Abstract
Purpose
To evaluate the ovarian response to controlled ovarian hyperstimulation (COH) in cancer patients according to an age-specific nomogram for the number of retrieved oocytes.
Methods
Retrospective observational study carried out in a University affiliated fertility clinic. Forty-eight patients with cancer underwent ovarian stimulation for oocyte cryopreservation. An age - specific nomogram for the number of retrieved oocytes was built with 1536 IVF cycles due to male factor exclusively, oocyte donation and age related fertility preservation. The number of oocytes retrieved in cancer patients was compared to the expected response according to the nomogram using the Z-score.
Results
The mean number of total retrieved oocytes in patients with cancer was 14.04 ± 8.83. After applying the Z-score to compare the number of retrieved oocytes between women with cancer and the expected response according to the age-specific nomogram, we did not observe a statistically significant difference (Z-score 0.23; 95 % CI [−0.13-0.60]).
Conclusion(s)
According to our results, patients with cancer exhibit an ovarian response as expected by age. Despite the limitation of the sample size, the obtained results should encourage oncologists for early referral of women with cancer to fertility specialists.
Keywords: Fertility preservation, Cancer, Controlled ovarian hyperstimulation, Ovarian response, Nomogram
Introduction
Fertility preservation (FP) is becoming an important issue in the approach of oncologic patients due to increased survival rates and delayed childbearing.
An estimated 1 out of 47 women will be diagnosed with cancer by age 40 years. The most common cancers in reproductive aged women are breast, melanoma, cervical, non-Hodgkin’s lymphoma and leukemia; the 5-year survival rate of these tumors ranges from 50 to 68 %, being 90 % for breast cancer [1].
The negative impact of cancer treatments on female fertility is well known and depends on patient’s age, dose and type of chemotherapeutic agent and field of irradiation. Alkylating agents, abdominal, pelvic or total body irradiation and bone marrow transplantation represent the maximum risk of gonadal damage [2].
The psychological burden related to this fertility loss is substantial in 77 % of patients [3]; thus, referral to the fertility specialist for FP counselling prior to the oncologic treatment start is recommended.
There are numerous strategies for FP in the female patient; the choice will depend on the age of the patient, social status, type of disease, treatment required and time availability before the onset of chemotherapy [4]. Combination of several strategies may improve the chances of success [5].
Oocyte cryostorage is one of the major approaches of FP as no surgery is required, it has already resulted in many live births and it can be achieved with mild stimulation protocols that last in average 12 days [6]. Even more, oocyte cryopreservation is no longer considered an experimental strategy for FP purposes [7].
However, concern has been raised regarding a potential negative impact of the cancer process itself on the ovarian function and the response to controlled ovarian hyperstimulation (COH), even before the start of oncologic treatment [8].
Whereas several studies have shown that malignancies, especially testicular cancer and lymphoma, in male patients adversely affect fertility before the initiation of the oncologic treatment [9], in women data are conflicting. An adverse influence of malignancy on oocyte biology was first reported by Pal et al. [10]. An increased catabolic state, malnutrition and increased stress hormone levels may affect the hypothalamic – gonadal axis and impair fertility; moreover, BRCA1 mutation has been linked to occult primary ovarian insufficiency [11]. A recent meta-analysis on ovarian performance of women with cancer undergoing COH for FP before oncologic treatment concludes that women with malignancy should expect a lower number of oocytes retrieved, compared with healthy age-matched patients [8]. Another systematic review states that malignancies in general do not affect the number of oocytes retrieved, although ovarian response was significantly lower in hormone-dependent neoplasias [12].
So far, studies investigating the effect of cancer on ovarian response have used age-matched patients as controls. Despite the usefulness of matching to control for confounding factors, not all the variability of the studied parameter may be taken into account when selecting controls; moreover, some authors refer incorrectly to “matching” just because the two groups are similar in the distribution of the matching variable [13].
The purpose of this study is to evaluate the ovarian response to COH in women with cancer and to compare it to the expected response according to an age-specific nomogram for the number of retrieved oocytes, using the standardized unit Z-score.
Material and methods
Study population and design
This is a retrospective observational study that includes 48 women with cancer that underwent COH for embryo or oocyte cryopreservation for FP between 2007 and 2012 in a university-affiliated clinic. Subjects that had received oncologic treatment before stimulation were excluded from the study.
Stimulation protocol
Depending upon the cycle day of the patient at the time of consultation, stimulation was initiated in the early follicular phase (classical stimulation protocol) or “at random”. In “random start” cycles, GnRH antagonist (0.25 mg/day) was administered for 3 days and stimulation was started afterwards.
Stimulation was carried out with rFSH (initial doses ranged from 200 UI to 375 IU/day, according to age and ovarian reserve tests; adjustments could be done according to subsequent response).
Letrozole 5 mg/day was added in all cases of hormone-dependent cancers from the stimulation start until the arrival of next menses.
GnRH antagonist (0.25 mg/day) was administered when a leading follicle of 14 mm was detected on the ultrasound scan until the day of ovulation trigger to prevent spontaneous premature LH surge.
When at least 3 follicles reached to 20 mm, ovulation was triggered with rhCG 250 μg or with GnRH agonist triptorelin 0.2 mg if there was a risk of ovarian hyperstimulation syndrome.
Oocyte retrival was performed transvaginally 36 h after ovulation trigger.
Outcomes
The main outcome was the number of retrieved oocytes, standardized by age and transformed into Z-score.
The following results were collected: basal FSH, AMH, antral follicle count (AFC), total gonadotropin consumption, duration of stimulation, peak serum estradiol level, number of retrieved oocytes and number of mature oocytes.
Statistical analysis
The expected ovarian response was calculated with an age-specific nomogram for the number of retrieved oocytes, which was built with 1536 IVF cycles due to male factor exclusively, oocyte donation and age-related fertility preservation; these cycles were performed at our Institution between 2010 and 2013 all under a GnRH antagonist protocol.
Construction of the curves for the nomogram was produced by GAMLSS method with R software [14, 15].
The Z-score was used to compare the number of retrieved oocytes between women with cancer and the expected response according to the nomogram.
The Z-score was defined as the number of oocytes retrieved in oncologic patients minus the mean number of oocytes retrieved in the reference population used to build the nomogram (at the same age) divided by the standard deviation (at the same age).
We calculated the 95 % confidence interval (CI) for the Z-scores. A confidence interval including zero entails that there aren’t statistically significant differences between the studied population and the reference population.
Analysis of variance (ANOVA) was performed to compare the means of Z-scores between the different variables taking into account letrozole use or not, time of stimulation start, type of ovulation trigger and the interaction among all of them.
Ethical approval
The study was approved by the Institution Review Board (IRB) (CIOG 18012012/01).
This report was prepared according to the STROBE statement [16].
Results
Forty-eight subjets underwent COH and oocyte retrieval before cancer treatment for FP purposes.
Stimulation was started in the early follicular phase in 79 % of cases, whereas 10 patients were not in the early follicular phase at the time of consultation and a “random start” cycle was performed. Letrozole was added in 28 cases (26 breast cancers and 2 endometrial cancers). Ovulation was triggered with rhCG in 29 cases and with GnRH agonist in the remaining 19 cases.
Gynaecological cancer accounted for 77 % of cases; haematological malignancies for 15 % and the remaining 8 % were malignancies of other origins (Table 1).
Table 1.
Cancer types
| Type of cancer | N = 48 |
|---|---|
| Gynecological - Breast - Ovarian cancer - Endometrial cancer - Endometrial stromal sarcoma - Cervical cancer |
37 26 7 2 1 1 |
| Haematological - Hodgkin lymphoma - Non-Hodgkin lymphoma - Leucemia |
7 5 1 1 |
| Other origins - Melanoma - Condrosarcoma - Ependimoma - Medullary astrocytoma |
4 1 1 1 1 |
Mean age of patients with cancer was 32.81 ± 4.07 years. Patients’ hormonal profile and AFC are shown in Table 2. The mean number of total retrieved oocytes was 14.04 ± 8.83. Mean duration of stimulation was 9.5 ± 2.33 days. Other stimulation parameters are displayed in Table 2.
Table 2.
Patients’ baseline characteristics and stimulation parameters
| Cancer patients | N = 48 |
|---|---|
| Age (y) | 32.81 ± 4.07 |
| AFC | 12.89 ± 5.6 |
| Basal FSH (IU/l) | 6.65 ± 2 |
| AMH (ng/ml) | 2.16 ± 1.49 |
| Total gonadotropin consumption (IU) | 3350 ± 964 |
| Peak serum E2 levels (pg/ml) | 1134 ± 756 |
| Duration of stimulation (days) | 9.5 ± 2.33 |
| No. of oocytes retrieved | 14.04 ± 8.83 |
| No. of MII oocytes | 11.38 ± 8.84 |
Values are expressed as mean ± standard deviation
AFC antral follicle count; E2 estradiol; MII metaphase II
The age–specific nomogram for the number of retrieved oocytes can be seen in Table 3, in which several centiles are shown as a function of age.
Table 3.
Age – specific nomogram for the number of retrieved oocytes (built with data from cycles due to male factor infertility, oocyte donation and age-related fertility preservation)
| Age (y) | 5th | 10th | 25th | 50th | 75th | 90th | 95th |
|---|---|---|---|---|---|---|---|
| 18 | 6 | 7 | 11 | 15 | 20 | 26 | 30 |
| 19 | 6 | 7 | 11 | 15 | 20 | 26 | 30 |
| 20 | 6 | 7 | 11 | 15 | 20 | 26 | 30 |
| 21 | 6 | 7 | 10 | 15 | 20 | 26 | 29 |
| 22 | 6 | 7 | 10 | 15 | 20 | 26 | 29 |
| 23 | 6 | 7 | 10 | 15 | 20 | 25 | 29 |
| 24 | 5 | 7 | 10 | 15 | 20 | 25 | 29 |
| 25 | 5 | 7 | 10 | 14 | 20 | 25 | 29 |
| 26 | 5 | 7 | 10 | 14 | 19 | 25 | 28 |
| 27 | 5 | 7 | 10 | 14 | 19 | 24 | 28 |
| 28 | 5 | 7 | 10 | 14 | 19 | 24 | 27 |
| 29 | 5 | 7 | 10 | 14 | 18 | 23 | 26 |
| 30 | 5 | 7 | 9 | 13 | 18 | 23 | 26 |
| 31 | 5 | 6 | 9 | 13 | 17 | 22 | 25 |
| 32 | 5 | 6 | 9 | 12 | 17 | 21 | 24 |
| 33 | 4 | 6 | 8 | 12 | 16 | 21 | 24 |
| 34 | 4 | 5 | 8 | 11 | 16 | 20 | 23 |
| 35 | 4 | 5 | 7 | 11 | 15 | 19 | 22 |
| 36 | 3 | 5 | 7 | 10 | 15 | 19 | 22 |
| 37 | 3 | 4 | 6 | 10 | 14 | 18 | 21 |
| 38 | 3 | 4 | 6 | 9 | 14 | 18 | 21 |
| 39 | 2 | 3 | 6 | 9 | 13 | 18 | 21 |
| 40 | 2 | 3 | 5 | 8 | 13 | 17 | 21 |
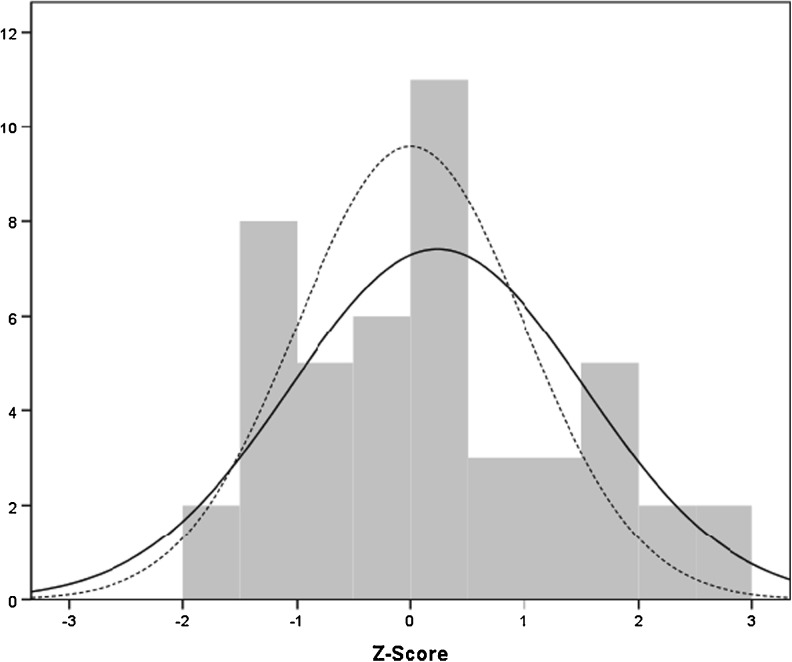
After applying the Z-score to compare the number of retrieved oocytes between women with cancer (in general) and the expected response according to the nomogram, we did not observe a statistically significant difference (Z-score 0.23; 95 % CI [−0.13–0.60]) (Fig. 1).
Fig. 1.
Z score. Continuous line: empiric response in patients with cancer. Discontinuous line: expected response according to the nomogram. Mean: 0.23. Standard deviation: 1.29. N = 48
We further applied the Z-score to compare the number of retrieved oocytes with each stimulation protocol (with letrozole in hormone-dependent cancers, without letrozole in non hormone-dependent cancers, follicular start, random start, rhCG trigger, GnRH agonist trigger) in cancer patients to the age-specific nomogram (Z-scores and CI shown in Table 4). Differences were not statistically significant except for the groups with letrozole (hormone-dependent tumors) and GnRH agonist trigger, in which the number of oocytes retrieved was significantly higher than the expected by age. The letrozole group continued to perform better than the expected by age after adjusting for the moment of stimulation start (Z-score 0.63; 95 % CI [0.16–1.10]) and the type of trigger (Z-score 0.70; 95 % CI [0.25–1.15]). Patients triggered with GnRH agonist continued to perform better than the expected by age after adjusting for the moment of stimulation start (Z-score 0.72; 95 % CI [0.12–1.32]) but the statistical significance was lost when we adjusted for the use or not of letrozole (Z-score 0.50; 95 % CI [−0.40–1.08]). The interaction among the three variables (letrozole use or not, time of stimulation start and type of trigger) was not statistically significant.
Table 4.
Z-scores and 95 % CI (compared with the age-specific nomogram)
| Cancer patients | Z-score | 95 % CI |
|---|---|---|
| All cancer patients (regardless of stimulation protocol) | 0.23 | −0.13–0.60 |
| Letrozole yes/no | ||
| - With letrozole (hormone-dependent tumors) - Without letrozole (non hormone-dependent tumors) |
0.71 −0.38 |
0.18–1.24 a
−0.80–0.03 |
| Start of stimulation | ||
| - Early follicular - Random start |
0.20 0.33 |
−0.21–0.63 −0.64–1.32 |
| Type of trigger | ||
| - rhCG - GnRH agonist |
−0.09 0.74 |
−0.57–0.38 0.15–1.32 a |
CI confidence interval
astatistically significant
Discussion
According to our results, ovarian response to COH in patients with cancer is as expected by age, as no significant differences were observed between the oocyte yield in women with malignancies and the expected one according to the age-specific nomogram.
The main aim of the study was to evaluate ovarian response in cancer patients and compare it to the expected one according to an age-specific nomogram; the other results obtained in each subgroup analysis should be interpreted with caution as more cases are needed in order to clarify whether they are due to the low number of cases, the type of malignancies or the stimulation protocol used. A potential increase of the gonadotrophin effect due to the use of aromatase inhibitors cannot be ruled out [17].
Previous studies assessing the ovarian reserve and the ovarian response in cancer patients have shown conflicting results. The majority of published studies regarding ovarian reserve have not demonstrated significant differences between patients with malignancy and their age–matched controls [18–21], although some authors have reported significantly lower AMH levels in patients with lymphoma [22] and lower AFC in women with cancer than in healthy controls [23]. Analyzing the ovarian reserve status was not the main goal of the current study but the observed data do not seem to reveal diminished ovarian reserve (Table 1).
As far as ovarian response is concerned, several studies have found a similar number of retrieved oocytes between cancer patients and the control group [18, 20, 21, 24–27], which is in agreement with the current findings. However, in one of the largest studies in which 223 cancer patients were included, patients with malignancy exhibited a weaker response to COH, especially those with hormone-dependent tumors. It is important to notice that in that study, patients with non hormone-dependent tumors showed a similar number of retrieved oocytes to controls [28]. Another study of the same group compared the ovarian response between oncologic patients and patients undergoing FP due to non oncological reasons and obtained comparable results in both groups; the main strength of that study is the big sample size, however, the comparison was done with an heterogeneous group that included age related fertility preservation, endometriosis and genetic diseases among others [29].
Two meta-analysis including some of the above mentioned studies conclude differently; in one of them 7 studies are included and the authors’ conclusion is that women with malignant disease should expect a lower number of oocytes retrieved after COH [8], whereas the other meta-analysis including 10 studies (the same seven of the former meta-analysis and 3 studies more recently published) concluded that malignancies do not affect the number of oocytes retrieved in general; even though women with hormone-dependent neoplasia have a significantly lower oocyte yield [12].
It is probable that the observed discrepancy between the different studies is due to the lack of larger sample sizes and heterogeneity in the malignancies included as well as the stimulation protocols used. The differences in the methodology used to choose the control groups may also influence the results.
The current study has some limitations, which are shared with most of the previously published ones, such as a small sample size, retrospective nature and type of cancer and protocol dependent bias; however it has some strengths related to the statistical methodology used to compare the ovarian response, that distinguish it from the studies published up to now.
The majority of the recent studies comparing the ovarian response in oncologic patients have used age-matched patients as controls and many do not specify how the matching was done (see Table 5). Matching technique is useful in case–control studies because it helps control confounding factors that may affect the studied variable and therefore, it makes groups more comparable. Nevertheless, some authors use the term “matched” to mean that the two groups are similar in the distribution of the matching variables, but not that there is individual matching of each case to his or her own control; such studies should not be considered as matched [13]. Moreover, the matching technique has a limitation related to the selection bias when choosing controls, as controls may not gather all the variability of the studied parameter and thus, groups would not be comparable any more. The main strength of the current study is that, in order to avoid this bias, a reference age-specific nomogram for the number of retrieved oocytes was built with an extensive number of IVF cycles downregulated with GnRH antagonists (as cancer patients) and performed in supposedly healthy women (exclusively male factor infertility, oocyte donation and age-related fertility preservation). Subsequently, we calculated the differences between the observed values and the expected ones according to age with the standardized Z-score.
Table 5.
Recent studies comparing ovarian response to COH in cancer patients
| Author | N | Comparison technique | Type of matching | No. of oocytes retrieved |
|---|---|---|---|---|
| Knopman et al. [26] | 28 cases 135 controls |
Age-matched controls Male factor infertility |
Ratio 1:4 | Differences NS |
| Noyes et al. [28] | 50 cases 32 controls |
Not matched | – | Differences NS |
| Quintero et al. [27] | 50 cases 50 controls |
Age-matched. Male factor/oocyte cryopreservation/oocyte donation |
Not specified | Differences NS |
| Das et al. [20] | 41 cases 48 controls |
Age-matched. Male factor infertility, 1st IVF cycles |
Not specified | Differences NS |
| Domingo et al. [30] | 223 cases 97 controls |
Age-matched Male factor infertility |
Not specified | Significant differences (lower in cancer patients) |
| Almog et al. [29] | 81 cases 81 controls |
Matched by age and closest date of stimulation Male factor infertility |
1:1 | Differences NS |
| G-Velasco et al. [31] | 355 oncological 560 non oncological |
Not matched | Differences NS | |
| Johnson et al. [22] | 50 cases 50 controls |
Matched by age, race, IVF cycle No., date of stimulation and fertilization method Male or tubal factor infertility and oocyte donors |
1:1 | Differences NS |
| Devesa et al. (current study) | 48 cases Age-specific nomogram (1536 cycles) |
Z-score 1st IVF cycles due to male factor infertility, oocyte donation and age-related fertility preservation |
Differences NS |
NS not significant
The authors consider that future studies should analyze each type of cancer separately and compare it to a nomogram in which the cycles included were performed with exactly the same protocol, in order to rule out both type of cancer and protocol dependent bias. Nevertheless, generally no statistically significant differences in the oocyte yield have been reported regarding the type of ovulation trigger [30], the time of stimulation start [31] or the use of letrozole [31, 32].
In conclusion, women with malignancy respond to COH as expected by age. This finding supports the role of oocyte cryopreservation as a means of FP and should encourage prompt referrals of patients from the oncology team to the reproductive endocrinologist after the diagnosis of cancer.
Acknowledgments
This work was performed under the auspices of “Catedra d’Investigació en Obstetricia i Ginecología” of Department of Obstetrics, Gynaecology and Reproductive Medicine; Hospital Universitari Quirón-Dexeus, Universitat Autònoma de Barcelona.
Footnotes
Capsule
Women with malignancy respond de COH as expected by age.
References
- 1.Matthews ML, Hurst BS, Marshburn PB, Usadi RS, Papadakis MA, Sarantou T. Cancer, fertility preservation, and future pregnancy: a comprehensive review. Obstet Gynecol Int. 2012;2012:953937. doi: 10.1155/2012/953937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Noyes N, Knopman JM, Melzer K, Fino ME, Friedman B, Westphal LM. Oocyte cryopreservation as a fertility preservation measure for cancer patients. Reprod Biomed Online. 2011;23:323–333. doi: 10.1016/j.rbmo.2010.11.011. [DOI] [PubMed] [Google Scholar]
- 3.Carter J, Chi DS, Brown CL, Abu-Rustum NR, Sonoda Y, Aghajanian C, et al. Cancer-related infertility in survivorship. Int J Gynecol Cancer. 2010;20:2–8. doi: 10.1111/IGC.0b013e3181bf7d3f. [DOI] [PubMed] [Google Scholar]
- 4.Martínez F, Devesa M, Coroleu B, Tur R, González C, Boada M, et al. Cancer and fertility preservation: Barcelona consensus meeting. Gynecol Endocrinol. 2013;29:285–291. doi: 10.3109/09513590.2012.743019. [DOI] [PubMed] [Google Scholar]
- 5.González C, Devesa M, Boada M, Coroleu B, Veiga A, Barri PN. Combined strategy for fertility preservation in an oncologic patient: vitrification of in vitro matured oocytes and ovarian tissue freezing. J Assist Reprod Genet. 2011;28:1147–1149. doi: 10.1007/s10815-011-9628-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Revelli A, Molinari E, Salvagno F, Delle Piane L, Dolfin E, Ochetti S. Oocyte cryostorage to preserve fertility in oncological patients. Obstet Gynecol Int. 2012;2012:525896. doi: 10.1155/2012/525896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mature oocyte cryopreservation: a guideline. Fertil Steril. 2013;99:37–43. [DOI] [PubMed]
- 8.Friedler S, Koc O, Gidoni Y, Raziel A, Ron-El R. Ovarian response to stimulation for fertility preservation in women with malignant disease: a systematic review and meta-analysis. Fertil Steril. 2012;97:125–133. doi: 10.1016/j.fertnstert.2011.10.014. [DOI] [PubMed] [Google Scholar]
- 9.van Casteren NJ, Boellaard WP, Romijn JC, Dohle GR. Gonadal dysfunction in male cancer patients before cy- totoxic treatment. Int J Androl. 2010;33:73–79. doi: 10.1111/j.1365-2605.2009.00956.x. [DOI] [PubMed] [Google Scholar]
- 10.Pal L, Leykin L, Schifren JL, Isaacson KB, Chang YC, Nikruil N, et al. Malignancy may adversely influence the quality and behaviour of oocytes. Hum Reprod. 1998;13:1837–1840. doi: 10.1093/humrep/13.7.1837. [DOI] [PubMed] [Google Scholar]
- 11.Oktay K, Kim JY, Barad D, Babayev SN. Association of BRCA1 mutations with occult primary ovarian insufficiency: a possible explanation for the link between infertility and breast/ovarian cancer risks. J Clin Oncol. 2010;28:240–244. doi: 10.1200/JCO.2009.24.2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tulandi T, Holzer H. Effects of malignancies on the gonadal function. Fertil Steril. 2012;98:813–815. doi: 10.1016/j.fertnstert.2012.05.010. [DOI] [PubMed] [Google Scholar]
- 13.Bland JM, Altman DG. Matching. BMJ. 1994;309:1128. doi: 10.1136/bmj.309.6962.1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.R: a language and environment for statistical computing. Vienna: R Foundation for Statistical Computing; 2008. [Google Scholar]
- 15.Rigby RA, Stasinopoulos DM. Generalized additive models for location, scale and shape (with discussion) Appl Stat. 2005;54:507–554. [Google Scholar]
- 16.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol. 2008;61:344–349. doi: 10.1016/j.jclinepi.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 17.Fatum M, McVeight E, Child T. The case for aromatase inhibitors use in Oncofertility patients. Should aromatase inhibitors be combined with gonadotropina treatment in Breast Cancer patients undergoing ovarian stimulation for fertiity preservation prior to chemotherapy? A debate. Hum Fertil. 2013;16:235–240. doi: 10.3109/14647273.2013.800650. [DOI] [PubMed] [Google Scholar]
- 18.Das M, Shehata F, Moria A, Holzer H, Son WY, Tulandi T. Ovarian reserve, response to gonadotropins, and oocyte maturity in women with malignancy. Fertil Steril. 2011;96:122–125. doi: 10.1016/j.fertnstert.2011.04.070. [DOI] [PubMed] [Google Scholar]
- 19.Moria A, Das M, Shehata F, Holzer H, Son W-Y, Tulandi T. Ovarian reserve and oocyte maturity in women with malignancy undergoing in vitro maturation treatment. Fertil Steril. 2011;95:1621–1623. doi: 10.1016/j.fertnstert.2010.12.041. [DOI] [PubMed] [Google Scholar]
- 20.Johnson LNC, Dillon KE, Sammel MD, Efymow BL, Mainigi MA, Dokras A, et al. Response to ovarian stimulation in patients facing gonadotoxic therapy. Reprod Biomed Online. 2013;26:337–344. doi: 10.1016/j.rbmo.2013.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Levin I, Almog B. Effect of cancer on ovarian function in patients undergoing in vitro fertilization for fertility preservation: a reappraisal. Curr Oncol. 2013;20:e1–e3. doi: 10.3747/co.20.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lawrenz B, Fehm T, von Wolff M, Soekler M, Huebner S, Henes J, et al. Reduced pretreatment ovarian reserve in premenopausal female patients with Hodgkin lymphoma or non-Hodgkin-lymphoma—evaluation by using antimüllerian hormone and retrieved oocytes. Fertil Steril. 2012;98:141–144. doi: 10.1016/j.fertnstert.2012.04.021. [DOI] [PubMed] [Google Scholar]
- 23.Ebbel E, Katz A, Kao CN, Cedars MI, Rosen MP. Reproductive aged women with cancer have a lower antral follicle count than expected. Fertil Steril. 2011;96:S199–S200. doi: 10.1016/j.fertnstert.2011.07.773. [DOI] [Google Scholar]
- 24.Knopman JM, Noyes N, Talebian S, Krey LC, Grifo JA, Licciardi F. Women with cancer undergoing ART for fertility preservation: a cohort study of their response to exogenous gonadotropins. Fertil Steril. 2009;91(4):1476–1478. doi: 10.1016/j.fertnstert.2008.07.1727. [DOI] [PubMed] [Google Scholar]
- 25.Quintero RB, Helmer A, Huang JQ, Westphal LM. Ovarian stimulation for fertility preservation in patients with cancer. Fertil Steril. 2010;93:865–868. doi: 10.1016/j.fertnstert.2008.10.007. [DOI] [PubMed] [Google Scholar]
- 26.Noyes N, Labella PA, Grifo J, Knopman JM. Oocyte cryopreservation: a feasible fertility preservation option for reproductive age cancer survivors. J Assist Reprod Genet. 2010;27:495–499. doi: 10.1007/s10815-010-9434-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Almog B, Azem F, Gordon D, Pauzner D, Amit A, Barkan G, et al. Effects of cancer on ovarian response in controlled ovarian stimulation for fertility preservation. Fertil Steril. 2012;98:957–960. doi: 10.1016/j.fertnstert.2012.06.007. [DOI] [PubMed] [Google Scholar]
- 28.Domingo J, Guillén V, Ayllón Y, Martínez M, Muñoz E, Pellicer A, et al. Ovarian response to controlled ovarian hyperstimulation in cancer patients is diminished even before oncological treatment. Fertil Steril. 2012;97:930–934. doi: 10.1016/j.fertnstert.2012.01.093. [DOI] [PubMed] [Google Scholar]
- 29.García-Velasco JA, Domingo J, Cobo A, Martínez M, Carmona L, Pellicer A. Five years’ experience using oocyte vitrification to preserve fertility for medical and nonmedical indications. Fertil Steril. 2013;99:1994–1999. doi: 10.1016/j.fertnstert.2013.02.004. [DOI] [PubMed] [Google Scholar]
- 30.Fauser BC, de Jong D, Olivennes F, Wramsby H, Tay C, Itskovitz-Eldor J, et al. Endocrine profiles after triggering of final oocyte maturation with GnRH agonist after Cotreatment with the GnRH antagonist Ganirelix during ovarian hyperstimulation for in vitro fertilization. J Clin Endocrinol Metab. 2002;87:709–715. doi: 10.1210/jcem.87.2.8197. [DOI] [PubMed] [Google Scholar]
- 31.Cakmak H, Rosen MP. Ovarian stimulation in cancer patients. Fertil Steril. 2013;99:1476–1484. doi: 10.1016/j.fertnstert.2013.03.029. [DOI] [PubMed] [Google Scholar]
- 32.Oktay K, Hourvitz A, Sahin G, Oktem O, Safro B, Cil A, et al. Letrozole reduces estrogen and gonadotropin exposure in women with breast cancer undergoing ovarian stimulation before chemotherapy. J Clin Endocrinol Metab. 2006;91:3885–3890. doi: 10.1210/jc.2006-0962. [DOI] [PubMed] [Google Scholar]