Abstract
A positive association of obesity with breast cancer incidence and mortality is well established. Recent reports indicate that adipose stromal cells (ASCs) play an important role in breast cancer development and progression by producing estrogens and tumor-promoting cytokines. Furthermore, circulating ASCs have been uniquely detected in obese individuals, which is likely due to increased tissue remodeling and cell mobilization. The number of circulating ASCs is even more prominent in obese patients with colon and prostate cancers, both of which are exacerbated by obesity. To determine whether a similar association exists for breast cancer, we collected blood samples from a cohort of breast cancer survivors and enumerated circulating ASCs by flow cytometry on the basis of the previously established ASC-associated immunophenotype (CD34+/CD31−/CD45−). We found significantly higher levels of circulating ASCs (p < 0.001) in breast cancer survivors with body mass index (BMI) ≥ 30 kg/m2 than their non-obese counterparts (BMI < 30). We also compared circulating ASCs before and after exercise of only the obese subjects enrolled in a 6-month individualized exercise program, but found no statistically significant difference, likely due to limited number of subjects in the study. Our findings suggest that circulating ASCs can serve as a potential biomarker for future studies of the impacts of obesity and physical activity on breast cancer recurrence and survival.
Keywords: circulating adipose stromal cells (ASC), obesity, breast cancer, exercise
INTRODUCTION
Breast cancer is the second leading cause of cancer-related death among women in the United States (1). Cancer survival is associated with significant decrements in health status and an increased risk of death from non-cancer causes (2). The “burden” of survival includes an increased risk of morbidity and mortality related to comorbid chronic diseases, such as type 2 diabetes and cardiovascular disease (3). According to the American Cancer Society, a cancer survivor is any person who has been diagnosed with cancer, from the time of diagnosis through the balance of life. In practice, the concept of survivorship is often associated with the period after active treatment ends (4). Epidemiological studies have shown that obesity increases breast cancer incidence and mortality, predominantly in postmenopausal women (5-7). Approximately 30-50% of breast cancer deaths may be attributed to excess body weight (overweight and obesity) (8). Excessive adiposity also contributes to deteriorated health of breast cancer survivors (9-13). According to the Centers for Disease Control and Prevention, the population of obese adult women has increased dramatically in the United States from approximately 16% in 1962 to 48% in 2008 (14). The dramatic increase in the prevalence of obesity, combined with the fact that over 75% of new cases of breast cancer occur in postmenopausal women (1), poses a pressing challenge on breast cancer prevention, treatment, and survival.
There is strong evidence that modifiable behavioral risk factors can influence the health of cancer survivors. In particular, physical activity is associated with diminished treatment-related side effects, enhanced quality of life (15, 16), and reduced risk of cancer recurrence and comorbidity (17-20). On the other hand, it is estimated that excess body weight and low physical activity together may account for one quarter to one third of all breast cancer cases (21). Though the benefits of exercise have been well documented, less than half of U.S. adults meet the recommendations for physical activity and a fourth report no activity at all (22, 23). Inactivity is even higher for women, as nearly 65% of women in the United States do not obtain sufficient amounts of physical activity (24). Substantial epidemiologic literature on physical activity and breast cancer (25, 26) supports a “probable” cancer-preventive role of physical activity in postmenopausal women, whereby habitual activity may lower risk by approximately 20% (27).
Reduced levels of fitness, loss of strength, treatment side effects, and the stressors of the cancer experience can make it more difficult for survivors who may want to increase their activity (28). A number of studies have examined the therapeutic value of exercise during primary cancer treatment (29-31). The evidence supports the paradigm that exercise is not only safe and feasible for cancer survivors, but it also can significantly improve physical functioning (29, 31-36). This applies to longer-term quality of life outcomes for survivors as well (33, 37-39). Increasing physical activity is perhaps the most easily modifiable and inexpensive method of ameliorating obesity (40) by increasing the expenditure side of the energy balance equation.
In evaluating obesity it is important to look at other variables beside body mass index (BMI). Using BMI has been criticized because BMI does not always reflect true adiposity (body fatness) (41-43). A safe, non-invasive way to assess body fatness is through the use of skinfold assessment (44). In this study we measured body composition both with BMI and body fatness estimates from skinfold assessments (42). It is important to account for body fatness, as small changes in composition of body fatness without corresponding changes in weight can do much to reduce risk as well as increase basal metabolism rate thereby increasing the energy expenditure (45).
Obesity is accompanied by expansion of white adipose tissue (WAT), which exerts a direct effect on tumor development and progression (46, 47) as a potent endocrine organ (48-50). WAT is composed of heterogeneous cell populations that include mature adipocytes, infiltrated monocytic cells, and perivascular adipocyte progenitors, which are also known as adipose stromal cells (ASCs). Among all the cell types in adipose tissue, ASCs serve as the major source of adipose tissue-derived estrogens (51, 52), a primary underlying factor for obesity-associated breast cancer risk among postmenopausal women (53, 54). Furthermore, recent studies have shown that WAT expansion is associated with ASC proliferation (55), and that number of ASCs per gram of WAT increases in obese individual (56, 57). ASCs share several common features with mesenchymal stem cells (MSCs) (58-61), such as the multipotent potential and the ability to produce a number of tumor-promoting cytokines and growth factors (51, 62-65). Indeed, several animal studies indicate that ASCs can home to tumor sites and promote tumor growth (47, 66-68).
Bellows et al. recently reported that a circulating cell population that shares the same immunophenotype as adipose tissue-derived ASCs was uniquely detected in the blood circulation of obese individuals (69, 70). This obesity-associated circulating cell population expressed high levels of the hematopoietic cell-specific marker (CD34+), but low levels of the endothelial- (CD31−) and macrophage/monocyte-specific (CD45−) markers. Intriguingly, the abundance of circulating ASCs was found to be even higher in obese colorectal and prostate cancer patients than their cancer-free obese controls (69, 71). Thus, circulating ASCs may not only serve as a novel biomarker for obesity-related pathological progression but also play an active role in cancer progression and metastasis. In light of the well-documented association of obesity with breast cancer, we compared in the current study the abundance of circulating ASCs in obese and non-obese breast cancer survivors. Although there is published work on the effect of exercise on circulating endothelial cells (72) and hematopoietic progenitor cells (73), similar studies on circulating ASCs have not been reported. Therefore, we sought to assess the impact of exercise on circulating ASCs among the obese breast cancer survivors.
MATERIALS AND METHODS
Recruitment
The subjects involved in the current report were part of a 6-month longitudinal study aimed at examining the impact of exercise on breast cancer survival. Upon approval of the protocol by the Institutional Review Board (IRB), participants were recruited with assistance from the Deriving Inspiration and Vitality through Activity (DIVA) Program from the ThriveWell® Cancer Foundation in San Antonio, Texas. The DIVA program is a self-referral program that offers support services for breast cancer survivors. Potential participants who called in to register for the DIVA program were screened for inclusion criteria. In addition, DIVA flyers advertising the study were distributed to local oncology clinics and relayed on radio and television announcements. Participants responding to these notices called the DIVA center to express interest and were screened for eligibility by research staff. Inclusion criteria were: female 18 years of age or older, history of breast cancer (invasive or ductal in situ), post treatment (chemo, radiation, surgery or combination) for at least 2 months, free of any absolute contra-indications for exercise testing as stated in the American College of Sports Medicine (ACSM) Guidelines for Exercise Testing and Prescription (42), able to read/write in English, and able to provide informed consent. Any subjects who had breast cancer relapse and/or new cancer diagnosis during the period of the study were withdrawn.
If interested in participating in the research, participants were asked to complete the Physical Activity Readiness Questionnaire (PAR-Q), as detailed in the ACSM's Guidelines. Physician's clearance for participating in an exercise program was required for any participants who answered “yes” to any of the seven questions listed on the PAR-Q prior to scheduling a “pre” intervention baseline appointment. Participants who answered “no” to all questions or had received physician's clearance to exercise were scheduled for an appointment at the South Texas Oncology and Hematology Center (START Center) DIVA location in San Antonio Texas. After providing informed consent, participants completed “pre” intervention baseline assessments in one session at the START Center location. A total of 130 potential participants expressed interest in the study, and 121 met the inclusion criteria. Of the ones who met the inclusion, 94 provided informed consent and completed the “pre” intervention baseline assessments. The first 12 obese and non-obese participants who had the baseline assessments were involved in the study of obesity-associated circulating ASCs (Table 1 and Fig. 1). Of the 94 participants who initially enrolled in the parent study, 72 completed one of the three 6-month exercise regimens as described below. Thirteen participants who completed the exercise program were obese, and had their pre- and post-exercise readings of circulating ASCs analyzed in this exercise-ASC study (Table 2 and Fig. 2).
Table 1.
Baseline Participant Characteristics Means (Standard Deviations) and Differences Between Groups by Mann-Whitney U test
| Obese (BMI≥30) n=12 | Non-Obese (BMI<30) n=12 | Mann-Whitney U p value | |
|---|---|---|---|
| Age (years) | 54.4 (8.8) | 56.9(9.8) | 0.63 |
| Co-Morbidity Index (CMI) | 2.4 (1.2) | 2.0 (1.8) | 0.55 |
| BMI (kg/m2) | 36.1 (3.2) | 27.1 (2.5) | <0.001 |
| % Body Fat | 37.2 (4.5) | 31.5 (2.7) | 0.002 |
| VO2 (ml O2/kg/min) | 17.8 (5.0) | 20.2 (4.8) | 0.38 |
| Arm Strength (kg) | 17.2 (4.1) | 19.9 (6.2) | 0.24 |
| Shoulder Strength (kg) | 27.9 (8.3) | 28.3 (8.2) | 0.67 |
| Sit-stand (repetitions) | 12.3 (2.3) | 13.8 (3.1) | 0.15 |
| Sit-reach (cm) | 29.8 (10.1) | 30.1 (7.7) | 0.98 |
Co-Morbidity Index (CMI) = sum of co-morbidities; BMI= body mass index; % body fat = adiposity estimated from three-site skinfold measures; VO2 = Cardiorespiratory function based on estimated VO2peak from submaximal cycle ergometry test; Arm Strength= force generated from arm isometric strength test; Shoulder Strength= force generated from shoulder isometric strength test; “Sit-reach” = lower body Flexibility test.
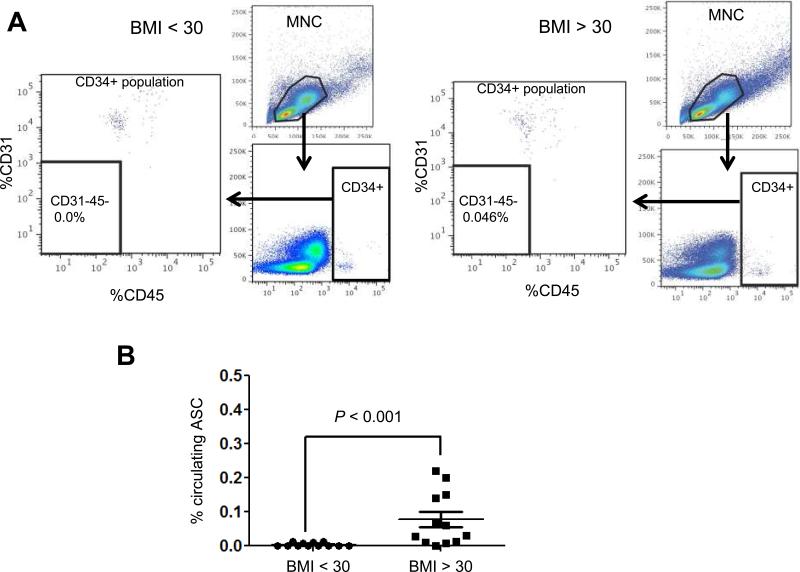
Figure 1. Obesity-associated circulating ASCs in breast cancer survivors.
(A) Representative raw data from one non-obese (BMI < 30) and one obese (BMI > 30) participant. Viable MNC (mono nuclear cell) populations (upper right panel) were analyzed for CD34 intensity (lower right panel), and the gated CD34+ sub-population was further assessed for CD31 and CD45 (left panel). Abundance of CD34+/CD31−/CD45− cells (% of total MNC) was numerated in each experiment. (B) Comparison of the abundance of circulating ASCs (CD34+/CD31−/CD45−) at baseline from the non-obese (n = 12) and obese (n = 12) breast cancer survivors. Mann-Whitney test was used to find the variability.
Table 2.
Change in circulating ASCs, body adiposity and fitness measures among obese participants (n=13) after 6 months of exercise.
| Exercise Group | ASC change | BMI change | COM Index | % Body-fat change | Responders Body-fat | VO2 change | Arm change | Shoulder change | Sit-stand change | Sit-reach change |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 0.31 | −1.40 | 1 | −2.92 | 1 | 5.51 | 12 | −3 | 1 | −2.5 |
| 3 | 0.00 | 2.20 | 2 | −0.36 | 0 | 1.33 | 5 | 6 | 8 | −1 |
| 2 | 0.44 | −2.00 | 4 | −6.91 | 1 | -- | -- | -- | -- | -- |
| 2 | −0.21 | −0.80 | 2 | −1.55 | 0 | 0.12 | 0 | 4 | 1 | −1 |
| 2 | −0.14 | 0.40 | 4 | −3.7 | 1 | 2.39 | 2 | 0 | 1 | 0 |
| 3 | 0.10 | −2.00 | 1 | −5.51 | 1 | −5.82 | −8 | −10 | 2 | 0 |
| 1 | −0.41 | −0.57 | 4 | −3.75 | 1 | 0.03 | −1 | 11 | −2 | 1.5 |
| 2 | 0.16 | 0.19 | 2 | −0.55 | 0 | −0.24 | 3 | 8 | 1 | 1 |
| 1 | −0.07 | 0.00 | 4 | −4.97 | 1 | 4.95 | 5 | −5 | 5 | 6.5 |
| 3 | 0.64 | 0.59 | 0 | −0.43 | 0 | −0.43 | −5 | −1 | −3 | 0.5 |
| 2 | −0.01 | 0.88 | 4 | −0.64 | 0 | 0.84 | 2 | 5 | −1 | 2.5 |
| 2 | −0.09 | 3.67 | 3 | −2.13 | 1 | −1.00 | −1 | 4 | 1 | −5 |
| 3 | 0.00 | −0.30 | 2 | −3.56 | 1 | 0.85 | −8 | −13 | 1 | −0.5 |
| Mean change (SD) | 0.06 (0.28) | 0.066 (1.6) | 2.54 (1.4) | −2.84 (2.1) | N/A | 0.71 (2.9) | 0.50 (5.7) | 0.50 (7.3) | 1.25 (2.9) | 0.17 (2.8) |
| ASC loss* | −0.16 (0.14) | 0.60 (1.6) | N/A | −2.79 (1.6) | 1.22 (2.14) | 1.17 (2.3) | 3.17 (5.3) | 0.83 (2.4) | 0.75 (3.8) | |
| ASC gain** | 0.25 (0.24) | −0.49 (1.5) | −3.28 (2.6) | 0.29 (3.7) | −0.80 (8.1) | −4.20 (8.4) | 1.80 (3.6) | −0.70 (1.2) |
Exercise Group: 1=YE, 2=CE, 3=C (see text for definitions). Responders = body fat loss ≥2%. -- = data not collected. Rows with loss in circulating ASCs are highlighted in blue. N/A= not applicable.
mean change (SD) in variables for participants with net ASC loss;
mean change (SD) in variable for participants with net ASC gain.
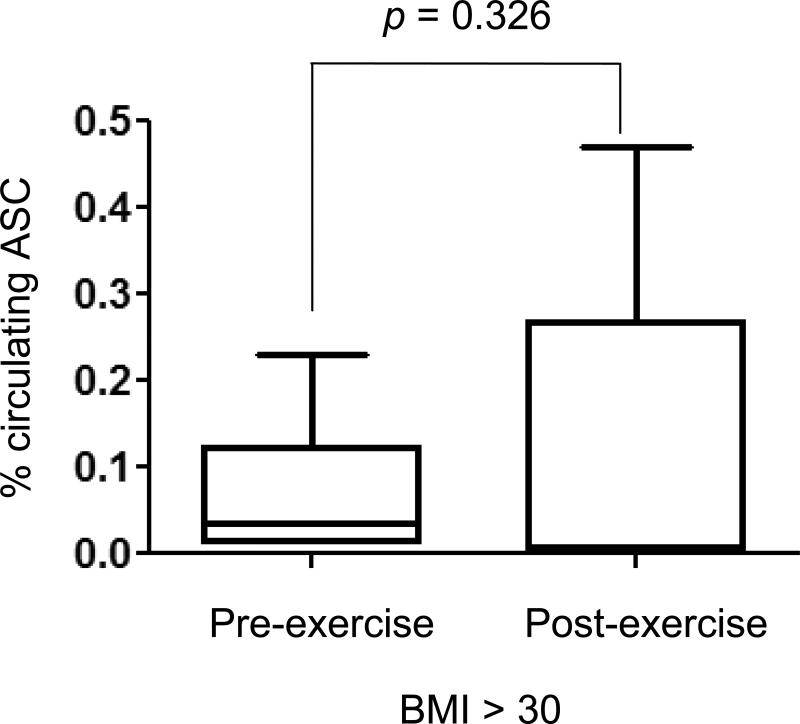
Figure 2. Enumeration of circulating ASCs before and after exercise.
Thirteen obese breast cancer survivors who completed one of the three six-month exercise regimens were assessed for their circulating ASC readings before (pre-) and after the 6-month programs (post-). Mann-Whitney test was used to find the variability.
After obtaining informed consent, assessment procedures were explained to the participants and were conducted in the order listed. The assessments involved: height/weight, venipuncture, anthropometric measurements, and a comprehensive fitness assessment that included tests for cardiorespiratory function, muscular strength, flexibility, and body fat composition. In addition we assessed level of co-morbidity for participants.
Prior to any physical assessments, using responses from the medical history information a co-morbidity index was calculated with information provided from the participants regarding previous diagnoses in addition to their diagnosis of breast cancer. The index was included to assess presence of other health issues that could affect participants’ level of engagement in exercise, including exercise assessments performed at baseline. The information, along with results from the exercise tests, was also used to identify any constraining issues in participating in specific exercises. A total of 17 items were included (diagnosis of a heart attack, heart failure, heart condition, circulation problems, blood clots, hypertension, stroke, lung problems, diabetes, kidney problems, rheumatoid arthritis, osteoarthritis, anemia, thyroid problems, neuropathy, fibromyalgia and hepatitis). The index was calculated by adding the number of “yes” responses to individual diagnoses (range of score, 0-17). In addition, participants were asked about any secondary cancers and if they had been given a diagnosis of lymphedema. The information on secondary cancers and lymphedema was noted but treated separately from the co-morbidity index. Patients with lymphedema require specific resistance training protocols (74-78), so this information allowed for further tailoring of exercise prescriptions at baseline and during the intervention period.
Height (cm) and weight (kg) were measured using a wall-mounted Stadiometer (Seca 644 Handrail Scale). This was used to calculate participant's BMI (kg/m2). For body composition, three-site (triceps, supra-ilium, and quadricep) skinfold assessments (79) (43) were performed. Measurements were summed, and body density (Db) calculated according to the ACSM-recommended formula (42):
Population-specific formulas were used for conversion of Db to % body fat. We chose to do skinfold assessment to estimate body fatness, as BMI does not account for the “quality” of weight. In some exercise programs, body mass index can remain constant coupled with gains in muscle mass and loss of adiposity (42). For cardiorespiratory function, a sub-maximal ramped cycle ergometer test with a Lode Corival Cycle (Groningen, Netherlands) and a metabolic cart (Parvo True One®, Sandy, UT) was used for measuring VO2 (ml O2/kg/min). During testing we also employed Borg's rating of perceived exertion (RPE) 6-20 scale (80). We asked participants to especially be aware of when they self-reported being at RPE level > 12, which corresponds to the anchor between “fairly light” and “somewhat hard” (~65-70% of maximum effort) (81). To estimate VO2max linear regression were used regressing 30 second averages of heart rate on VO2 and extrapolating VO2 to age predicted maximum heart rate (42). Arm and shoulder muscular strength was tested using a strength dynamometer system with a sensor reporting peak force (kg) generated during voluntary maximal isometric contraction. The larger peak force measured of two trials was used as the strength measure. For leg strength, participants performed a functional timed “sit-to-stand” test (82). The number of times the person could stand and sit back down in a 30-second time period was used as the measure for leg strength. For flexibility, participants performed a “sit-and-reach” test that assesses hip and lower back flexibility were measured using a “sit-and-reach” flexibility box.
Exercise Prescriptions and documentation
After providing informed consent and completing the baseline assessments, participants were randomized to either: a comprehensive, individualized exercise program (CE) (aerobic, strength, flexibility), a structured Yoga class (YE), or no specific regimen (C) where participants could choose their exercise activities. All participants were asked to fill out an exercise log and a weekly physical activity questionnaire to document their activity. We used a form of adaptive randomization called “minimization”, which is similar to stratification in that participant characteristics are used to assign them to the treatment conditions. For randomization group assignment was based on age, BMI, and result of the exercise test (estimated VO2max). Regardless of assigned group, participants were encouraged to exercise 3 times per week for approximately one hour per session, with a targeted intensity of at least “12” on their RPE scale (80). We instructed participants to exercise at this level as a threshold for being at an intensity high enough to gain the benefits of exercise (i.e. moderate-to-vigorous intensity) (42). If participants wished to exercise more than three hours, they were neither encouraged nor discouraged to do so, but were asked to log their activities.
Isolation of Circulating ASCs
ASCs were collected from the blood samples by following a previously published protocol (83). In brief, blood samples (16 ml) were collected in blue top tiger tubes (BD Biosciences, USA) and were stored on ice. The tubes were centrifuged at room temperature for 25 min with no brake at 1,600 g. Fuzzy layers containing mononuclear cells, which were located just above the gel barrier, were collected in 15 ml conical tubes. FcR-blocking reagent (Miltenyi Biotec, USA) was added to the mononuclear cells (MNCs) according to the manufacturer's recommendation (20 μl per 107 cells) and incubated on ice for 10 min. Each sample was aliquoted into 5 tubes and various antibodies were added in each tube. After 30 min incubation on ice, cells were washed twice with ice-cold 1XPBS and were resuspended in a final volume of 400 μl of ice-cold PBS. ASC analysis was conducted with an LSR-II flow cytometer and the FACSDiva Software (BD Bioscience). Cells were gated to exclude cell clumps, contaminating polymorphonuclear cells, red blood cells, platelets, endothelial microparticles, and cell debris. Viable MNCs (>1000,000 per sample) were then used to enumerate individual populations. For fluorescence-activated cell sorting (FACS) on MNCs, fluorescein anisothiocyanate–conjugated CD31 antibody (clone WM59) and eFluor–conjugated CD45 antibody (clone 2D1) were purchased from e-Bioscience, and phycoerythrin-conjugated CD34 antibody (clone 563), along with appropriate isotype control immunoglobulin G were purchased from BD Bioscience. Cells with the immunophenotype of CD34+/CD31−/CD45− were identified as circulating ASCs per published work (70).
Statistical Analysis
Data presented in each figure represents a mean value of the percentage expression of the ASCs in the blood in obese vs. non-obese participants at baseline, and % body fat responders (≥2% body fat loss) vs. non-responders after 6 months of exercise. The mean values ± standard deviation (SD) and statistical analyses were calculated and plotted using SPSS (Version 20, 2012, IBM Corp). Due to the small “n” in each comparison group, a non-parametric approach (Mann-Whitney test) was used to compare continuous variables. Difference for all tests were considered significant when p < 0.05.
RESULT AND DISCUSSION
To determine a potential association between obesity and circulating ASCs among breast cancer survivors, we identified a subset of individuals who participated in a larger cohort study of exercise and breast cancer survivorship (84). All women included in this subset averaged 56.3 years old (SD=8.1) with a mean time from breast cancer diagnosis to study entry of 4.7 years (SD=3.7; range 1-12 years; data not shown). The majority (77%) of participants were ≥24 months post-treatment; 63% had invasive breast cancer, 41% of whom were diagnosed at Stage II, followed by 24% at Stage 0-I. Over 70% were unaware of their cancer subtype, and 44% had non-BRCA-1/2-related disease (56% did not know their BRCA-1/2 carrier status). Therapy regimen frequencies ranged from 79% for radiotherapy to 94% for chemotherapy and 100% for surgery, with the majority having received a combination, and 46% reported receiving hormonal therapy (data not shown).
To determine whether obesity is associated with circulating ASCs among breast cancer survivors, we first collected baseline blood samples from the women prior to their being randomized into the exercise programs. We included the first 12 non-obese and 12 obese participants in the parent study, with ages varying from 42-78 years and a mean age of 56.9 (SD=9.8) (non-obese) and 54.4 (SD=8.8) years (obese) (Table 1). Comorbidity index did not significantly differ between obese and non-obese participants. The average BMI of the non-obese subjects was 27.1 (SD=2.5), and that of the obese group was 36.1 (SD=3.2), p < 0.001. Similarly, as expected the participants differed significantly in % body fatness with the obese participants averaging 37.2% body fat (SD=4.5) to the non-obese 31.5% body fat (SD = 2.7), p = 0.002. Obese and non-obese participants did not significantly differ with regards to cardiorespiratory function, shoulder, leg or arm strength, or flexibility at baseline.
To analyze the counts of circulating ASCs, we enumerated the CD34+/CD31−/CD45− population following an established gating protocol for multiparametric flow cytometry (83). Representatives of the raw data from one obese and non-obese participant are shown in Fig. 1A. As shown in Fig. 1B, the mean percentage for the non-obese group (BMI < 30) was 0.004 ± 0.002 %. In contrast, the mean percentage of circulating ASCs in obese subjects (BMI ≥ 30) was 0.077 ± 0.0017 %. This represents a 19-fold statistically significant increase (p < 0.001) in the circulating ASCs for the obese individuals. Increased number of circulating ASCs was first linked to cancer-free obese individuals by Bellows et al. (70). Similar observations were reported for obese colorectal and prostate cancer patients (69, 71). In this regard, our finding of circulating ASCs among obese breast cancer survivors further validates the previous reports and extends the association between circulating ASCs and obesity to a women's cancer type that has been long associated with obesity.
The obese individuals in our analysis of circulating ASCs also participated in a larger randomized controlled exercise study. Therefore, we asked whether these participants experienced any exercise-associated changes in circulating ASCs. Of the 72 participants who completed the parent study, a total of 13 obese participants completed one of the three six-month exercise regimens, and had both pre- and post-exercise readings of circulating ASCs available (Table 2). For the current study, we did not stratify this group into different types of exercise programs due to the limited number of obese participants who completed the parent study. We detected decreases of circulating ASCs in six, and increases in seven individuals following the exercise program. As a whole, there was no significant difference between the pre- and post-exercise ASC readings (Fig. 2). However, those with reduced ASC levels had an average body fat decrease of 2.79 %, coupled with a slight increase in cardiorespiratory function of 1.22 ml O2/kg/min. Moreover these participants also improved in functional strength and flexibility fitness tests as well as shown in Table 2. Though the numbers are too small to generalize any conclusions, our pilot results suggest a potential interaction between fitness changes (body fatness, cardiorespiratory function) and ASC changes. We plan to conduct larger, more randomized controlled trials to further address this question.
We recognize several limitations in the current study and intend to address them in our future research. One caveat of the exercise-related study is the small sample size. Due to a high attrition rate from the obese participants in the parent exercise study, we were unable to determine any statistically significant exercise-associated changes. Therefore, this part of the study was underpowered, which precludes us from establishing a definitive relationship between physical activity and the circulating ASC numbers for the breast cancer survivors. The issue of high attrition rate could be alleviated by shorter exercise program duration. For example, instead of 6 months, 12-16 weeks as adopted in other similar kinds of exercise research (84-87) could be considered for future studies. In addition, as all subjects included in the study were breast cancer survivors, we were unable to discern whether the past disease history could contribute to the elevated baseline levels of circulating ASCs in the obese subjects. Accrual of obese women with no history of breast cancer would shed light on this important question. Lastly, despite the common immunophenotype shared by circulating ASCs and their adipose tissue-derived counterparts, it was difficult to pinpoint the true origin of circulating ASCs as detected in the current and previously published studies (69-71). Comparison of the gene expression signature between these two sources of human ASCs, preferably from the same individual, may reveal additional commonality and potential distinctions between these two cell populations.
In conclusion, our work shows a significant association between circulating ASCs and obesity among breast cancer survivors. Additional work is needed to validate the notion that circulating ASCs can be used as a biomarker for exercise-enhanced cancer survival. Mounting evidence has pointed to the diagnostic and prognostic values of circulating tumor cells (CTCs) for multiple cancer types including breast cancer (88-90). In this regard, circulating ASCs could provide a complementary prognostic marker for obese cancer patients. From the mechanistic perspective, it will be of importance to determine whether circulating ASCs may serve as an “accomplice” of CTCs by facilitating the latter in metastasizing to distant organs, as has been suggested by a recent animal study (91).
ACKNOWLEDGMENTS
This work was funded by grants to Dr. Li from NIH (CA161349) and Cancer Prevention Research Institute of Texas (RP110524); to Dr. Ramirez from Susan G. Komen for the Cure (SAB08-00005); to Dr. Ghosh from San Antonio Area Foundation and ThriveWell Cancer Foundation; and to Dr. Hughes from the National Cancer Institute (K22 CA 154626, U54 CA153511). The content is solely the responsibility of the authors and does not necessarily represent the official views of the above-mentioned funding agencies. The authors gratefully acknowledge the support of the Cancer Therapy and Research Center at The University of Texas Health Science Center - San Antonio, an NCI-designated Cancer Center (P30CA054174). The authors also gratefully acknowledge the support of Dr. Amy Lang, the ThriveWell™ Cancer Foundation, and the START Center for Cancer Care.
Footnotes
CONFLICT OF INTEREST
The authors declare to comply with all requirements for publication. There is no conflict of interest.
REFERENCES
- 1.Nunez NP, Perkins SN, Smith NC, et al. Obesity accelerates mouse mammary tumor growth in the absence of ovarian hormones. Nutrition and cancer. 2008;60:534–541. doi: 10.1080/01635580801966195. [DOI] [PubMed] [Google Scholar]
- 2.Baade PD, Fritschi L, Eakin EG. Non-cancer mortality among people diagnosed with cancer (Australia). Cancer Causes Control. 2006;17:287–297. doi: 10.1007/s10552-005-0530-0. [DOI] [PubMed] [Google Scholar]
- 3.Demark-Wahnefried W, Pinto BM, Gritz ER. Promoting health and physical function among cancer survivors: potential for prevention and questions that remain. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2006;24:5125–5131. doi: 10.1200/JCO.2006.06.6175. [DOI] [PubMed] [Google Scholar]
- 4.American Cancer Society . Cancer Treatment & Survivorship Facts & Figures 2012-2013. American Cancer Society; Atlanta, GA: 2012. p. 44. [Google Scholar]
- 5.McTiernan A. Behavioral risk factors in breast cancer: can risk be modified? Oncologist. 2003;8:326–334. doi: 10.1634/theoncologist.8-4-326. [DOI] [PubMed] [Google Scholar]
- 6.Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348:1625–1638. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- 7.Bianchini F, Kaaks R, Vainio H. Overweight, obesity, and cancer risk. Lancet Oncol. 2002;3:565–574. doi: 10.1016/s1470-2045(02)00849-5. [DOI] [PubMed] [Google Scholar]
- 8.Petrelli JM, Calle EE, Rodriguez C, Thun MJ. Body mass index, height, and postmenopausal breast cancer mortality in a prospective cohort of US women. Cancer Causes Control. 2002;13:325–332. doi: 10.1023/a:1015288615472. [DOI] [PubMed] [Google Scholar]
- 9.Champ CE, Volek JS, Siglin J, Jin L, Simone NL. Weight gain, metabolic syndrome, and breast cancer recurrence: are dietary recommendations supported by the data? Int J Breast Cancer. 2012;2012:506868. doi: 10.1155/2012/506868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hampton T. Breast cancer symposium highlights risk, recurrence, and research trials. JAMA. 2012;307:348–350. doi: 10.1001/jama.2012.30. [DOI] [PubMed] [Google Scholar]
- 11.Ligibel J. Obesity and breast cancer. Oncology (Williston Park) 2011;25:994–1000. [PubMed] [Google Scholar]
- 12.Protani M, Coory M, Martin JH. Effect of obesity on survival of women with breast cancer: systematic review and meta-analysis. Breast Cancer Res Treat. 2010;123:627–635. doi: 10.1007/s10549-010-0990-0. [DOI] [PubMed] [Google Scholar]
- 13.Sparano JA, Wang M, Zhao F, et al. Obesity at diagnosis is associated with inferior outcomes in hormone receptor-positive operable breast cancer. Cancer. 2012 doi: 10.1002/cncr.27527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of obesity in the United States, 2009-2010. NCHS Data Brief. 2012:1–8. [PubMed] [Google Scholar]
- 15.Speck RM, Courneya KS, Masse LC, Duval S, Schmitz KH. An update of controlled physical activity trials in cancer survivors: a systematic review and meta-analysis. Journal of cancer survivorship : research and practice. 2010;4:87–100. doi: 10.1007/s11764-009-0110-5. [DOI] [PubMed] [Google Scholar]
- 16.Speed-Andrews AE, Courneya KS. Effects of exercise on quality of life and prognosis in cancer survivors. Curr Sports Med Rep. 2009;8:176–181. doi: 10.1249/JSR.0b013e3181ae98f3. [DOI] [PubMed] [Google Scholar]
- 17.LaCroix AZ, Leveille SG, Hecht JA, Grothaus LC, Wagner EH. Does walking decrease the risk of cardiovascular disease hospitalizations and death in older adults? J Am Geriatr Soc. 1996;44:113–120. doi: 10.1111/j.1532-5415.1996.tb02425.x. [DOI] [PubMed] [Google Scholar]
- 18.Helmrich SP, Ragland DR, Leung RW, Paffenbarger RS., Jr. Physical activity and reduced occurrence of non-insulin-dependent diabetes mellitus. The New England journal of medicine. 1991;325:147–152. doi: 10.1056/NEJM199107183250302. [DOI] [PubMed] [Google Scholar]
- 19.Giovannucci E, Ascherio A, Rimm EB, Colditz GA, Stampfer MJ, Willett WC. Physical activity, obesity, and risk for colon cancer and adenoma in men. Ann Intern Med. 1995;122:327–334. doi: 10.7326/0003-4819-122-5-199503010-00002. [DOI] [PubMed] [Google Scholar]
- 20.Giovannucci EL, Liu Y, Leitzmann MF, Stampfer MJ, Willett WC. A prospective study of physical activity and incident and fatal prostate cancer. Archives of internal medicine. 2005;165:1005–1010. doi: 10.1001/archinte.165.9.1005. [DOI] [PubMed] [Google Scholar]
- 21.Neilson HK, Friedenreich CM, Brockton NT, Millikan RC. Physical activity and postmenopausal breast cancer: proposed biologic mechanisms and areas for future research. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2009;18:11–27. doi: 10.1158/1055-9965.EPI-08-0756. [DOI] [PubMed] [Google Scholar]
- 22.Pate RR, Pratt M, Blair SN, et al. Physical activity and public health. A recommendation from the Centers for Disease Control and Prevention and the American College of Sports Medicine. JAMA. 1995;273:402–407. doi: 10.1001/jama.273.5.402. [DOI] [PubMed] [Google Scholar]
- 23.Blair SN, Cheng Y, Holder JS. Is physical activity or physical fitness more important in defining health benefits? Med Sci Sports Exerc. 2001;33:S379–399. doi: 10.1097/00005768-200106001-00007. discussion S419-320. [DOI] [PubMed] [Google Scholar]
- 24.Ainsworth BE, Sternfeld B, Slattery ML, Daguise V, Zahm SH. Physical activity and breast cancer: evaluation of physical activity assessment methods. Cancer. 1998;83:611–620. doi: 10.1002/(sici)1097-0142(19980801)83:3+<611::aid-cncr3>3.3.co;2-u. [DOI] [PubMed] [Google Scholar]
- 25.Monninkhof EM, Elias SG, Vlems FA, et al. Physical activity and breast cancer: a systematic review. Epidemiology. 2007;18:137–157. doi: 10.1097/01.ede.0000251167.75581.98. [DOI] [PubMed] [Google Scholar]
- 26.Friedenreich CM, Cust AE. Physical activity and breast cancer risk: impact of timing, type and dose of activity and population subgroup effects. British journal of sports medicine. 2008;42:636–647. doi: 10.1136/bjsm.2006.029132. [DOI] [PubMed] [Google Scholar]
- 27.Warburton DE, Katzmarzyk PT, Rhodes RE, Shephard RJ. Evidence-informed physical activity guidelines for Canadian adults. Can J Public Health. 2007;98(Suppl 2):S16–68. [PubMed] [Google Scholar]
- 28.Doyle C, Kushi LH, Byers T, et al. Nutrition and physical activity during and after cancer treatment: an American Cancer Society guide for informed choices. CA: A Cancer Journal for Clinicians. 2006;56:323–353. doi: 10.3322/canjclin.56.6.323. [DOI] [PubMed] [Google Scholar]
- 29.Schmitz KH, Holtzman J, Courneya KS, Masse LC, Duval S, Kane R. Controlled physical activity trials in cancer survivors: a systematic review and meta-analysis. Cancer Epidemiology, Biomarkers and Prevention. 2005;14:1588–1595. doi: 10.1158/1055-9965.EPI-04-0703. [DOI] [PubMed] [Google Scholar]
- 30.Courneya KS. Exercise in cancer survivors: an overview of research. Medicine and Science in Sports and Exercise. 2003;35:1846–1852. doi: 10.1249/01.MSS.0000093622.41587.B6. [DOI] [PubMed] [Google Scholar]
- 31.Knols R, Aaronson NK, Uebelhart D, Fransen J, Aufdemkampe G. Physical exercise in cancer patients during and after medical treatment: a systematic review of randomized and controlled clinical trials. Journal of Clinical Oncology. 2005;23:3830–3842. doi: 10.1200/JCO.2005.02.148. [DOI] [PubMed] [Google Scholar]
- 32.McTiernan A, Ulrich C, Slate S, Potter J. Physical activity and cancer etiology: associations and mechanisms. Cancer Causes and Control. 1998;9:487–509. doi: 10.1023/a:1008853601471. [DOI] [PubMed] [Google Scholar]
- 33.Courneya KS, Mackey JR, Bell GJ, Jones LW, Field CJ, Fairey AS. Randomized controlled trail of exercise training in postmenopausal breast cancer survivors: cardiopulmonary and quality of life outcomes. Journal of Clinical Oncology. 2003;21:1660–1668. doi: 10.1200/JCO.2003.04.093. [DOI] [PubMed] [Google Scholar]
- 34.Basen-Engquist K, Hughes D, Perkins H, Shinn E, Taylor CC. Dimensions of physical activity and their relationship to physical and emotional symptoms in breast cancer survivors. Journal of cancer survivorship : research and practice. 2008;2:253–261. doi: 10.1007/s11764-008-0067-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hughes DC, Leung P, Naus MJ. Using single-system analyses to assess the effectiveness of an exercise intervention on quality of life for Hispanic breast cancer survivors: a pilot study. Soc Work Health Care. 2008;47:73–91. doi: 10.1080/00981380801970871. [DOI] [PubMed] [Google Scholar]
- 36.Schmitz KH, Courneya KS, Matthews C, et al. American College of Sports Medicine roundtable on exercise guidelines for cancer survivors. Medicine & Science In Sports & Exercise. 2010;42:1409–1426. doi: 10.1249/MSS.0b013e3181e0c112. [DOI] [PubMed] [Google Scholar]
- 37.Segal R, Evans W, Johnson D, et al. Structured exercise improves physical functioning in women with stages I and II breast cancer: results of a randomized controlled trial. Journal of Clinical Oncology. 2001;19:657–665. doi: 10.1200/JCO.2001.19.3.657. [DOI] [PubMed] [Google Scholar]
- 38.Segal RJ, Reid RD, Courneya KS, et al. Resistance exercise in men receiving androgen deprivation therapy for prostate cancer. Journal of Clinical Oncology. 2003;21:1653–1659. doi: 10.1200/JCO.2003.09.534. [DOI] [PubMed] [Google Scholar]
- 39.Pinto BM, Clark MM, Maruyama NC, Feder SI. Psychological and fitness changes associated with exercise participation among women with breast cancer. Psycho-oncology. 2003;12:118–126. doi: 10.1002/pon.618. [DOI] [PubMed] [Google Scholar]
- 40.Pate RR, Pratt M, Blair SN, et al. Physical activity and public health: a recommendation from the centers for disease control and prevention and the American College of Sports Medicine. JAMA: Journal of the American Medical Association. 1995;273:402–407. doi: 10.1001/jama.273.5.402. [DOI] [PubMed] [Google Scholar]
- 41.Lavie CJ, De Schutter A, Patel DA, Romero-Corral A, Artham SM, Milani RV. Body composition and survival in stable coronary heart disease: impact of lean mass index and body fat in the “obesity paradox”. Journal of the American College of Cardiology. 2012;60:1374–1380. doi: 10.1016/j.jacc.2012.05.037. [DOI] [PubMed] [Google Scholar]
- 42.American College of Sports Medicine . ACSM's Guidelines for Exercise Testing and Prescription. Lippincott Williams & Wilkins; Philadelphia, PA: 2009. [Google Scholar]
- 43.Jackson AS, Moss RM. Understanding Exercise for Health and Fitness. Kendall/Hunt Publishing Company; Dubuque, Iowa: 1997. [Google Scholar]
- 44.Jackson AS, Ellis KJ, McFarlin BK, Sailors MH, Bray MS. Cross-validation of generalised body composition equations with diverse young men and women: the Training Intervention and Genetics of Exercise Response (TIGER) Study. The British journal of nutrition. 2009;101:871–878. doi: 10.1017/S0007114508047764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Demark-Wahnefried W. Diet: Energy Balance and Adiposity. In: American Society of Clinical Oncology, editor. ASCO Curriculum: Cancer Prevention. American Society of Clinical Oncology; Alexandria, VA: 2007. pp. 3–33. [Google Scholar]
- 46.Vona-Davis L, Howard-McNatt M, Rose DP. Adiposity, type 2 diabetes and the metabolic syndrome in breast cancer. Obesity reviews : an official journal of the International Association for the Study of Obesity. 2007;8:395–408. doi: 10.1111/j.1467-789X.2007.00396.x. [DOI] [PubMed] [Google Scholar]
- 47.Zhang Y, Daquinag A, Traktuev DO, et al. White adipose tissue cells are recruited by experimental tumors and promote cancer progression in mouse models. Cancer research. 2009;69:5259–5266. doi: 10.1158/0008-5472.CAN-08-3444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Roberts DL, Dive C, Renehan AG. Biological mechanisms linking obesity and cancer risk: new perspectives. Annual review of medicine. 2010;61:301–316. doi: 10.1146/annurev.med.080708.082713. [DOI] [PubMed] [Google Scholar]
- 49.Khandekar MJ, Cohen P, Spiegelman BM. Molecular mechanisms of cancer development in obesity. Nat Rev Cancer. 2011;11:886–895. doi: 10.1038/nrc3174. [DOI] [PubMed] [Google Scholar]
- 50.Grossmann ME, Ray A, Nkhata KJ, et al. Obesity and breast cancer: status of leptin and adiponectin in pathological processes. Cancer metastasis reviews. 2010;29:641–653. doi: 10.1007/s10555-010-9252-1. [DOI] [PubMed] [Google Scholar]
- 51.Ghosh S, Kang T, Wang H, Hu Y, Li R. Mechanical phenotype is important for stromal aromatase expression. Steroids. 2011;76:797–801. doi: 10.1016/j.steroids.2011.02.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nichols JE, Bulun SE, Simpson ER. Effects of conditioned medium from different cultured cell types on aromatase expression in adipose stromal cells. J Soc Gynecol Investig. 1995;2:45–50. [PubMed] [Google Scholar]
- 53.Bulun SE, Chen D, Moy I, Brooks DC, Zhao H. Aromatase, breast cancer and obesity: a complex interaction. Trends Endocrinol Metab. 2012;23:83–89. doi: 10.1016/j.tem.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Reed MJ. The role of aromatase in breast tumors. Breast Cancer Res Treat. 1994;30:7–17. doi: 10.1007/BF00682737. [DOI] [PubMed] [Google Scholar]
- 55.Joe AW, Yi L, Even Y, Vogl AW, Rossi FM. Depot-specific differences in adipogenic progenitor abundance and proliferative response to high-fat diet. Stem Cells. 2009;27:2563–2570. doi: 10.1002/stem.190. [DOI] [PubMed] [Google Scholar]
- 56.Maumus M, Sengenes C, Decaunes P, et al. Evidence of in situ proliferation of adult adipose tissue-derived progenitor cells: influence of fat mass microenvironment and growth. J Clin Endocrinol Metab. 2008;93:4098–4106. doi: 10.1210/jc.2008-0044. [DOI] [PubMed] [Google Scholar]
- 57.Daquinag AC, Zhang Y, Kolonin MG. Vascular targeting of adipose tissue as an anti-obesity approach. Trends Pharmacol Sci. 2011;32:300–307. doi: 10.1016/j.tips.2011.01.004. [DOI] [PubMed] [Google Scholar]
- 58.Chantrain CF, Feron O, Marbaix E, DeClerck YA. Bone marrow microenvironment and tumor progression. Cancer Microenviron. 2008;1:23–35. doi: 10.1007/s12307-008-0010-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Galie M, Konstantinidou G, Peroni D, et al. Mesenchymal stem cells share molecular signature with mesenchymal tumor cells and favor early tumor growth in syngeneic mice. Oncogene. 2008;27:2542–2551. doi: 10.1038/sj.onc.1210920. [DOI] [PubMed] [Google Scholar]
- 60.Karnoub AE, Dash AB, Vo AP, et al. Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature. 2007;449:557–563. doi: 10.1038/nature06188. [DOI] [PubMed] [Google Scholar]
- 61.Bhowmick NA, Neilson EG, Moses HL. Stromal fibroblasts in cancer initiation and progression. Nature. 2004;432:332–337. doi: 10.1038/nature03096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ghosh S, Dean A, Walter M, et al. Cell density-dependent transcriptional activation of endocrine-related genes in human adipose tissue-derived stem cells. Experimental cell research. 2010;316:2087–2098. doi: 10.1016/j.yexcr.2010.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Walter M, Liang S, Ghosh S, Hornsby PJ, Li R. Interleukin 6 secreted from adipose stromal cells promotes migration and invasion of breast cancer cells. Oncogene. 2009;28:2745–2755. doi: 10.1038/onc.2009.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Blaber SP, Webster RA, Hill CJ, et al. Analysis of in vitro secretion profiles from adipose-derived cell populations. Journal of translational medicine. 2012;10:172. doi: 10.1186/1479-5876-10-172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Klopp AH, Zhang Y, Solley T, et al. Omental adipose tissue-derived stromal cells promote vascularization and growth of endometrial tumors. Clinical cancer research : an official journal of the American Association for Cancer Research. 2012;18:771–782. doi: 10.1158/1078-0432.CCR-11-1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Muehlberg FL, Song YH, Krohn A, et al. Tissue-resident stem cells promote breast cancer growth and metastasis. Carcinogenesis. 2009;30:589–597. doi: 10.1093/carcin/bgp036. [DOI] [PubMed] [Google Scholar]
- 67.Prantl L, Muehlberg F, Navone NM, et al. Adipose tissue-derived stem cells promote prostate tumor growth. Prostate. 2010;70:1709–1715. doi: 10.1002/pros.21206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zimmerlin L, Donnenberg AD, Rubin JP, Basse P, Landreneau RJ, Donnenberg VS. Regenerative therapy and cancer: in vitro and in vivo studies of the interaction between adipose-derived stem cells and breast cancer cells from clinical isolates. Tissue Eng Part A. 2011;17:93–106. doi: 10.1089/ten.tea.2010.0248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bellows CF, Zhang Y, Chen J, Frazier ML, Kolonin MG. Circulation of progenitor cells in obese and lean colorectal cancer patients. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2011;20:2461–2468. doi: 10.1158/1055-9965.EPI-11-0556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bellows CF, Zhang Y, Simmons PJ, Khalsa AS, Kolonin MG. Influence of BMI on level of circulating progenitor cells. Obesity. 2011;19:1722–1726. doi: 10.1038/oby.2010.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ribeiro R, Monteiro C, Silvestre R, et al. Human periprostatic white adipose tissue is rich in stromal progenitor cells and a potential source of prostate tumor stroma. Experimental biology and medicine. 2012;237:1155–1162. doi: 10.1258/ebm.2012.012131. [DOI] [PubMed] [Google Scholar]
- 72.Cesari F, Sofi F, Gori AM, et al. Physical activity and circulating endothelial progenitor cells: an intervention study. European journal of clinical investigation. 2012;42:927–932. doi: 10.1111/j.1365-2362.2012.02670.x. [DOI] [PubMed] [Google Scholar]
- 73.Kroepfl JM, Pekovits K, Stelzer I, et al. Exercise increases the frequency of circulating hematopoietic progenitor cells, but reduces hematopoietic colony-forming capacity. Stem cells and development. 2012;21:2915–2925. doi: 10.1089/scd.2012.0017. [DOI] [PubMed] [Google Scholar]
- 74.Ahmed RL, Thomas W, Yee D, Schmitz KH. Randomized Controlled Trial of Weight Training and Lymphedema in Breast Cancer Survivors. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2006;24:2765–2772. doi: 10.1200/JCO.2005.03.6749. [DOI] [PubMed] [Google Scholar]
- 75.Schmitz KH. Balancing lymphedema risk: exercise versus deconditioning for breast cancer survivors. Exercise and sport sciences reviews. 2010;38:17–24. doi: 10.1097/JES.0b013e3181c5cd5a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Schmitz KH, Ahmed RL, Troxel A, et al. Weight lifting in women with breast-cancer-related lymphedema. The New England journal of medicine. 2009;361:664–673. doi: 10.1056/NEJMoa0810118. [DOI] [PubMed] [Google Scholar]
- 77.Schmitz KH, Ahmed RL, Troxel AB, et al. Weight lifting for women at risk for breast cancer-related lymphedema: a randomized trial. JAMA : the journal of the American Medical Association. 2010;304:2699–2705. doi: 10.1001/jama.2010.1837. [DOI] [PubMed] [Google Scholar]
- 78.Schmitz KH, Troxel AB, Cheville A, et al. Physical Activity and Lymphedema (the PAL trial): assessing the safety of progressive strength training in breast cancer survivors. Contemporary clinical trials. 2009;30:233–245. doi: 10.1016/j.cct.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Baumgartner TA, Jackson AS. Measurement for Evaluation in Physical Education and Exercise Science. Wm. C. Brown Communications, Inc.; Dubuque, Iowa: 1995. [Google Scholar]
- 80.Borg G. Borg's Perceived Exertion and Pain Scales. Human Kinetics; Champaign, IL: 1998. [Google Scholar]
- 81.Borg G. Ratings of perceived exertion and heart rates during short-term cycle exercise and their use in a new cycling strength test. International journal of sports medicine. 1982;3:153–158. doi: 10.1055/s-2008-1026080. [DOI] [PubMed] [Google Scholar]
- 82.Rikli RE, Jones CJ. Development and validation of a functional fitness test for community-residing older adults. Journal of aging and physical activity. 1999:129–161. [Google Scholar]
- 83.Duda DG, Cohen KS, Scadden DT, Jain RK. A protocol for phenotypic detection and enumeration of circulating endothelial cells and circulating progenitor cells in human blood. Nat Protoc. 2007;2:805–810. doi: 10.1038/nprot.2007.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Cuevas B, Hughes DC, Long Parma D, et al. Motivation, Exercise and Stress in Breast Cancer Survivors. Supportive Care in Cancer. 2013 doi: 10.1007/s00520-013-2038-6. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sazlina SG, Browning CJ, Yasin S. Promoting physical activity in sedentary elderly Malays with type 2 diabetes: a protocol for randomised controlled trial. BMJ Open. doi: 10.1136/bmjopen-2012-002119. 22012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Jane ML, Ho CC, Chen SC, Huang YC, Lai CH, Liaw YP. A Simple Method for Increasing High-Density Lipoprotein Cholesterol Levels: A Pilot Study of Combination Aerobic and Resistance Exercise Training. Int J Sports Physiol Perform. 2012 [PubMed] [Google Scholar]
- 87.Davis CL, Pollock NK, Waller JL, et al. Exercise dose and diabetes risk in overweight and obese children: a randomized controlled trial. JAMA. 2012;308:1103–1112. doi: 10.1001/2012.jama.10762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wang X, Sun Q, Mao F, Shen S, Huang L. CTCs hemodialysis: Can it be a new therapy for breast cancer? Med Hypotheses. 2012 doi: 10.1016/j.mehy.2012.06.025. [DOI] [PubMed] [Google Scholar]
- 89.Bidard FC, Fehm T, Ignatiadis M, et al. Clinical application of circulating tumor cells in breast cancer: overview of the current interventional trials. Cancer Metastasis Rev. 2012 doi: 10.1007/s10555-012-9398-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sinha G. Circulating tumor cells in early-stage breast cancer. J Natl Cancer Inst. 2012;104:1693–1694. doi: 10.1093/jnci/djs478. [DOI] [PubMed] [Google Scholar]
- 91.Zhang Y, Daquinag AC, Amaya-Manzanares F, Sirin O, Tseng C, Kolonin MG. Stromal progenitor cells from endogenous adipose tissue contribute to pericytes and adipocytes that populate the tumor microenvironment. Cancer Res. 2012;72:5198–5208. doi: 10.1158/0008-5472.CAN-12-0294. [DOI] [PubMed] [Google Scholar]