. [DOI] [PubMed] [Google Scholar]
- 6.Price JF, Thomas AK, Grenier M, Eidem BW, O'Brian Smith E, Denfield SW, Towbin JA, Dreyer WJ. B-type natriuretic peptide predicts adverse cardiovascular events in pediatric outpatients with chronic left ventricular systolic dysfunction. Circulation. 2006;114:1063–1069. doi: 10.1161/CIRCULATIONAHA.105.608869. [DOI] [PubMed] [Google Scholar]
- 7.Wei CM, Heublein DM, Perrella MA, Lerman A, Rodeheffer RJ, McGregor CG, Edwards WD, Schaff HV, Burnett JC., Jr Natriuretic peptide system in human heart failure. Circulation. 1993;88:1004–1009. doi: 10.1161/01.cir.88.3.1004. [DOI] [PubMed] [Google Scholar]
- 8.Lechner E, Schreier-Lechner EM, Hofer A, Gitter R, Mair R, Biebl A, Tulzer G. Aminoterminal brain-type natriuretic peptide levels correlate with heart failure in patients with bidirectional Glenn anastomosis and with morbidity after the Fontan operation. J Thorac Cardiovasc Surg. 2009;138:560–564. doi: 10.1016/j.jtcvs.2009.03.005. [DOI] [PubMed] [Google Scholar]
- 9.Ohye RG, Sleeper LA, Mahony L, Newburger JW, Pearson GD, Lu M, Goldberg CS, Tabbutt S, Frommelt PC, Ghanayem NS, Laussen PC, Rhodes JF, Lewis AB, Mital S, Ravishankar C, Williams IA, Dunbar-Masterson C, Atz AM, Colan S, Minich LL, Pizarro C, Kanter KR, Jaggers J, Jacobs JP, Krawczeski CD, Pike N, McCrindle BW, Virzi L, Gaynor JW. Comparison of shunt types in the Norwood procedure for single-ventricle lesions. N Engl J Med. 2010;362:1980–1992. doi: 10.1056/NEJMoa0912461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hehir DA, Dominguez TE, Ballweg JA, Ravishankar C, Marino BS, Bird GL, Nicolson SC, Spray TL, Gaynor JW, Tabbutt S. Risk factors for interstage death after stage 1 reconstruction of hypoplastic left heart syndrome and variants. J Thorac Cardiovasc Surg. 2008;136:94–99. 99 e91–99 e93. doi: 10.1016/j.jtcvs.2007.12.012. [DOI] [PubMed] [Google Scholar]
- 11.Kogon BE, Ramaswamy V, Todd K, Plattner C, Kirshbom PM, Kanter KR, Simsic J. Feeding difficulty in newborns following congenital heart surgery. Congenit Heart Dis. 2007;2:332–337. doi: 10.1111/j.1747-0803.2007.00121.x. [DOI] [PubMed] [Google Scholar]
- 12.Koch A, Zink S, Singer H. B-type natriuretic peptide in paediatric patients with congenital heart disease. Eur Heart J. 2006;27:861–866. doi: 10.1093/eurheartj/ehi773. [DOI] [PubMed] [Google Scholar]
- 13.Holmgren D, Westerlind A, Berggren H, Lundberg PA, Wahlander H. Increased natriuretic peptide type B level after the second palliative step in children with univentricular hearts with right ventricular morphology but not left ventricular morphology. Pediatr Cardiol. 2008;29:786–792. doi: 10.1007/s00246-008-9201-8. [DOI] [PubMed] [Google Scholar]
- 14.Hsu DT, Zak V, Mahony L, Sleeper LA, Atz AM, Levine JC, Barker PC, Ravishankar C, McCrindle BW, Williams RV, Altmann K, Ghanayem NS, Margossian R, Chung WK, Border WL, Pearson GD, Stylianou MP, Mital S. Enalapril in infants with single ventricle: results of a multicenter randomized trial. Circulation. 2010;122:333–340. doi: 10.1161/CIRCULATIONAHA.109.927988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hsu DT, Mital S, Ravishankar C, Margossian R, Li JS, Sleeper LA, Williams RV, Levine JC, McCrindle BW, Atz AM, Servedio D, Mahony L. Rationale and design of a trial of angiotensin-converting enzyme inhibition in infants with single ventricle. Am Heart J. 2009;157:37–45. doi: 10.1016/j.ahj.2008.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Santos H, Cauliez B, Tron C, Brunel V, Lavoinne A. Is heparin plasma suitable for the determination of B-type natriuretic peptide on the Beckman-Coulter Access 2? Clinical chemistry and laboratory medicine : CCLM / FESCC. 2010;48:399–401. doi: 10.1515/CCLM.2010.069. [DOI] [PubMed] [Google Scholar]
- 17.Shimizu H, Aono K, Masuta K, Asada H, Misaki A, Teraoka H. Stability of brain natriuretic peptide (BNP) in human blood samples. Clinica chimica acta; international journal of clinical chemistry. 1999;285:169–172. doi: 10.1016/s0009-8981(99)00112-6. [DOI] [PubMed] [Google Scholar]
- 18.Hsu DT, Mital S, Ravishankar C, Margossian R, Li JS, Sleeper LA, Williams RV, Levine JC, McCrindle BW, Atz AM, Servedio D, Mahony L. Rationale and design of a trial of angiotensin-converting enzyme inhibition in infants with single ventricle. Am Heart J. 2009;157:37–45. doi: 10.1016/j.ahj.2008.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koch A, Singer H. Normal values of B type natriuretic peptide in infants, children, and adolescents. Heart. 2003;89:875–878. doi: 10.1136/heart.89.8.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Atz AM, Zak V, Breitbart RE, Colan SD, Pasquali SK, Hsu DT, Lu M, Mahony L, Paridon SM, Puchalski MD, Geva T, McCrindle BW. Factors associated with serum brain natriuretic peptide levels after the Fontan procedure. Congenit Heart Dis. 2011;6:313–321. doi: 10.1111/j.1747-0803.2011.00496.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eerola A, Jokinen E, Sairanen H, Pihkala J. During treatment protocol for univentricular heart serum levels of natriuretic peptides decrease. Eur J Cardiothorac Surg. 2010;38:735–740. doi: 10.1016/j.ejcts.2010.03.056. [DOI] [PubMed] [Google Scholar]
- 22.Koch AM, Zink S, Singer H. B-type natriuretic peptide in patients with systemic right ventricle. Cardiology. 2008;110:1–7. doi: 10.1159/000109399. [DOI] [PubMed] [Google Scholar]
- 23.Koch AM, Zink S, Singer H, Dittrich S. B-type natriuretic peptide levels in patients with functionally univentricular hearts after total cavopulmonary connection. Eur J Heart Fail. 2008;10:60–62. doi: 10.1016/j.ejheart.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 24.Holmgren D, Westerlind A, Berggren H, Lundberg PA, Wahlander H. Increased natriuretic peptide type B level after the second palliative step in children with univentricular hearts with right ventricular morphology but not left ventricular morphology. Pediatric cardiology. 2008;29:786–792. doi: 10.1007/s00246-008-9201-8. [DOI] [PubMed] [Google Scholar]
- 25.Lowenthal A, Camacho BV, Lowenthal S, Natal-Hernandez L, Liszewski W, Hills NK, Fineman JR, Bernstein HS. Usefulness of B-Type Natriuretic Peptide and N-Terminal Pro-B-Type Natriuretic Peptide as Biomarkers for Heart Failure in Young Children With Single Ventricle Congenital Heart Disease. Am J Cardiol. 2011 doi: 10.1016/j.amjcard.2011.10.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Comas F, Sivori G, Ithuralde A, Garcia-Nani MA, Balestrini M, Seara C, Garcia-Delucis P, Nojek C, Ithuralde M. Functional univentricular heart: immediate and long term results, in the different stages of sequential correction. Arch Cardiol Mex. 2011;81:82–86. [PubMed] [Google Scholar]
- 27.d'Udekem Y, Xu MY, Galati JC, Lu S, Iyengar AJ, Konstantinov IE, Wheaton GR, Ramsay JM, Grigg LE, Millar J, Cheung MM, Brizard CP. Predictors of survival after single-ventricle palliation: the impact of right ventricular dominance. Journal of the American College of Cardiology. 2012;59:1178–1185. doi: 10.1016/j.jacc.2011.11.049. [DOI] [PubMed] [Google Scholar]
- 28.Kunii Y, Kamada M, Ohtsuki S, Araki T, Kataoka K, Kageyama M, Nakagawa N, Seino Y. Plasma brain natriuretic peptide and the evaluation of volume overload in infants and children with congenital heart disease. Acta Med Okayama. 2003;57:191–197. doi: 10.18926/AMO/32809. [DOI] [PubMed] [Google Scholar]
- 29.Oosterhof T, Tulevski II, Vliegen HW, Spijkerboer AM, Mulder BJ. Effects of volume and/or pressure overload secondary to congenital heart disease (tetralogy of fallot or pulmonary stenosis) on right ventricular function using cardiovascular magnetic resonance and B-type natriuretic peptide levels. Am J Cardiol. 2006;97:1051–1055. doi: 10.1016/j.amjcard.2005.10.047. [DOI] [PubMed] [Google Scholar]
- 30.Paul MA, Backer CL, Binns HJ, Mavroudis C, Webb CL, Yogev R, Franklin WH. B-type natriuretic peptide and heart failure in patients with ventricular septal defect: a pilot study. Pediatric cardiology. 2009;30:1094–1097. doi: 10.1007/s00246-009-9503-5. [DOI] [PubMed] [Google Scholar]
- 31.Xanthakis V, Larson MG, Wollert KC, Aragam J, Cheng S, Ho J, Coglianese E, Levy D, Colucci WS, Michael Felker G, Benjamin EJ, Januzzi JL, Wang TJ, Vasan RS. Association of novel biomarkers of cardiovascular stress with left ventricular hypertrophy and dysfunction: implications for screening. Journal of the American Heart Association. 2013;2:e000399. doi: 10.1161/JAHA.113.000399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nagaya N, Nishikimi T, Goto Y, Miyao Y, Kobayashi Y, Morii I, Daikoku S, Matsumoto T, Miyazaki S, Matsuoka H, Takishita S, Kangawa K, Matsuo H, Nonogi H. Plasma brain natriuretic peptide is a biochemical marker for the prediction of progressive ventricular remodeling after acute myocardial infarction. Am Heart J. 1998;135:21–28. doi: 10.1016/s0002-8703(98)70338-2. [DOI] [PubMed] [Google Scholar]
- 33.Eindhoven JA, van den Bosch AE, Jansen PR, Boersma E, Roos-Hesselink JW. The usefulness of brain natriuretic peptide in complex congenital heart disease: a systematic review. Journal of the American College of Cardiology. 2012;60:2140–2149. doi: 10.1016/j.jacc.2012.02.092. [DOI] [PubMed] [Google Scholar]
- 34.Goldberg DJ, French B, McBride MG, Marino BS, Mirarchi N, Hanna BD, Wernovsky G, Paridon SM, Rychik J. Impact of oral sildenafil on exercise performance in children and young adults after the fontan operation: a randomized, double-blind, placebo-controlled, crossover trial. Circulation. 2011;123:1185–1193. doi: 10.1161/CIRCULATIONAHA.110.981746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Paridon SM, Mitchell PD, Colan SD, Williams RV, Blaufox A, Li JS, Margossian R, Mital S, Russell J, Rhodes J. A cross-sectional study of exercise performance during the first 2 decades of life after the Fontan operation. Journal of the American College of Cardiology. 2008;52:99–107. doi: 10.1016/j.jacc.2008.02.081. [DOI] [PubMed] [Google Scholar]
- 36.Hall EK, Glatz AC, Quartermain MD, Ravishankar C, Kaufman B, Cohen MS, Hanna BD, Goldberg DJ. Brain-type natriuretic peptide correlates with right heart pressures in a cross section of pediatric heart transplant patients. Pediatric transplantation. 2011;15:70–74. doi: 10.1111/j.1399-3046.2010.01421.x. [DOI] [PubMed] [Google Scholar]
- 37.Jan SL, Fu YC, Hwang B, Lin SJ. B-type natriuretic peptide in children with atrial or ventricular septal defect: a cardiac catheterization study. Biomarkers : biochemical indicators of exposure, response, and susceptibility to chemicals. 2012;17:166–171. doi: 10.3109/1354750X.2011.649494. [DOI] [PubMed] [Google Scholar]