Abstract
Main purpose and research question
To determine whether the true fusogen Syncytin-1 and its receptor (ASCT-2) is present in human gametes using qRT-PCR, immunoblotting and immunofluorescence.
Methods
Donated oocytes and spermatozoa, originating from a fertility center in tertiary referral university hospital, underwent qRT-PCR, immunoblotting and immunofluorescence analyzes.
Results
Quantitative RT-PCR of sperm samples from sperm donors showed that syncytin-1 is present in all samples, however, protein levels varied between donors. Syncytin-1 immunoreactivity predominates in the sperm head and around the equatorial segment. The receptor ASCT-2 is expressed in the acrosomal region and in the sperm tail. Moreover, ASCT-2, but not syncytin-1, is expressed in oocytes and the mRNA level increases with increasing maturity of the oocytes.
Conclusions
Syncytin and its receptor are present in human gametes and localization and temporal appearance is consistent with a possible role in fusion between oocyte and sperm.
Keywords: ASCT-2, Fertilization, Oocyte, Sperm, Syncytin
Introduction
The process of fertilization has been an area of intense research for decades. Fertilization consists of a multitude of steps in which mature gametes meet and fuse at the correct time and place. Spermatozoa and cumulus- oocyte complex interaction result in adhesion, binding and penetration of the zona pellucida (ZP) by the spermatozoon. This is followed by interaction, adhesion and fusion of the inner acrosomal membrane of the spermatozoon and the oocyte plasma membrane (the oolemma) allowing genetic material of the haploid gametes to merge and form the totipotent diploid zygote [1].
The spermatozoa must undergo the process of capacitation, before they gain the capacity to interact and bind to the ZP. During capacitation, among other changes, decapacitating factors are shed and the plasma membrane is remodelled to form membrane rafts on the apical surface of the sperm head where ZP-binding proteins are concentrated [2, 3]. Capacitation enables the spermatozoa to perform the acrosome reaction (AR), in which the plasma membrane and outer acrosomal membrane fuse to release the acrosomal content [1]. The fertilization process therefore consists of two independent membrane fusion events, i.e. the AR and the fusion between the membrane of the spermatozoon and the oolemma. While SNARE fusion proteins (SNARE is an acronym derived from “SNAP (Soluble NSF Attachment Protein REceptor”)) are involved during membrane fusion in the AR [4, 5], the fusogenic molecules involved in fusion of the spermatozoon and oocyte have eluded identification [6–8].
Following ZP penetration the fertilizing spermatozoon adheres in a parallel manner to the oolemma at the equatorial segment [1]. This is the initial step leading to actual membrane fusion, hereby establishing cytoplasmic continuity. The adhesion protein Izumo-1 is located on the acrosomal region of the spermatozoon (demonstrated in mouse and human) and is only accessible following the AR [9]. Izumo-1 is essential for gamete adhesion before fusion of the inner acrosomal membrane and oolemma [9]. On the oolemma the tetraspanins CD9 and CD81 form tetraspanin webs which interact with Izumo-1, however only CD9-/- and CD81-/- double knockout mice are completely infertile, suggesting a capacity for one of the two to partly compensate for the other [10]. Tetraspanin webs are, among a multitude of properties, involved in cell-cell adhesion and viral infections [11], but is not known to have any membrane fusion capabilities [12].
Besides Izumo-1 and the tetraspanins CD9 and CD81, several other molecules have been suggested to participate in membrane fusion, on the oolemma (integrins, glucosyl phosphatidylinositol (GPI-1 anchored protein)) and spermatozoon (disintegrin and a metalloprotease (ADAM) (e.g. the disintegrins fertilin β and cyritestin)) [6–8].
The viral envelope protein Syncytin is an example of a fusogen [12, 13]. Syncytins participate in cell fusion in the placenta (trophoblast cells) [14–16], in muscles (myoblasts) [17], bone marrow (osteoclasts) [18] and in cancer [19, 20]. Syncytin-1 (ERVW-1), which is expressed in humans, binds to a neutral amino acid transporter (the D-type retroviral receptor: ASCT-2 (SLC1A5)) and employs another transporter (ASCT-1 (SLC1A4)) as an auxiliary receptor [14, 21].
We therefore decided to investigate whether the fusogen Syncytin-1 and its receptor ASCT-2 were present in human spermatozoa and oocytes.
Material & methods
Oocytes and semen samples
Oocytes were obtained from patients attending infertility treatment at the fertility clinic of Rigshospitalet, University Hospital of Copenhagen, Denmark. The inclusion criteria were indication for IVF or ICSI treatment, female age between 25 and 37 years (both inclusive) and regular menstrual cycles (21–35 days, both inclusive). Exclusion criteria were: medical or genetic conditions known to interfere with IVF treatment. The couple or single woman was informed both orally and with written patient information material. The oral information was given no later than 2 days before oocyte pickup. Couples could choose to donate 1) immature oocytes; MI or GV and/or 2) MII oocytes still not fertilized on the day of embryo transfer (embryo transfer conducted on day 2 after oocyte pickup). The couple or the donor woman was informed about all aspects of the study and written consent was obtained from all participants before inclusion in the study. Semen samples were obtained from donors with a priori known normal semen quality. Semen was recovered via a sperm bank, in which donors received a fee for each sample delivered.
Hormonal treatment of oocyte donors
Long protocols using down-regulation with GnRH-agonists (Synarela, Pharmacia, Denmark; Suprefact, Aventis Pharma, Denmark) for at least 14 days or short protocol using GnRH-antagonists (Orgalutran, Organon, Copenhagen, Denmark; Cetrotide, MerckSerono, Copenhagen, Denmark) were applied. Patients were stimulated with recombinant FSH (Puregon, Organon; Gonal-F, Copenhagen, Serono) or urine derived FSH (Menopur, Ferring, Copenhagen, Denmark) and ovulation induced by urine derived hCG (Profasi, Serono, Pregnyl, Organon) or Ovitrelle (MerckSerono, Denmark). Oocyte aspiration was performed according to the standard procedure of the clinic.
Collection of oocytes and sperm cells
Immature oocytes and surplus spermatozoa were donated on the day of oocyte pickup. Unfertilized oocytes from IVF and ICSI were donated on the day of transfer, approximately 48 h after oocyte pickup. For protein analysis, both pooled oocytes, single oocytes and spermatozoa pellets were placed in Eppendorf tubes, respectively, with 5–200 μl RIPA lysis buffer and stored at −20 °C until analysis. For RNA analysis both pooled and single oocytes were placed in microtubes containing 20 μl TRIzol (Invitrogen, Carlsbad, CA, USA) and stored at −20 °C until analysis. Semen samples were centrifuged (5 min at 16,000 g) and dissolved directly in lysis buffer (Macherey-Nagel, Düren, Germany). For immunocytochemistry, spermatozoa were spread on SuperFrost plus slides (Thermo Scientific, Walldorf, Germany), fixed in 1 % paraformaldehyde and stored in a dark at room temperature until further analysis.
Immunocytochemistry
Fixed spermatozoa were hydrated in PBS and processed for immunostaining. Initially, samples were blocked with 10 % goat serum and incubated overnight at 4 °C with mouse monoclonal anti-syncytin-1 (human, 7E3, 5 μg/ml) [17] or polyclonal rabbit anti-ASCT2 (10 μg/ml, Abcam, Cambridge, UK). The following day, samples were washed three times in PBS and incubated with species-specific antibodies labelled with Alexa-594 or Alexa-488 (5 μg/ml, Molecular Probes, Eugene, OR, USA), respectively. Nuclei were stained with bisbenzimide (Hoechst 33 258; Sigma, St. Louis, MO, USA). The specimens were mounted on glass slides using DAKO fluorescent mounting medium (DAKO, Glostrup, Denmark). Controls, including use of isotype-matched monoclonal antibodies, and pre-absorption of the polyclonal ASCT-2 antibody as well as conventional staining controls were included.
SDS-PAGE and immunoblotting
Extraction, SDS-PAGE and electroblotting were performed as described by Mortensen [22]. Antibodies to syncytin-1 (7E3 mouse monoclonal anti-human, 1.5 μg/ml) or ASCT-2 (rabbit polyclonal anti-human 10 μg/ml) were used in combination with chemiluminescent detection and the intensities of the bands were quantitated using NIH Image software [22]. Syncytin-1 protein levels were reported relative to β-actin protein levels to adjust for loading effects.
RNA purification
Total RNA from oocytes was isolated using TRIzol (Invitrogen) according to the manufacturer’s recommendations with a few modifications. Phase separation was facilitated using the max-tract High Density tubes (Qiagen, Hilden, Germany), 0.5 μL glycogen (Roche, Mannheim, Germany) was used as carrier for the precipitation of RNA, and the dried RNA-pellet was directly used in the reversed transcription (RT).
Total RNA from spermatozoa was isolated by dissolving a pellet of sperm directly in lysis buffer and following the procedure of the Nucleospin® RNA II kit (Macherey-Nagel, Düren, Germany) using on-column DNA digestion. The RNA concentration was measured using a NanoDrop instrument (Thermo Fisher Scientific, Wilmington, USA).
RNA from whole testicular tissue was obtained from commercial vendors (Clonetech, Saint-Germain-en-Laye, France and Ambion, Paisley, UK).
Quantitative real-time RT-PCR
The RNA-pellet was dissolved in a total volume of 8 μl, containing 16 ng/μL random hexamer primers (Fermentas, St. Leon, Germany), 8 ng/μL (μM) oligo dT primers (Fermentas), and 0.05 μL RNase H Minus, Point Mutant (Promega, Madison, WI, USA). RNA was incubated at 70 °C to denature RNA secondary structure and then quickly chilled on ice to let the primers anneal to the RNA. 1× buffer (Promega), 0.5 mM dNTPs (Fermentas), 200 U M-MLV Reverse Transcriptase (Promega), and 0.025 μL RNase H Minus, Point Mutant (Promega) were added to a final volume of 12.5 μL. The reverse transcription was performed at 42 °C for 1 h and the enzyme was inactivated at 95 °C for 5 min. Quantitative RT-PCR was performed using LightCycler® Fast Start DNA Master SYBR Green I and the LightCycler® Real-Time PCR system (Roche, Mannheim, Germany). Primers used for syncytin-1 were: right: CCCCATCGTATAGGAGTCTT and left: CCCCATCAGACATACCAGTT, producing an amplicon of 207 bp, while primers for ASCT-2 were: right: CCGCTTCTTCAACTCCTTCAA and left: ACCCACATCCTCCATCTCCA, producing an amplicon of 121 bp. The identity of the amplicons was confirmed by sequencing. Results were normalized to GAPDH (right: CAGGTGGTCTCCTCTGACTT and left: TGCTGTAGCCAAATTCGTTGT, producing an amplicon of 127 bp).
BeWo-cells, which have previously been shown to express syncytin-1 [23], were used as positive control, and a no template control was made in all runs, using water instead of cDNA. An intron spanning GAPDH-primer was used in the qRT-PCR to identify possible genomic DNA contamination. The relative expression was calculated using the ddCt-method, with GAPDH as reference gene.
RT-PCR
cDNA synthesis was performed by adding a 4:1 mixture of oligo-dT20 and random hexamers (0.5 μg/μl), 5× cDNA synthesis buffer (650 mM Tris–HCl pH: 8.3; 25 mM MgCl2; 100 mM KCl2), 25 mM dNTP mix (GE Healthcare, Brondby, Denmark) and AMV reverse transcriptase (Affymetrix, Cleveland, USA) to 1 ug of heat denaturated (65 ° C) RNA. cDNA synthesis was performed by incubating the samples at 42 ° C for 1 h. Finally 0.1 % triton X-100 was added and cDNA denaturated at 95 ° C for 1 min. PCR was performed by adding 10× DDRT buffer (100 mM Tris–HCl pH 8.4; 500 mM KCL; 18 mM MgCl2; 1 % Triton), 2.5 mM dNTP mix, Taq polymerase (GE Healthcare) and primers at a concentration of 1 pmol/μl. Cycle conditions were: 1 cycle of 5 min at 95 °C; 40 cycles of 30 s at 95 ° C, 1 min at 64 ° C, 1 min at 72 ° C and 1 cycle of 5 min at 72 °C. Representative bands from each primer combination were excised and sequenced for verification (Eurofins MWG, Germany).
Results
Syncytin-1 and ASCT-2 is expressed in human sperm
Reverse transcriptase-polymerase chain reaction (RT-PCR) of RNA isolated from eight different donors showed that syncytin-1 was present in all samples (data not shown). Genomic contamination was avoided by on-column DNase digestion during RNA purification and the use of intron-spanning primers. The syncytin-1 receptor, ASCT-2, was also detectable in most donor ejaculates, however in some donors at a very low level. ASCT-2, but not Syncytin-1 expression was detected in RNA from whole testicular tissue. The identity of the bands corresponding to syncytin-1 and ASCT-2 were verified by sequencing.
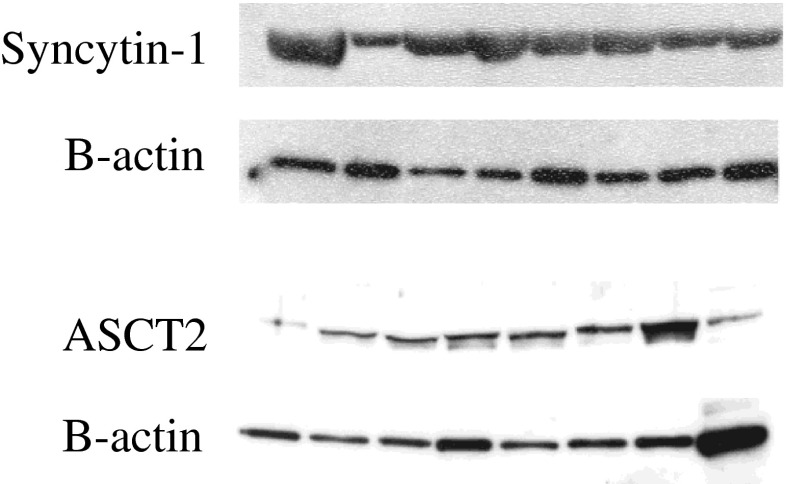
Immunoblotting using an antiserum against the extracellular domain of syncytin-1 revealed an immunoreactive band around 60 kDa in extracts of eight different donors corresponding in size to a syncytin-1 component previously demonstrated to be present in the placenta [17, 15]. The protein level of syncytin-1 varied among the different semen donors (Fig. 1). ASCT-2 was detected in all semen samples, but appeared less abundant than syncytin-1. A similar variation in protein levels between samples was observed, despite using equal amount of protein in each well.
Fig. 1.
Expression of syncytin-1 and its receptor ASCT-2 by human sperm. Western blot showing syncytin-1 and ASCT-2 immunoreactive bands in samples from eight different sperm donors, respectively. Below, reprobing for β-actin is shown
Syncytin-1 and ASCT-2 is localized at the acrosomal region and equatorial segment of the spermatozoa
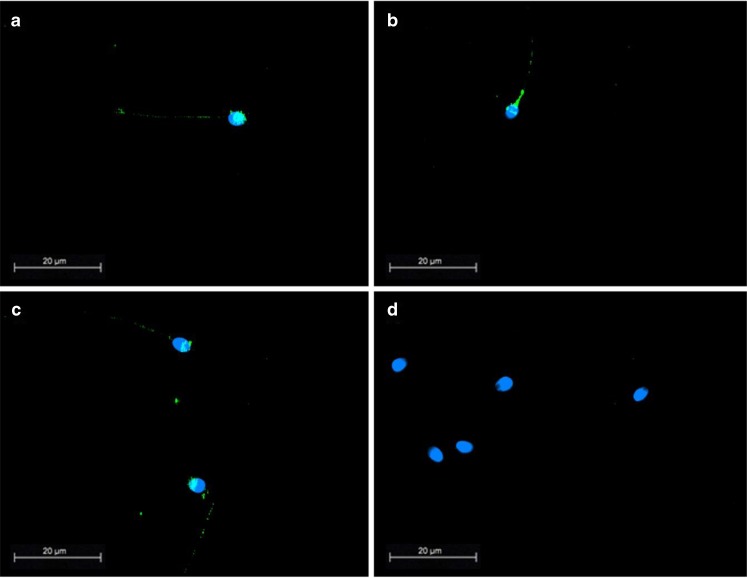
Immunofluorescence staining of fixed spermatozoa from eight different donors showed that syncytin-1 immunoreactivity was predominately present in the acrosomal region of the spermatozoa (Fig. 2). If the acrosomal region was not stained, syncytin-1 was localized at the equatorial segment only. Slight staining of the midpiece and tail was also noted. There was no obvious difference in the syncytin-1 localisation between the different donors.
Fig. 2.
Localization of syncytin-1 in human sperm. Immunofluorescence images of fixed representative sperm samples from different sperm donors stained with a monoclonal antibody raised to the extracellular domain of syncytin-1 (a–c). Note staining of Syncytin-1 in the head of the spermatozoa and around the equatorial segment. Slight staining of the midpiece and tail also noted. d shows an isotype matched control. (Scalebar 20 μm, objective: 100×/1.30 PL Fluotar)
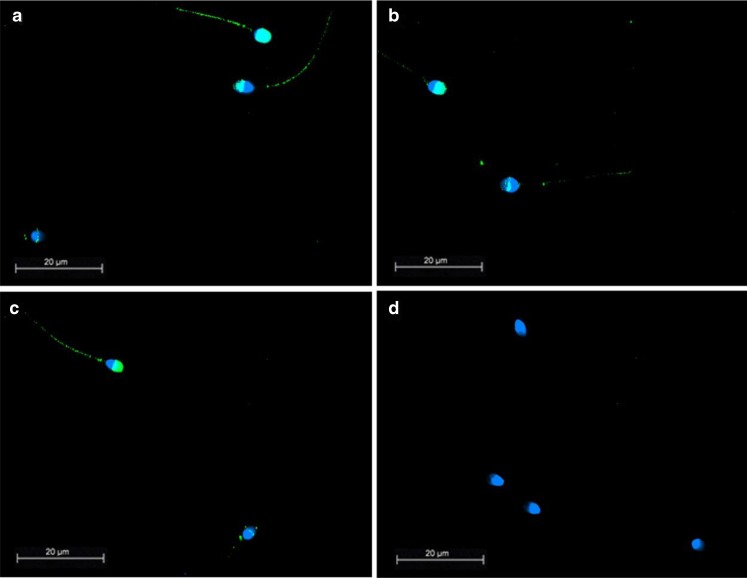
ASCT-2 was predominately localized to the acrosomal region and in the tail region (Fig. 3). Controls, including use of type-matched monoclonal antibodies and pre-absorption of the polyclonal ASCT-2 antibody, as well as conventional staining controls were all negative.
Fig. 3.
Localization of ASCT-2 in human sperm. Immunofluorescence images of fixed representative sperm samples from different sperm donors stained with an anti-ASCT-2 (a–c). Note staining in the acrosomal region of the spermatozoa and scarce staining of the tail. d shows a control with pre-absorption of the primary antibody. (Scalebar 20 μm, objective: 100×/1.30 PL Fluotar)
The receptor ASCT-2 is expressed in human oocytes
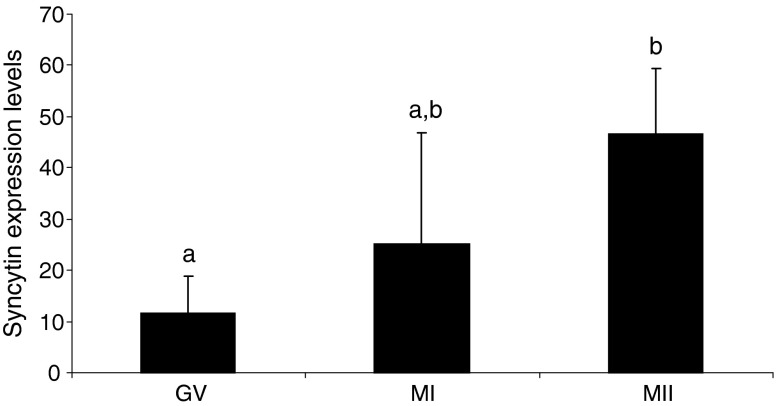
A total of 80 oocytes (GV: 20, MI: 25, MII: 35) were included in the analysis. Quantitative RT-PCR showed that ASCT-2 was expressed in all of the evaluated stages of oocyte maturation (Fig. 4). There was significantly higher expression of ASCT-2 in mature MII stage oocytes compared to the immature GV-stage (p = 0.027). However, expression of syncytin-1 was not detected in any of the oocytes examined.
Fig. 4.
Expression of ASCT-2 in human oocytes. Quantiatative RT-PCR of ASCT-2 in oocytes at different developmental stages showed that the mRNA level increases as the oocytes mature. Chi-square test was applied to evaluate potential statistical significant differences, level of significance p ≤ 0.05. a,b indicates significant difference, p < 0.027
Discussion
Membrane fusion plays an essential role in mammalian fertilization, however a specific fusogen involved in fertilization has yet to be identified. Our findings demonstrate the presence of the fusogen syncytin-1 and its receptor ASCT-2 in human gametes. Syncytin-1 is predominantly localized at the acrosomal region of the spermatozoa. However, in a subset of cells it is exclusively localized to the equatorial segment. The process of capacitation and AR can be induced in vitro during gradient centrifugation, media exposure etc., however the extent varies between species [24], and only a smaller fraction of human spermatozoa will undergo the AR reaction in vitro [25, 26]. The observed localization of syncytin-1, equatorial segment or acrosomal region, respectively could depend upon whether the spermatozoa had undergone the AR or not. Localization of a fusogen at the equatorial segment is supported by electron microscopy findings demonstrating gamete membrane fusion being initiated at the equatorial segment [27, 1]. Moreover, the fusogen (syncytin-1) and it’s receptor (ASCT-2) is expressed in spermatozoa and oocytes, respectively. In contrast to spermatozoa expressing syncytin-1, none of the analyzed oocytes expressed the fusogen, but only it’s receptor.
Syncytin belongs, as mentioned, to a family of endogenous retroviruses [13]. Introduction of syncytin expression in a cell line prevents retrovirus infection [28], suggesting that co-expression of syncytin and its receptor will block either of the two [29]. Co-expression of syncytin-1 and ASCT-2 on spermatozoa could therefore function as a regulator of syncytin, preventing membrane fusion at the acrosomal region until the correct time and place.
In oocytes, we find that the ASCT-2 mRNA level significantly related to oocyte maturity from the germinal vesicle to the metaphase 2 stage. Due to shortage of fresh metaphase II (MII) oocytes we evaluated ASCT-2 mRNA in MII oocytes 48 h after oocyte pickup, which potentially also could affect expression level. Since syncytin-1 is present and localized at the right place in spermatozoa and ASCT-2 is present in the mature oocytes, this suggests that the fusogen syncytin-1 and its receptor could be involved in membrane fusion of the human gametes.
Previous studies have demonstrated the essential involvement of the tetraspanins CD9 and CD81 in fertilization [30]. CD9 and CD 81 have been shown also to participate in membrane fusion during retrovirus infection [31]. All together the demonstration of syncytin-1 on the equatorial segment of spermatozoa supports the model suggested by Nixon et al. [6], linking documented membrane adhesion factors of importance to membrane fusion during fertilization.
The finding from the present study may potentially have significant impact on clinical decision making in assisted reproduction programs. Assuming that the presence of Syncytin-1 on spermatozoa is a necessity for gamete membrane fusion during fertilization, analysis for its presence may directly impact clinical decisions like choice of treatment strategy. A high level of Syncytin-1, enabling gamete membrane fusion, would be supportive of insemination or standard in vitro fertilization, whereas low level or absence of Syncytin-1 would indicate the use of ICSI treatment.
Further, the presence of Syncytin-1 may be used as a diagnostic tool when evaluating a man’s reproductive potential. Consequently, it may also be used to decide if a man should be used as sperm donor or not.
The presence of the receptor on the oocytes may be utilized in pharmaceutical or culture related interventions as an indicator of the developmental competence of the oocytes.
In conclusion, syncytin-1 and ASCT-2 are expressed in human gametes with localization and temporal appearance consistent with a possible role in fusion between oocyte and spermatozoon. Moreover, it is the first time a true fusogen has been identified in human gametes.
Reduced or total failure of syncytin-1 or ASCT-2 expression in spermatozoa or oocytes respectively, could potentially cause fertilization failure, which would be circumvented by ICSI. However, future functional studies are needed to examine whether the fusogen syncytin-1 is essential for human fertilization.
Acknowledgments
Ethical statement
All participating persons gave their informed consent prior inclusion in the study. The Danish Ethical committee approval was obtained before the study was initiated (J. nr H-B-2008-150).
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Capsule Membrane fusion is an important part of fertilization. Here we present, for the first time, the presence of a true fusogen Syncytin-1 and its receptor on human gametes.
This work was conducted at The Fertility Clinic, Rigshospitalet and at the Faculty of Life Science, University of Copenhagen
References
- 1.Yanagimachi R. Mammalian fertilization. In: Knobil E, Neill J, editors. The physiology of reproduction. 2. New York: Raven; 1994. pp. 189–317. [Google Scholar]
- 2.van Gestel RA, Brewis IA, Ashton PR, Brouwers JF, Gadella BM. Multiple proteins present in purified porcine sperm apical plasmamembranes interact with the zona pellucida of the oocyte. Mol Hum Reprod. 2007;13:445–54. doi: 10.1093/molehr/gam030. [DOI] [PubMed] [Google Scholar]
- 3.Nixon B, Mitchell LA, Anderson AL, Mclaughlin EA, O’bryan MK, Aitken RJ. Proteomic and functional analysis of human sperm detergent resistant membranes. J Cell Physiol. 2011;226:2651–65. doi: 10.1002/jcp.22615. [DOI] [PubMed] [Google Scholar]
- 4.Blas GAD, Roggero CM, Tomes CN, Mayorga LS. Dynamics of SNARE assembly and disassembly during sperm acrosomal exocytosis. PLoS Biol. 2005;3:e323. doi: 10.1371/journal.pbio.0030323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rodriguez F, Bustos MA, Zanetti MN, Ruete MC, Mayorga LS, Tomes CN. alfa-SNAP prevents docking of the acrosome during sperm exocytosis because it sequesters monomeric syntaxin. PLoS One. 2011;6:e21925. doi: 10.1371/journal.pone.0021925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nixon B, Aitken RJ, McLaughlin EA. New insights into the molecular mechanisms of sperm-egg interaction. Cell Mol Life Sci. 2007;64:1805–23. doi: 10.1007/s00018-007-6552-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sutovsky P. Sperm–egg adhesion and fusion in mammals. Expert Rev Mol Med. 2009;11:e11. doi: 10.1017/S1462399409001045. [DOI] [PubMed] [Google Scholar]
- 8.Evans JP. Sperm-egg interaction. Annu Rev Physiol. 2012;74:477–502. doi: 10.1146/annurev-physiol-020911-153339. [DOI] [PubMed] [Google Scholar]
- 9.Inoue N, Ikawa M, Isotani A, Okabe M. The immunoglobulin superfamily protein Izumo is required for sperm to fuse with eggs. Nature. 2005;434:234–8. doi: 10.1038/nature03362. [DOI] [PubMed] [Google Scholar]
- 10.Rubinstein E, Ziyyat A, Prenant M, Wrobel E, Wolf JP, Levy S, et al. Reduced fertility of female mice lacking CD81. Dev Biol. 2006;290:351–8. doi: 10.1016/j.ydbio.2005.11.031. [DOI] [PubMed] [Google Scholar]
- 11.Yunta M, Lazo PA. Tetraspanin proteins as organisers of membrane microdomains and signalling complexes. Cell Signal. 2003;15:559–64. doi: 10.1016/S0898-6568(02)00147-X. [DOI] [PubMed] [Google Scholar]
- 12.Oren-Suissa M, Podbilewicz B. Cell fusion during development. Trends Cell Biol. 2007;17:537–46. doi: 10.1016/j.tcb.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 13.Weissenhorn W, Hinz A, Gaudin Y. Virus membrane fusion. FEBS Lett. 2007;581:2150–5. doi: 10.1016/j.febslet.2007.01.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blond JL, Lavillette D, Cheynet V, Bouton O, Oriol G, Chapel-Fernandes S. An envelope glycoprotein of the human endogenous retrovirus HERV-W is expressed in the human placenta and fuses cells expressing the type D mammalian retrovirus receptor. J Virol. 2000;74:3321–9. doi: 10.1128/JVI.74.7.3321-3329.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mi S, Lee X, Li X, Veldman GM, Finnerty H, Racie L, et al. Syncytin is a captive retroviral envelope protein involved in human placental morphogenesis. Nature. 2000;403:785–9. doi: 10.1038/35001608. [DOI] [PubMed] [Google Scholar]
- 16.Dupressoir A, Vernochet C, Bawa O, Harper F, Pierron G, Opolon P, et al. Syncytin-A knockout mice demonstrate the critical role in placentation of a fusogenic, endogenous retrovirus-derived, envelope gene. Proc Natl Acad Sci U S A. 2009;106:12127–32. doi: 10.1073/pnas.0902925106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bjerregaard B, Talts JF, Larsson LI. The endogenous envelope protein syncytin is involved in myoblast fusion. In: Larsson LI, editor. Cell fusions, regulation and control. New York: Springer; 2011. pp. 267–75. [Google Scholar]
- 18.Søe K, Andersen TL, Hobolt-Pedersen AS, Bjerregaard B, Larsson LI, Delaissé JM. Involvement of human endogenous retroviral syncytin-1 in human osteoclast fusion. Bone. 2011;48:837–46. doi: 10.1016/j.bone.2010.11.011. [DOI] [PubMed] [Google Scholar]
- 19.Bjerregaard B, Holck S, Christensen IJ, Larsson LI. Syncytin is involved in breast cancer-endothelial cell fusions. Cell Mol Life Sci. 2006;63:1906–11. doi: 10.1007/s00018-006-6201-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Strick R, Ackermann S, Langbein M, Swiatek J, Schubert SW, Hashemolhosseini S, et al. Proliferation and cell-cell fusion of endometrial carcinoma are induced by the human endogenous retroviral syncytin-1 and regulated by TGF-beta. J Mol Med. 2007;85:23–38. doi: 10.1007/s00109-006-0104-y. [DOI] [PubMed] [Google Scholar]
- 21.Lavillette D, Marin M, Ruggieri A, Mallet F, Cosset FL, Kabat D. The envelope glycoprotein of human endogenous retrovirus type W uses a divergent family of amino acid transporters/cell surface receptors. J Virol. 2000;76:6442–52. doi: 10.1128/JVI.76.13.6442-6452.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mortensen K, Lichtenberg J, Thomasen PD, Larsen LI. Spontaneous fusion between cancer cells and endothelial cells. Cell Mol Life Sci. 2004;61:2125–31. doi: 10.1007/s00018-004-4200-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yu C, Shen K, Lin M, Chen P, Lin C, Chang GD, et al. GCMa regulates the syncytin-mediated trophoblastic fusion. J Biol Chem. 2002;277:50062–8. doi: 10.1074/jbc.M209316200. [DOI] [PubMed] [Google Scholar]
- 24.Ikawa M, Inoue N, Benham AM, Okabe M. Fertilization: a sperm’s journey to and interaction with the oocyte. J Clin Invest. 2010;120:984–94. doi: 10.1172/JCI41585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mallet PJ, Stock CE, Fraser LR. Acrosome loss in human sperm incubated in vitro under capacitating conditions. Int J Androl. 1985;8:357–64. doi: 10.1111/j.1365-2605.1985.tb00848.x. [DOI] [PubMed] [Google Scholar]
- 26.Green S, Fishel S, Rowe P. The incidence of spontaneous acrosome reaction in homogeneous populations of hyperactivated human spermatozoa. Hum Reprod. 1999;14:1819–22. doi: 10.1093/humrep/14.7.1819. [DOI] [PubMed] [Google Scholar]
- 27.Bedford JM, Cooper GW. Membrane fusion events in the fertilization of vertebrate eggs. In: Poste G, Nicholson GL, editors. Cell surface reviews. Amsterdam: Elsevier/North-Holland Biomedical press; 1978. pp. 65–125. [Google Scholar]
- 28.Ponferrada VG, Mauck BS, Wooley DP. The envelope glycoprotein of human endogenous retrovirus HERV-W induces cellular resistance to spleen necrosis virus. Arch Virol. 2003;148:659–75. doi: 10.1007/s00705-002-0960-x. [DOI] [PubMed] [Google Scholar]
- 29.Potgens AJG, Drewlo S, Kokozidou M, Kaufmann P. Syncytin: the major regulator of trophoblast fusion? Recent developments and hypotheses on its action. Hum Reprod Update. 2004;10:487–96. doi: 10.1093/humupd/dmh039. [DOI] [PubMed] [Google Scholar]
- 30.Rubinstein E, Ziyyat A, Wolf JP, Le Naour F, Boucheix C. The molecular players of sperm-egg fusion in mammals. Semin Cell Dev Biol. 2006;17:254–63. doi: 10.1016/j.semcdb.2006.02.012. [DOI] [PubMed] [Google Scholar]
- 31.Gordon-Alonso M, Yañez-Mó M, Barreiro O, Álvarez S, Muñoz-Fernández MÁ, Valenzuela-Fernández A, et al. Tetraspanins CD9 and CD81 modulate HIV-1-induced membrane fusion. J Immunol. 2006;177:5129–37. doi: 10.4049/jimmunol.177.8.5129. [DOI] [PubMed] [Google Scholar]