Abstract
Purpose
To evaluate the association between serum progesterone (P) levels on the day of embryo transfer (ET) and pregnancy rates in fresh donor IVF/ICSI cycles.
Methods
Fresh donor cycles with day 3 ET from 10/2007 to 8/2012 were included (n = 229). Most cycles (93 %) were programmed with a gonadotropin releasing hormone (GnRH) agonist; oral, vaginal or transdermal estradiol was used for endometrial priming, and intramuscular P was used for luteal support (50–100 mg/day). Recipient P levels were measured at ET, and P dose was increased by 50–100 % if <20 ng/mL per clinic practice. The main outcome measure was rate of live birth (> = 24 weeks gestational age). Generalized estimating equations were used to account for multiple cycles from the same recipient, adjusted a priori for recipient and donor age.
Results
Mean recipient serum P at ET was 25.5 ± 10.1 ng/mL. Recipients with P < 20 ng/mL at ET, despite P dose increases after ET, were less likely to achieve clinical pregnancy (RR = 0.75, 95 % CI = 0.60–0.94, p = 0.01) and live birth (RR = 0.77, 95 % CI = 0.60–0.98, p = 0.04), as compared to those with P ≥ 20 ng/mL. P dose increases were more often required in overweight and obese recipients.
Conclusions
Serum P levels on the day of ET in fresh donor IVF/ICSI cycles were positively correlated with clinical pregnancy and live birth rates. An increase in P dose after ET was insufficient to rescue pregnancy rates. Overweight and obese recipients may require higher initial doses of P supplementation. Future research is needed to define optimal serum P at ET and the interventions to achieve this target.
Keywords: Donor oocyte, Embryo transfer, Implantation, IVF/ICSI, Progesterone, Pregnancy rate
Introduction
In cycles using cryopreservation embryo transfer, donor oocyte or gestational carrier, supplemental progesterone (P) is required for endometrial gland formation and successful trophoblast invasion, as endogenous P levels are likely inadequate. In a natural cycle, the corpus luteum typically produces 25 to 50 mg of P daily, which is equivalent to the pharmacologic dose administered intramuscularly to patients in prepared IVF cycles [1]. Following spontaneous ovulation, the midluteal serum P concentration is typically between 10 and 20 ng/mL [2]. Similar levels of serum P are achieved approximately 4 h after administration of 50 mg of intramuscular (IM) P [1]. In our current clinical practice, if recipient P on the day of embryo transfer (ET) is <20 ng/mL, then the dose of IM P is increased by 50 to 100 % (i.e., from 50 to 75–100 mg per day).
While much research has focused on identifying optimal P regimens for luteal support following ET, few studies have addressed the predictive value of serum P level on the day of ET [3, 4]. P has been shown to have a quiescent effect on the uterus, as high serum P on the day of fresh ET has been associated with decreased uterine contractility and subsequently higher implantation rates [5]. Furthermore, while pre-ovulatory rises in serum P may lead to premature luteinization and impaired endometrial receptivity [6–9], inadequate duration of P supplementation prior to transfer may likewise negatively affect implantation [10].
The goal of the present study was to determine a threshold value for serum P on the day of ET in donor egg recipients. A secondary goal was to identify those patients who may require higher initial doses of P supplementation.
Materials and methods
Institutional Review Board approval was obtained from our institution.
Study population and design
All fresh donor IVF/ICSI cycles between 1/2007 and 8/2012 at our institution having a day 3 ET were reviewed for serum P level at ET (n = 246). Records with missing data or cycles with preimplantation genetic diagnosis were excluded. Likewise, cycles with luteal support other than IM P were excluded, as prepared cycles supplemented with micronized vaginal progesterone gel (Crinone 8 %) for cryopreservation embryo transfer have lower live birth rates than those supplemented with IM P [11]. The final study population included 229 donor-recipient pairs meeting our inclusion criteria.
Cycles were partitioned into tertiles (T) according to P concentration (T1: 8.19–20.00 ng/mL; T2: 20.08–28.46 ng/mL; T3 28.63–60.00 ng/mL). As our institution’s historical threshold value for increasing progesterone supplementation (20 ng/mL) was the same as the cut-off between T1 and T2 (20.00 ng/mL), cycles were dichotomized according to serum P < 20 ng/mL or P ≥ 20 ng/mL at ET (i.e., T1 was compared to T2/T3).
Clinical and laboratory protocols
Controlled ovarian hyperstimulation for donors was performed with downregulation protocols using gonadotropin releasing hormone (GnRH) agonist, GnRH antagonists, or a poor responder protocol using very low GnRH agonist in one patient [12, 13]. Follicular and serum estradiol (E2) monitoring was performed per standard protocol. Oocyte retrieval occurred approximately 36 h after IM injection with hCG 10,000 IU (Pregnyl®; N.V. Organon, The Netherlands) when the two lead follicles were 17–18 mm in diameter. Standard insemination and ICSI was performed within 4 to 6 h after retrieval, as previously described [14]. Day 3 embryo morphology was scored according to blastomere number, degree of fragmentation and extent of asymmetry as described by Racowsky et al. [15]. Briefly, each embryo was assigned a fragmentation score of 0 %, 1–9 %, 10–25 %, 26–50 %, or >50 % and a symmetry score of perfect, moderate asymmetry or severe asymmetry. A good-quality embryo was defined as having ≥8 cells with <10 % fragmentation and either no asymmetry or moderate asymmetry.
Embryo transfer was performed on day 3 under ultrasound guidance using a Wallace catheter (Marlow/Cooper Surgical, Shelton, CT, USA). ET characteristics were qualitatively graded by the transferring physician according to difficulty (easy, some difficulty or extreme difficulty). Blood on the catheter tip at time of transfer was also used as a proxy for a difficult transfer.
Endometrial preparation and progesterone support
Endometrial preparation of the recipients was programmed with or without a luteal GnRH agonist. E2 supplementation was initiated with the luteal GnRH agonist, which was begun 10 days before the start of controlled ovarian hyperstimulation; in patients not receiving a luteal GnRH agonist, E2 was initiated with controlled ovarian hyperstimulation. Fixed dose oral, vaginal or transdermal E2 was used for endometrial priming to achieve a target E2 level of ≥180 pg/mL, regardless of whether a luteal GnRH agonist was used. In patients with a serum E2 below this target, either the same mode of E2 was continued at a higher dose, or a different mode of delivery (e.g., 1–2 mg vaginal E2 twice daily or 0.3 mg transdermal patches every other day) was used to achieve the target. IM P in oil was used for luteal support at a dose of 50–100 mg per day, beginning in the evening 4 days before ET (locally compounded at Village Fertility, Waltham, Massachusetts, or Freedom Fertility, Byfield, Massachusetts). In cycles without luteal GnRH down regulation, serum P was checked prior to the start of IM P to ensure that the recipient had not ovulated. IM P and estradiol were continued until 10 weeks’ of completed gestation or negative hCG.
Serum P was measured in all recipients on the day of ET and, when <20 ng/mL, the dose of IM P was increased by 50–100 %. In those patients who received additional P supplementation, serum P levels were rechecked 3 to 4 days following ET. All serum P levels on the day of ET were assayed using the Roche Elecsys 2010 Electrochemiluminescence immunoassay, Progesterone II in our on-site laboratory; results were expressed as ng/mL. All serum E2 levels on the day of ET were measured using the Roche Elecsys 2010 Electrochemiluminescence immunoassay, Estradiol II in our on-site laboratory; results were expressed as pg/mL. Intra- and inter-assay coefficients of variation for the P and E2 assays were ≤5.5 % and <10 %, respectively. This laboratory is inspected by the Joint Commission of American Pathology and is compliant with Clinical Laboratory Improvement Amendments (CLIA) standards.
Outcome variables
Clinical outcomes were assessed per transfer and included: Not pregnant, defined by negative serum hCG (<3 mIU/mL); biochemical pregnancy, defined as hCG ≥3 mIU/mL but with no intra- or extrauterine sac visualized on transvaginal ultrasound; ectopic pregnancy, defined by extrauterine pregnancy visualized on ultrasound or confirmed intraoperatively; clinical pregnancy, defined as at least one intrauterine gestational sac on ultrasound; spontaneous abortion, defined as loss of a clinical pregnancy prior to 24 weeks’ gestational age; live birth, defined as birth of a viable infant after 23 weeks and 6 days’ gestational age; and multiple birth, defined as delivery of two or more viable infants.
Statistical analyses
Relative risks (RR), 95 % confidence intervals (CI), and 2-sided Wald P-values were obtained from generalized estimating equations to account for multiple cycles from the same recipient. Donor and recipient ages at cycle start were included in the model a priori. Other covariates tested as potential confounders included: Recipient parity, recipient’s prior miscarriage history, donor and recipient body mass index (BMI), whether the oocyte donor was anonymous or known to the recipient, primary infertility diagnosis, use of donor sperm, age of sperm source (i.e., donor or paternal age), duration of E2 priming, mode of E2 delivery (oral, vaginal or transdermal), cumulative progesterone dose, donor serum E2 level on day of hCG trigger and recipient E2 level on day of ET, use of ICSI or assisted hatching, programmed cycle with GnRH agonist down regulation, quality and number of embryos transferred and ET difficulty.
The addition of the above covariates to the base model did not change the effect estimate for progesterone by greater than 10 % and therefore none was included in the final model [16]. Stepwise regression (likewise using entry and stay criteria of 0.10) was then used to identify whether any demographic characteristics could predict those patients who required an increase in P dose. Differences were considered significant with P < .05, and the P-values were two sided. Analyses were performed using Statistical Analysis Software (SAS) Version 9.2 (SAS Institute, Inc, Cary, NC).
Results
Patient demographics, cycle parameters and transfer characteristics
Demographic and cycle parameters for the 229 cycles meeting inclusion criteria are shown in Table 1. Mean donor and recipient and ages were 27.1 ± 4.1 and 41.5 ± 4.5 years, respectively. The mean age of the individual providing sperm (i.e., partner or sperm donor) was 42.2 ± 6.6 years and the average total motile count was 88.3 ± 77.2 million. Donor and testicular sperm were rarely used (5.2 % and 2.2 %, respectively).
Table 1.
Patient demographics and cycle characteristics among 229 fresh donor oocyte cycles
| Characteristic | |
|---|---|
| Oocyte donor age (years) | 27.1 ± 4.0 |
| Oocyte donor BMI (kg/m2) | 23.1 ± 3.2 |
| Oocyte donor known to recipient | 50 (21.8 %) |
| Recipient age (years) | 41.5 ± 4.5 |
| Recipient BMI (kg/m2) | 24.1 ± 4.5 |
| Recipient race | |
| White | 174 (76.0 %) |
| Black | 11 (4.8 %) |
| Asian | 18 (7.9 %) |
| Hispanic | 4 (1.7 %) |
| American Indian | 1 (0.4 %) |
| Unknown or declined | 21 (9.2 %) |
| Recipient gravidity | |
| 0 | 93 (40.6 %) |
| ≥1 | 136 (59.4 %) |
| Recipient parity | |
| 0 | 174 (76.0 %) |
| ≥1 | 55 (24.0 %) |
| Primary infertility diagnosis | |
| Diminished ovarian reserve | 214 (93.4 %) |
| Other | 15 (6.6 %) |
| Sperm donor | 12 (5.2 %) |
| Testicular sperm | 5 (2.2 %) |
| Luteal GnRH agonist useda | 213 (93.0 %) |
| Mode of E2 delivery to recipient | |
| Oral | 191 (83.4 %) |
| Vaginal | 13 (5.7 %) |
| Combination oral and vaginal | 13 (5.7 %) |
| Transdermal | 12 (5.2 %) |
| Cumulative IM progesterone dose (mg) prior to embryo transfer | 183.8 ± 33.3 |
| ICSI | 78 (34.1 %) |
| Number embryos transferred | |
| 1 | 16 (7.0 %) |
| 2 | 195 (85.2 %) |
| 3 or more | 18 (7.8 %) |
| Number of good quality embryos transferredb | |
| 0 | 27 (11.8 %) |
| 1 | 44 (19.2 %) |
| 2 | 158 (69.0 %) |
| Total | 229 |
Values represent mean ± standard deviation, or n (%)
BMI body mass index, ICSI intracytoplasmic sperm injection
aLuteal gonadotropin releasing hormone (GnRH) agonist was used prior to endometrial priming in recipient
bA good-quality embryo was defined as having ≥8 cells with <10 % fragmentation and either no asymmetry or moderate asymmetry
Most cycles (93 %) were prepared with a luteal GnRH agonist. The mean donor serum E2 at hCG trigger was 2,301 ± 953 pg/mL. The most common route of E2 for priming of recipient endometrium was oral (83.4 %), compared to transvaginal (5.7 %), combined oral and transvaginal (5.7 %), or transdermal (5.2 %). The mean cumulative E2 dose administered to recipients prior to transfer was 161.6 ± 49.5 mg over a mean duration of 27.6 ± 3.8 days. The mean recipient serum E2 at transfer, checked in 109 patients (48 %), was 439.5 ± 223.0 pg/mL. The most common cumulative dose of IM P used prior to transfer was 175 mg (85.5 %), followed by 200 mg (6.6 %).
Most cycles had two embryos transferred (85.2 % versus 7.8 % with > = 3 embryos and 7.0 % with 1 embryo). Among cycles in which ≥3 embryos were transferred (n = 13), 30.8 % had two good-quality embryos transferred, while 61.5 % had no good-quality embryos transferred. Known donors were used in 53.8 % of these cycles, as compared to 19.9 % in cycles with ≤2 embryos transferred. Two good-quality embryos were transferred in 69.0 % of all cycles. The transfer difficulty was qualitatively rated by the transferring physician as ‘easy’ (compared to ‘some difficulty’ or ‘extreme difficulty’) in 87.4 % of cases. The presence of blood on the catheter tip as a proxy for difficult transfer was observed in 18.9 % of transfers.
Clinical outcomes
The overall live birth rate per transfer in the study cohort was 60.3 % (138/229). The mean serum P level at transfer for cycles resulting in a live birth was 26.7 ± 10.1 ng/mL, as compared with 23.8 ± 9.8 ng/mL for cycles resulting in no live birth.
Patients with serum P < 20 ng/mL (n = 75) were compared to those with serum P ≥ 20 ng/mL (n = 154), as 20 ng/mL is the historical value below which patients receive additional supplementation in our program. The 20 ng/mL value also represented the cut-off value between the first and second tertile in the distribution of serum P at ET in this study. Cycles with serum P < 20 ng/mL at ET were significantly less likely to result in clinical pregnancy (56.0 % vs. 73.4 %; RR = 0.75, 95 % CI = 0.60–0.94, P = .01) and live birth (50.7 % vs. 64.9 %; RR = 0.77, 95 % CI = 0.60–0.98, P = .04), as compared to those with P ≥ 20 ng/mL at ET (Table 2). Rates of biochemical pregnancy, ectopic pregnancy, spontaneous abortion and multiple birth were not significantly different between the two P groups (data not shown). Serum P at ET was positively correlated with live birth rate, with an R2 value of 0.14 according to multivariate analysis (Fig. 1). The likelihood of live birth increased with serum P level at ET in a dose-dependent manner (P = .02).
Table 2.
Association between serum progesterone concentration at embryo transfer and IVF outcomes among 229 fresh egg donor cycles
| Outcome | P a < 20 ng/ml (n = 75) | P >= 20 ng/ml (n = 154) (Referent group) | RR (95 % CI) | p-value |
|---|---|---|---|---|
| Clinical pregnancyb | 42 (56.0 %) | 113 (73.4 %) | 0.75 (0.60–0.94) | 0.01 |
| Spontaneous abortionc | 4 (5.3 %) | 13 (8.4 %) | 0.80 (0.27–2.33) | 0.68 |
| Live birthd | 38 (50.7 %) | 100 (64.9 %) | 0.77 (0.60–0.98) | 0.04 |
| Multiple birthe | 14 (18.7 %) | 40 (26.0 %) | 0.72 (0.42–1.25) | 0.24 |
aP = progesterone, measured on the day of embryo transfer
bClinical pregnancy was defined as the presence of at least one intrauterine gestational sac
cSpontaneous abortion was defined as loss of a clinical pregnancy prior to 24 weeks’ gestational age
dLive birth was defined as birth of a viable infant after 23 weeks and 6 days’ gestational age
eMultiple birth was defined as delivery of two or more viable infants
Fig. 1.
Association between percent live birth and serum progesterone range (ng/mL) on the day of embryo transfer in fresh donor oocyte cycles. The likelihood of live birth increased with serum P level at ET in a dose-dependent manner (P = .02). Live birth is defined as delivery of live infant after 23 weeks and 6 days’ gestational age
Subanalysis of serum estradiol and estradiol to progesterone ratio at ET
Serum E2 was measured at ET in 109 recipients (48 %). When multivariate analysis was restricted to these 109 cycles, addition of serum E2 to the base model did not change the effect estimate for progesterone by greater than 10 % and was thus not included in the final model. Live birth rates were similar between the highest tertile (T3 range: 470–1,311 pg/mL) and lowest tertile (T1 range: 131–313 pg/mL) of E2 at ET (58.3 % versus 55.6 % live birth/transfer, respectively). Subanalysis of the E2:P ratio at transfer was further limited to cycles prepared with oral estradiol (n = 86, 79 %), consistent with prior studies [17, 18]. A large difference in live birth rate was observed when comparing the outcome of cycles within the highest quartile of E2:P ratio (Q4 range: 20.8–54.2) to the lowest quartile (Q1 range: 3.8–11.5) (35 % versus 76 % live birth/transfer, respectively).
Subanalysis of cycles requiring progesterone dose adjustment
Supplemental P doses were increased in 32.8 % of cycles (75 of 229). At clinicians’ discretion, three patients with P < 20 ng/mL did not receive increased supplementation (serum P of 8.67, 18.56 and 19.55 ng/mL) and were not included in the following subanalysis, and three patients with P ≥ 20 ng/mL did receive increased supplementation (serum P of 20.08, 20.58 and 20.6 ng/mL) and were included in the following subanalysis.
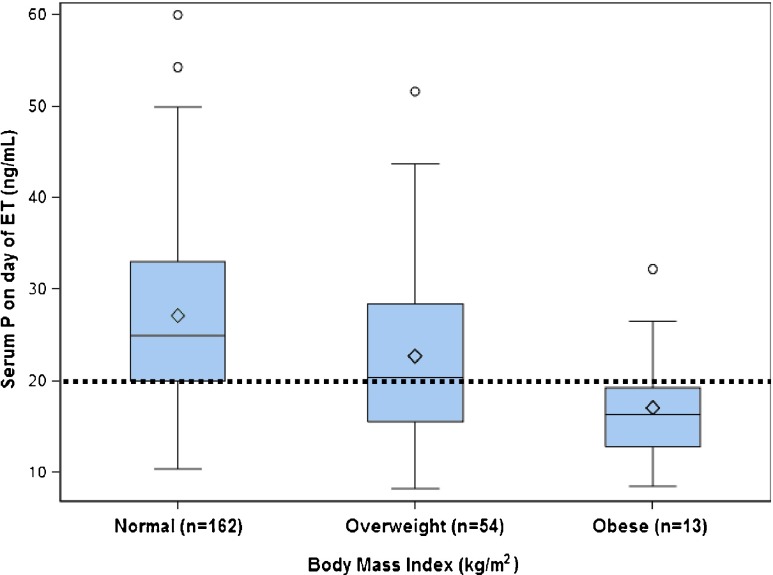
Of the 75 cycles receiving additional supplementation, 39 (52 %) resulted in live birth, similar to the baseline rate of live birth in the group with initial serum P < 20 ng/mL (50.7 %). Multivariate analysis revealed that BMI was the only predictor of an initial serum P < 20 ng/mL (Table 3; Fig. 2). The mean BMI of patients who required a dose increase in supplemental P was 26.1 ± 5.5 kg/m2, while those who did not had a mean BMI of 23.2 ± 3.6 kg/m2. Overweight recipients (BMI 25–29.9 kg/m2) were 82 % more likely to require P dose increases as compared to recipients with a normal BMI (RR = 1.82, 95%CI = 1.23–2.70, P = .003). Patients with BMI ≥ 30 kg/m2 were 2.7 times more likely to require a P dose increase as compared to recipients with a normal BMI (RR = 2.72, 95 % CI = 1.74–4.25, P < .0001). Importantly, BMI was not an independent predictor of live birth in multivariate analysis; rather, this variable only predicted which patients had an increased risk of a serum P < 20 ng/mL on the day of ET.
Table 3.
Comparison of fresh donor oocyte cycles that did or did not require supplemental progesterone dose adjustments according to body mass index
| Recipient BMI (kg/m2) | No P dose increase (n = 154) | P dose increased (n = 75) | RR (95 % CI) | P-value |
|---|---|---|---|---|
| Normal (<25) | 121 (74.7 %) | 41 (25.3 %) | Referent | Referent |
| Overweight (25–29.9) | 29 (53.7 %) | 25 (46.3 %) | 1.82 (1.23–2.70) | 0.003 |
| Obese (≥30) | 4 (30.8 %) | 9 (69.2 %) | 2.72 (1.74–4.25) | <0.0001 |
Supplemental intramuscular progesterone was increased by 50–100 % of the starting dose if serum progesterone was <20 ng/mL on the day of embryo transfer
BMI body mass index, P progesterone
Fig. 2.
Boxplots of serum progesterone level (ng/mL) on day of embryo transfer in fresh donor oocyte cycles according to body mass index. White diamond represents the mean. Box middle line represents the median. Box lower and upper limits represent the 25th and 75th percentiles, respectively. Fences represent 1.5 times the interquartile range. The open circles represent data outliers. The dotted line represents P = 20 ng/mL, below which patients received additional P supplementation
Discussion
This study was undertaken to evaluate the association between serum progesterone (P) levels on the day of ET and clinical outcome in fresh donor IVF/ICSI cycles with a day 3 embryo transfer. The principal finding was that embryo recipients with serum P < 20 ng/mL on the day of ET, despite increased P dosing after ET, had a significantly lower likelihood of live birth, compared to those with serum P ≥ 20 ng/mL at ET. Furthermore, overweight and obese patients were more likely to have low levels of P that require dose increases with supplemental IM P. Despite the additional P supplementation, recipients with P < 20 ng/mL at ET had persistently lower rates of clinical pregnancy and live birth, suggesting that additional supplementation after ET was insufficient to rescue pregnancy rates. The results of this study suggest that a subset of embryo recipients—those with P < 20 ng/mL at ET—may benefit from earlier intervention to increase the dose of P or higher starting doses of P in future cycles.
There are several possible mechanisms to explain these observations. As described previously, P has a quiescent effect on the uterus, and higher concentrations of serum P have been correlated with less uterine contractility by sonographic assessment and higher implantation rates [5]. Thus, patients with serum P ≥ 20 ng/mL may have dampened myometrial activity, thereby a lower risk of expelling the transferred embryo(s). Alternatively, serum P may be related to the timely development of the so-called ‘window of implantation,’ by blocking proliferative effects of estrogen in the endometrium and inducing the transcriptional program required for stromal decidualization and trophoblast invasion [19]. This ‘window of implantation’ is dependent on the function of a specific profile of growth and transcription factors, membrane receptors, cell adhesion molecules and glycoproteins, all known to be modulated upstream by P [19]. Knockout mouse models of several of these regulators—including uterine-specific COUP-TFII, a transcription factor activated by hedgehog proteins whose expression is induced by P—show impaired decidualization and implantation [20]. Progesterone has also been shown to induce maternal immunosuppression via the action of Hoxa-10, which is necessary for the development of immune tolerance to the allogeneic embryo [21]. Ultimately, in those cycles in our study with low serum P at ET, there may be irreversible disturbances in the endometrial molecular and immunologic environment that cannot be overcome by later dose adjustments in supplemental P. Alternatively, though extensive multivariate analysis attempted to identify other possible confounders, lower progesterone levels at ET may reflect an independent underlying physiologic difference between patients with low versus high P at ET that has not yet been identified and may result in reduced success of treatment. Follow up studies designed to obtain adequate progesterone levels at ET in all patients would clarify this issue.
Our study identified a subset of heavier recipients (either overweight or obese at cycle start), who are more likely to have serum P < 20 ng/mL at ET, requiring increased doses of IM P. In patients with higher BMIs, IM P injections may not adequately penetrate the subcutaneous tissue into the muscle, or their serum P levels may be lower due to a larger volume of distribution and the highly lipophilic properties of P. Of note, BMI was not an independent predictor of live birth in this study, consistent with a recent meta-analysis of 4,758 donor oocyte recipients (live birth in obese patients vs. non-obese patients: RR 0.91, 95 % CI 0.65–1.27) [22]. In our study, patients with serum P ≥ 20 ng/mL at ET, regardless of BMI, were more likely to obtain clinical pregnancy or live birth than patients with serum P < 20 ng/mL. Rather, BMI only predicted the risk of needing a dose adjustment in supplemental P. These results suggest that recipients with higher BMIs would likely benefit from higher doses of P from the start of supplementation, but not a prolonged course of supplementation prior to ET [10]. Further pharmacokinetic research is needed to define optimal dosing for these patients, in order to obtain serum P levels ≥20 ng/mL reliably.
While this study used serum P of ≥20 ng/mL as the threshold value, which reflects our existing practice pattern and the cut-off between the first and second tertile in the overall distribution of serum P in our study cohort, the optimal serum P at ET remains unknown. Interestingly, our results showed that the likelihood of live birth increased with serum P level at ET in a dose-dependent manner (P = .02), and did not suggest an upper limit of serum P at ET above which pregnancy rates declined. Ultimately, however, further research is required regarding target serum P at ET in embryo recipients, and the dosing regimens necessary to achieve this.
The effect of serum E2 at ET on live birth rates was difficult to assess in this study. Serum E2 levels were obtained in less than half of recipients, and the factors determining whether or not this measure was performed (e.g., mode of supplemental E2, use of a luteal GnRH agonist, year of embryo transfer) may have introduced confounding. When restricted to patients with a recorded E2 at transfer, however, mulitvariate analysis suggested that E2 did not alter the effect estimate of progesterone significantly. Furthermore, live birth rates were similar across all tertiles of E2 at ET. Ultimately, due to inconsistency with which E2 measurements were obtained at ET, residual confounding could remain, but confounding by E2 was not observed in these statistical analyses. Our findings are also consistent with previous reports of donor cycles prepared with oral estradiol, in which the serum E2 on day 9, day 12, and the day of oocyte retrieval did not correlate with pregnancy rates [17, 18].
There appeared to be a large difference in live birth rate comparing the outcome of cycles within the highest quartile of E2:P ratio (Q4 range: 20.8–54.2) to the lowest quartile (Q1 range: 3.8–11.5) (35 % versus 76 % live birth/transfer, respectively). This is in line with the primary finding of this study that low serum P (thereby increasing the E2:P ratio) was associated with lower live birth rates. The mean serum P of the highest quartile of E2:P ratio was 16.1 ± 5.1 ng/mL, while the mean serum P of the lowest quartile of E2:P ratio was 36.6 ± 8.7 ng/mL. Ultimately, however, as analysis was restricted to cycles using oral estradiol and only a total of 86 women contributed to these data across all quartiles, the highest and lower quartile represented only a total of 8 and 16 live births, respectively. Therefore, we are unable to speculate regarding statistical significance, but suggest that these findings warrant further investigation in a larger study population.
We acknowledge several limitations of this study. First, the database used in this study has multiple contributors. To address the possibility of data entry errors, data outliers, including P at ET <20 ng/mL, were verified in the electronic medical record, in which laboratory values are directly reported. Second, this study was restricted to fresh donor oocyte cycles, and applicability to fresh autologous IVF cycles or cryopreserved cycles remains unclear. Next, endometrial stripe thickness is not measured in recipients during fresh donor cycles in our program, and as a result, this variable was not available for analysis. Also, as this was a retrospective study, endometrial biopsies from mock cycles were not obtained; thus, serum P levels could not be correlated with endometrial histology or ultrastructural markers of uterine receptivity. Finally, these results are not generalizable to patients using methods of luteal support other than IM P, as serum P concentrations do not reflect the in situ endometrial concentration from vaginally-delivered P due to the first uterine pass effect [23].
In summary, the results of this study with donor oocyte cycles and day 3 ET identify a subset of recipients—those with serum P < 20 ng/mL at ET—with significantly lower clinical pregnancy and live birth rates. Additional research is needed to define further the optimal serum P level at ET in fresh donor oocyte cycles, and to assess the relevance of this variable in cryopreserved cycles. Moreover, rigorous evaluation is required of interventions used to achieve that threshold, which could then be applied in prospective trials. Until then, clinicians may consider prescribing higher initial doses of IM P for recipients with a BMI ≥25 kg/m2 or those who have already undergone a failed donor cycle with a serum P < 20 ng/mL at ET.
Acknowledgments
Financial support
None
Financial disclosures
P.C. Brady: none
D.J. Kaser: none
E.S. Ginsburg: royalties from UpToDate
R.K. Ashby: none
S.A. Missmer: Associate Editor of Human Reproduction
K.F. Correia: none
C. Racowsky: Board member of ASRM; royalties from UpToDate
Footnotes
Capsule Serum progesterone levels at embryo transfer in fresh donor IVF/ICSI cycles were positively correlated with live birth. Overweight and obese recipients were more likely to have low serum progesterone at transfer.
References
- 1.Strauss JF, Williams CJ. The ovarian life cycle. In: Strauss JF, Barbieri RL, editors. Reproductive endocrinology, 6th edn. Philadelphia; 2009. p. 155–90.
- 2.Sallam HN, Sallam A, Ezzeldin F, Agamia AF, Abou-Ali A. Reference values for the midluteal plasma progesterone concentration: evidence from human menopausal gonadotropin-stimulated pregnancy cycles. Fertil Steril. 1999;71:711–4. doi: 10.1016/S0015-0282(98)00531-7. [DOI] [PubMed] [Google Scholar]
- 3.van der Linden M, Buckingham K, Farquhar C, Kremer JA, Metwally M. Luteal phase support for assisted reproduction cycles. Cochrane Database Syst Rev. 2011;5 doi: 10.1002/14651858.CD009154.pub2. [DOI] [PubMed] [Google Scholar]
- 4.Glujovsky D, Pesce R, Fiszbajn G, Sueldo C, Hart RJ, Ciapponi A. Endometrial preparation for women undergoing embryo transfer with frozen embryos or embryos derived from donor oocytes. Cochrane Database Syst Rev. 2010 Jan 20;(1):CD006359. [DOI] [PubMed]
- 5.Fanchin R, Righini C, Olivennes F, Taylor S, de Ziegler D, Frydman R. Uterine contractions at the time of embryo transfer alter pregnancy rates after in-vitro fertilization. Hum Reprod. 1998;13:1968–74. doi: 10.1093/humrep/13.7.1968. [DOI] [PubMed] [Google Scholar]
- 6.Check JH, Wilson C, Choe JK, Amui J, Brasile D. Evidence that high serum progesterone (P) levels on day of human chorionic gonadotropin (hCG) injection have no adverse effect on the embryo itself as determined by pregnancy outcome following embryo transfer using donated eggs. Clin Exp Obstet Gynecol. 2010;37:179–80. [PubMed] [Google Scholar]
- 7.Ochsenkühn R, Arzberger A, von Schönfeldt V, Gallwas J, Rogenhofer N, Crispin A, et al. Subtle progesterone rise on the day of human chorionic gonadotropin administration is associated with lower live birth rates in women undergoing assisted reproductive technology: a retrospective study with 2,555 fresh embryo transfers. Fertil Steril. 2012;98:347–54. doi: 10.1016/j.fertnstert.2012.04.041. [DOI] [PubMed] [Google Scholar]
- 8.Huang R, Fang C, Xu S, Yi Y, Liang X. Premature progesterone rise negatively correlated with live birth rate in IVF cycles with GnRH agonist: an analysis of 2,566 cycles. Fertil Steril. 2012;98:664–70. doi: 10.1016/j.fertnstert.2012.05.024. [DOI] [PubMed] [Google Scholar]
- 9.Xu B, Li Z, Zhang H, Jin L, Li Y, Ai J, et al. Serum progesterone level effects on the outcome of in vitro fertilization in patients with different ovarian response: an analysis of more than 10,000 cycles. Fertil Steril. 2012;97:1321–7. doi: 10.1016/j.fertnstert.2012.03.014. [DOI] [PubMed] [Google Scholar]
- 10.Prapas Y, Prapas N, Jones EE, Duleba AJ, Olive DL, Chatziparasidou A, et al. The window for embryo transfer in oocyte donation cycles depends on the duration of progesterone therapy. Hum Reprod. 1998;13:720–3. doi: 10.1093/humrep/13.3.720. [DOI] [PubMed] [Google Scholar]
- 11.Kaser DJ, Ginsburg ES, Missmer SA, Correia K, Racowsky C. Intramuscular progesterone versus 8 % Crinone vaginal gel for luteal phase support for day 3 cryopreserved embryo transfer. Fertil Steril. 2012;98:1464–9. doi: 10.1016/j.fertnstert.2012.08.007. [DOI] [PubMed] [Google Scholar]
- 12.Tummon IS, Daniel SA, Kaplan BR, Nisker JA, Yuzpe AA. Randomized, prospective comparison of luteal leuprolide acetate and gonadotropins versus clomiphene citrate and gonadotropins in 408 first cycles of in vitro fertilization. Fertil Steril. 1992;58:563–8. [PubMed] [Google Scholar]
- 13.Cheung LP, Lam PM, Lok IH, Chiu TT, Yeung SY, Tjer CC, et al. GnRH antagonist versus long GnRH agonist protocol in poor responders undergoing IVF: a randomized controlled trial. Hum Reprod. 2005;20:616–21. doi: 10.1093/humrep/deh668. [DOI] [PubMed] [Google Scholar]
- 14.Reichman DE, Jackson KV, Racowsky C. Incidence and development of zygotes exhibiting abnormal pronuclear disposition after identification of two pronuclei at the fertilization check. Fertil Steril. 2010;94:965–70. doi: 10.1016/j.fertnstert.2009.04.018. [DOI] [PubMed] [Google Scholar]
- 15.Racowsky C, Combelles CMH, Nureddin A, Pan Y, Finn A, Miles L, et al. Day 3 and day 5 morphological predictors of embryo viability. Reprod BioMed Online. 2003;6:323–31. doi: 10.1016/S1472-6483(10)61852-4. [DOI] [PubMed] [Google Scholar]
- 16.Greenland S. Modeling and variable selection in epidemiologic analysis. Am J Public Health. 1989;79:340–9. doi: 10.2105/AJPH.79.3.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Noyes N, Hampton BS, Berkeley A, Licciardi F, Grifo J, Krey L. Factors useful in predicting the success of oocyte donation: a 3-year retrospective analysis. Fertil Steril. 2001;76:92–7. doi: 10.1016/S0015-0282(01)01823-4. [DOI] [PubMed] [Google Scholar]
- 18.Remohí J, Ardiles G, García-Velasco JA, Gaitán P, Simón C, Pellicer A. Endometrial thickness and serum oestradiol concentrations as predictors of outcome in oocyte donation. Hum Reprod. 1997;12:2271–6. doi: 10.1093/humrep/12.10.2271. [DOI] [PubMed] [Google Scholar]
- 19.Halasz M, Szekeres-Bartho J. The role of progesterone in implantation and trophoblast invasion. J Reprod Immunol. 2013;97(1):43–50. doi: 10.1016/j.jri.2012.10.011. [DOI] [PubMed] [Google Scholar]
- 20.Kurihara I, Lee DK, Petit FG, Jeong J, Lee K, Lydon JP, et al. COUP-TFII mediates progesterone regulation of uterine implantation by controlling ER activity. PLoS Genet. 2007;3:e102. doi: 10.1371/journal.pgen.0030102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yao MW, Lim H, Schust DJ, Choe SE, Farago A, Ding Y, et al. Gene expression profiling reveals progesterone-mediated cell cycle and immunoregulatory roles of Hoxa-10 in the preimplantation uterus. Mol Endocrinol. 2003;17:610–27. doi: 10.1210/me.2002-0290. [DOI] [PubMed] [Google Scholar]
- 22.Jungheim ES, Schon SB, Schulte MB, DeUgarte DA, Fowler SA, Tuuli MG. IVF outcomes in obese donor oocyte recipients: a systematic review and meta-analysis. Hum Reprod. Hum Reprod. 2013;28:2720–7. [DOI] [PMC free article] [PubMed]
- 23.Bulletti C, de Ziegler D, Flamigni C, Giacomucci E, Polli V, Bolelli G, et al. Targeted drug delivery in gynaecology: the first uterine pass effect. Hum Reprod. 1997;12:1073–9. doi: 10.1093/humrep/12.5.1073. [DOI] [PubMed] [Google Scholar]