Abstract
Varicocele is a pathologic enlargement of the pampiniform venous plexus within the spermatic cord, a condition that is a common cause of impaired sperm production and decreased quality of sperm. While varicocele is the most common surgically correctable risk factor for male infertility, not all males with varicocele experience infertility. In fact, most men with varicocele have normal spermatogenesis. Despite its prevalence, the molecular mechanisms of varicocele and its effect on testicular function are yet to be completely understood. We postulate that men with varicocele-associated infertility could have preexisting genetic lesions or defects in molecular mechanisms that make them more susceptible to varicocele-mediated testicular injury affecting spermatogenesis.
Keywords: DNA damage, Male infertility, Spermatogenesis, Varicocele, Varicocelectomy
Introduction
Varicocele is a pathologic enlargement of the pampiniform venous plexus within the spermatic cord. Typically, a varicocele is observed during the physical examination as an asymmetry in scrotal size and is graded according to the Dubin and Amelar classification system [1]—grade I varicocele is palpable only during the Valsalva maneuver; grade II varicocele is palpable in the standing position, and; grade III varicocele is visible without palpation. Subclinical varicocele is neither visible nor palpable in either the standing position or during the Valsalva maneuver, but is detectable by other studies, such as the Doppler ultrasound. A male who presents with varicocele is often asymptomatic; only rarely is varicocele associated with scrotal pain [2]. Nevertheless, varicocele is the most commonly identified and most common surgically correctable risk factor for male infertility [3]. Notably, however, not all males with varicocele present with infertility. Therefore, it is possible, and perhaps likely, that different intrinsic susceptibilities exist among men with varicocele. These susceptibilities could be responsible for whether varicocele impairs spermatogenesis, leading to infertility.
Currently, there are three widely supported etiologies of varicocele. First, differences in the drainage of the left and right testicular veins are thought to contribute to varicocele formation. Whereas the right testicular vein enters directly into the inferior vena cava, the left testicular vein joins the left renal vein at a right angle. This anatomic difference and unique angle of insertion is hypothesized to lead to increased hydrostatic pressure, resulting in enlargement of the pampiniform venous plexus [4]. Second, a complete absence or functional deficiency of venous valves in the testicular venous system is thought to permit retrograde blood flow that contributes to the pathogenesis of varicocele. Third, the so-called “nutcracker effect,” whereby the renal vein is compressed between the aorta and superior mesenteric artery, is thought to increase hydrostatic pressure in the testicular venous system, also resulting in dilatation of the venous plexus in the spermatic cord [5].
The pathophysiologic mechanisms that lead to varicocele-associated infertility are yet to be completely understood. The five following mechanisms are thought to contribute to the pathogenesis of varicocele affecting testicular function: (1) hypoperfusion leading to hypoxia, (2) heat stress, (3) oxidative stress, (4) hormonal imbalances, and (5) exogenous toxicants. Still, these five mechanisms alone do not provide a complete understanding of the effect of varicocele on spermatogenesis. That not all males with varicocele present with infertility leads us to believe that molecular and genetic factors could in fact play a role in understanding the pathogenesis of varicocele-associated infertility. In fact, hereditary studies suggest a genetic component, as varicoceles are found in significantly higher rates in the first-degree relatives of men with varicocele as compared to controls [4, 6]. In aiming to elucidate the molecular mechanisms of varicocele-associated infertility, this review hopes to uncover a thorough understanding of the processes that underlie the development of infertility among a subset of males with varicocele.
Hypoperfusion and hypoxia
In the testes, blood pressure in pre-capillary arterioles and post-capillary venules is very low in comparison to many other tissues in the body. Consequently, the testicular microenvironment is highly sensitive to even minimal changes in blood pressure [7]. In men with varicocele, the pathologic enlargement of the pampiniform venous plexus causes pooling of blood and reversal of flow through the testicular venous system. Such venous stasis and retrograde flow likely impairs the countercurrent heat exchange system in the testes, leading to elevated temperatures in the scrotum. Further, any increase in venous pressure elicits a compensatory vasoconstriction of pre-capillary arterioles as a mechanism to downregulate arterial flow in order to maintain homeostasis with regards to intratesticular pressure [8]. This pre-capillary arteriolar vasoconstriction leads to hypoperfusion of the testes, thereby decreasing delivery of oxygen and nutrients to the cells of the testicular microenvironment.
In fact, a study by Lee et al. reported that, in men with varicocele, testicular veins expressed increased levels of hypoxia-inducible factor 1α (HIF-1α), confirming that the cells of the testicular microenvironment are exposed to a decrease in oxygen delivery [9]. Hypoxia-inducible factors are well known for their ability to promote cell survival in hypoxic conditions. In the setting of low oxygen levels, hypoxia-inducible factors can drive the biosynthesis of new vessels through which larger quantities of oxygen may be delivered to the hypoxic tissues and can drive cells toward metabolic pathways that do not require oxygen to generate energy. These functions promote cell survival under hypoxic conditions. However, hypoxia-inducible factors promote apoptosis, or programmed cell death, in the setting of low oxygen levels. Whether hypoxia-inducible factors promote cell survival or cell death in the setting of low oxygen levels seems to depend upon the tissue in which they are expressed and the severity of the hypoxia. A study by Wang et al. used terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) to determine the degree of apoptosis within the testes of control and experimentally-induced varicocele rats. The researchers found that testicular HIF-1α levels, in response to varicocele-induced hypoxia, were associated with increased levels of germ cell apoptosis [10]. Therefore, we posit that the HIF-1α expressed in the testicular tissues in response to varicocele-induced hypoxia promotes germ cell apoptosis, thereby contributing to male infertility.
Heat stress
In the spermatic cord, the testicular artery is surrounded by several testicular veins, each of which contributes to the pampiniform plexus. In 1959, Dahl and Herrick proposed that the inflowing arterial blood from the testicular artery is cooled by the outflowing venous blood in the pampiniform plexus, and that this countercurrent heat exchange mechanism is responsible for maintaining the scrotal temperature a few degrees below the body’s core temperature as a means to optimize the environment for normal testicular function [11]. Enlargement of the pampiniform venous plexus that leads to venous stasis and retrograde flow in men with varicocele likely alters the countercurrent heat exchange system in the testes, thereby impeding the cooling of arterial blood and leading to scrotal hyperthermia. In fact, elevated intrascrotal and intratesticular temperatures have been demonstrated in both humans with varicocele and experimental varicocele models [12, 13]. Such elevation in temperature within the testicular microenvironment likely contributes to the deleterious effects that varicocele has on normal sperm physiology [14–16].
In the testes, many of the enzymes responsible for DNA synthesis function in a temperature-dependent manner and exhibit optimal activity at the lower temperatures of the normal testicular environment. Elevated temperatures associated with varicocele impose heat stress that could disrupt the function of these enzymes [17–19]. In fact, a study by Fujisawa et al. has demonstrated that men with varicocele who present with infertility exhibit decreased activity of topoisomerase I and DNA polymerase, two key enzymes involved in the synthesis of DNA [18, 19]. Protein synthesis in sperm, too, has been shown to exhibit a temperature dependency [17–19]. Recently, numerous studies have used newer research technologies to discover differences in the expression of key proteins involved in normal sperm physiology. For example, a study by De Amicis et al. found that “varicocele sperm” exhibited reduced expression of phosphatidylinositol 3-kinase (PI3K), a key protein involved in the regulation of capacitation, acrosome reaction, and fertilization. Additionally, the researchers demonstrated that PI3K exhibits subcellular compartmentalization within human sperm, which is compromised in men with varicocele. Whereas “healthy sperm” expresses PI3K in the sperm head membrane and nucleus and in the entire tail, expression of PI3K in “varicocele sperm” was confined to the sperm head [20]. Given that elevated temperatures can disrupt protein synthesis within sperm, we posit that the heat stress imposed on sperm as a consequence of varicocele impacts the synthesis of key proteins necessary for normal sperm physiology.
In 2013, a study by Hosseinifar et al. compared the sperm protein profiles between men with and without varicocele [21]. The researchers identified decreased proteomic expression of HSPA5, a gene that encodes for heat shock protein 5, in men with varicocele. Similarly, a study by Lima et al. used semiquantitative real-time polymerase chain reaction (RT-PCR) analysis of human ejaculated sperm to show that adolescents with varicocele and oligozoospermia express significantly lower quantities of the HSPA2 gene in comparison to adolescents with varicocele and normal sperm concentrations [22]. Downregulation of HSPA5 and HSPA2 in men with varicocele as compared to controls suggests that decreased levels of heat shock proteins lead to increased susceptibility of the testes to elevated temperatures.
Additionally, the Hosseinifar et al. study also found that men with varicocele exhibit a downregulation of the expression of the ATP5D gene that encodes the delta (δ) subunit of the catalytic core of the mitochondrial adenosine triphosphate synthase (ATPase), from which sperm cells derive energy to drive the flagellar motor. This downregulation of ATP5D leads to a dysfunctional ATPase complex, which significantly impairs sperm motility [21]. Taken together, these findings suggest that differences in the expression of many genes—including those whose protein products protect the cell against elevated temperatures and are responsible for many of the sperm’s normal physiologic functions, including motility—could contribute to the susceptibility of sperm cells to varicocele-induced heat stress, thereby contributing to varicocele-associated infertility.
Oxidative stress
Reactive oxygen species (ROS) are byproducts of various metabolic pathways in the cell that serve as key regulators in vital cellular events [23, 24]. In the male reproductive tract, ROS play important roles in sperm function. ROS-mediated signal transduction pathways are necessary for capacitation, hyperactivation, and acrosomal reaction, all processes by which sperm attains its functional maturity [23, 25–27]. Further, ROS-mediated lipid peroxidation of membrane lipids facilitates sperm adhesion to the oocyte in the process of fertilization [28]. However, increases in ROS to levels beyond those of physiologic conditions could lead to oxidative stress. Normally, though, an antioxidant defense system that consists of free radical scavengers, chain-breaking antioxidants, and enzymes that break down ROS and their metabolites reduces the oxidant load and confers protection against such oxidative stress. Therefore, that semen samples from infertile males with varicocele exhibit elevated levels of ROS [29, 30] and that varicocele is associated with an increased DNA fragmentation index (DFI), that improves post-varicocelectomy [31] suggest that an imbalance between the oxidant load and the natural antioxidant defense system could play a role in development of varicocele-associated infertility.
Elevated levels of ROS impair sperm function by a variety of mechanisms. By targeting each of the four classes of biological macromolecules—carbohydrates, lipids, nucleic acids, and proteins—ROS induce oxidative stress that disrupts normal sperm physiology. ROS target polyunsaturated fatty acids (PFAs) in the lipid membrane of sperm, thereby inducing a chain reaction of lipoperoxide (radical) formation whose product is malondialdehyde, a known marker of lipid peroxidation from oxidative injury [32, 33]. Such oxidative modifications have detrimental effects on the sperm lipid membranes, specifically on membrane fluidity, which dampens sperm motility and leads to suboptimal fertilization [24]. ROS also target key proteins in the cell, specifically in the mitochondria. ROS-induced oxidative damage to mitochondrial proteins destroy the mitochondrial membrane potential, leading to the loss of sperm motility [34]. Perhaps most importantly, ROS-mediated damage to sperm nucleic acids. One of the potential mechanisms by which varicocele contributes to infertility is the increased production of ROS in the sperm head, leading to high levels of oxidative stress that mediates damage to DNA.
Oxidative stress has the potential to induce various types of DNA damage—DNA base pair oxidation, single- or double-stranded DNA breaks, chromosomal rearrangements, and gene mutations, which includes deletions, point mutations, and polymorphisms [35–37]. The integrity of nuclear DNA, and consequently the maintenance of functional DNA in sperm, is of the highest importance in fertility. Therefore, sperm have defense mechanisms in place against damaging oxidative species. Sperm tightly condense their DNA to protect its structure during transit through both the male and female reproductive tracts, express antioxidants to help counteract any oxidative species, and have enzymatic mechanisms to repair damaged DNA. Yet, these protective mechanisms can be overwhelmed, especially in the presence of excessive ROS, leaving sperm with defective DNA. Further understanding of the types of DNA damage present in males with varicocele and how they impact the fertility potential could help determine which subset of patients are most suitable to undergo surgical repair of varicocele.
Still, as new research technologies continue to emerge, much remains to be explored in a continued effort to understand the role of oxidative stress in the development of varicocele-associated infertility. Proteomic expression analysis in men with varicocele demonstrated decreased expression of SOD1, a gene that encodes for the superoxide dismutase 1 enzyme responsible for counteracting free superoxide radicals in cells [21]. Other studies have also found gene expression differences in men with varicocele contributing to imbalances in the pro- and anti-oxidant species in sperm. Like SOD1, glutathione S-transferases are a family of cytosolic and mitochondrial enzymes that function in the removal of oxidative species from cells. Deletions in the glutathione S-transferase M1 (GST-M1) and GST-T1 enzymes in men with varicocele have been associated with impaired sperm motility and elevated levels of 8-hydroxy-2′-deoxyguanosine in sperm DNA, a marker of DNA damage [38, 39]. Certain genetic lesions disrupt the balance of pro- and antioxidant species in cells. We posit that, in men with varicocele, such lesions could lead to greater ROS exposure that mediates more significant oxidative damage to the testes, contributing to varicocele-associated infertility.
Hormonal imbalances
Leydig cells, in response to stimulation by luteinizing hormone (LH), synthesize and secrete testosterone, which signals locally in the testes (paracrine signaling) and distally throughout the body (endocrine signaling). Since testosterone is necessary for proper spermatogenesis, it has been postulated that disturbances in Leydig cell function, and therefore in testosterone levels, could contribute to the pathophysiology of varicocele-associated infertility. A study by Rajfer et al. used control and experimentally-induced varicocele rats to determine whether impaired testosterone biosynthesis was associated with varicocele. The researchers found decreased activity of 17,20-desmolase and 17-α-hydroxylase, both key enzymes in the testosterone biosynthesis pathway, leading to decreased intratesticular testosterone levels [40]. We suggest that varicocele could contribute to changes in the testicular microenvironment that disrupt Leydig cell function and lower intratesticular testosterone levels, contributing to varicocele-associated infertility.
Testosterone binds to androgen receptor (AR) to form a complex that binds DNA and controls the activity of many androgen response elements. AR expression is significantly decreased in infertile men with varicocele as compared to both infertile men without varicocele and controls. A study by Zalata et al. indicates that AR expression is highly correlated with sperm count, sperm motility, and sperm morphology [41]. Genetic lesions in AR can disrupt AR-mediated testosterone signaling pathways. We speculate that, in men with varicocele, such lesions could lead to impaired testosterone function in the setting of normal testosterone levels, which could contribute to varicocele-associated infertility.
Exogenous toxicants
Recently, researchers have explored the contribution of occupational and environmental exposures to varicocele-associated infertility. That there is a well-established association between cigarette smoking and lowered semen quality [42] has led to the hypothesis that exposure to toxicants in cigarette smoke, including cadmium (Cd2+), could exacerbate the effects of varicocele in smokers, leading to infertility. In fact, researchers have not only reported elevated Cd2+ levels in the testes [43], testicular veins [44], and seminal fluid [45] of men with varicocele, but have also found that Cd2+ levels predict impaired testicular function in men with varicocele [43, 46]. Unexpectedly, however, elevated Cd2+ levels in men with varicocele were unaffected by smoking status [32]. Thus, it is possible that lesions in the genetic and molecular mechanisms that control Cd2+ homeostasis could explain the elevated Cd2+ levels in men with varicocele and could contribute to varicocele-associated infertility.
While the mechanism by which Cd2+ accumulates in the testes remains unknown, this process is likely to occur in two stages. Cd2+ must first disrupt the blood-testis barrier to enter the testicular interstitium and must then enter the developing sperm cells of the seminiferous tubular epithelium. Cd2+ entry could occur through the alpha1 L-type voltage dependent calcium channels (L-VDCC α1) expressed on developing sperm cells in the testes [47]. While L-VDCC α1 control calcium (Ca2+) homeostasis in the testes, these Ca2+ channels are incompletely cation specific, permitting other divalent cations, like Cd2+, to enter into the cells of the seminiferous tubular epithelium. Transport of Cd2+ through L-VDCC α1, then, could modulate Cd2+-mediated testicular damage. A study by Benoff et al. found that microdeletions in exons 7–8 in the cation-conducting pore of L-VDCC α1 alter the structure of the gate, thereby further decreasing the selectivity of the cation-conducting pore. This L-VDCC α1 variant more easily permits Cd2+ flux at the expense of Ca2+ [47], which could result in a disturbance in Ca2+ homeostasis within developing sperm cells that mediates apoptosis and disrupts Ca2+-mediated sperm functions such as the acrosomal reaction. We posit, then, that microdeletions in L-VDCC α1 in men with varicocele could contribute to varicocele-associated infertility.
Conclusion
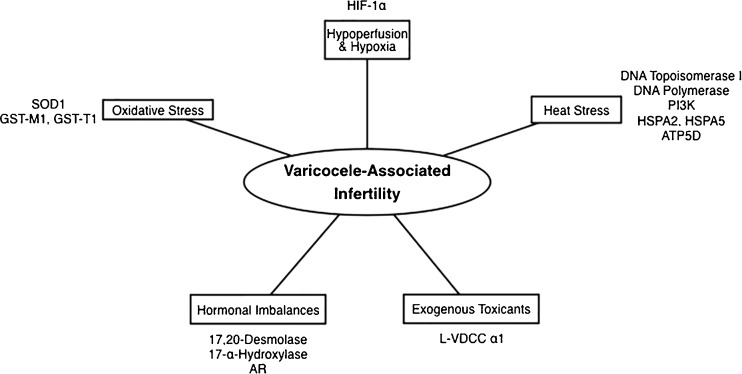
Varicocele is a pathologic enlargement of the testicular veins within the pampiniform plexus that can affect testicular function and lead to infertility. While the pathophysiologic mechanisms that lead to such varicocele-associated infertility are not completely understood, the five following mechanisms are thought to contribute to the effect of varicocele on testicular function: (1) hypoperfusion leading to hypoxia, (2) heat stress, (3) oxidative stress, (4) hormonal imbalances, and (5) exogenous toxicants. We hypothesize that molecular and genetic differences could exist among men with varicocele, and that these differences could be responsible for whether or not varicocele is associated with male infertility (Fig. 1).
Fig. 1.
Summary of the molecular and genetic mechanisms identified to date that could contribute to the pathogenesis of varicocele-associated infertility. HIF-1α hypoxia-inducible factor-1α, PI3K phosphatidylinositol 3-kinase, HSPA2/5 heat shock protein A2/5, ATP5D adenosine triphosphate synthase (ATPase), delta subunit, L-VDCC α1 L-type voltage dependent calcium channel, α1 isotype, AR androgen receptor, SOD1 superoxide dismutase 1, GST-M1/T1 glutathione S-transferase M1/T1
Still, continued research efforts could lead to a deeper understanding of the pathophysiology of varicocele-associated infertility and could lead to established clinical guidelines that determine which patients with varicocele will benefit from intervention. We expect that this review will incite research efforts that continue to elucidate the molecular and genetic basis of varicocele-associated infertility.
Acknowledgments
Disclosure statement
The authors have no conflicts of interest to declare.
Author contributions
M.M.S., R.R., and D.J.L. contributed equally in writing the manuscript.
Footnotes
Capsule Varicocele and male infertility.
References
- 1.Dubin L, Amelar RD. Varicocele size and results of varicocelectomy in selected subfertile men with varicocele. Fertil Steril. 1970;21(8):606–9. doi: 10.1016/s0015-0282(16)37684-1. [DOI] [PubMed] [Google Scholar]
- 2.Clarke BG. Incidence of varicocele in normal men and among men of different ages. JAMA. 1966;198:1121–2. doi: 10.1001/jama.1966.03110230137039. [DOI] [PubMed] [Google Scholar]
- 3.Skoog SJ, Roberts KP, Goldstein M, Pryor JL. The adolescent varicocele: what’s new with an old problem in young patients? Pediatrics. 1997;100(1):112–21. doi: 10.1542/peds.100.1.112. [DOI] [PubMed] [Google Scholar]
- 4.Eisenberg ML, Lipshultz LI. Varicocele-induced infertility: newer insights into its pathophysiology. Indian J Urol. 2011;27:58–64. doi: 10.4103/0970-1591.78428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Naughton CK, Nangia AK, Agarwal A. Pathophysiology of varicoceles in male infertility. Hum Reprod Update. 2011;7:461–72. doi: 10.1093/humupd/7.5.473. [DOI] [PubMed] [Google Scholar]
- 6.Raman JD, Walmsley K, Goldstein M. Inheritance of varicoceles. Urology. 2005;65(6):1186–9. doi: 10.1016/j.urology.2004.12.057. [DOI] [PubMed] [Google Scholar]
- 7.Sweeney TE, Rozum JS, Desjardins C, Gore RW. Microvascular pressure distribution in the hamster testis. Am J Physiol. 1991;260(5 Pt 2):H1581–9. doi: 10.1152/ajpheart.1991.260.5.H1581. [DOI] [PubMed] [Google Scholar]
- 8.Gat Y, Zukerman Z, Chakraborty J, Gornish M. Varicocele, hypoxia and male infertility. Fluid Mechanics analysis of the impaired testicular venous drainage system. Hum Reprod. 2005;20(9):2614–9. doi: 10.1093/humrep/dei089. [DOI] [PubMed] [Google Scholar]
- 9.Lee JD, Jeng SY, Lee TH. Increased expression of hypoxia-inducible factor-1alpha in the internal spermatic vein of patients with varicocele. J Urol. 2006;175(3 Pt 1):1045–8. doi: 10.1016/S0022-5347(05)00417-9. [DOI] [PubMed] [Google Scholar]
- 10.Wang H, Sun Y, Wang L, Xu C, Yang Q, Liu B, et al. Hypoxia-induced apoptosis in the bilateral testes of rats with left-sided varicocele: a new way to think about varicocele. J Androl. 2010;31:299–305. doi: 10.2164/jandrol.108.007153. [DOI] [PubMed] [Google Scholar]
- 11.Dahl EV, Herrick JF. A vascular mechanism for maintaining testicular temperature by counter-current exchange. Surg Gynaecol Obstet. 1959;108(6):697–705. [PubMed] [Google Scholar]
- 12.Goldstein M, Eid JF. Elevation of intratesticular and scrotal skin surface temperature in men with varicocele. J Urol. 1989;142(3):743–5. doi: 10.1016/s0022-5347(17)38874-2. [DOI] [PubMed] [Google Scholar]
- 13.Green KF, Turner TT, Howards SS. Varicocele: reversal of the testicular blood flow and temperature effects by varicocele repair. J Urol. 1984;131(6):1208–11. doi: 10.1016/s0022-5347(17)50874-5. [DOI] [PubMed] [Google Scholar]
- 14.Paduch DA, Skoog SJ. Current management of adolescent varicocele. Rev Urol. 2001;3(3):120–33. [PMC free article] [PubMed] [Google Scholar]
- 15.Gorelick JI, Goldstein M. Loss of fertility in men with varicocele. Fertil Steril. 1993;59(3):613–6. [PubMed] [Google Scholar]
- 16.Zorgniotti AW, Macleod J. Studies in temperature, human semen quality, and varicocele. Fertil Steril. 1973;24(11):854–63. [PubMed] [Google Scholar]
- 17.Fujisawa M, Yoshida S, Kojima K, Kamidono S. Biochemical changes in testicular varicocele. Arch Androl. 1989;22(2):149–59. doi: 10.3109/01485018908986765. [DOI] [PubMed] [Google Scholar]
- 18.Fujisawa M, Yoshida S, Matsumoto O, Kojima K, Kamidono S. Decrease of topoisomerase I activity in the testes of infertile men with varicocele. Arch Androl. 1988;21(1):45–50. doi: 10.3109/01485018808986732. [DOI] [PubMed] [Google Scholar]
- 19.Fujisawa M, Yoshida S, Matsumoto O, Kojima K, Kamidono S. Deoxyribonucleic acid polymerase activity in the testes of infertile men with varicocele. Fertil Steril. 1988;50(5):795–800. doi: 10.1016/s0015-0282(16)60318-7. [DOI] [PubMed] [Google Scholar]
- 20.De Amicis F, Perrotta I, Santoro M, Guido C, Morelli C, Cesario MG, et al. Human sperm anatomy: different expression and localization of phosphatidylinositol 3-kinase in normal and varicocele human spermatozoa. Ultrastruct Pathol. 2013;37(3):176–82. doi: 10.3109/01913123.2013.763881. [DOI] [PubMed] [Google Scholar]
- 21.Hosseinifar H, Gourabi H, Salekdeh GH, Alikhani M, Mirshahvaladi S, Sabbaghian M, et al. Study of sperm protein profile in men with and without varicocele using two-dimensional gel electrophoresis. Urology. 2013;81:293–300. doi: 10.1016/j.urology.2012.06.027. [DOI] [PubMed] [Google Scholar]
- 22.Lima SB, Cenedeze MA, Bertolla RP, Filho PA, Oehninger S, Cedenho AP. Expression of the HSPA2 gene in ejaculated spermatozoa from adolescents with and without varicocele. Fertil Steril. 2006;86:1659–63. doi: 10.1016/j.fertnstert.2006.05.030. [DOI] [PubMed] [Google Scholar]
- 23.Griveau JF, Le Lannou D. Reactive oxygen species and human spermatozoa: physiology and pathology. Int J Androl. 1997;20(2):61–9. doi: 10.1046/j.1365-2605.1997.00044.x. [DOI] [PubMed] [Google Scholar]
- 24.Sharma RK, Agarwal A. Role of reactive oxygen species in male infertility. Urology. 1996;48(6):835–50. doi: 10.1016/S0090-4295(96)00313-5. [DOI] [PubMed] [Google Scholar]
- 25.de Lamirande E, Gagnon C. Capacitation-associated production of superoxide anion by human spermatozoa. Free Radic Biol Med. 1995;18(3):487–95. doi: 10.1016/0891-5849(94)00169-K. [DOI] [PubMed] [Google Scholar]
- 26.de Lamirande E, Lamothe G. Reactive oxygen-induced reactive oxygen formation during human sperm capacitation. Free Radic Biol Med. 2009;46(4):502–10. doi: 10.1016/j.freeradbiomed.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 27.Agarwal A, Nallella KP, Allamaneni SS, Said TM. Role of antioxidants in treatment of male infertility: an overview of the literature. Reprod Biomed Online. 2004;8(6):616–27. doi: 10.1016/S1472-6483(10)61641-0. [DOI] [PubMed] [Google Scholar]
- 28.Kodama H, Kuribayashi Y, Gagnon C. Effect of sperm lipid peroxidation on fertilization. J Androl. 1996;17(2):151–7. [PubMed] [Google Scholar]
- 29.Weese DL, Peaster ML, Himsl KK, Leach GE, Lad PM, Zimmern PE. Stimulated reactive oxygen species generation in the spermatozoa of infertile men. J Urol. 1993;149(1):64–7. doi: 10.1016/s0022-5347(17)36000-7. [DOI] [PubMed] [Google Scholar]
- 30.Sharma RK, Pasqualotto FF, Nelson DR, Thomas AJ, Argawal A. The reactive oxygen species-total antioxidant capacity score is a new measure of oxidative stress to predict male infertility. Hum Reprod. 1999;14:2801–7. doi: 10.1093/humrep/14.11.2801. [DOI] [PubMed] [Google Scholar]
- 31.Li F, Yamaguchi K, Okada K, Matsushita K, Ando M, Chiba K, et al. Significant improvement of sperm DNA quality after microsurgical repair of varicocele. Syst Biol Reprod Med. 2012;58(5):274–7. doi: 10.3109/19396368.2012.692431. [DOI] [PubMed] [Google Scholar]
- 32.Agarwal A, Hamada A, Esteves SC. Insight into oxidative stress in varicocele-associated male infertility: part 1. Nat Rev Urol. 2012;12:678–90. doi: 10.1038/nrurol.2012.197. [DOI] [PubMed] [Google Scholar]
- 33.Koksal IT, Tefekli A, Usta M, Erol H, Abbasoglu S, Kadioglu A. The role of reactive oxygen species in testicular dysfunction associated with varicocele. BJU Int. 2000;86(4):549–52. doi: 10.1046/j.1464-410X.2000.00755.x. [DOI] [PubMed] [Google Scholar]
- 34.Aitken RJ, Baker MA. Oxidative stress, sperm survival and fertility control. Mol Cell Endocrinol. 2006;250(1–2):66–9. doi: 10.1016/j.mce.2005.12.026. [DOI] [PubMed] [Google Scholar]
- 35.Spiropoulos J, Turnbull DM, Chinnery PF. Can mitochondrial DNA mutations cause sperm dysfunction? Mol Hum Reprod. 2002;8:719–21. doi: 10.1093/molehr/8.8.719. [DOI] [PubMed] [Google Scholar]
- 36.Sharma RK, Said T, Agarwal A. Sperm DNA damage and its clinical relevance in assessing reproductive outcome. Asian J Androl. 2004;6(2):139–48. [PubMed] [Google Scholar]
- 37.Aitken RJ, Krausz C. Oxidative stress, DNA damage and the Y chromosome. Reproduction. 2001;122(4):497–506. doi: 10.1530/rep.0.1220497. [DOI] [PubMed] [Google Scholar]
- 38.Chen SS, Chang LS, Chen HW, Wei YH. Polymorphisms of glutathione S-transferase M1 and male infertility in Taiwanese patients with varicocele. Hum Reprod. 2002;17(3):718–25. doi: 10.1093/humrep/17.3.718. [DOI] [PubMed] [Google Scholar]
- 39.Ichioka K, Nagahama K, Okubo K, Soda T, Ogawa O, Nishiyama H. Genetic polymorphisms in glutathione S-transferase T1 affect the surgical outcome of varicocelectomies in infertile patients. Asian J Androl. 2009;11(3):333–41. doi: 10.1038/aja.2008.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rajfer J, Turner TT, Rivera F, Howards SS, Sikka SC. Inhibition of testicular testosterone biosynthesis following experimental varicocele in rats. Biol Reprod. 1987;36(4):933–7. doi: 10.1095/biolreprod36.4.933. [DOI] [PubMed] [Google Scholar]
- 41.Zalata AA, Mokhtar N, Badawy Ael N, Othman G, Alghobary M, Mostafa T. Androgen receptor expression relationship with semen variables in infertile men with varicocele. J Urol. 2013;189(6):2243–7. doi: 10.1016/j.juro.2012.11.112. [DOI] [PubMed] [Google Scholar]
- 42.Vine MF, Tse CK, Hu P, Truong KY. Cigarette smoking and semen quality. Fertil Steril. 1996;65(4):835–42. doi: 10.1016/s0015-0282(16)58223-5. [DOI] [PubMed] [Google Scholar]
- 43.Benoff SH, Millan C, Hurley IR, Napolitano B, Marmar JL. Bilateral increased apoptosis and bilateral accumulation of cadmium in infertile men with left varicocele. Hum Reprod. 2004;19(3):616–27. doi: 10.1093/humrep/deh139. [DOI] [PubMed] [Google Scholar]
- 44.Jeng SY, Wu SM, Lee JD. Cadmium accumulation and metallothionein overexpression in internal spermatic vein of patients with varicocele. Urology. 2009;73(6):1231–5. doi: 10.1016/j.urology.2009.01.008. [DOI] [PubMed] [Google Scholar]
- 45.Benoff S, Hurley IR, Barcia M, Mandel FS, Cooper GW, Hershlag A. A potential role for cadmium in the etiology of varicocele-associated infertility. Fertil Steril. 1997;67(2):336–47. doi: 10.1016/S0015-0282(97)81921-8. [DOI] [PubMed] [Google Scholar]
- 46.Benoff S, Marmar JL, Hurley IR. Molecular and other predictors for infertility in patients with varicoceles. Front Biosci (Landmark Ed) 2009;14:3641–72. doi: 10.2741/3478. [DOI] [PubMed] [Google Scholar]
- 47.Benoff S, Goodwin LO, Millan C, Hurley IR, Pergolizzi RG, Marmar JL. Deletions in L-type calcium channel alpha1 subunit testicular transcripts correlate with testicular cadmium and apoptosis in infertile men with varicoceles. Fertil Steril. 2005;83(3):622–34. doi: 10.1016/j.fertnstert.2004.07.976. [DOI] [PubMed] [Google Scholar]