Abstract
Background
Cardiac troponin T is independently associated with cardiovascular events and mortality in patients with chronic kidney disease (CKD). Serum levels of high sensitivity cardiac troponin T (hs-TnT) reflect subclinical myocardial injury in ambulatory patients. We sought to determine the distribution and predictors of hs-TnT in CKD patients without overt cardiovascular disease (CVD).
Methods
We studied 2464 participants within the multi-ethnic Chronic Renal Insufficiency Cohort (CRIC) who did not have self-reported CVD. We considered renal and non-renal factors as potential determinants of hs-TnT, including demographics, comorbidities, left ventricular (LV) mass, serologic factors, estimated glomerular filtration rate (eGFR) and albumin to creatinine ratio.
Results
Hs-TnT was detectable in 81% of subjects, and the median (IQR) hs-TnT was 9.4 pg/ml (4.3-18.3). Analysis was performed using Tobit regression, adjusting for renal and non-renal factors. After adjustment, lower eGFR was associated with higher expected hs-TnT; participants with eGFR < 30 ml/min/1.73 m2 had 3-fold higher expected hs-TnT compared to subjects with eGFR > 60. Older age, male gender, black race, LV mass, diabetes and higher blood pressure all had strong, independent associations with higher expected hs-TnT.
Conclusions
Knowledge of the determinants of hs-TnT in this cohort may guide further research on the pathology of heart disease in patients with CKD and help to stratify sub-groups of CKD patients at higher cardiovascular risk.
Keywords: Troponin T, Chronic kidney disease, Cardiovascular disease
Background
Studies have shown that lower estimated glomerular filtration (eGFR) and higher albumin to creatine ratio (ACR) are strong, independent risk factors for incident heart failure [1-3]. However, how chronic kidney disease (CKD) leads to heart failure is not fully understood; specifically, we lack information on which biological mediators of CKD initiate myocardial injury and whether certain subgroups of patients with CKD are more susceptible to myocardial injury. High sensitivity cardiac troponin T (hs-TnT) independently predicts cardiovascular mortality in populations with or without cardiac disease [4-6], and predicts cardiovascular events [7,8] and all-cause mortality [9] in patients with CKD and end-stage renal disease [10]. Since the highly sensitive assay detects much lower levels of myocardial injury than prior assays, it may be useful for studying the earliest stages of heart disease in subjects with CKD. Our group has shown that hs-TnT is independently associated with left ventricular hypertrophy (LVH) in participants of the CRIC cohort who do not have self-reported cardiovascular disease (CVD) [11]. Additional culprits in the CKD milieu thought to contribute to the development of CVD include inflammation, anemia, and deranged mineral metabolism. Whether these factors are associated with subclinical myocardial injury (as measured by hs-TnT) in a CKD cohort without overt CVD has not been previously studied.
We sought to examine renal and non-renal predictors of subclinical myocardial injury using hs-TnT in subjects without self-reported CVD in the Chronic Renal Insufficiency Cohort (CRIC). First, we hypothesized that lower estimated glomerular filtration rate (eGFR) and higher urine albumin-creatinine ratio (ACR) would be independently associated with higher hs-TnT concentration. Second, we hypothesized that elevated fibroblast growth factor 23 (FGF-23) concentrations, hyperphosphatemia, and anemia would be associated with higher hs-TnT independently of eGFR.
Methods
The Chronic Renal Insufficiency Cohort (CRIC) Study was designed to investigate risk factors for progression of CKD, cardiovascular disease and overall mortality in persons with CKD. Participants were recruited between June 2003 and March 2007 at seven centers (Ann Arbor, Michigan; Baltimore, Maryland; Chicago, Illinois; Cleveland, Ohio; New Orleans, Louisiana; Philadelphia, Pennsylvania; and Oakland, California). Investigators recruited 3939 racially and ethnically diverse individuals between the ages of 21 to 74 years with eGFR between 20 and 70 ml/min/1.73 m2 by simplified MDRD equation [12]. Exclusion criteria were as follows: polycystic kidney disease, use of immunosuppression within the last 6 months, institutionalization, inability to consent, enrollment in other studies, pregnancy, New York Heart Association class III to IV heart failure, HIV, cirrhosis, myeloma, renal cancer, recent chemotherapy, organ transplant, or dialysis treatment within the last month [13]. The Institutional Review Board at each study site approved the protocol and participants gave written, informed consent. For this analysis, participants with known self-reported cardiovascular disease, peripheral vascular disease, or heart failure were excluded.
Kidney function was measured using cystatin-based eGFR (eGFRcys) and creatinine-based eGFR using the MDRD equation (eGFRcr). Compared to creatinine, cystatin has been shown to be a better marker of kidney function at higher eGFR [14], and cystatin has a stronger association with cardiovascular outcomes than creatinine-based eGFR (eGFRcr) [15,16]. In CRIC, eGFRcys has a wider distribution of values than eGFRcr because enrollment was based upon a fixed range of eGFR [17]. Samples for cystatin were processed using a Siemens BNII nephelometer at the CRIC central laboratory, with a coefficient of variation (CV) of 4.9%. Values were corrected for drift over time by standardization to calibrator lot 51 and reagent lot 40. Cystatin-c based eGFR was calculated by the CKD-EPI equation [18], and eGFR was categorized as < 30, 30–44, 45–59, and ≥ 60 ml/min/1.73 m2. Urine samples were collected for spot albumin-creatinine ratios (ACR), and ACR was categorized as < 30, 30–299, 300–999, and ≥ 1000 mg/g.
Information on demographics and clinical history was obtained by self-report on questionnaires administered at the baseline visit. Diabetes was defined as documented medical history, current or previous use of diabetic medications, or elevated fasting blood glucose. Blood pressure was averaged over three measurements performed in a standardized fashion in a seated position at rest using a calibrated sphygmomanometer. Echocardiograms were performed at one year after enrollment and were read at the core echocardiography laboratory (University of Pennsylvania) using guidelines of the American Society of Echocardiography [19]. Left ventricular mass index was derived by the area-length method, indexed to height2.7[19]. Hs-TnT was measured at Rapid Response Laboratories, University of Maryland Medical Center with the Roche Elecsys immunoassay (Roche Diagnostics, Indianapolis, Indiana), which has an analytical range of 3 to 10,000 ng/L, and a coefficient of variation of 9% at 13.5 ng/L (the 99th percentile in a healthy reference group) [20].
Statistical analysis
Of 2505 subjects without CVD, 2464 had hs-TnT measurements, and of these 474 (19%) were undetectable (< 3 pg/ml). We first categorized hs-TnT as undetectable (< 3 pg/ml), Tertile 1 (3–8.6 pg/ml), Tertile II (8.7-17 pg/ml), and Tertile III (17–739 pg/ml). We compared characteristics of participants in these four categories using ANOVA or Kruskal-Wallis tests for continuous variables and Chi-squared tests for categorical variables.
We next performed multivariable regression analysis in order to identify factors with independent associations with hs-TnT. Because such a large proportion of hs-TnT values were undetectable, we used tobit regression, which is designed to handle left-censored values. Undetectable values were assigned the level 1.5 pg/ml. Because hs-TnT is right-skewed, the outcome was log-transformed for analysis. Coefficients were then exponentiated to yield the estimated multiplicative increase attributable to each factor; e.g., an exponentiated coefficient of 1.3 represents a 30% higher hs-TnT in the exposed compared with the unexposed. Initially, the regression model included all candidate covariates with p-value < 0.05 by univariate analysis. Model 1 was built using backwards stepwise selection until only covariates significant at p < 0.05 remained. Model 2 was built by adding LV mass and ejection fraction to Model 1. All analyses were performed using STATA 11 (StataCorp LP, College Station, TX).
Results
In this subgroup of CRIC study participants without CVD, hs-TnT was detectable in 81% of subjects, and median (IQR) of hs-TnT was 9.4 pg/ml (4.3-18.3) (Figure 1). Increased age, male gender, black race and Hispanic ethnicity were associated with higher TnT, as were diabetes and hypertension. Subjects with higher hs-TnT had lower LDL, lower HDL, higher tryglycerides, lower hemoglobin, higher phosphorus, higher FGF-23, lower eGFRcys and higher ACR (Table 1).
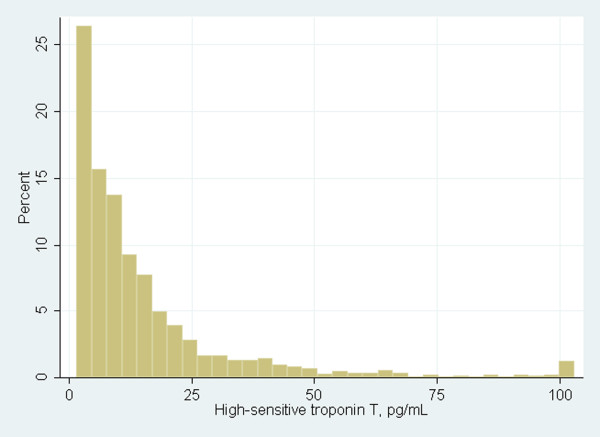
Figure 1.
Distribution of high sensitivity troponin t among chronic renal insufficiency cohort participants without self-reported cardiovascular disease. Legend: Hs-TnT was detectable in 81% of subjects; median (IQR) of hs-TnT was 9.4 pg/ml (4.3-18.3). All hs-TnT values greater than 100 pg/ml were truncated in one bar at 100.
Table 1.
Characteristics of chronic renal insufficiency cohort participants with high sensitivity troponin t measurements
| |
Undetectable |
Tertile I |
Tertile II |
Tertile III |
P |
|---|---|---|---|---|---|
| |
(hs-TnT < 3) |
(3.00-8.63) |
(8.67-17.03) |
(17.05-738.7) |
|
| (n=474) | (n=666) | (n=661) | (n=663) | ||
|
Hs-TnT (pg/ml)* |
1.5 (1.5-1.5) |
5.7 (4.6-7.1) |
11.9 (10.1-14.2) |
30.0 (21.4-45.3) |
< 0.001 |
|
Age |
51.5 (11.8) |
55.7 (11.8) |
60.3 (10.7) |
59.2 (11.1) |
< 0.001 |
|
Female |
75.5 |
53.8 |
42.7 |
29.1 |
< 0.001 |
|
Race |
|
|
|
|
< 0.001 |
|
Non-Hispanic White |
53.2 |
49.9 |
45.1 |
29.1 |
|
|
Non-Hispanic Black |
33.8 |
35.6 |
38.0 |
45.4 |
|
|
Hispanic |
8.4 |
9.0 |
13.9 |
21.4 |
|
|
Other |
4.6 |
5.6 |
3.0 |
4.1 |
|
|
Diabetes |
14.8 |
29.1 |
45.1 |
68.3 |
< 0.001 |
|
Hypertension |
68.6 |
82.1 |
91.4 |
94.7 |
< 0.001 |
|
Current smoking |
13.1 |
10.4 |
11.8 |
11.0 |
0.526 |
|
SBP (mmHg) † |
124.1 (16.9) |
128.3 (17.3) |
132.9 (19.5) |
140.5 (20.9) |
< 0.001 |
|
DBP (mmHg) ‡ |
75.1 (12.1) |
75.5 (11.9) |
74.3 (12.0) |
76.8 (11.8) |
0.010 |
|
BMI (kg/m
2
) § |
30.6 (8.5) |
31.2 (7.9) |
32.3 (8.1) |
32.5 (7.5) |
< 0.001 |
|
HDL (mg/dL) || |
54.1 (17.7) |
50.9 (15.2) |
49.1 (16.2) |
46.4 (15.4) |
< 0.001 |
|
LDL (mg/dL) |
108.9 (30.4) |
107.7 (34.1) |
102.4 (34.7) |
98.7 (36.3) |
< 0.001 |
|
Triglycerides (mg/dL) |
144.2 (114.1) |
155.2 (115.1) |
149.3 (91.1) |
161.3 (108.0) |
< 0.001 |
|
Total cholesterol (mg/dL) |
193.7 (37.6) |
192.3 (41.4) |
184.3 (44.8) |
182.0 (45.6) |
< 0.001 |
|
Serum albumin (g/dL) |
4.2 (0.4) |
4.1 (0.4) |
4.1 (0.4) |
3.9 (0.5) |
< 0.001 |
|
hsCRP (mg/L) # |
4.9 (8.3) |
4.9 (8.8) |
5.4 (8.7) |
5.7 (9.8) |
0.015 |
|
Hemoglobin (g/dL) |
13.2 (1.5) |
13.2 (1.8) |
13.0 (1.8) |
12.2 (1.8) |
< 0.001 |
|
Phosphate (mg/dL) |
3.6 (0.6) |
3.6 (0.5) |
3.6 (0.6) |
3.9 (0.7) |
< 0.001 |
|
FGF-23 (RU/ml)** |
104.7 (73.8-160.2) |
114.6 (81.3-182.5) |
137.4 (93.3-208.8) |
171.7 (113.9-293.0) |
< 0.001 |
|
eGFRcys †† (ml/min/1.73 m
2
) |
66.1 (22.7) |
55.9 (19.7) |
49.5 (16.3) |
40.9 (16.2) |
< 0.001 |
|
ACR (μg/mg) ψ |
11.2 (5.0-86.9) |
21.3 (5.4-201.7) |
30.5 (7.9-279.7) |
289.7 (31.4-1393.2) |
< 0.001 |
|
LV mass (g/m
2.7
) Ω |
43.8 (10.2) |
46.1 (10.4) |
50.3 (12.8) |
55.4 (13.8) |
< 0.001 |
| Ejection fraction (%) | 55.7 (6.3) | 55.9 (6.4) | 55.8 (7.4) | 54.3 (7.3) | < 0.001 |
Mean (SD) or median (IQR) are reported for continuous variables and percentage for categorical variables. P values are obtained using ANOVA or Kruskal-Wallis tests for continuous variables and Chi-squared tests for categorical variables. * High sensitivity troponin T. † Systolic blood pressure. ‡ Diastolic blood pressure. § Body mass index. || High density lipoprotein. Low density lipoprotein. # High sensitivity c-reactive protein. ** Fibroblast growth factor 23. †† Cystatin-based estimated glomerular filtration rate. ψ Albumin to creatinine ratio. Ω Left ventricular.
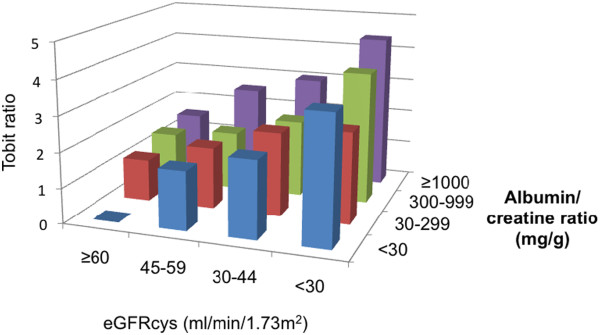
After multivariate adjustment, the strongest renal predictor of hs-TnT was eGFRcys. All eGFRcys categories below the referent group of > 60 ml/min/1.73 m2 were associated with incrementally higher hs-TnT. Subjects with eGFRcys < 30 ml/min/1.73 m2 had almost a 3-fold higher hs-TnT than the referent group with eGFRcys > 60 ml/min/1.73 m2. ACR did not show a linear association with hs-TnT; only those subjects with ACR ≥ 1000 mg/g had higher hs-TnT compared to those with ACR < 30 mg/g. Associations of phosphate and FGF-23 with hs-TnT were significantly attenuated by adjustment, and hemoglobin lost significance with adjustment (compared to referent category of hemoglobin > 14, hemoglobin <=11 had 16% higher hs-TnT, p=0.07) (Table 2). We found an additive effect between eGFRcys and ACR when we further categorized subjects by both of these factors. After multivariate adjustment, those in the lowest eGFR/highest ACR category (eGFRcys< 30 ml/min/1.73 m2 and ACR ≥ 1000) had more than a 4-fold increase in hs-TnT compared to the referent group (eGFRcys> 60 ml/min/1.73 m2 and ACR< 30) (Figure 2).
Table 2.
Associations between renal factors and high sensitivity troponin t
| |
|
Unadjusted |
Model 1 |
Model 2 |
|||
|---|---|---|---|---|---|---|---|
| N* | Tobit ratio (95% CI) | P | Tobit ratio (95% CI) | P | Tobit ratio (95% CI) | P | |
|
eGFRcys* (ml/min/1.73 m
2
) |
|
|
|
|
|
|
|
| ≥ 60 |
757 |
Reference |
- |
Reference |
|
Reference |
- |
| 45-59 |
684 |
2.11 (1.86-2.41) |
< 0.001 |
1.51 (1.35-1.69) |
< 0.001 |
1.50 (1.34-1.67) |
< 0.001 |
| 30-44 |
705 |
3.02 (2.66-3.44) |
< 0.001 |
1.97 (1.74-2.22) |
< 0.001 |
1.96 (1.74-2.21) |
< 0.001 |
| < 30 |
311 |
5.27 (4.47-6.21) |
< 0.001 |
2.90 (2.47-3.41) |
< 0.001 |
2.83 (2.41-3.33) |
< 0.001 |
|
ACR (μg/mg) † |
|
|
|
|
|
|
|
| < 30 |
1115 |
Reference |
- |
Reference |
|
Reference |
- |
| 30-299 |
582 |
1.58 (1.39-1.80) |
< 0.001 |
1.08 (0.98-1.20) |
0.130 |
1.06 (0.96-1.19) |
0.214 |
| 300-999 |
338 |
1.94 (1.66-2.28) |
< 0.001 |
1.09 (0.96-1.25) |
0.191 |
1.08 (0.95-1.23) |
0.238 |
| ≥ 1000 |
325 |
3.61 (3.08-4.23) |
< 0.001 |
1.56 (1.35-1.81) |
< 0.001 |
1.53 (1.32-1.77) |
< 0.001 |
|
Phosphate (mg/dL) |
|
|
|
|
|
|
|
| < 3 |
265 |
Reference |
- |
Reference |
|
Reference |
- |
| 3-3.9 |
1427 |
1.08 (0.90-1.28) |
0.419 |
1.02 (0.90-1.17) |
0.739 |
1.02 (0.90-1.17) |
0.711 |
| 4-4.9 |
646 |
1.59 (1.31-1.93) |
< 0.001 |
1.06 (0.91-1.23) |
0.445 |
1.06 (0.92-1.24) |
0.412 |
| ≥ 5 |
81 |
3.51 (2.52-4.90) |
< 0.001 |
1.35 (1.04-1.76) |
0.023 |
1.39 (1.08-1.81) |
0.012 |
|
FGF-23 ‡ |
|
|
|
|
|
|
|
| Quartile I |
606 |
Reference |
|
Reference |
|
Reference |
|
| Quartile II |
606 |
1.45 (1.25-1.68) |
< 0.001 |
1.07 (0.96-1.20) |
0.230 |
1.08 (0.96-1.21) |
0.206 |
| Quartile III |
606 |
2.11 (1.82-2.45) |
< 0.001 |
1.20 (1.06-1.35) |
0.004 |
1.17 (1.04-1.33) |
0.011 |
| Quartile IV | 606 | 2.62 (2.26-3.04) | < 0.001 | 1.22 (1.06-1.40) | 0.004 | 1.17 (1.02-1.34) | 0.021 |
*N’s are # observations among participants with TnT measurements, with 7 observation excluded.
Model 1 adjusted for: age (per SD), female, race, diabetes, SBP categories, albumin (per SD), eGFR_CysC, ACR, and phosphate and FGF-23. Model 2 adjusted for: Model 1 + LV mass, ejection fraction.
eGFRcys=cystatin-based estimated glomerular filtration rate. † albumin to creatinine ratio. ‡ fibroblast growth factor 23.
Figure 2.
Joint associations of categories of estimated glomerular filtration rate and albumin to creatinine ratio with high sensitivity troponin t in adjusted tobit regression analyses. Legend: After multivariate adjustment, lower eGFR and higher albuminuria were associated with higher levels of hs-TnT. Final Tobit model adjusted for: age (per SD), female, race, diabetes, SBP categories, albumin (per SD), LV mass, ejection fraction, eGFRcys, ACR, and phosphate and FGF-23 (per SD). SD: standard deviation. SBP: systolic blood pressure. LV: left ventricular. eGFRcys: cystatin- based estimated glomerular filtration rate. ACR: albumin to creatinine ratio. FGF-23: fibroblast growth factor 23.
Several non-renal covariates remained strong predictors of hs-TnT after multivariable adjustment. Increased age was associated with higher hs-TnT, and hs-TnT was substantially lower in women compared to men. Compared to non-Hispanic whites, non-Hispanic blacks had significantly higher hs-TnT; although Hispanic participants had higher TnT levels, the association was not significant. Higher LV mass index was associated with higher hs-TnT across all categories of LV mass index; lower ejection fraction was associated with higher hs-TnT mainly in the lowest category (≤ 35%). Diabetes, elevated SBP and lower serum albumin were independently associated with higher hs-TnT (Table 3). After adjustment, the following markers were not associated with higher hs-TnT (B coefficient per SD (95% CI)): hsCRP 0.98 (0.94-1.02), p=0.386; LDL 1.01 (0.97-1.06), p=0.510; HDL 1.04 (0.99-1.09), p=0.126; triglycerides 0.99 (0.95-1.03), p=0.597. The associations of renal and non-renal covariates were consistent across categories of renal function. Substitution of eGFRcr for eGFRcys did not change our findings.
Table 3.
Associations between non-renal factors and high sensitivity troponin t
| |
|
Unadjusted |
Model 1 |
Model 2 |
|||
|---|---|---|---|---|---|---|---|
| N* | Tobit ratio (95% CI) | P | Tobit ratio (95% CI) | P | Tobit ratio (95% CI) | P | |
|
Age (per SD) |
2,457 |
1.39 (1.32-1.46) |
< 0.001 |
1.31 (1.25-1.37) |
< 0.001 |
1.31 (1.25-1.37) |
< 0.001 |
|
Female |
1,190 |
0.41 (0.37-0.46) |
< 0.001 |
0.38 (0.35-0.41) |
< 0.001 |
0.39 (0.36-0.42) |
< 0.001 |
|
Race |
|
|
|
|
|
|
|
| Non-Hisp white |
1,075 |
Reference |
- |
Reference |
- |
Reference |
- |
| Non-Hisp Black |
946 |
1.51 (1.34-1.70) |
< 0.001 |
1.31 (1.20-1.43) |
< 0.001 |
1.26 (1.16-1.38) |
< 0.001 |
| Hispanic |
330 |
2.09 (1.77-2.47) |
< 0.001 |
1.06 (0.93-1.21) |
0.398 |
1.02 (0.89-1.16) |
0.768 |
| Other |
106 |
1.10 (0.84-1.44) |
0.492 |
1.03 (0.84-1.25) |
0.807 |
1.02 (0.84-1.24) |
0.854 |
|
Diabetes |
1,009 |
2.96 (2.68-3.28) |
< 0.001 |
1.87 (1.71-2.04) |
< 0.001 |
1.84 (1.69-2.00) |
< 0.001 |
|
Albumin (per SD) |
2,437 |
0.72 (0.68-0.76) |
< 0.001 |
0.92 (0.88-0.96) |
0.001 |
0.93 (0.89-0.98) |
0.003 |
|
SBP (mmHg)* |
|
|
|
|
|
|
|
| < 120 |
408 |
Reference |
- |
Reference |
- |
Reference |
- |
| 120-129 |
324 |
1.32 (1.09-1.61) |
0.005 |
1.03 (0.89-1.19) |
0.678 |
1.03 (0.89-1.19) |
0.706 |
| 130-139 |
321 |
1.69 (1.39-2.05) |
< 0.001 |
1.14 (0.98-1.32) |
0.081 |
1.12 (0.97-1.29) |
0.139 |
| 140-149 |
194 |
2.03 (1.62-2.55) |
< 0.001 |
1.24 (1.05-1.47) |
0.014 |
1.22 (1.03-1.44) |
0.023 |
| 150-159 |
131 |
2.71 (2.09-3.51) |
< 0.001 |
1.44 (1.19-1.76) |
< 0.001 |
1.38 (1.14-1.69) |
0.001 |
| ≥ 160 |
163 |
3.36 (2.64-4.27) |
< 0.001 |
1.35 (1.12-1.63) |
0.001 |
1.27 (1.06-1.53) |
0.011 |
|
LV mass (g/m
2.7
) † |
|
|
|
|
|
|
|
| < 40 |
474 |
Reference |
- |
|
|
Reference |
- |
| 40-59 |
1,165 |
1.94 (1.68-2.24) |
< 0.001 |
|
|
1.17 (1.05-1.30) |
0.006 |
| 60-79 |
284 |
3.89 (3.20-4.72) |
< 0.001 |
|
|
1.39 (1.19-1.63) |
< 0.001 |
| 80-99 |
41 |
3.77 (2.49-5.72) |
< 0.001 |
|
|
1.47 (1.04-1.97) |
0.018 |
| ≥ 100 |
10 |
4.85 (2.15-10.95) |
< 0.001 |
|
|
1.87 (1.02-3.42) |
0.043 |
|
Ejection fraction (%) |
|
|
|
|
|
|
|
| > 50 |
1,845 |
Reference |
- |
|
|
Reference |
- |
| 46-50 |
197 |
1.12 (0.92-1.38) |
0.253 |
|
|
1.13 (0.98-1.31) |
0.087 |
| 36-45 |
130 |
1.33 (1.04-1.69) |
0.023 |
|
|
1.13 (0.95-1.34) |
0.184 |
| ≤ 35 | 22 | 1.93 (1.09-3.41) | 0.024 | 1.64 (1.09-2.48) | 0.018 | ||
*N’s are # observations among participants with TnT measurements, with 7 observation excluded.
Model 1 adjusted for: age (per SD), female, race, diabetes, SBP categories, albumin (per SD), eGFR_CysC, ACR, and phosphate and FGF-23. Model 2 adjusted for: Model 1 + LV Mass, ejection fraction. * Systolic blood pressure. † Left ventricular.
Discussion
In this study of participants without CVD selected from the CRIC cohort, over 80% had detectable levels of hs-TnT. Lower eGFR, higher LV mass, increased age, male gender, black race, diabetes and higher blood pressure were independently associated with higher hs-TnT. Interpreting these associations may help us understand which subjects with CKD are most susceptible to developing coronary disease or heart failure.
The median hs-TnT in our cohort (9 pg/ml) was comparable to subjects with stable coronary artery disease (5–6 pg/ml [21]) and heart failure (13–17 pg/ml [22]). This degree of chronic, low-level troponin release has been attributed to various mechanisms in addition to macrovascular atherosclerotic disease. Left ventricular hypertrophy (LVH) is common in this population, and is associated with higher levels of hs-TnT [11]. Chronic kidney disease may lead to myocardial injury via endothelial dysfunction and microvascular disease caused by elevated levels of asymmetric dimethylarginine (ADMA) [23] or mediators of oxidative stress [24]. Alternatively, the association between lower eGFR and higher TnT could be in part due to reduced renal clearance of troponin T. Troponin T fragments small enough to be cleared by the kidneys have been found in serum of patients with ESRD [25]. However, we also show that LV mass is associated with hs-TnT independently of eGFR; furthermore, 5% of persons with eGFR< 30 and 10% with eGFR between 30–45 had undetectable hs-TnT, demonstrating that impaired clearance is unlikely to be solely responsible for detectable levels of hs-TnT.
Similar to the Dallas Heart Study in which hs-TnT was studied in a community-based cohort [4] we found that older age, male gender, black race and diabetes were independently associated with elevated hs-TnT. While it seems intuitive that older age and diabetes would be associated with microvascular injury and thus lead to higher hs-TnT, the explanation for male gender and black race is less obvious. Male gender and black race are associated with higher LV mass index [26-28], but in our study these associations persisted after adjustment for LV mass index. African Americans have been found to have a higher risk of endothelial dysfunction [29,30], and this may explain the higher levels of hs-TnT among them in our cohort.
Prior studies have shown FGF-23 to have strong, independent associations with structural cardiovascular disease including LVH [31], coronary artery disease [32], and carotid intima-media thickness [33]; cardiovascular events [34]; and overall mortality [13,35] in cohorts with prevalent CVD and all stages of CKD [13]. One role of FGF-23 in cardiovascular disease is via induction of left ventricular hypertrophy; FGF-23 injected into mice at concentrations of 800 RU/ml resulted in LVH [36]. In addition, the growth factor was associated with myocardial injury (hs-TnT) in a CKD cohort, an association that was only mildly attenuated by adjustment for LVH [37]. In contrast, we observed only a weak association of FGF-23 with hs-TnT levels with or without adjustment for LV mass index. The fact that we excluded CRIC participants with history of CVD may account for these differences. While FGF-23 does not appear to be a predominant factor for subclinical myocardial injury in CRIC subjects without CVD, it may yet prove crucial in the development of heart failure in this cohort via induction of LVH.
Strengths of our study include the large study cohort, broad range of eGFRcys and ethnic diversity represented by CRIC. The high percentage of participants with detectable hs-TnT increased our power to detect cross-sectional associations. There are also several important limitations. Subjects with known cardiovascular disease were excluded based on history, not left heart catheterization; some subjects with undetected coronary artery disease may have been included. Since our analysis is cross-sectional, we cannot assume a causal relationship between factors associated with hs-TnT and hs-TnT or incident CVD. We cannot separate the relative importance of hs-TnT production vs. clearance as determinants of serum concentrations.
Conclusions
We conclude that in the absence of CVD, the most important renal predictor of higher hs-TnT in the CRIC cohort is lower eGFRcys. Non-renal predictors include age, male gender, black race, diabetes, and higher systolic blood pressure. Further studies will evaluate whether hs-TnT is associated with adverse outcomes in CRIC.
Abbreviations
CRIC: Chronic renal insufficiency cohort; CKD: Chronic kidney disease; CVD: Cardiovascular disease; Hs-TnT: High sensitivity troponin T; SBP: Systolic blood pressure; DBP: Diastolic blood pressure; BMI: Body mass index; LDL: Low density lipoprotein; hsCRP: High sensitive C-reactive protein; FGF-23: Fibroblast growth factor 23; eGFRcys: Cystatin-based estimated glomerular filtration; eGFRcr: Creatinine-based estimated glomerular filtration; MDRD: Modification of diet in renal disease; ACR: Albumin to creatinine ratio; LV: Left ventricular; LVH: Left ventricular hypertrophy; SD: Standard deviation; IQR: Interquartile range; ADMA: Asymmetric dimethylarginine.
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
RD participated in the formulation of hypotheses, statistical analyses and drafted the paper. YL conducted statistical analyses. MS is responsible for study conception and design as well as supervising manuscript completion. The remaining authors were all involved in critically revising the manuscript content and format. All authors have given final approval of the version to be published.
Pre-publication history
The pre-publication history for this paper can be accessed here:
Contributor Information
Ruth F Dubin, Email: ruth.dubin@ucsf.edu.
Yongmei Li, Email: yongmeil@hotmail.com.
Jiang He, Email: jhe@tulane.edu.
Bernard G Jaar, Email: bjaar@jhmi.edu.
Radhakrishna Kallem, Email: krishna.kallem@uphs.upenn.edu.
James P Lash, Email: jplash@uic.edu.
Gail Makos, Email: gmakos@scsp.net.
Sylvia E Rosas, Email: sylvia.rosas@uphs.upenn.edu.
Elsayed Z Soliman, Email: esoliman@wakehealth.edu.
Ray R Townsend, Email: townsend@mail.med.upenn.edu.
Wei Yang, Email: weiyang@mail.med.upenn.edu.
Alan S Go, Email: Alan.S.Go@kp.org.
Martin Keane, Email: keanem@mail.med.upenn.edu.
Christopher deFilippi, Email: cdefilip@medicine.umaryland.edu.
Rakesh Mishra, Email: rakesh.mishra@ucsf.edu.
Myles Wolf, Email: MWolf2@med.miami.edu.
Michael G Shlipak, Email: michael.shlipak@ucsf.edu.
Acknowledgements
We are indebted to the CRIC Study Investigators who are listed as authors, as well as the following CRIC Study Investigators: Lawrence J. Appel, MD, MPH, Harold I. Feldman, MD, MSCE, John W. Kusek, PhD, Akinlolu Ojo, MD, PhD, and Mahboob Rahman, MD.
Support Funding for the CRIC Study was obtained under a cooperative agreement from National Institute of Diabetes and Digestive and Kidney Diseases (U01DK060990, U01DK060984, U01DK061022, U01DK061021, U01DK061028, U01DK060980, U01DK060963, and U01DK060902). This project was supported by M.S.’s R01 DK066488 award (principal investigator M.S.). R.D.is supported by K23 DK092354. In addition, this work was supported in part by: the University of Pennsylvania CTRC CTSA UL1 RR-024134, Johns Hopkins University UL1 RR-025005, University of Maryland GCRC M01 RR-16500, Clinical and Translational Science Collaborative of Cleveland, UL1TR000439 from the National Center for Advancing Translational Sciences (NCATS) component of the National Institutes of Health and NIH roadmap for Medical Research, Michigan Institute for Clinical and Health Research (MICHR) UL1RR024986, University of Illinois at Chicago CTSA UL1RR029879, Tulane University Translational Research in Hypertension and Renal Biology P30GM103337, Kaiser Permanente Northern California NIH/NCRR UCSF-CTSI UL1 RR-024131.
References
- Sarnak MJ, Katz R, Stehman-Breen CO, Fried LF, Jenny NS, Psaty BM, Newman AB, Siscovick D, Shlipak MG. Cardiovascular Heatlh Study. Cystatin C concentration as a risk factor for heart failure in older adults. Ann Intern Med. 2005;14:497–505. doi: 10.7326/0003-4819-142-7-200504050-00008. [DOI] [PubMed] [Google Scholar]
- Waheed S, Matsushita K, Sang Y, Hoogeveen R, Ballantyne C, Coresh J. Combined association of albuminuria and cystatin C-based estimated GFR with mortality, coronary heart disease, and heart failure outcomes: the atherosclerosis risk in communities (ARIC) study. Am J Kidney Dis. 2012;14:207–216. doi: 10.1053/j.ajkd.2012.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ix JH, Shlipak MG, Chertow GM, Whooley MA. Association of cystatin C with mortality, cardiovascular events, and incident heart failure among persons with coronary heart disease: data from the heart and soul study. Circulation. 2007;14:173–179. doi: 10.1161/CIRCULATIONAHA.106.674358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lemos JA, Drazner MH, Omland T, Ayers CR, Khera A, Rohatgi A, Hashim I, Berry JD, Das SR, Morrow DA, McGuire DK. Association of troponin T detected with a highly sensitive assay and cardiac structure and mortality risk in the general population. JAMA. 2010;14:2503–2512. doi: 10.1001/jama.2010.1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egstrup M, Schou M, Tuxen CD, Kistorp CN, Hildebrandt PR, Gustafsson F, Faber J, Goetze JP, Gustafsson I. Prediction of outcome by highly sensitive troponin T in outpatients with chronic systolic left ventricular heart failure. Am J Cardiol. 2012;14:552–557. doi: 10.1016/j.amjcard.2012.04.033. [DOI] [PubMed] [Google Scholar]
- Latini R, Masson S, Anand IS, Missov E, Carlson M, Vago T, Angelici L, Barlera S, Parrinello G, Maggioni AP, Tognoni G, Cohn JN. ValHeFT Investigators. Prognostic value of very low plasma concentrations of troponin T in patients with stable chronic heart failure. Circulation. 2007;14:1242–1249. doi: 10.1161/CIRCULATIONAHA.106.655076. [DOI] [PubMed] [Google Scholar]
- McMurray JJ, Uno H, Jarolim P, Desai AS, de Zeeuw D, Eckardt KU, Ivanovich P, Levey AS, Lewis EF, McGill JB, Parfrey P, Parving HH, Toto RM, Solomon SD, Pfeffer MA. Predictors of fatal and nonfatal cardiovascular events in patients with type 2 diabetes mellitus, chronic kidney disease, and anemia: an analysis of the trial to reduce cardiovascular events with aranesp (darbepoetin-alfa) therapy (TREAT) Am Heart J. 2011;14:748–755. doi: 10.1016/j.ahj.2011.07.016. e3. [DOI] [PubMed] [Google Scholar]
- Scheven L, de Jong PE, Hillege HL, Lambers Heerspink HJ, van Pelt LJ, Kootstra JE, Bakker SJ, Gansevoort RT. PREVEND study group. High-sensitive troponin T and N-terminal pro-B type natriuretic peptide are associated with cardiovascular events despite the cross-sectional association with albuminuria and glomerular filtration rate. Eur Heart J. 2012;14:2272–2281. doi: 10.1093/eurheartj/ehs163. [DOI] [PubMed] [Google Scholar]
- Landray MJ, Emberson JR, Blackwell L, Dasgupta T, Zakeri R, Morgan MD, Ferro CJ, Vickery S, Ayrton P, Nair D, Dalton RN, Lamb EJ, Baigent C, Townend JN, Wheeler DC. Prediction of ESRD and death among people with CKD: the chronic renal impairment in birmingham (CRIB) prospective cohort study. Am J Kidney Dis. 2010;14:1082–1094. doi: 10.1053/j.ajkd.2010.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGill D, Talaulikar G, Potter JM, Koerbin G, Hickman PE. Over time, high-sensitivity TnT replaces NT-proBNP as the most powerful predictor of death in patients with dialysis-dependent chronic renal failure. Clin Chim Acta. 2010;14:936–939. doi: 10.1016/j.cca.2010.03.004. [DOI] [PubMed] [Google Scholar]
- Mishra RK, Li Y, Defilippi C, Fischer MJ, Yang W, Keane M, Chen J, He J, Kallem R, Horwitz EJ, Rafey M, Raj DS, Go AS, Shlipak MG. CRIC study investigators. Association of cardiac troponin T with left ventricular structure and function in CKD. Am J Kidney Dis. 2013;14:701–709. doi: 10.1053/j.ajkd.2012.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman HI, Appel LJ, Chertow GM, Cifelli D, Cizman B, Daugirdas J, Fink JC, Franklin-Becker ED, Go AS, Hamm LL, He J, Hostetter T, Hsu CY, Jamerson K, Joffe M, Kusek JW, Landis JR, Lash JP, Miller ER, Mohler ER 3rd, Muntner P, Ojo AO, Rahman M, Townsend RR, Wright JT. Chronic Renal Insufficiency Cohort (CRIC) Study Investigators. The chronic renal insufficiency cohort (CRIC) study: design and methods. JASN. 2003;14:S148–S153. doi: 10.1097/01.ASN.0000070149.78399.CE. [DOI] [PubMed] [Google Scholar]
- Isakova T, Xie H, Yang W, Xie D, Anderson AH, Scialla J, Wahl P, Gutierrez OM, Steigerwalt S, He J, Schwartz S, Lo J, Ojo A, Sondhemier J, Hsu CY, Lash J, Leonard M, Kusek JW, Feldman HI, Wolf M. Chronic Renal Insufficiency Cohort (CRIC) Study Group. Fibroblast growth factor 23 and risks of mortality and end-stage renal disease in patients with chronic kidney disease. JAMA. 2011;14:2432–2439. doi: 10.1001/jama.2011.826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins BA, Nelson RG, Ostrander BE, Blouch KL, Krolewski AS, Myers BD, Warram JH. Detection of renal function decline in patients with diabetes and normal or elevated GFR by serial measurements of serum cystatin C concentration: results of a 4-year follow-up study. J Am Soc Nephrol. 2005;14:1404–1412. doi: 10.1681/ASN.2004100854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shlipak MG, Sarnak MJ, Katz R, Fried LF, Seliger SL, Newman AB, Siscovick DS, Stehman-Breen C. Cystatin C and the risk of death and cardiovascular events among elderly persons. N Engl J Med. 2005;14:2049–2060. doi: 10.1056/NEJMoa043161. [DOI] [PubMed] [Google Scholar]
- Shlipak MG, Wassel Fyr CL, Chertow GM, Harris TB, Kritchevsky SB, Tylavsky FA, Satterfield S, Cummings SR, Newman AB, Fried LF. Cystatin C and mortality risk in the elderly: the health, aging, and body composition study. JASN. 2006;14:254–261. doi: 10.1681/ASN.2005050545. [DOI] [PubMed] [Google Scholar]
- Hsu CY, Propert K, Xie D, Hamm L, He J, Miller E, Ojo A, Shlipak M, Teal V, Townsend R, Weir M, Wilson J, Feldman H. CRIC Investigators. Measured GFR does not outperform estimated GFR in predicting CKD-related complications. JASN. 2011;14:1931–1937. doi: 10.1681/ASN.2010101077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens LA, Coresh J, Schmid CH, Feldman HI, Froissart M, Kusek J, Rossert J, Van Lente F, Bruce RD, Zhang YL, Greene T, Levey AS. Estimating GFR using serum cystatin C alone and in combination with serum creatinine: a pooled analysis of 3,418 individuals with CKD. Am J Kidney Dis. 2008;14:395–406. doi: 10.1053/j.ajkd.2007.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA. et al. Recommendations for chamber quantification: a report from the american society of Echocardiography’s guidelines and standards committee and the chamber quantification writing group, developed in conjunction with the European association of echocardiography, a branch of the European society of cardiology. JASE. 2005;14:1440–1463. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Giannitsis E, Kurz K, Hallermayer K, Jarausch J, Jaffe AS, Katus HA. Analytical validation of a high-sensitivity cardiac troponin T assay. Clin Chem. 2010;14:254–261. doi: 10.1373/clinchem.2009.132654. [DOI] [PubMed] [Google Scholar]
- Omland T, de Lemos JA, Sabatine MS, Christophi CA, Rice MM, Jablonski KA, Tjora S, Domanski JM, Gersh BJ, Rouleau JL, Pfeffer MA, Braunwald E. A sensitive cardiac troponin T assay in stable coronary artery disease. N Engl J Med. 2009;14:2538–2547. doi: 10.1056/NEJMoa0805299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masson S, Anand I, Favero C, Barlera S, Vago T, Bertocchi F, Maggioni AP, Tavazzi L, Tognoni G, Cohn JN, Latini R. Serial measurement of cardiac troponin T using a highly sensitive assay in patients with chronic heart failure: data from 2 large randomized clinical trials. Circulation. 2012;14:280–288. doi: 10.1161/CIRCULATIONAHA.111.044149. [DOI] [PubMed] [Google Scholar]
- Kajimoto H, Kai H, Aoki H, Yasuoka S, Anegawa T, Aoki Y, Ueda S, Okuda S, Imaizumi T. Inhibition of eNOS phosphorylation mediates endothelial dysfunction in renal failure: new effect of asymmetric dimethylarginine. Kidney Int. 2012;14:762–768. doi: 10.1038/ki.2011.476. [DOI] [PubMed] [Google Scholar]
- Del Vecchio L, Locatelli F, Carini M. What we know about oxidative stress in patients with chronic kidney disease on dialysis–clinical effects, potential treatment, and prevention. Semin Dial. 2011;14:56–64. doi: 10.1111/j.1525-139X.2010.00819.x. [DOI] [PubMed] [Google Scholar]
- Diris JH, Hackeng CM, Kooman JP, Pinto YM, Hermens WT, van Dieijen-Visser MP. Impaired renal clearance explains elevated troponin T fragments in hemodialysis patients. Circulation. 2004;14:23–25. doi: 10.1161/01.CIR.0000113713.67092.C5. [DOI] [PubMed] [Google Scholar]
- Drazner MH, Dries DL, Peshock RM, Cooper RS, Klassen C, Kazi F, Willett D, Victor RG. Left ventricular hypertrophy is more prevalent in blacks than whites in the general population: the Dallas heart study. Hypertension. 2005;14:124–129. doi: 10.1161/01.HYP.0000169972.96201.8e. [DOI] [PubMed] [Google Scholar]
- Hinderliter AL, Light KC, Willis PW. Left ventricular mass index and diastolic filling. Relation to blood pressure and demographic variables in a healthy biracial sample. Am J Hypertens. 1991;14:579–585. doi: 10.1093/ajh/4.7.579. [DOI] [PubMed] [Google Scholar]
- Meijs MF, Bots ML, Cramer MJ, Vonken EJ, Velthuis BK, van der Graaf Y, Spiering W, Mali WP, Doevendans PA. Differences in determinants of left ventricular mass assessed by cardiac magnetic resonance imaging across subjects with and without previous symptomatic atherosclerotic disease. Int J Cardiol. 2010;14:145–150. doi: 10.1016/j.ijcard.2008.08.008. [DOI] [PubMed] [Google Scholar]
- Perregaux D, Chaudhuri A, Rao S, Airen A, Wilson M, Sung BH, Dandona P. Brachial vascular reactivity in blacks. Hypertension. 2000;14:866–871. doi: 10.1161/01.HYP.36.5.866. [DOI] [PubMed] [Google Scholar]
- Campia U, Choucair WK, Bryant MB, Waclawiw MA, Cardillo C, Panza JA. Reduced endothelium-dependent and -independent dilation of conductance arteries in African Americans. J Am Coll Cardiol. 2002;14:754–760. doi: 10.1016/S0735-1097(02)02015-6. [DOI] [PubMed] [Google Scholar]
- Gutierrez OM, Januzzi JL, Isakova T, Laliberte K, Smith K, Collerone G, Sarwar A, Hoffman U, Coglianese E, Christenson R, Wang TJ, deFilippi C, Wolf M. Fibroblast growth factor 23 and left ventricular hypertrophy in chronic kidney disease. Circulation. 2009;14:2545–2552. doi: 10.1161/CIRCULATIONAHA.108.844506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanbay M, Nicoleta M, Selcoki Y, Ikizek M, Aydin M, Eryonucu B, Duranay M, Akcay A, Armutcu F, Covic A. Fibroblast growth factor 23 and fetuin A are independent predictors for the coronary artery disease extent in mild chronic kidney disease. Clin J Am Soc Nephrol. 2010;14:1780–1786. doi: 10.2215/CJN.02560310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balci M, Kirkpantur A, Gulbay M, Gurbuz OA. Plasma fibroblast growth factor-23 levels are independently associated with carotid artery atherosclerosis in maintenance hemodialysis patients. Hemodial Int. 2010;14:425–432. doi: 10.1111/j.1542-4758.2010.00480.x. [DOI] [PubMed] [Google Scholar]
- Kendrick J, Cheung AK, Kaufman JS, Greene T, Roberts WL, Smits G, Chonchol M. FGF-23 associates with death, cardiovascular events, and initiation of chronic dialysis. J Am Soc Nephrol. 2011;14:1913–1922. doi: 10.1681/ASN.2010121224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez OM, Mannstadt M, Isakova T, Rauh-Hain JA, Tamez H, Shah A, Smith K, Lee H, Thadhani R, Juppner J, Wolf M. Fibroblast growth factor 23 and mortality among patients undergoing hemodialysis. N Engl J Med. 2008;14:584–592. doi: 10.1056/NEJMoa0706130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faul C, Amaral AP, Oskouei B, Hu MC, Sloan A, Isakova T, Gutierrez OM, Aguillon-Prada R, Lincoln J, Hare JM, Mundel P, Morales A, Scialla J, Fischer M, Soliman EZ, Chen J, Go AS, Rosas SE, Nessel L, Townsend RR, Feldman HI, St Joh Sutton M, Ojo A, Gadegbeku C, Di Marco GS, Reuter S, Kentrup D, Tiemann K, Brand M, Hill JA. et al. FGF23 induces left ventricular hypertrophy. J Clin Invest. 2011;14:4393–4408. doi: 10.1172/JCI46122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith K, Defilippi C, Isakova T, Gutierrez OM, Laliberte K, Seliger S, Kelley W, Duh SH, Hise M, Christenson R, Wolf M, Januzzi J. Fibroblast growth factor 23, high-sensitivity cardiac troponin, and left ventricular hypertrophy in CKD. Am J Kidney Dis. 2013;14:67–73. doi: 10.1053/j.ajkd.2012.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]