Abstract
Parabens and phthalates are potential endocrine disruptors frequently used in personal care/beauty products, and the developing fetus may be sensitive to these chemicals. We measured urinary butyl-paraben (BP), methyl-paraben (MP), propyl-paraben (PP), mono-n-butyl phthalate (MBP), and monoethyl phthalate (MEP) concentrations up to three times in 177 pregnant women from a fertility clinic in Boston MA. Using linear mixed models, we examined the relationship between self-reported personal care product use in the previous 24 hours and urinary paraben and phthalate metabolite concentrations. Lotion, cosmetic, and cologne/perfume use were associated with the greatest increases in the molar sum of phthalate metabolite and paraben concentrations, although the magnitude of individual biomarker increases varied by product used. For example, women who used lotion had BP concentrations 111% higher (95% confidence interval [CI]:41%, 216%) than non-users, while their MBP concentrations were only 28% higher (CI:2%, 62%). Women using/cologne/perfume had MEP concentrations 167% (CI:98%, 261%) higher than non-users, but BP concentrations were similar. We observed a monotonic dose-response relationship between the total number of products used and urinary paraben and phthalate metabolite concentrations. These results suggest that questionnaire data may be useful for assessing exposure to a mixture of chemicals from personal care products during pregnancy.
Keywords: Endocrine disruptors, Epidemiology, Mixtures, Parabens, Phthalates
Introduction
Personal care products often contain both parabens and phthalates, which are suspected endocrine-disrupting compounds (EDCs) (1, 2). Individuals may be simultaneously exposed to both chemical classes since they use multiple products that contain these chemicals (e.g., lotion and cosmetics) and/or both classes of chemicals may be found in a single product. Women of reproductive age have higher urinary concentrations of phthalate metabolites and parabens than men, suggesting that personal care product use may be an important source of exposure among women (3–5). This may be of particular concern to the developing fetus which is often more sensitive to the effects of EDCs than adults (6).
Derivatives of para-hydroxybenzoic acid, including butyl-paraben (BP), ethyl-paraben (EP), methyl-paraben (MP), and propyl-paraben (PP), are commonly used as antimicrobial agents to increase personal care and beauty product shelf life (7). Parabens are suspected of having weak estrogenic and anti-androgenic activity that may increase the risk of adverse health outcomes in experimental animals, including altered gonadal hormone signaling or metabolism and spermatogenesis (8–12). The relative biological activity of the different parabens increases with the alkyl side chain length, but is several orders of magnitude lower than that of estradiol (8).
Phthalic acid esters like dibutyl phthalate (DBP) and diethyl phthalate (DEP) are used to retain scents in cologne/perfumes and deliver agents in aerosols (13). DBP has well documented anti-androgenic effects in rats, while DEP does not (14). Some phthalates may have very weak estrogenic activity (15). Human studies suggest that fetal DBP and DEP exposure may increase the risk of adverse health outcomes during early childhood (16–19).
Prior studies have confirmed that some personal care products may be modifiable sources of phthalate or paraben exposures; however, few studies have considered exposure to mixtures of both parabens and phthalates (20–24). As mixtures, some EDCs may act additively or multiplicatively to increase the risk of adverse health effects (14, 25).
Using repeated urinary biomarker concentrations and questionnaires we characterized the relationship between DBP, DEP, BP, MP, and PP exposure and personal care product use in pregnant women. We focused on these phthalates and parabens because of their known or suspected presence in personal care products (7, 13). Because exposure may occur as a mixture, we used self-reported personal care product use as a surrogate of exposure to both classes of chemicals to determine whether the total personal care product use was associated with higher paraben and phthalate exposure.
Material and Methods
Participants
The Environment and Reproductive Health [EARTH] Study recruited women 18 to 45 years old and male partners seeking evaluation and treatment for infertility at the Massachusetts General Hospital Fertility Center in Boston between March 2005 and March 2011. The present analysis is from a larger prospective pre-conception open-cohort study designed to examine the relationship between environmental chemical exposures and fertility/pregnancy outcomes. Details of the methods and participants have been previously described (26, 27). Women were eligible for the present analysis if they had a live birth, provided at least one urine sample during pregnancy, completed a questionnaire at the time of urine collection about their personal care product use in the past 24 hours, and had complete covariate data.
The Human Studies Institutional Review Boards of the Massachusetts General Hospital (MGH), Harvard School of Public Health (HSPH), and the Centers for Disease Control and Prevention (CDC) approved this study. Study protocols were explained and all questions were answered by a trained research nurse before participants provided informed consent.
Urine Sample Collection and Analytic Measurements
Women provided up to three spot urine samples during pregnancy at routine clinic visits. The time of sample collection was recorded on a standardized form. Before aliquoting and storing samples at −80° C, urine specific gravity (SG) was measured using a handheld refractometer (National Instrument Company Inc, Baltimore, MD) that was calibrated with deionized water prior to each use. Samples were shipped overnight on dry ice to the CDC for analysis.
We measured the total concentration of two phthalate metabolites and three parabens: mono-n-butyl phthalate (MBP), monoethyl phthalate (MEP), BP, MP, and PP, using previously described solid phase extraction-high performance liquid chromatography-isotope dilution tandem mass spectrometry methods and quality control procedures (3, 28–30). We did not measure ethyl paraben concentrations in this study because of its relatively low frequency of detection in the US population (4). The limits of detection (LOD) for MBP, MEP, BP, MP, and PP were in the low μg/L range (~0.1 to ~1 μg/L). All paraben and phthalate metabolite concentrations were specific-gravity (SG) standardized (median SG: 1.015) using a modification of a previously described formula (20). Concentrations below the LOD were given a value of the LOD/√2 (31). Because the analytic standard for MEP was of inadequate purity, we applied a correction factor of 0.66 to MEP concentrations (32).
We characterized biomarker concentrations in three different ways. First, we examined each individual paraben or phthalate metabolite. Second, we calculated the sum of the molar concentrations of urinary phthalate metabolites and parabens. Finally, we created a relative estrogenicity equivalency factor (EEF) summary measure using an approach outlined by Safe and Shirai and results from in vitro yeast reporter assay data from Harris and Routledge (8, 10, 15, 33). We assumed that MBP, MEP, BP, MP, and PP were 10,000,000; 2,000,000; 10,000; 2,500,000; and 30,000 times less potent than 17β-estradiol, respectively. We calculated the EEF (μmol/L) as follows:
where [MBP], [MEP], [BP], [MP], and [PP] are the micromolar concentrations (μmol/L) of the chemicals. The final EEF is expressed in units of estrogenic activity relative to estradiol. We did not create a similar summary measure for the anti-androgenic activity of MEP and MBP because MEP does not possess anti-androgenic activity in rodent models and comparable data for parabens and phthalates do not exist (14). All of these measures were log10-transformed to satisfy regression model assumptions.
Personal Care Product Use
Women completed a questionnaire at the time of urine collection that asked if and when they used 13 personal care products that are applied to the skin or scalp in the past 24 hours. These included deodorant, shampoo, conditioner/crème rinse, hairspray/hair gel, other hair care products (e.g., mousse, hair bleach, relaxer, perm), shaving cream, cologne/perfume, bar soap, liquid soap/body wash, hand/body lotion, colored cosmetics (hair dye, foundation, blush, eye shadow, eye liner, or mascara), suntan/sunblock lotion, and nail polish.
We used two approaches to characterize women’s personal care product use. First, we examined use of each product separately (yes vs. no). Second, we summed the total number of products used in the last 24 hours and categorized women into three product user groups corresponding to the bottom 30% (low users, 0–5 products), middle 41% (medium users, 6–7 products), and top 29% (high users, 8–11 products) of product users. We calculated the number and proportion of women using individual products within these three categories to better understand the patterns of product use among low, medium, and high product users.
Covariates
We obtained women’s age at baseline, race, and education using a standardized questionnaire. A trainer research nurse recorded women’s weight and height at each study visit, which was used to compute body mass index (BMI). The estimated date of conception was calculated using one of three dating methods: oocyte retrieval date, which was abstracted from medical records; crown rump length, which was measured during a fetal ultrasound between 6–8 weeks gestation; or woman’s report of last menstrual period. When more than one dating method was available, priority was given to retrieval date > ultrasound > last menstrual period. The date of conception was used to compute the week of pregnancy each urine sample was collected.
Statistical Analysis
We calculated univariate characteristics (counts, proportions, means, and standard deviations [SD]) of demographic factors and examined the distribution of urinary phthalate and paraben concentrations using box and whisker plots. We examined the correlation between pairs of log10-transformed urinary biomarker concentrations using Pearson correlation coefficients.
We began our statistical analyses by examining personal care product use in relation to each phthalate metabolite and paraben concentration using linear mixed models with an unstructured covariance matrix and random intercept for each woman to account for repeated measurements. Urinary phthalate metabolite or paraben concentrations were the outcome in these models and we calculated the percent change in biomarker or summary measure concentration with the various characterizations of personal care product use by exponentiating the beta coefficients from these models. We also computed the covariate-adjusted least squares geometric mean (GM) biomarker concentrations among women with and without personal care product use.
All models were adjusted for weeks of gestation (time-varying), maternal age at enrollment, education, race, and BMI (time-varying). Models examining individual personal care products were also adjusted for the total number of other personal care products used, not counting the one being examined.
As a sensitivity analysis, we examined whether urinary phthalate metabolite and paraben concentrations varied according to the time since the product was last used by calculating geometric mean concentrations according to categories of no product use, product use >6–24 hours ago, and product use in the last 6 hours. We also adjusted our primary analyses for the time of day of personal care product use and season of sample collection to determine if adjustment for other activities that co-vary with personal care product use or calendar time biased our results.
Results
A total of 266 women had a live birth and 205 of these women (77%) provided at least one urine sample and completed a product use questionnaire during pregnancy. One-hundred and seventy-seven women (67%) who had complete covariate, personal care product questionnaire, and urinary phthalate metabolite data together provided 391 urine samples. Most women provided 2 (41%) or 3 (40%) urine samples. Complete data and urinary paraben concentrations were available for 170 (64%) women.
Women included in the primary analyses were on average 35.7 years old (standard deviation [SD]: 4.0) and predominately non-Hispanic (97%), Caucasian (91%), college educated (95%), and had normal BMI (67%). Most women conceived with in vitro fertilization methods (63%), while the rest conceived naturally (18%) or with intrauterine insemination (19%). On average, within each trimester, urine samples and questionnaires were collected at 5.6 (SD: 1.6; range: 1.4–12.3), 20.0 (SD: 3.5; range 12.1–29.3), and 33.5 (SD: 2.5, range: 25.6, 38.4) weeks since conception.
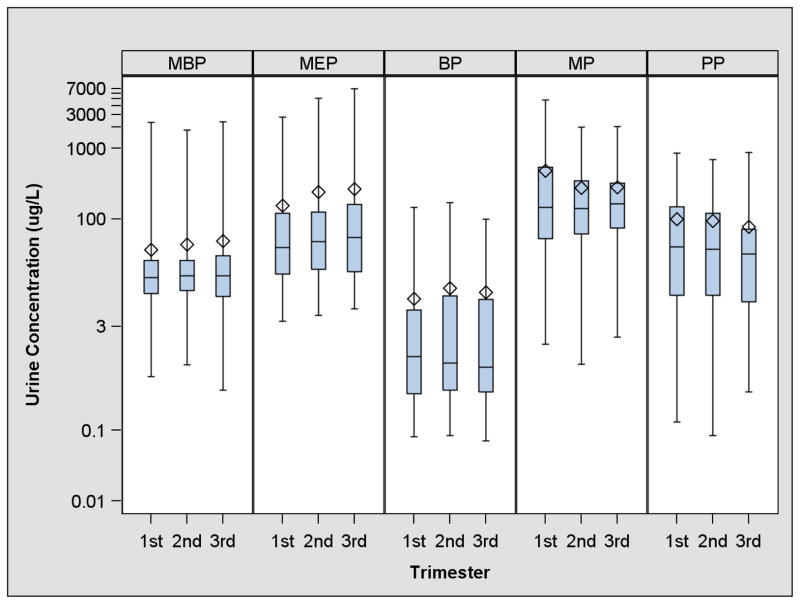
The range of urinary phthalate metabolite and paraben concentrations spanned approximately 3–4 orders of magnitude with median molar biomarker concentrations decreasing in order of MP>MEP>PP>MBP>BP (Figure 1, Supplemental Table 1, and Supplemental Figure 1). Detection frequencies ranged from 72% for BP to 100% for MEP. Urinary MP and PP concentrations were strongly correlated (Pearson R=0.87), while BP was weakly correlated with MP and PP (Pearson R=0.29–0.31). MEP and MBP concentrations were also weakly correlated (Pearson R=0.26). The other correlations between urinary phthalate metabolite and paraben concentrations were generally weak (Pearson R=0.05–0.38).
Figure 1.
Box and whisker plots of specific gravity adjusted urinary phthalate metabolite and paraben concentrations (μg/L) during pregnancy among women with a live birth from the EARTH Study1
1-MBP: monobutyl phthalate, MEP: monoethyl phthalate, BP: butyl paraben MP: methyl paraben, PP: propyl paraben.
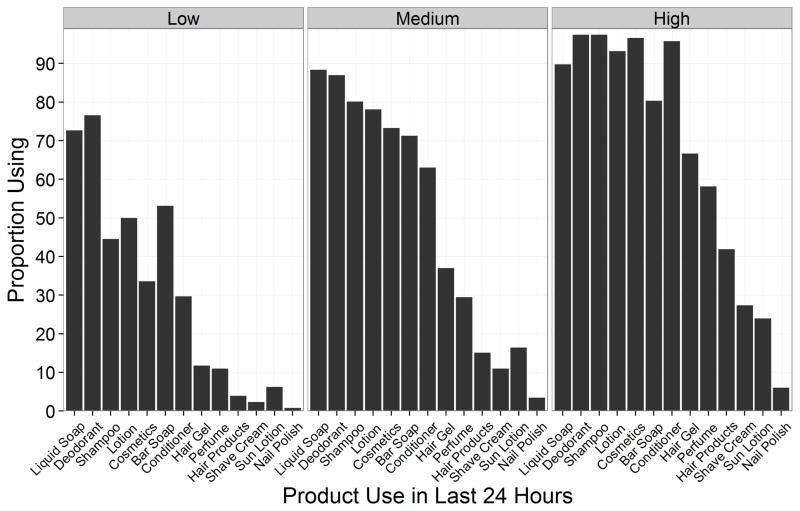
Among the 13 personal care products queried for in our survey, women reported using liquid soap (85%) and deodorant (84%) most frequently in the last 24 hours (Table 1 and Figure 2). Sun lotion (14%) and nail polish (4%) were the least frequently used personal care products. The proportion of women using specific products changed across product use categories (0–5, 6–7, and 8–11 products) (Figure 2). The majority of women used deodorant, bar soap, liquid soap, or lotion in all three categories, but the proportion of women using these products increased across the three categories of use. The use of products like shampoo, conditioner, hair gel, other hair products, cologne/perfume, cosmetics, or sun lotion increased considerably from the lowest to highest categories.
Table 1.
Adjusted change in specific gravity standardized urinary phthalate metabolite and paraben concentrations according to personal care product use in the previous 24 hours among 177 pregnant women with a live birth from the EARTH Study 1,2
| Phthalate metabolites | Parabens | Molar Sum2 | ||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| N using product3 (%) | % Change MBP (95% CI) | % Change MEP (95% CI) | N using product3 (%) | % Change BP (95% CI) | % Change MP (95% CI) | % Change PP (95% CI) | % Change (95% CI) | |
| Liquid Soap | 150 (85) | 20 (−8, 57) | −9 (−36, 30) | 144 (85) | −5 (−41, 53) | −19 (−45, 20) | −33 (−59, 9) | −13 (−37, 21) |
| Deodorant | 149 (84) | 12 (−20, 58) | 14 (−28, 79) | 144 (85) | 26 (−32, 134) | 13 (−30, 84) | 40 (−24, 159) | 29 (−14, 95) |
| Shampoo | 134 (76) | 2 (−20, 29) | −29 (−48, −3) | 129 (76) | −21 (−48, 21) | −16 (−40, 19) | −3 (−37, 51) | −11 (−34, 18) |
| Lotion | 127 (72) | 28 (2, 62) | 50 (11, 103) | 122 (72) | 111 (41, 216) | 144 (76, 238) | 221 (113, 382) | 80 (37, 138) |
| Cosmetics | 120 (68) | 16 (−10, 49) | 53 (9, 113) | 115 (68) | 89 (21, 198) | 66 (17, 137) | 105 (31, 220) | 66 (23, 124) |
| Bar Soap | 118 (67) | 3 (−18, 29) | 10 (−19, 49) | 112 (66) | −2 (−35, 48) | 14 (−17, 58) | 41 (−6, 112) | 17 (−11, 54) |
| Conditioner | 111 (63) | 12 (−10, 39) | −27 (−45, −3) | 107 (63) | −17 (−44, 23) | −15 (−38, 17) | −21 (−47, 18) | −16 (−35, 10) |
| Hair Gel | 74 (42) | 9 (−14, 38) | 30 (−5, 79) | 73 (43) | 112 (38, 225) | 41 (1, 97) | 28 (−16, 96) | 28 (−4, 71) |
| Cologne/perfume | 55 (31) | 18 (−7, 49) | 167 (98, 261) | 54 (32) | −10 (−41, 37) | 45 (4, 102) | 48 (−2, 125) | 54 (16, 104) |
| Hair Products | 35 (20) | −13 (−33, 14) | 2 (−29, 47) | 33 (19) | 33 (−18, 118) | −4 (−35, 42) | −7 (−43, 52) | −7 (−33, 29) |
| Shave Cream | 26 (15) | 7 (−22, 47) | 11 (−27, 69) | 26 (15) | 17 (−33, 105) | −19 (−48, 26) | 12 (−37, 96) | −5 (−35, 39) |
| Sun Lotion | 24 (14) | −10 (−32, 20) | 13 (−23, 66) | 24 (14) | −14 (−48, 43) | 49 (−1, 123) | 43 (−14, 139) | 38 (−2, 95) |
| Nail Polish | 7 (4) | 88 (14, 211) | 152 (30, 388) | 7 (4) | −11 (−63, 114) | 5 (−50, 121) | 42 (−44, 258) | 51 (−19, 180) |
Separate models for each predictor and outcome. Models adjusted for maternal race (white vs. non-white), education (graduate school vs. no graduate school), age (years), body mass index (continuous, time-varying), weeks gestation (time varying), and number of other personal care products used (continuous, time-varying).
Log10-transformed specific gravity adjusted monobutyl phthalate (MBP), monoethyl phthalate (MEP), butyl paraben (BP), methyl paraben (MP), and propyl paraben (PP) concentrations were summed.
Number and percentage of women using the product when they provided their first pregnancy urine sample. Sample size for the total molar sum models is the same as the phthalate metabolites.
Statistically significant (p<0.05) findings are bolded.
Figure 2.
Proportion of visits women reported using specific personal products in the last 24-hours according to categorical personal care product use among pregnant women with a live birth from the EARTH study1
1-Categories are defined as 0–5 (low), 6–7 (medium), and 8–11 (high) products used in the last 24 hours. Numbers are based on the 391 visits completed by 177 women (n=128, 146, and 117 for low, medium, and high use, respectively).
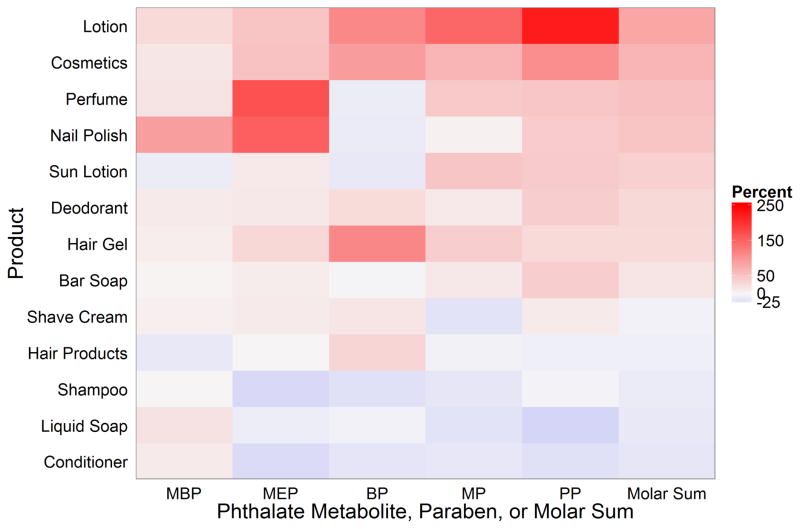
On average, women who used lotion, cosmetics, cologne/perfume, nail polish, sun lotion, or hair gel had total molar urinary phthalate metabolite and paraben concentrations 28 to 80% higher than non-users of these products (Table 1). The pattern of metabolite increases varied according to the product used. Lotion users had urinary BP and PP concentrations 2 to 3 times higher compared to non-users, but their urinary MBP concentrations were only 28% higher (95% Confidence Interval [CI]: 2, 62) (Figure 3 and Table 1). Cologne users had MEP and MP concentrations 167% (CI: 98, 261%) and 45% (CI: 4, 102%) higher than non-users. The small number (n=7) of women reporting nail polish use in the last 24 hours had higher urinary concentrations of MBP (88; CI: 14, 211) and MEP (152; CI: 30, 388), but not of parabens, compared to non-users.
Figure 3.
Adjusted percent change in specific gravity standardized urinary phthalate metabolite and paraben concentrations with personal care product use in the last 24 hours among pregnant women with a live birth from the EARTH study1,2,3
1-Separate models for each predictor and outcome. Models adjusted for maternal race (white vs. non-white), education (graduate school vs. no graduate school), age (years), body mass index (continuous, time-varying), weeks gestation (time varying), and number of other personal care products used (continuous, time-varying).
2-MBP: monobutyl phthalate, MEP: monoethyl phthalate, BP: butyl paraben, MP: methyl paraben, PP: propyl paraben.
3-Products are sorted in order of the largest (top) to smallest (bottom) change in the phthalate and paraben molar sum concentrations.
Women who reported using shampoo, conditioner, other hair products, and liquid soap had slightly lower total molar phthalate metabolite and paraben concentrations compared to non-users, although the CI of these estimates included the null value. Adjusted GM urinary phthalate metabolite and paraben concentrations according to product use are shown in Supplemental Table 3.
After weighting the parabens and phthalate metabolites concentrations by their relative estrogenicity, the rank order of the paraben and phthalate metabolites changed (Supplemental Figures 1 and 2). Urinary PP concentrations were now the highest, followed by BP, MBP, MEP, and MBP concentrations (Supplemental Figure 2). Log10-transformed EEF and total molar sum concentrations were highly correlated (Pearson R=0.90). When we examined the relationship between personal care product use and the EEF, the results were relatively similar to those we observed when we used molar sum of phthalate metabolites and parabens (Table 2). However, deodorant and sun lotion use were now associated with the 3rd and 4th largest change in the EEF, respectively, although the CI of the sun lotion estimate included the null value.
Table 2.
Adjusted change in specific gravity standardized Estrogenicity Equivalence Factor (EEF) according to personal care product use in the last 24 hours among pregnant women with a live birth from the EARTH study1,2
| Product | N using product (%)3 | % Change in Estrogenicity Equivalence Factor2 |
|---|---|---|
| Liquid Soap | 150 (85) | −21 (−49, 23) |
| Deodorant | 149 (84) | 75 (0, 206) |
| Shampoo | 134 (76) | −6 (−37, 40) |
| Lotion | 127 (72) | 145 (68, 256) |
| Cosmetics | 120 (68) | 91 (27, 188) |
| Bar Soap | 118 (67) | 22 (−16, 77) |
| Conditioner | 111 (63) | −18 (−43, 17) |
| Hair Gel | 74 (42) | 39 (−6, 105) |
| Cologne/perfume | 55 (31) | 40 (−5, 106) |
| Hair Products | 35 (20) | −6 (−40, 47) |
| Shave Cream | 26 (15) | 15 (−32, 95) |
| Sun Lotion | 24 (14) | 56 (−3, 149) |
| Nail Polish | 7 (4) | 31 (−44, 205) |
Separate models for each predictor. Models adjusted for maternal race (white vs. non-white), education (graduate school vs. no graduate school), age (years), body mass index (continuous, time-varying), weeks gestation (time varying), and number of other personal care products used (continuous, time-varying).
The estrogenicity equivalence factor (EEF) (μmol/L) is computed by weighting monobutyl phthalate (MBP), monoethyl phthalate (MEP), butyl paraben (BP), methyl paraben (MP), and propyl paraben (PP) according to their potency relative to 17β-estradiol. MBP, MEP, BP, MP, and PP were assumed to be 10,000,000; 2,000,000; 10,000; 2,500,000; and 30,000 times, respectively, less potent than17β-estradiol. The weighted sum was computed by converting μg/L concentrations to μmol/L, weighting, and then summing.
Number and percentage of women using the product when they provided their first pregnancy urine sample.
Statistically significant (p<0.05) findings are bolded.
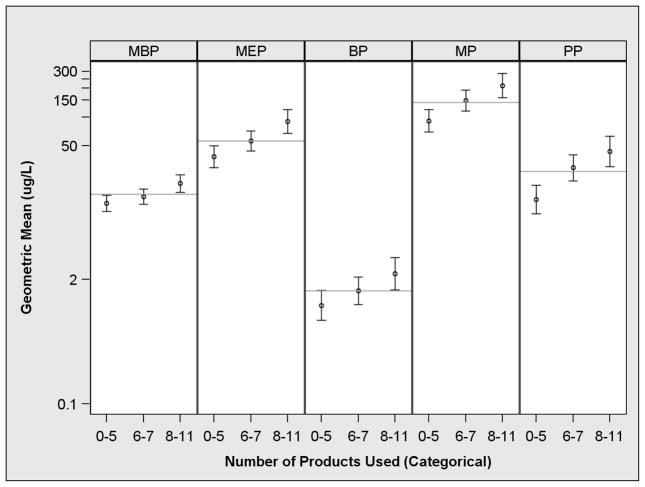
Women who reported using more personal care products had higher urinary concentrations of all the phthalate metabolites and parabens (Figure 4 and Supplemental Table 4). The largest relative increases were observed for PP followed by MP>MEP>BP>MBP. Compared to low product users, PP concentrations were 219% higher (CI: 94, 423) in high users, whereas MBP concentrations were 61% (CI: 22, 111) higher. Urinary MBP concentrations among low and medium product users were relatively similar.
Figure 4.
Adjusted geometric mean specific gravity standardized urinary phthalate metabolite and paraben concentrations according to the number of personal care product use in the last 24 hours among pregnant women with a live birth from the EARTH study1,2,3
1-Separate models for each predictor and outcome. Models adjusted for maternal race (white vs. non-white), education (graduate school vs. no graduate school), age (years), body mass index (continuous, time-varying), and weeks gestation (time varying).
2-MBP: monobutyl phthalate, MEP: monoethyl phthalate, BP: butyl paraben, MP: methyl paraben, and PP: propyl paraben.
3-Grey line represents the overall geometric mean. Error bars are the 95% CI of the geometric mean.
The time between product use and urine sample collection influenced urinary phthalate metabolite and paraben concentrations (Supplemental Figure 3). For almost all of the products, biomarker concentrations were highest among women who used the product in the last 6 hours, lower for those using in the last 6–24 hours, and lowest among non-users. Of note were the higher urinary MEP (GM: 183 μg/L; CI: 96, 348) and BP (GM: 4.2 μg/L; CI: 1.8, 9.7) concentrations among women who used shave cream in the last 6 hours compared to women who did not use shave cream (MEP GM: 55 μg/L; CI: 46, 66 and BP GM: 1.5μg/L; CI: 1.1, 1.9) or used it >6 hours before the urine sample collection (MEP GM: 48 μg/L; CI: 30, 76 and BP GM: 1.7 μg/L; CI: 0.9, 3.1).
Adjusting for the time of day the product was used or the season of the year the sample was collected did not substantively change most of our results (results not shown). Adjusting for time of day attenuated the relationship between sun lotion use and urinary MP concentrations, while strengthening the relationship with urinary PP concentrations.
Discussion
There is a growing interest in determining the health effects associated with the wide range of environmental exposures experienced across the life course that make up the ‘exposome’ and we attempted to characterize a component of exposures related to personal care product use (34). In this cohort, individual and summary measures of urinary phthalate metabolite and paraben concentrations were higher in pregnant women who reported using lotion, cosmetics, cologne/perfume, hair gel, and nail polish in the last 24 hours compared to women not using these products. Among lotion users these increases were due to higher concentrations of multiple chemicals (i.e., all three parabens). In contrast, the increases among cosmetic users were due to higher concentrations of both phthalate metabolites and parabens.
The concentrations of and correlations between urinary phthalate metabolites and parabens observed in this study were similar to other studies conducted in Japan, the Netherlands, France, Spain, and the United States (4, 5, 10, 21, 35–39). The high correlation between MP and PP is likely due to their joint use as antimicrobials in foods and personal care products.
Parabens are used in thousands of personal care products, typically at levels less than 1% w/w (7). Despite their relatively low concentrations and the wide degree of variability in paraben levels by brand and product formulation, we observed higher paraben and phthalate metabolite concentrations in the urine of users of lotion, cosmetics, and hair gel, compared to non-users. Some phthalates are used at relatively high concentrations in some products like cologne/perfume (1, 13, 38, 40). Consistent with our findings, two surveys have detected DEP and parabens in some of the same products (1, 38). Despite previous studies detecting at least one paraben and phthalate in all 13 products examined in this study (1), we observed null or modestly negative associations between some products and urinary paraben or phthalate concentrations. We speculate that this may be due to the relatively short dermal contact time of certain products (e.g., liquid soap, shampoo, and conditioner), reduced statistical precision due to few individuals with or without product use (e.g., liquid soap and sun lotion), or low paraben/phthalate concentrations in the specific products used by our participants.
Several epidemiological studies have examined the relationships between self-reported product use and urinary paraben and/or phthalate metabolite concentrations. Three epidemiological studies of men and women have reported higher urinary MEP concentrations among cologne/cologne/perfume users compared to non-users (20, 21, 41). The 167% increase observed in this study is similar to the results of Parlett et al. and Just et al. reporting MEP concentrations 129 and 192% higher among cologne/perfume users compared to non-users, respectively. Consistent with our findings related to lotion, two studies of non-pregnant Mexican women and pregnant Puerto Ricans observed increased urinary MEP and paraben concentrations among lotion users, respectively (42, 43). The study of Puerto Rican women also reported higher urinary paraben concentration among women using cosmetics. A prior study of pregnant women in New York City did not report higher MBP concentrations among women who used nail polish or polish remover; however, they asked about use in the last 48 hours, whereas we asked about use in the last 24 hours (21). Another study of pregnant women observed higher urinary MBP concentrations among lotion and nail polish users compared to non-users (24). However, they did not observe higher MEP concentrations among cologne/perfume users. Janjua and colleagues in Denmark applied body lotion containing DBP (2%), DEP (2%), and BP (2%) every day to 26 male volunteers for a week (22). Metabolites of all three chemicals rose dramatically in 24-hour urine samples collected after lotion application. Discrepancies in the findings across these studies could be due to differences in questionnaire design, timing of urine sample collection in relation to product use, product reformulation over time, and the types or brands of products used by the study participants in their respective source populations.
The higher concentrations of urinary phthalate metabolites and parabens among women using more personal care products suggests that the number of personal care products used may be a sensitive, but non-specific indicator of exposure to these phthalate diesters and parabens. While the use of questionnaire data is appealing for epidemiological studies, many personal care products contain other chemicals that may have endocrine disrupting properties, thus, reducing the specificity of a questionnaire (1). However, questionnaires are simple to administer and inexpensive compared to biological monitoring. Therefore, in studies with very large sample sizes (e.g., n>1,000) or limited budgets, questionnaires may be useful surrogates for ranking exposure to multiple EDCs found in personal care products. Future studies using questionnaires could reduce exposure misclassification by obtaining information about the time since a product was last used since we observed higher urinary biomarker concentrations among women who used certain personal care products in the last 6 hours compared to women who used them >6 hours ago. Questionnaires should be designed to take into account the biological half-life of the compounds of interest, as well as the variability in exposure patterns.
Our results were similar when we used either the total molar sum or the biologically-weighted EEF. Compared to the total molar sum, which assumes that different chemicals have equivalent toxicity, the EEF for these five chemicals is a biologically based estimate of the relative total estrogenic potential and may provide a more relevant estimate of exposure than a molar sum. However, the absolute EEF among lotion users was still orders of magnitude below that of estradiol. While the anti-androgenic activity of DBP has been described in relation to other phthalates, animal data do not suggest that DEP is anti-androgenic and there are not comparable data for parabens, thus limiting our ability to create a biologically weighted anti-androgenic sum for these phthalates and parabens (44).
The toxic equivalency approach we employed summarizing for parabens and phthalates has been used for dioxin-like compounds, where toxicity data from animal studies has been applied to human studies (33, 45). One limitation of this approach in epidemiological studies is the assumption that all the chemicals within a class act via a single mechanism that is equivalent in both humans and the experimental system used to estimate relative toxicity. Chemicals can have multiple mechanisms of toxicity and the assumption of a single mechanism is questionable when different chemical classes are being examined, especially when extrapolating from in vitro to in vivo models. For the present study, it may not be appropriate to sum urinary phthalate metabolite and paraben concentrations if they act through multiple or different biological pathways. Statistically, these summary measures may not provide additional information beyond a simple sum if the individual chemicals are highly correlated, the relative potencies of the chemicals are similar, or one chemical’s concentration is orders of magnitude higher than the others. Additional multidisciplinary research is needed to determine if and how different chemical exposures can be summarized to better characterize the potential human health hazard related to mixtures.
Our study is limited by sources of variability in the measurement of personal care product use and urinary biomarker concentrations. We did not collect information about the amount or brand of products used. Prior studies have reported relatively high variability in the amount of personal care product applied by individuals and this may be one factor that contributes to the within-person variability of urinary biomarker concentrations (26, 27, 46, 47). Differences in product formulations might result in even higher or lower exposures for users of specific brands (7). These products may also contain other endocrine disrupting chemicals like triclosan or benzophenone (1). Future studies could use new strategies to estimate the chemical content from product labels and combine this with the amount and frequency of product use (48).
Our previous work in this cohort did not suggest that pregnancy-induced changes in metabolism or excretion were responsible for systematic changes in these paraben or phthalate metabolite concentrations before or during pregnancy, (49, 50). However, urinary phthalate metabolite and paraben concentrations have moderate within-person variability during pregnancy due in part to the short biological half-life of these compounds (<24 hours) (26, 27). This may necessitate multiple spot urine samples to classify gestational exposure. Indeed, a strength of the current study was the repeated urine measurements, allowing us to incorporate this variability into our estimates.
Conclusions
Pregnant women in this cohort who reported using lotion, cosmetics, hair gel, and cologne/perfume had higher concentrations of a mixture of phthalate metabolites and parabens in their urine compared to non-users of these products. These findings are consistent with prior studies documenting the presence of phthalates and parabens in personal care products and epidemiological studies observing higher biomarkers of exposure among users of personal care products. Self-reported product use questionnaires may be a sensitive, but non-specific technique to quantify exposure to a mixture of endocrine disrupting compounds in epidemiological studies. Future studies should continue to explore human exposure to chemical mixtures and the relevance of these mixtures to human health.
Supplementary Material
Acknowledgments
Funding: This work was funded by NIEHS grants T32 ES007069, R01 ES009718, P30 ES000002, and R00 ES020346.
We acknowledge the technical assistance of M. Silva, E. Samandar, J. Preau, X. Ye, X. Zhou, R. Hennings, and J. Tao [Centers for Disease Control and Prevention (CDC)] in measuring the urinary concentrations of phthalate metabolites and parabens.
Footnotes
Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the CDC
Conflict of Interest: The authors declare no conflicts of interest.
References
- 1.Dodson RE, Nishioka M, Standley LJ, Perovich LJ, Brody JG, Rudel RA. Endocrine disruptors and asthma-associated chemicals in consumer products. Environmental health perspectives. 2012;120(7):935–43. doi: 10.1289/ehp.1104052. Epub 2012/03/09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shen X, Wu S, Yan C. Impacts of low-level lead exposure on development of children: recent studies in China. Clin Chim Acta. 2001;313(1–2):217–20. doi: 10.1016/s0009-8981(01)00675-1. [DOI] [PubMed] [Google Scholar]
- 3.Calafat AM, Wong LY, Ye X, Reidy JA, Needham LL. Concentrations of the sunscreen agent benzophenone-3 in residents of the United States: National Health and Nutrition Examination Survey 2003–2004. Environmental health perspectives. 2008;116(7):893–7. doi: 10.1289/ehp.11269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Calafat AM, Ye X, Wong LY, Bishop AM, Needham LL. Urinary concentrations of four parabens in the U.S. population: NHANES 2005–2006. Environmental health perspectives. 2010;118(5):679–85. doi: 10.1289/ehp.0901560. Epub 2010/01/09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Silva MJ, Barr DB, Reidy JA, Malek NA, Hodge CC, Caudill SP, et al. Urinary levels of seven phthalate metabolites in the U.S. population from the National Health and Nutrition Examination Survey (NHANES) 1999–2000. Environmental health perspectives. 2004;112(3):331–8. doi: 10.1289/ehp.6723. Epub 2004/03/05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rice D, Barone S., Jr Critical periods of vulnerability for the developing nervous system: evidence from humans and animal models. Environmental health perspectives. 2000;108 (Suppl 3):511–33. doi: 10.1289/ehp.00108s3511. Epub 2000/06/15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Andersen A. Final Amended Report on the Safety Assessment of Methylparaben, Ethylparaben, Propylparaben, Isopropylparaben, Butylparaben, Isobutylparaben, and Benzylparaben as used in Cosmetic Products. International Journal of Toxicology. 2008;27(Suppl 4):1–82. doi: 10.1080/10915810802548359. [DOI] [PubMed] [Google Scholar]
- 8.Routledge EJ, Parker J, Odum J, Ashby J, Sumpter JP. Some alkyl hydroxy benzoate preservatives (parabens) are estrogenic. Toxicology and applied pharmacology. 1998;153(1):12–9. doi: 10.1006/taap.1998.8544. Epub 1999/01/06. [DOI] [PubMed] [Google Scholar]
- 9.Boberg J, Taxvig C, Christiansen S, Hass U. Possible endocrine disrupting effects of parabens and their metabolites. Reproductive toxicology (Elmsford, NY. 2010;30(2):301–12. doi: 10.1016/j.reprotox.2010.03.011. [DOI] [PubMed] [Google Scholar]
- 10.Shirai S, Suzuki Y, Yoshinaga J, Shiraishi H, Mizumoto Y. Urinary Excretion of Parabens in Pregnant Japanese Women. Reproductive toxicology (Elmsford, NY) 2012 doi: 10.1016/j.reprotox.2012.07.004. Epub 2012/07/14. [DOI] [PubMed] [Google Scholar]
- 11.Oishi S. Effects of propyl paraben on the male reproductive system. Food Chem Toxicol. 2002;40(12):1807–13. doi: 10.1016/s0278-6915(02)00204-1. Epub 2002/11/07. [DOI] [PubMed] [Google Scholar]
- 12.Oishi S. Effects of butyl paraben on the male reproductive system in mice. Arch Toxicol. 2002;76(7):423–9. doi: 10.1007/s00204-002-0360-8. Epub 2002/07/12. [DOI] [PubMed] [Google Scholar]
- 13.Koo HJ, Lee BM. Estimated exposure to phthalates in cosmetics and risk assessment. Journal of toxicology and environmental health. 2004;67(23–24):1901–14. doi: 10.1080/15287390490513300. [DOI] [PubMed] [Google Scholar]
- 14.Howdeshell KL, Wilson VS, Furr J, Lambright CR, Rider CV, Blystone CR, et al. A mixture of five phthalate esters inhibits fetal testicular testosterone production in the sprague-dawley rat in a cumulative, dose-additive manner. Toxicol Sci. 2008;105(1):153–65. doi: 10.1093/toxsci/kfn077. Epub 2008/04/16. [DOI] [PubMed] [Google Scholar]
- 15.Harris CA, Henttu P, Parker MG, Sumpter JP. The estrogenic activity of phthalate esters in vitro. Environmental health perspectives. 1997;105(8):802–11. doi: 10.1289/ehp.97105802. Epub 1997/08/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miodovnik A, Engel SM, Zhu C, Ye X, Soorya LV, Silva MJ, et al. Endocrine disruptors and childhood social impairment. Neurotoxicology. 2011;32(2):261–7. doi: 10.1016/j.neuro.2010.12.009. Epub 2010/12/25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Whyatt RM, Liu X, Rauh VA, Calafat AM, Just AC, Hoepner L, et al. Maternal prenatal urinary phthalate metabolite concentrations and child mental, psychomotor, and behavioral development at 3 years of age. Environmental health perspectives. 2012;120(2):290–5. doi: 10.1289/ehp.1103705. Epub 2011/09/07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Swan SH, Main KM, Liu F, Stewart SL, Kruse RL, Calafat AM, et al. Decrease in anogenital distance among male infants with prenatal phthalate exposure. Environmental health perspectives. 2005;113(8):1056–61. doi: 10.1289/ehp.8100. Epub 2005/08/05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Engel SM, Miodovnik A, Canfield RL, Zhu C, Silva MJ, Calafat AM, et al. Prenatal phthalate exposure is associated with childhood behavior and executive functioning. Environmental health perspectives. 2010;118(4):565–71. doi: 10.1289/ehp.0901470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Duty SM, Ackerman RM, Calafat AM, Hauser R. Personal care product use predicts urinary concentrations of some phthalate monoesters. Environmental health perspectives. 2005;113(11):1530–5. doi: 10.1289/ehp.8083. Epub 2005/11/03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Just AC, Adibi JJ, Rundle AG, Calafat AM, Camann DE, Hauser R, et al. Urinary and air phthalate concentrations and self-reported use of personal care products among minority pregnant women in New York city. Journal of exposure science & environmental epidemiology. 2010;20(7):625–33. doi: 10.1038/jes.2010.13. Epub 2010/04/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Janjua NR, Frederiksen H, Skakkebaek NE, Wulf HC, Andersson AM. Urinary excretion of phthalates and paraben after repeated whole-body topical application in humans. International journal of andrology. 2008;31(2):118–30. doi: 10.1111/j.1365-2605.2007.00841.x. [DOI] [PubMed] [Google Scholar]
- 23.Romero-Franco M, Hernandez-Ramirez RU, Calafat AM, Cebrian ME, Needham LL, Teitelbaum S, et al. Personal care product use and urinary levels of phthalate metabolites in Mexican women. Environ Int. 2011 doi: 10.1016/j.envint.2011.02.014. Epub 2011/03/25. [DOI] [PubMed] [Google Scholar]
- 24.Buckley JP, Palmieri RT, Matuszewski JM, Herring AH, Baird DD, Hartmann KE, et al. Consumer product exposures associated with urinary phthalate levels in pregnant women. Journal of exposure science & environmental epidemiology. 2012 doi: 10.1038/jes.2012.33. Epub 2012/07/05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Silva E, Rajapakse N, Kortenkamp A. Something from “nothing”--eight weak estrogenic chemicals combined at concentrations below NOECs produce significant mixture effects. Environmental science & technology. 2002;36(8):1751–6. doi: 10.1021/es0101227. Epub 2002/05/08. [DOI] [PubMed] [Google Scholar]
- 26.Braun JM, Smith KW, Williams PL, Calafat AM, Berry K, Ehrlich S, et al. Variability of Urinary Phthalate Metabolite and Bisphenol A Concentrations before and during Pregnancy. Environmental health perspectives. 2012 doi: 10.1289/ehp.1104139. Epub 2012/01/21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smith KW, Braun JM, Williams PL, Ehrlich S, Correia KF, Calafat AM, et al. Predictors and Variability of Urinary Paraben Concentrations in Men and Women, Including before and during Pregnancy. Environmental health perspectives. 2012 doi: 10.1289/ehp.1104614. Epub 2012/06/23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Silva MJ, Samandar E, Preau JL, Jr, Reidy JA, Needham LL, Calafat AM. Quantification of 22 phthalate metabolites in human urine. Journal of chromatography. 2007;860(1):106–12. doi: 10.1016/j.jchromb.2007.10.023. [DOI] [PubMed] [Google Scholar]
- 29.Ye X, Bishop AM, Needham LL, Calafat AM. Automated on-line column-switching HPLC-MS/MS method with peak focusing for measuring parabens, triclosan, and other environmental phenols in human milk. Anal Chim Acta. 2008;622(1–2):150–6. doi: 10.1016/j.aca.2008.05.068. Epub 2008/07/08. [DOI] [PubMed] [Google Scholar]
- 30.Ye X, Bishop AM, Reidy JA, Needham LL, Calafat AM. Parabens as urinary biomarkers of exposure in humans. Environmental health perspectives. 2006;114(12):1843–6. doi: 10.1289/ehp.9413. Epub 2006/12/23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hornung RW, Reed LD. Estimation of Average Concentration in the Presence of Nondetectable Values. Applied Occupational and Environmental Hygiene. 1990;5(1):46–51. [Google Scholar]
- 32.CDC CfDCaP. NHANES-Whats New. 2012 [cited 2012 January 2012]; Available from: http://www.cdc.gov/nchs/nhanes/new_nhanes.htm#Jan12.
- 33.Safe SH. Hazard and risk assessment of chemical mixtures using the toxic equivalency factor approach. Environmental health perspectives. 1998;106 (Suppl 4):1051–8. doi: 10.1289/ehp.98106s41051. Epub 1998/08/14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wild CP. Complementing the genome with an “exposome”: the outstanding challenge of environmental exposure measurement in molecular epidemiology. Cancer Epidemiol Biomarkers Prev. 2005;14(8):1847–50. doi: 10.1158/1055-9965.EPI-05-0456. Epub 2005/08/17. [DOI] [PubMed] [Google Scholar]
- 35.Adibi JJ, Whyatt RM, Williams PL, Calafat AM, Camann D, Herrick R, et al. Characterization of phthalate exposure among pregnant women assessed by repeat air and urine samples. Environmental health perspectives. 2008;116(4):467–73. doi: 10.1289/ehp.10749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Casas L, Fernandez MF, Llop S, Guxens M, Ballester F, Olea N, et al. Urinary concentrations of phthalates and phenols in a population of Spanish pregnant women and children. Environ Int. 2011;37(5):858–66. doi: 10.1016/j.envint.2011.02.012. Epub 2011/03/29. [DOI] [PubMed] [Google Scholar]
- 37.Ye X, Pierik FH, Hauser R, Duty S, Angerer J, Park MM, et al. Urinary metabolite concentrations of organophosphorous pesticides, bisphenol A, and phthalates among pregnant women in Rotterdam, the Netherlands: the Generation R study. Environmental research. 2008;108(2):260–7. doi: 10.1016/j.envres.2008.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shen HY, Jiang HL, Mao HL, Pan G, Zhou L, Cao YF. Simultaneous determination of seven phthalates and four parabens in cosmetic products using HPLC-DAD and GC-MS methods. Journal of separation science. 2007;30(1):48–54. doi: 10.1002/jssc.200600215. Epub 2007/02/23. [DOI] [PubMed] [Google Scholar]
- 39.Philippat C, Mortamais M, Chevrier C, Petit C, Calafat AM, Ye X, et al. Exposure to Phthalates and Phenols during Pregnancy and Offspring Size at Birth. Environmental health perspectives. 2011 doi: 10.1289/ehp.1103634. Epub 2011/09/09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hubinger JC. A survey of phthalate esters in consumer cosmetic products. Journal of cosmetic science. 2010;61(6):457–65. Epub 2011/01/19. [PubMed] [Google Scholar]
- 41.Parlett LE, Calafat AM, Swan SH. Women’s exposure to phthalates in relation to use of personal care products. Journal of exposure science & environmental epidemiology. 2012 doi: 10.1038/jes.2012.105. Epub 2012/11/22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Meeker JD, Cantonwine D, Rivera-Gonzalez LO, Ferguson KK, Mukherjee B, Calafat AM, et al. Distribution, variability and predictors of urinary concentrations of phenols and parabens among pregnant women in Puerto Rico. Environmental science & technology. 2013 doi: 10.1021/es400510g. Epub 2013/03/09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Romero-Franco M, Hernandez-Ramirez RU, Calafat AM, Cebrian ME, Needham LL, Teitelbaum S, et al. Personal care product use and urinary levels of phthalate metabolites in Mexican women. Environ Int. 2011;37(5):867–71. doi: 10.1016/j.envint.2011.02.014. Epub 2011/03/25. [DOI] [PubMed] [Google Scholar]
- 44.Howdeshell KL, Furr J, Lambright CR, Wilson VS, Ryan BC, Gray LE., Jr Gestational and lactational exposure to ethinyl estradiol, but not bisphenol A, decreases androgen-dependent reproductive organ weights and epididymal sperm abundance in the male long evans hooded rat. Toxicol Sci. 2008;102(2):371–82. doi: 10.1093/toxsci/kfm306. Epub 2007/12/22. [DOI] [PubMed] [Google Scholar]
- 45.Sagiv SK, Thurston SW, Bellinger DC, Tolbert PE, Altshul LM, Korrick SA. Prenatal organochlorine exposure and behaviors associated with attention deficit hyperactivity disorder in school-aged children. American journal of epidemiology. 2010;171(5):593–601. doi: 10.1093/aje/kwp427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Loretz L, Api AM, Barraj L, Burdick J, Davis de A, Dressler W, et al. Exposure data for personal care products: hairspray, spray perfume, liquid foundation, shampoo, body wash, and solid antiperspirant. Food Chem Toxicol. 2006;44(12):2008–18. doi: 10.1016/j.fct.2006.06.029. Epub 2006/08/22. [DOI] [PubMed] [Google Scholar]
- 47.Loretz LJ, Api AM, Barraj LM, Burdick J, Dressler WE, Gettings SD, et al. Exposure data for cosmetic products: lipstick, body lotion, and face cream. Food Chem Toxicol. 2005;43(2):279–91. doi: 10.1016/j.fct.2004.09.016. Epub 2004/12/29. [DOI] [PubMed] [Google Scholar]
- 48.Bennett DH, Wu XM, Teague CH, Lee K, Cassady DL, Ritz B, et al. Passive sampling methods to determine household and personal care product use. Journal of exposure science & environmental epidemiology. 2012;22(2):148–60. doi: 10.1038/jes.2011.40. Epub 2011/12/23. [DOI] [PubMed] [Google Scholar]
- 49.Braun JM, Smith KW, Williams PL, Calafat AM, Berry K, Ehrlich S, et al. Variability of urinary phthalate metabolite and bisphenol A concentrations before and during pregnancy. Environmental health perspectives. 2012;120(5):739–45. doi: 10.1289/ehp.1104139. Epub 2012/01/21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Smith KW, Braun JM, Williams PL, Ehrlich S, Correia KF, Calafat AM, et al. Predictors and variability of urinary paraben concentrations in men and women, including before and during pregnancy. Environmental health perspectives. 2012;120(11):1538–43. doi: 10.1289/ehp.1104614. Epub 2012/06/23. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.