Abstract
Postoperative infection and sepsis remain a major cause of morbidity among patients undergoing surgery. Maintenance of strict asepsis is essential if post-operative infection and its consequences are to be minimized. In developing countries maintenance of asepsis in most operation theatres is limited to fumigation and mopping. Clinical trials have confirmed that 80–90 % of bacterial contaminants found in wound after surgery come from microbes present in air of operating theatre. A study was conducted to evaluate the microbiological contamination of maxillofacial operation theatres in India and its correlation to weekly fumigation. A total of 6,723 culture plates, including 2,241 air and 4,482 swab samples were studied. Samples were collected at prefumigation, midcycle and post-fumigation stages and were cultured over three different medias. Predominant bacteria identified were Staphylococcus aureus (76 out of 83 samples by active air sampling) followed by Streptococcus while Aspergillus was the main fungal isolate. Formaldehyde based fumigation decreased the colony forming units (cfu/m3) of all organisms in different samples and was found to be highly effective against Fungi and E. coli. Literature suggests that for conventional operating theatres the bioload should not exceed 35 cfu/m3 in an empty theatre. In our study the cfu levels were always lower immediately after fumigation was carried out; however they moved up beyond this limit as the days passed. This implies that once a week fumigation with formaldehyde is less than optimal to achieve acceptable levels of disinfection.
Keywords: Fumigation, Colony forming unit, Formaldehyde, Air sampler, Settle plate method
Introduction
Hospital indoor air contains a diverse range of microbial population. Ever since Lister described aseptic techniques in 1866, the environment in which major surgical operations are carried out has been a matter of intense debate among the surgical fraternity [1]. Post-operative infection and sepsis remain a major cause of morbidity among patients undergoing surgery. Maintenance of strict asepsis is essential if post-operative wound and its consequences are to be minimized. Hospital associated infections add to 57 % of additional hospital days and 42 % of extra expenses. In India incidence of post-operative infections in various hospitals is relatively higher (10–25 %) [2] than UK (5 %) [3] and USA (5–6 %) [4]. The total number of bacteria carrying particles falling in the wound has been calculated to be approximately 270/cm3/h. The risk of infection depends on how many of these bacteria are viable at the time of wound closure, their virulence, the precise site of lodgment and integrity of patient’s host defense [5].
Many dental hospitals in developing countries often do not have well designed and properly ventilated operation theatres; technologies like the use of clean air is not a regular practice thereby making overall environmental control suboptimal. The only means of maintaining acceptable levels of disinfection in such operation theatres is to follow stringent protocols of fumigation and mopping of the surfaces [6]. Any laxity in the chain of disinfection could lead to increased colonization of the theatre air and surfaces putting the patients to a greater risk of infectious complications. In such circumstances it becomes imperative to establish regularly the efficacy of disinfection protocols by employing appropriate microbiological methods.
The maxillofacial operation theatre is different from the other operation theatres. In addition to the shedding of micro-organisms from the skin of the theatre personnel and the patient, there are additional sources of contamination in the maxillofacial theatre including decayed enamel, dentin, pulp, cementum, bone, blood, pus and saliva (with its abundant microflora). Also, generation of aerosols due to frequent use of rotary instruments like air-rotors, micro-motors and oscillating saws could lead to distinctive microbiological contamination [7].
In India, oral and maxillofacial surgery as a branch still primarily remains a dental specialization, unlike the developed world where dual qualification (medical and dental) requirement has made the specialization better integrated with the medical specialties. Lack of dedicated microbiology and infection control teams in dental hospitals without associated medical colleges may lead to laxity in adequate checks and balances to counter microbial contamination in aseptic environments.
Due to the uniqueness of the maxillofacial operation theatre in terms of aforesaid reasons, it needs to be evaluated discretely. There have been a few studies in India to evaluate the bacterial contamination of operation theatres, but none has focused on the oral and maxillofacial theatres.
The present study was aimed at monitoring the microbiological status of maxillofacial operation theatre in a developing country with respect to contamination of its air and different surfaces.
Materials and Methods
The study was carried out in the maxillofacial operation theatre of a teaching dental hospital in India over a period of 18 months (November 2009–April 2011). The dental wing of the hospital has one major operating room only. The hospital routine for sterilization of the operating room consisted of high level disinfection every Saturday by formaldehyde gas generated by addition of KMnO4 and 40 % liquid formalin. The operating room was sealed off for 24 h following formaldehyde fumigation and liquid ammonia solution was used to neutralize the irritant effects of formaldehyde 2 h before surgery. Samples were collected in the empty theatre before fumigation (prefumigation), 36 h after the fumigation process was complete (post-fumigation) and on Wednesday evening (midcycle).
Air Sampling
Air sampling was done by the centrifugal air sampler method (active air sampling) and settle plate method (passive air sampling). Three different media, blood agar, nutrient agar and potato dextrose agar (PDA) were employed to culture the microorganisms.
Surface Sampling
Sterile wet swabs were taken from different surfaces in the operation theatre:
operating table;
instrument trolley
light;
shelf;
floor; and
instrument surface.
The data was recorded and evaluated on the basis of:
date, method and site of collection of samples and
type of the micro-organisms identified, and their colony forming units (cfu).
Results
A total of 6,723 culture plates were studied. Samples collected included 2,241 air and 4,482 swab samples. In all the methods 83 samples each were collected at prefumigation, midcycle and post-fumigation stages. Each sample was inoculated on the three media.
The results were tabulated (Tables 1, 2, 3) and analyzed (Figs. 1, 2).
Table 1.
Type and frequency of (predominant) microorganisms identified in 83 air samples by air sampler
| Blood agar | Nutrient agar | PDA | |||||
|---|---|---|---|---|---|---|---|
| E. coli (%) | Staph (%) | Strep β hemolyticus (%) | E. coli (%) | Staph (%) | Strep (%) | Asp | |
| Prefumigation (83) | 6 (7.2) | 23 (27.7) | 54 (65.1) | 6 (7.2) | 57 (68.7) | 20 (24.1) | 83 (100) |
| Midcycle (83) | – | 24 (28.9) | 59 (71.1) | – | 60 (72.3) | 23 (27.7) | 83 (100) |
| Post-fumigation (83) | – | 76 (91.6) | 7 (8.4) | – | 61 (73.5) | 22 (26.5) | 0 |
Table 2.
Type and frequency of microorganisms on air sampling by settle plate method
| Blood agar | Nutrient agar | PDA | ||||||
|---|---|---|---|---|---|---|---|---|
| E. coli (%) | Gram positive colonies giving pigments (%) | Staph (%) | Strep β (%) | E. coli (%) | Staph (%) | Strep (%) | Asp | |
| Prefumigation | 7 (8.4) | 5 (6) | 21 (25.3) | 50 (60.2) | 7 (8.4) | 55.7 (66.7) | 20.3 (24.1) | 83 (100) |
| Midcycle | – | – | 25 (30.1) | 58 (69.9) | – | 65 (78.3) | 18 (21.7) | 83 (100) |
| Post-fumigation | – | – | 70.3 (84.7) | 12.6 (15.3) | – | 66.3 (79.9) | 16.6 (20.1) | 0 |
Table 3.
Type and frequency of microorganisms identified on surface swab sampling
| Blood agar | Nutrient agar | PDA | |||
|---|---|---|---|---|---|
| Staph (%) | Strep β (%) | Staph (%) | Strep (%) | Asp (%) | |
| Prefumigation | 25 (30.1) | 58 (69.9) | 64 (77.1) | 19 (22.9) | 83 (100) |
| Midcycle | 23 (27.7) | 60 (72.35) | 65 (78.3) | 18 (21.7) | 83 (100) |
| Post-fumigation | 75 (90.4) | 8 (9.6) | 67 (80.7) | 16 (19.3) | 0 |
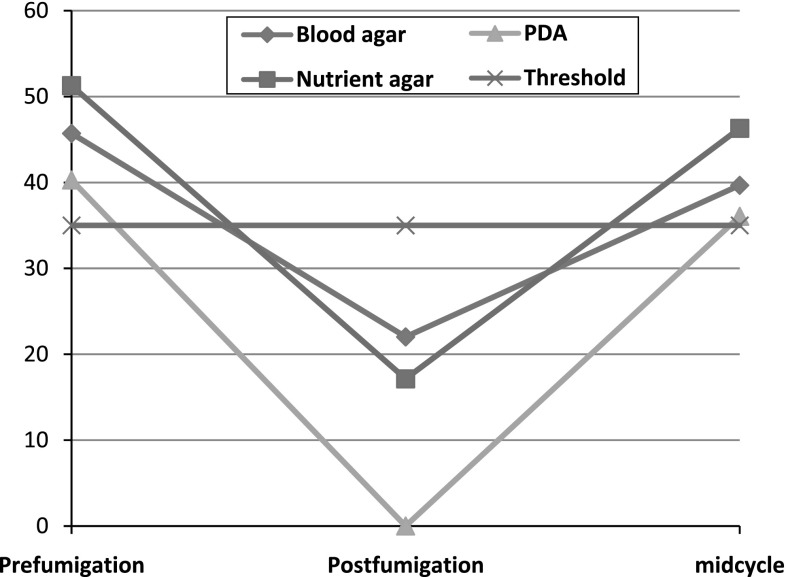
Fig. 1.
Average cfu at different times using air sampler on different culture media
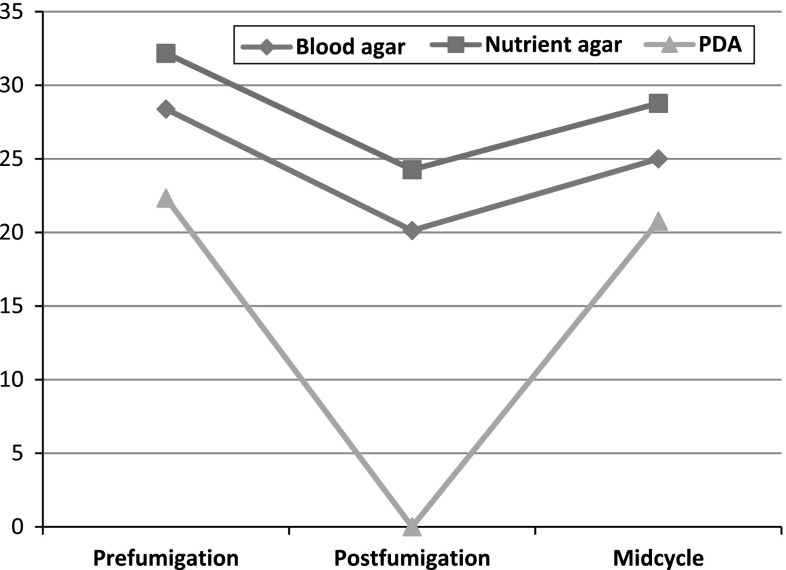
Fig. 2.
Average cfu on settle plate sampling using different media at different times
Overall, the mostly commonly isolated organisms were S. aureus, Streptococcus β hemolyticus, E. coli and Aspergillus.
S. aureus seemed to be the most resistant (to fumigation) among the predominant bacteria identified in air and surface swab samples. It was identified on 76 out of 83 samples in post-fumigation period as compared to 23 times in prefumigation by active air sampling (Table 1). Although the frequency of identification of S. aureus increased in post-fumigation period as compared to prefumigation, the average number of colony forming units decreased in post-fumigation period (Fig. 1). Identical findings were observed in both settle plate air sampling and surface swab sampling (Tables 2, 3). The frequency of isolation of S. aureus was 70.3 and 75 post-fumigation as compared to 21 and 25 out of 83 times in prefumigation period respectively.
The average number of cfu always decreased post-fumigation as compared to prefumigation (Fig. 2).
Discussion
Surgical site infections are the third most frequent cause of hospital infections [8]. They have been responsible for increasing the expenditure, morbidity and mortality related to surgeries. Even in hospitals with most modern facilities, rigorous protocols of preoperative preparation and antibiotic prophylaxis, they continue to be a major problem.
Microbiological contamination of air in the operating room is generally considered to be a risk factor for surgical site infections. According to Pasquarella et al. [9] microbiological quality of air may be considered as a mirror of the hygienic condition of the operation theatres. Clinical trials have confirmed that 80–90 % of bacterial contaminants found in wound after surgery come from colony forming units present in air of operating theatre [10]. When the number of cfu is reduced, there is corresponding reduction in the incidence of wound infection [11]. It has been observed that counts in the range of 700–1,800 cfu/m3 were related to significant risk of infection and the risk was slight when they were below 180 cfu/m3 [12].
Evaluation of the quality of air/surfaces in operating theatres can be performed by microbiological sampling consisting of measuring the amount of micro-organisms on a surface or in a determined air volume [13, 14]. Many different methods for environmental monitoring have been used and can be divided into four groups; the count of colony forming units (cfu) per cubic meter of air; the count of cfu on settle plates; measurement of chemical components of microbial cells per meter cube; the count under the microscope.
According to Hoffman et al. [15] aerobic cultures on non-selective medium should not exceed 35 bacterial/fungal particles per cubic meter by active air sampling. In our study although the cfu levels were always lower than 35 microbial particles post fumigation on all types of media, they climbed up above this limit as the days passed by after the fumigation was carried out (Fig. 1). According to Kelkar et al. [14] air sampling was done in an ophthalmic OT by settle plate method on blood agar and presence of unacceptably high bacterial count (>10 cfu) was seen in 3.26 % samples.
According to Dharan and Pittet [16] countries have set threshold limits for conventionally ventilated operating theatres, although most recommend 20 air changes per hour to obtain 50–150 cfu/m3. It is recommended that for conventional operating theatres the bioload should not exceed 35 cfu/m3 in an empty theatre [17] or 180 cfu/m3 during an operation [12]. Although these values never crossed 55 cfu at any time in our sampling, the study is limited by the fact that the sampling was always carried out in empty theatres.
As far as settle plate method of air sampling is concerned it has certain limitations for example it is not a satisfactory method of detecting bacteria in very small suspended particles [18] and it is not considered to be as accurate a method as active air sampling by air sampler. According to Fischer et al. the acceptable limits of microbial count in air by settle plate sampling is 5–8 cfu in resting operation theatre and 61–90 cfu in the operation theatre during activity [9]. For aseptic rooms, values ranging from 5 to 90 cfu/m3 have been put forward. The pattern of counts in our study with settle plate method was similar to the counts obtained by active air sampling. They always came down post fumigation and started to increase as the time from fumigation increased. The values ranged from 0 to 35 cfu, remaining above the 5–8 cfu range given by Fischer at all times except when they approached zero on PDA media post fumigation (Fig. 2). However the values did conform to the range given for aseptic rooms. According to Osaro et al. [19], air sampling was done by settle plate method on nutrient agar and PDA. The minimum and maximum cfu were 15 and 39 on nutrient agar and 8 and 18 on PDA respectively. This is comparable to the results obtained in our study wherein the minimum and maximum cfu on nutrient agar was 10 and 41 while on PDA it ranged from 0 to 33.
S. aureus and β haemolytic streptococci are important healthcare associated pathogens and can persist in environment for extended periods; when healthcare personals are heavily colonized with these microorganisms, they can be shed and infection can occur. Staphylococcus is known to be carried in nasopharynx, throat, skin, cuts, boils, nails, and as such can easily contribute to the microflora in the environment. E. coli which are coliforms makes up to 10 % of microorganism of humans and are used as indicator organisms for microbial monitoring of the operation theatres [20].
Multiple reservoirs have been reported as being responsible for microbial load in the operating theatre, including unfiltered air, ventilation system, surgical team and contaminated antiseptic solutions. The operating room layouts, operating room etiquette, sterilization of instruments and sterile surgical protocols directly affect the incidence of post-operative infections. All these factors including environmental disinfection need the most critical monitoring. Operating rooms in developing countries often do not adhere to standards for physical parameters. Most conventional operating theatres in hospitals in India are equipped with window mounted air conditioning units. These air conditioners are installed more for comfort than for clean air delivery and the filters utilized in these units can act as nidus for growth and proliferation of pathogenic fungi [21].
Amongst the bacterial isolates E. coli, Staphylococcus and Proteus were observed to be the most prevailing isolates while Aspergillus and Fusarium were the fungal isolates most commonly found in the study [19]. According to Javed et al. [12] who conducted air sampling by settle plate method on blood agar, Staphylococcus was isolated from all the air samples obtained from various OTs except ENT. Bacterial count in air ranged from 6,500 to 15,730 cfu. In our study the predominant bacteria identified in all the samples were Staphylococcus aureus and Streptococcus while Aspergillus was the main fungal isolate.
Formaldehyde based fumigation decreased the cfu/m3 of all organisms in different samples and was found to be highly effective against Fungi and E. coli. It was observed that in prefumigation and midcycle sampling by air sampler the cfu/m3 were greater than the acceptable limits (35 cfu/m3) but in post-fumigation sampling this value decreased to levels below the acceptable limits. In the settle plate method the cfu were greater than the acceptable limits (<5–8 cfu) in all types of sampling except PDA cultures which showed no growth in post-fumigation sampling. The colony forming units of Staphylococcus decreased like all other microorganisms in the post fumigation period. However the frequency of identification of S. aureus proportionately increased at the expense of other microbes suggesting that they are comparatively more resistant to fumigation than the others.
The rate of colonization of the maxillofacial operation theatre by Staphylococcus and Streptococcus β hemolyticus appears to be very fast and remained above the acceptable limits on settle plate sampling. This implies that once a week fumigation in absence of recommended environmental controls (e.g. 15–20/h air changes) is less than the optimal and needs to be increased.
Since it is necessary to seal the operation theatre for 24–48 h after the formaldehyde fumigation, more than one operation theatres would be required to carry out surgical procedures. If the same is not possible due to resource constraints, a mid weekly fumigation with a disinfectant (e.g. Quaternary ammonium compound) that does not require sealing for 24–48 h could be added to the conventional weekend fumigation with formaldehyde. This calls for further studies to investigate the efficacy of quaternary ammonium compounds in this context.
References
- 1.Smith C. Hospital operating theatre environment and the assessment of filters for use in associated ventilation plants. Ann Occup Hyg. 1975;17:303–314. doi: 10.1093/annhyg/17.3-4.303. [DOI] [Google Scholar]
- 2.Ajaz M, Qadri GJ, Tabish SA, et al. Incidence of nosocomial infection in postoperative patients at a teaching hospital at Kashmir. JK Pract. 2004;11:38–40. [Google Scholar]
- 3.Meers PD, Ayliffe GAJ, Emmerson AM, et al. Report on the national survey of infection in hospitals, 1980. J Hosp Infect. 1981;2(supplement):29–34. doi: 10.1016/S0195-6701(81)80007-2. [DOI] [Google Scholar]
- 4.Raymond DP, Pelletier SJ, Crabtree TD, Schulman AM, Pruett TL, Sawyer RG. Surgical infection and the aging population. Am Surg. 2001;67(9):827–832. [PubMed] [Google Scholar]
- 5.Kaur N, Hans C. Air bacterial isolations from operation theatres in a tertiary care hospital in India. J Clin Diagn Res. 2007;1(2):87–89. [Google Scholar]
- 6.Rao TV, Chithra VN (2008) Surveillance and sterilisation of operation theatres in the developing world. http://www.articlesbase.com/medicinearticles/surveillance-sterilization-anddisinfection-of-operation-theatres-inthe-developing-world-327599.html. Accessed 4 Nov 2012
- 7.Rautemaa R, Nordberg A, Wuolijoki-Saaristo K, Meurman JH. Bacterial aerosols in dental practice—a potential hospital infection problem? J Hosp Infect. 2006;64:76–81. doi: 10.1016/j.jhin.2006.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention. Guideline for prevention of surgical site infection (1999) Infect Control Hosp Epidemiol 20:247–278 [DOI] [PubMed]
- 9.Pasquarella C, Pitzurra O, Savino A. The index of microbial air contamination. J Hosp Infect. 2000;46:241–256. doi: 10.1053/jhin.2000.0820. [DOI] [PubMed] [Google Scholar]
- 10.Michla Y, Holliday M, Geuld K, Weir DJ, McCaskie AW. The impact on bacterial contamination of hip arthroplasty wounds of personal protection helmet system. J Bone Joint Surg Br. 2006;88B(III):412. [Google Scholar]
- 11.Howarth FH. Prevention of airborne infection during surgery. Lancet. 1985;1:386–388. doi: 10.1016/S0140-6736(85)91399-6. [DOI] [PubMed] [Google Scholar]
- 12.Javed I, Hafeez R, Zubair M, Anwar MS, Tayyib M, Husnain S. Microbiological surveillance of operation theatres and ICUs of a tertiary care hospital, Lahore. Biomedica. 2008;24:99–102. [Google Scholar]
- 13.Landrin A, Bissery A, Kac G. Monitoring air sampling in operation theatres: can particle counting replace microbiological sampling. J Hosp Infect. 2005;61:27–29. doi: 10.1016/j.jhin.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 14.Kelkar U, Kelkar S, Bal AM, Kulkarni S, Kulkarni S. Microbiological evaluation of various parameters in ophthalmic operation rooms. The need to establish guidelines. Indian J Ophthalmol. 2003;51:171–176. [PubMed] [Google Scholar]
- 15.Hoffman PN, Williams J, Stacey A, et al. Microbiological commissioning and monitoring of operation theatre suites. J Hosp Infect. 2002;52:1–28. doi: 10.1053/jhin.2002.1237. [DOI] [PubMed] [Google Scholar]
- 16.Dharan S, Pittet D. Environmental controls in operating theatres. J Hosp Infect. 2002;51:79–84. doi: 10.1053/jhin.2002.1217. [DOI] [PubMed] [Google Scholar]
- 17.Grand H (2009) Victorian infection control professionals association biennial state conference 2009
- 18.Nakhla LS, Cummings RF. A comparative evaluation of a new centrifugal air sampler (RCS) with a slit air sampler (SAS) in hospital environment. J Hosp Infect. 1981;2:261–266. doi: 10.1016/0195-6701(81)90048-7. [DOI] [PubMed] [Google Scholar]
- 19.Osaro EF, Ufuoma IO, Dorcas AO. Hospital indoor airborne microflora in private and government owned hospitals in Benin City, Nigeria. World J Med Sci. 2008;3:34–38. [Google Scholar]
- 20.Prescott LM, Harley JP, Klein DA. Microbiology. 6. New York: McGraw Hill Co.; 2005. [Google Scholar]
- 21.Kelkar U, Bal AM, Kulkarni S. Fungal contamination of air conditioning units in operating theatres in India. J Hosp Infect. 2005;60:81–84. doi: 10.1016/j.jhin.2004.10.011. [DOI] [PubMed] [Google Scholar]