Abstract
Introduction
Dental infection has plagued humankind for as long as our civilization has been a fight against microorganisms by man dates back to ancient civilization. The discoveries of antibiotics are encouraging trends towards conquest of the microbial infection.
Materials and Methods
This study emphasizes the detection of pathogenic microorganisms by microbiological examination and culture of specimens representative of the infection, importance of early and correct diagnosis of infections, prompt treatment and supportive care.
Results
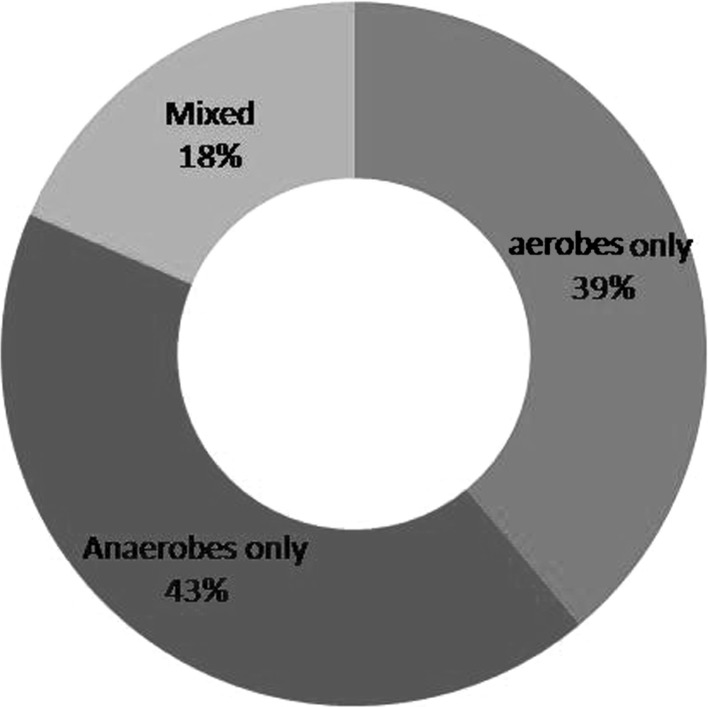
The age group most commonly involved was in the third and fourth decades of life. Extraction followed by incision and drainage was done. The most commonly involved space was submandibular followed by buccal space. Thirty isolates were obtained. 43 % of the strains were strict anaerobes and 39 % were aerobes, with mixed growth was seen in 18.52 %. Amongst aerobes alpha hemolytic Streptococcus aureus and Peptostreptococcus as anaerobes were the most predominant followed by Bacteroides and Prevotella. Mixed aerobic and anaerobic isolates were obtained from 18.52 % of total cases. Overall resistance to Penicillin was 22 %, amongst aerobes.
Conclusion
Amoxicillin and Clavulanic acid combination performed better, as 100 % strains were sensitive to it. The results of this study saw a changing trend in terms of predominance of anaerobic bacteria over aerobic ones.
Keywords: Odontogenic space infection, Microbiology, Antibiotics, Culture sensitivity
Introduction
Facial space infection has been recognized and described since the time of Galen in the second century. A fight against microorganisms by man dates back to ancient civilization. Ancient Indians used chaulmoogra oil to cure leprosy [1]. Despite all these, even after centuries and endless research, mankind has not been successful in eradicating microbial infections in total [2–5]. The discoveries of sulfonamides by Domgk and Penicillin by Alexander Fleming [1] are encouraging trends towards conquest of the microbial infection. Although Penicillin was considered the long awaiting panacea for dental infection, the bacteriological spectrum of the oral flora and the understanding of its complexities have undergone rapid evolution since Penicillin was introduced [1], microorganisms are still a step ahead. The newer and more potent antibiotics too have faced a stiff resistance.
Treatment of localized infection was probably the first primitive surgical procedure performed, and it most likely involved the opening of bulging abscesses with sharp stones or pointed sticks. Even today the principle remains the same though the technique has improved [1]. Greater numbers of medically compromised patients with altered defense mechanisms, the change of oral microbial flora toward more resistant forms, and the altered efficacy of conventional antibiotic therapy have increased the potential for serious sequelae of dental infections [5]. Therefore an understanding of the pathogenic and proper management of oral infections is of critical importance to the dental practitioner.
Since microorganisms vary from region to region as do their susceptibilities [1] it is of vital importance that such studies are to be done in India and should be compared with western literature. This will help in monitoring the continuous evolution of microorganisms and their susceptibility to commonly used drugs. This study is to emphasize the detection of pathogenic microorganisms by microbiological examination and culture of specimens representative of the infection, importance of early and correct diagnosis of infections, prompt treatment and supportive care.
Material and Methods
Thirty patients referred to the Oral and Maxillofacial Surgery Department of Modern Dental College and Research Centre, Indore over a period of two and a half years (May 2008–November 2010) with specific complaints of infectious symptoms diagnosed as orofacial space infection of odontogenic origin irrespective of their age and sex formed the study group.
Inclusion criteria: Patients with maxillofacial infections of odontogenic origin which have to be treated with extraction and incision and drainage.
Exclusion criteria: Immune-compromised (systemic disease or metabolic disorder, congenital defects or primary immune-deficiencies, iatrogenic and social factors), pregnancy and history of allergy to any drugs.
Specimen Collection
Extra oral approach was preferred to eliminate contamination with oral flora. The extra oral sites were prepared with germicidal soap, alcohol, povidone iodine or a combination of these. Intraoral sites were prepared with chlorexidine. Disposable syringes (5 ml) with disposable needle of 18 gauge were used to aspirate the pus from the abscess. In cases where the abscess was deep and the site difficult to reach, a sterile cotton swab was used to collect the sample. After collection it was immediately transferred into fluid thioglycollate medium and sent to microbiology department for further investigation.
Approach to Identification [6]
Pus samples were processed as follows:
Smear studies of gram staining
Aerobic culture
Anaerobic culture
Culture: Culture was done for both aerobic & anaerobic bacteria.
Aerobic culture: For aerobic culture the samples were inoculated on Mac-Conkeys agar, blood agar, and nutrient broth. After overnight inoculation the plates were observed for colony formation. The colonies were identified by gram staining and biochemical tests. For gram positive cocci catalase, bacitracin sensitivity, optochin sensitivity, coagulase test and growth in 6.5 % sodium chloride were used. For gram negative bacilli oxidase test, catalase test, indole test, urease test, citrate test and triple sugar iron were used.
If no growth was observed after the first culture, subcultures from nutrient broth was made on Mac-Conkey’s agar, blood agar and looked for growth after overnight incubation. Growth was identified using appropriate biochemical tests.
Anaerobic culture: For anaerobic culture, sample was inoculated into plain blood agar, kanamycin and vancomycin blood agar, bile esculin agar and incubated anaerobically using gas pack, in anaerobic jar for 47–72 h. The plates were observed for colony formation.
The colonies were identified by gram’s stain morphology, hemolysis, and sensitivity to antibiotics like Penicillin, vancomycin, kanamycin, colistin, growth in bile, indole test, pigmentation, lipase, catalase and sodium polyethanol sulphonate and colonies were tested for aero-tolerance.
If no growth was observed after first culture, subculture was done from Brain heart infusion broth on plain blood agar, bile esculin agar and identified as mentioned above.
Antibiotic sensitivity was done by Kirby-Bauer disk diffusion method for the following drugs:
Penicillin G
Ampicillin
Amoxycillin
Amoxycillin-Clavulanic acid
Cotrimaxazole
Cefotaxime
Cephalexin
Gatifloxacillin
Gentamycin
Amikacin
Doxycycline
Metronidazole
Erythromycin
Roxythromycin
Clindamycin
All patients were started with empirical antibiotics in the form of Amoxicillin 500 mg PO q 8 h for adults and Metronidazole PO 400 mg q 8 h. Severely ill patients were started with Cefotaxime IV 1 g q 12 h and Metronidazole IV 500 mg q 8 h.
Causative teeth were identified by clinical, radiological examination and were extracted. Incision and drainage was carried out under aseptic conditions as follows: All the patients were taken under local anesthesia. Incision was given using no 11 blade. Stab incision was made at most dependent area. Thorough exploration of all the portions of abscess cavity was done by blunt dissection using Lister’s sinus forceps. The corrugated rubber drain was placed if required and stabilized with sutures. Drain was kept in place for less than 48 h [7].
After culture and sensitivity, depending on the clinical course of the disease appropriate antibiotics were given.
Results
Extraction followed by incision and drainage was done. In few cases only extraction was done. Needle aspiration and collection of the samples by a swab through an incision was done and subjected to a series of tests and clinical efficacy of antibiotics was analysed.
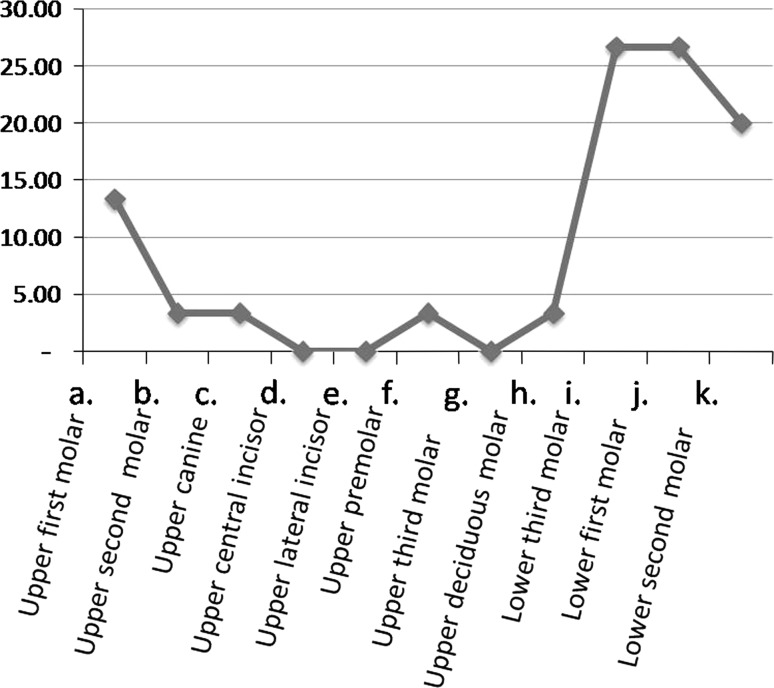
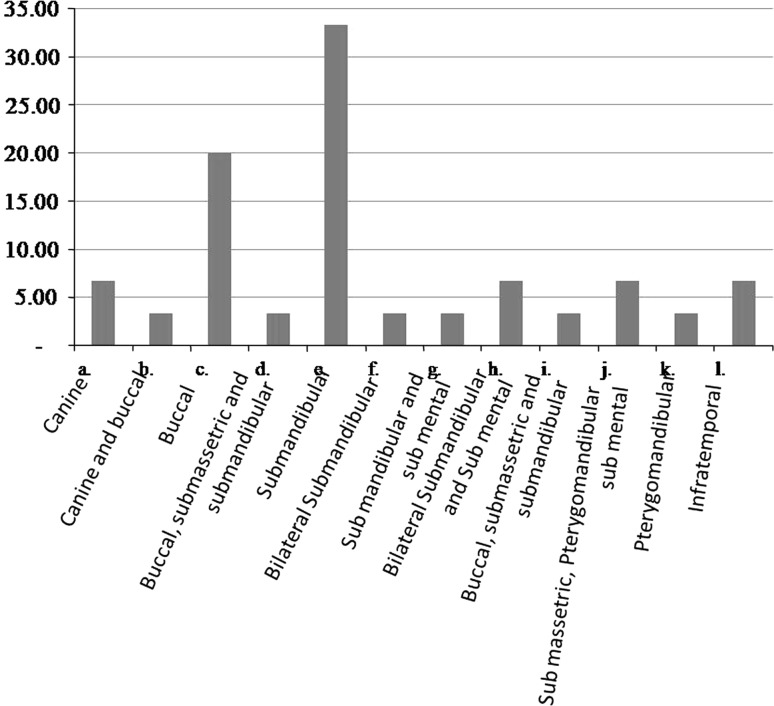
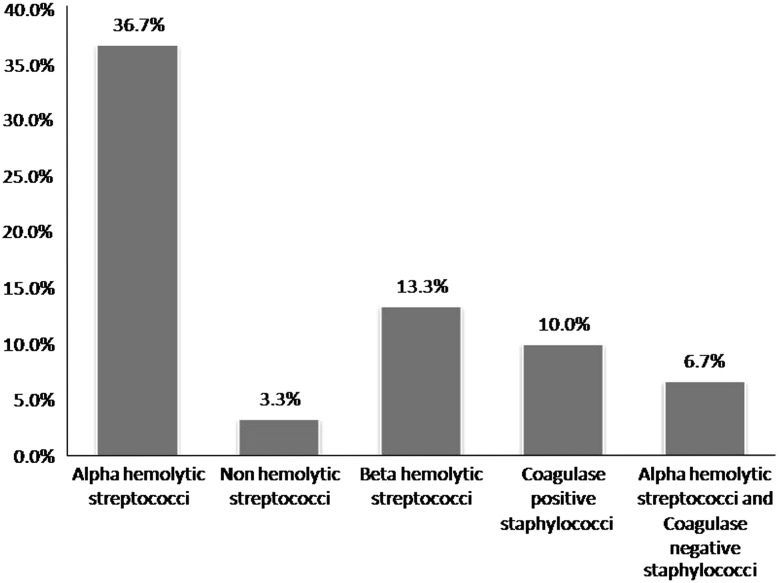
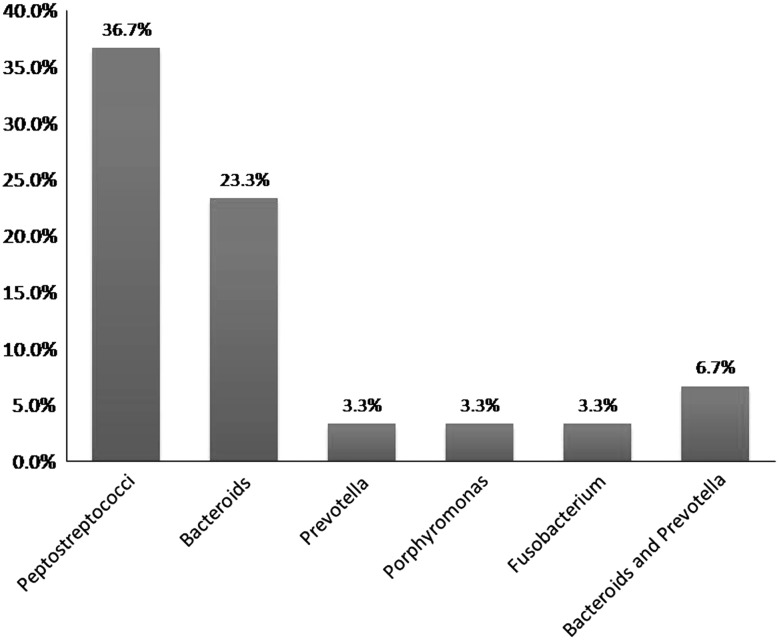
The age group most commonly involved was in the third and fourth decades of life. The mean age group was 32.43. Males were more commonly involved than females. Mandibular first molar was the most common causative tooth followed by mandibular third molar (Fig. 1). The most commonly involved space was submandibular followed by buccal space (Fig. 2). Out of 30 cases, pathogens were isolated in 28 cases and 2 cases yielded negative culture. Thirty isolates were obtained. 22(42.59 %) of the strains were strict anaerobes and 21(38.89 %) were aerobes, with mixed growth was seen in 10(18.52 %). Out of 30 patients 22 were receiving antibiotic therapy for at least 48 h prior to incision, 2 patients revealed no growth. The microorganisms were divided into two broad groups of aerobes and anaerobes which further constituted gram positive cocci and bacilli, and gram negative cocci and bacilli. Amongst aerobes, gram positive cocci included Streptococcus aureus, Enterococcus faecalis, and coagulase negative Staphylococcus aureus. Anaerobic gram positive cocci were Peptostreptococcus anaerobius and P. asaccharolyticus. Gram positive bacilli (12.90 %) and Gram negative cocci were not found. Gram negative bacilli consisted of Prevotella (25.81 %). Distribution of bacterial isolates is illustrated in Fig. 3. Amongst aerobes alpha hemolytic Streptococcus aureus were the most predominant isolates (36.7 %, Fig. 4). Amongst anaerobes, Peptostreptococcus predominated and accounted for 36.7 % followed by Bacteroides and Prevotella (6.7 %, Fig. 5). Mixed aerobic and anaerobic isolates were obtained from 18.52 % of total cases.
Fig. 1.
Frequencies of causative teeth
Fig. 2.
Site distribution of oro-facial space infection
Fig. 3.
Types of isolates
Fig. 4.
Prevalence of aerobic bacteria in the sample
Fig. 5.
Prevalence of anaerobic bacteria in the sample
Overall resistance to Penicillin was 22 %, amongst aerobes. Amoxicillin and Clavulanic acid combination performed better, as 100 % strains were sensitive to it. Results showed very low sensitivity to macrolide group. Only 36.66 % organisms were found sensitive to Erythromycin. Cefuroxime, a second generation Cephalosporin was less effective with just 46.66 % bacterial strains susceptible to it, whereas Cefotaxime (third generation Cephalosporin) was found to be highly effective (83 % sensitivity).Contrary to belief, Ciprofloxacin had 83.33 % sensitivity amongst microorganisms, which is comparable to Cefotaxime. This shows their usefulness in the current scenario. As expected, Amikacin combination were effective on all organisms tested for sensitivity.
Discussion
Origin of maxillofacial infection could be from a periapical lesion, periodontal condition, pericoronal problem, post surgical infection or direct trauma resulting in epithelial breach. Of these odontogenic ones are most commonly encountered [8–12]. Mostly an ignored or ill-treated decayed tooth becomes the root cause of a serious and life threatening infection. In a country like India where healthcare providers are inadequate in number and facilities are less, ignorance to a dental problem adds to the worsening condition. Complications such as retropharyngeal spread and intracranial extension or mediastinal spread and airway obstruction indicate the potentially serious nature of these infections [1, 13–23].
Presentation of patient condition is dictated by complex microflora, involved tooth and anatomic routes of spread [1]. Incision and drainage is the primary treatment for sure, but understanding of involved microorganisms and sensitivity pattern constitutes an important part of it. Many a times even after proper surgical treatment patient condition fails to improve, one of the important reasons for this is resistant bacterial strains and selection of wrong antibiotics [1, 24].
In our study 30 patients with orofacial odontogenic space infections were considered. The age group most commonly involved was in the third and fourth decades of life. The mean age group was 32.43. This finding is comparable to the age distribution reported by Hunt et al. [2], and Virolainen et al. [3]. Males were more commonly involved than females. This finding can be compared to the sex distribution given by Goldberg et al. [8]. Females outnumbered males in the study conducted by Hunt [2]. Mandibular first molar was the most common causative tooth followed by mandibular third molar. This finding can be compared with the study by Storoe et al. [12] where third molar was the commonest tooth followed by second and first molars. The most commonly involved space was submandibular followed by buccal space. This finding was similar to the findings of Storoe [12], Goldberg et al. [8], Wang [25], Akst [26].
Microorganisms
The concept of a mixed aerobic anaerobic infection is an important one relative to odontogenic infections. Odontogenic infection is due to the interdependent and synergistic metabolism of a variety of microorganisms. The roles played by different microorganisms in the group may be difficult to establish but at least in some cases, it has been demonstrated that individual members of the group produce metabolites that are essential for the growth of other microorganisms in the group. They produce substances that create a favorable pH in the environment, or they consume oxygen and facilitate anaerobic growth.
The microbial residents of the oral cavity constitute one of the most varied floras in the human body. The variation relates to the many microenvironments in the oral cavity, such as the various surfaces of the teeth, gingival sulcus, tongue, and buccal mucosa. Each organism has a unique set of conditions that permit the organisms to establish residency and thrive, including receptors for selective adherence, appropriate nutrients and oxygen tension or simply physical protection from unfavorable conditions. For these reasons an understanding of the nature of the oral flora and its dynamics is important in oro-facial infections environment, or they consume oxygen and facilitate anaerobic growth.
According to literature available aerobic predominance was seen initially which later on turned towards anaerobes. Results by various studies are contradictory till today. In our study anaerobic growth was present in 42.59 % of all cases. Presence of high number of anaerobes could have the following explanations:
As mentioned earlier, there were many patients who took medications prior to their presentation to the hospital OPD or casualty. The drug most commonly used was Amoxicillin which mainly acts on aerobes. This could be the reason why we got less number of aerobic population.
Patient presentation at a later stage could be one of the reasons, as it is seen that in initial stages aerobes predominate and when within a closed space available oxygen is utilized, anaerobes take over [1].
A recent study by Thomas [27] demonstrated that aerobic organisms outnumbered anaerobes by almost 2:1 ratio; our study had exactly opposite results. Aerobic organisms were only 38.89 % of positive cases. According to this equation anaerobes dominated by 2:1 ratio. This finding can also be explained by above mentioned reasons. Gram positive aerobic organisms were S. aureus, Enterococcus faecalis and coagulase negative S. aureus.
On the other hand gram positive anaerobes included P. anaerobius and P. asaccharolyticus. Gram negative organisms found were 100 % anaerobes (P.intermedia and P. melaninogenica). In this study 2 (6.56 %) samples were negative for any growth. Reason could be attributed to the following factors:
Sample collection and other technical errors (contamination of specimen while collecting pus) eg. contact with skin or mucosa during collection of sample.
Preoperative self medication by the patient—73.3 % (22 out of 30) patients had taken antibiotics before they reported to the hospital. Of these, 6.66 % (2 out of 30) revealed no growth.
Gram positive cocci were observed in 61.29 % of the gram stain smear study, negative rods in 25.7 % and positive rods in 12.90 % of the cases. These findings were similar to the findings of Aderhold et al. [4] and Konow et al. [5].
Alpha hemolytic streptococci were found in 36.7 % among aerobes, a finding similar to that of the study by Goldberg et al. [8], Aderhold et al. [4]. Beta hemolytic streptococci were present in 13.3 % of the aerobes isolated. Streptococci were the most common aerobes isolated. This finding was similar to the study conducted by Aderhold et al. [4].
Anaerobic bacteria were present in 42.59 % of the total number of isolates. Most common anaerobe isolated was peptococci, (36.7 %) followed by bacteroids (23.3 %) which was similar to the study conducted by Goldberg et al. [8], Aderhold et al. [4].
Antibiotic Sensitivity
Various literature reports that many species of organisms were resistant to Penicillin [27–29]. However most patients were clinically well by the time Penicillin resistance was discovered. There are three likely explanations for this: (1) in vitro resistance does not necessarily imply in vivo resistance, particularly in mixed infections; (2) the source of infection had usually been removed and/or surgical incision and drainage accomplished which even without antibiotics can effect a cure in many cases; (3) the possibility that in interdependent, synergistic, mixed infections, as long as one bacterial species is sensitive to Penicillin, the entire pathogenic complex may be rendered nonpathogenic.
In the present study Penicillin resistance was seen commonly in anaerobes (46 %). Gentamicin and Amikacin had highest percentage of resistance, a finding similar to studies done by Aderhold et al. [4].
Aminoglycosides are effective in controlling infections due to aerobic gram negative rods which are quite rarely encountered by oral and maxillofacial surgeons. Staphylococci were most frequently resistant to Penicillin’s among aerobes. All the organisms were found sensitive to Clindamycin and Amoxicillin–Clavulanate. Most anaerobes were sensitive to Metronidazole. Organisms were sensitive to Cephalosporins to a higher degree than to Penicillins.
Sensitivity test was performed only for aerobic organisms, as there is still a controversy with anaerobic sensitivity and lack of consensus on standard method [2, 4].
Much has been published about Penicillin resistant organisms [27]. Recently Thomas [30] quoted Penicillin resistance to be seen in 19 % of all strains. We found it in 10 % of total strains isolated and amongst 38.89 % of aerobes. The resistant strains came from Streptococcus group, as expected. Even though a large population of microbes is sensitive to this drug, allergy is the main hinderance to its use.
Semisynthetic Penicillins like Amoxicillin with Clavulanic acid have proved to be better than Penicillin alone. Our study found the same. 23.33 % organisms with sensitivity data were susceptible to this combination. This supported to the findings of Thomas [30].
Since 1980s the effectiveness of Erythromycin has decreased and its use in the treatment of maxillofacial infections has become very limited. We found only 38.89 % of total isolates and 36.33 % of aerobes responding to Erythromycin. This coincides with almost all the results available in the literature [11].
Starting from first generation, the Cephalosporins have travelled a long distance reaching fifth generation at present. The reason for this evolution is obvious: resistant infections. This study saw a difference in the sensitivity of second and third generation Cephalosporins too. Cefuroxime (second generation) had very low sensitivity as compared to Cefotaxime (third generation). Where cefuroxime was effective against 33.33 % organisms with sensitivity data, Cefotaxime had a very high percentage of 83.33 %.
One of the first drugs from the quinolone group, Ciprofloxacin has been replaced by its advanced versions like Gatifloxacin and Moxifloxacin these days. Still, the studies done in the last 5 years show their relevance in current scenario [12, 29]. We too found Ciprofloxacin as potent as third generation Cephalosporins. Looking at all these datas in support, the importance of this drug can not be ignored.
Resistant infections of hospital ICUs are being treated by higher antibiotics like Amikacin these days. In case of maxillofacial infections when we encounter life threatening infections, one has to switch to these options as a last resort. This study revealed 100 % sensitivity of all microbes to Amikacin. Same has been postulated from past observations by series of case reports of lethal infections like cervical necrotizing fasciitis [10].
Conclusion
The results of this study clearly indicate that there is a change in pattern as anaerobes, but not aerobes, dominated the bacterial population in contrast to the recent studies. Mixed growth was not found in significant number with respect to the current literature. Gram positives were much more in number against gram negative organisms. Aerobes found were only gram positive cocci whereas anaerobic population consisted of both Gram positive cocci and gram negative bacilli. Most commonly isolated organisms were Peptostreptococcus and Streptococcus aureus. Penicillin resistance was within the expected limits as mentioned before. Ciprofloxacin and Cefotaxime had good effectiveness against organisms.
In the end, it would be apt to state that our study saw a changing trend in terms of predominance of anaerobic bacteria over aerobic ones. Other findings such as population of gram positive organisms, most commonly isolated bacteria and Penicillin resistance were same as seen in current observations.
Importance of thorough drainage of the infected site cannot be overlooked and this should be supported by proper antibiotic therapy based on culture and sensitivity reports. Time to time analysis of bacterial strains and resistance pattern should be a continuous process, so that we do not lag behind the latest changes. To achieve this goal, extensive research with a larger sample size is required to come to a conclusion.
Conflict of interest
None declared.
Ethical approval
Not required.
References
- 1.Topazian RG, Goldberg MH, Hupp JR. Oral and maxillofacial infections. 4. Philadelphia: W.B.Saunders; 2002. [Google Scholar]
- 2.Hunt DE, King TJ, Fuller GE. Antibiotic susceptibility of bacteria isolated from oral infections. J Oral Surg. 1978;36:527–529. [PubMed] [Google Scholar]
- 3.Virolainen E, Haapaniemi J, Aitasalo K, Suonpaa J. Deep neck infections. Int J Oral Surg. 1979;8:407–411. doi: 10.1016/S0300-9785(79)80078-2. [DOI] [PubMed] [Google Scholar]
- 4.Aderhold L, Knothe H, Frenkel G. Bacteriology of dentigenous pyogenic infections. Oral Surg. 1981;52:583–587. doi: 10.1016/0030-4220(81)90072-4. [DOI] [PubMed] [Google Scholar]
- 5.Konow LV, Nord CE, Nordenram A. Anaerobic bacteria in dentoalveolar infections. Int J Oral Surg. 1981;10:313–322. doi: 10.1016/S0300-9785(81)80027-0. [DOI] [PubMed] [Google Scholar]
- 6.Forbes BA. Baily and Scott’s diagnostic microbiology. St. Louis: C.V Mosby; 2000. [Google Scholar]
- 7.Flynn TR, Hoekstra W, Lawrence FR. The use of drains in oral and maxillofacial surgery. J Oral Maxillofac Surg. 1983;41:508. doi: 10.1016/0278-2391(83)90241-0. [DOI] [PubMed] [Google Scholar]
- 8.Goldberg MH, Nemarich AN, Marco WP. Complications after mandibular third molar surgery: a statistical analysis of 500 consecutive procedures in private practice. J Am Dent Assoc. 1985;111:277–279. doi: 10.14219/jada.archive.1985.0098. [DOI] [PubMed] [Google Scholar]
- 9.Allen D, Loughnan TE, Ord RA. A re-evaluation of the role of tracheostomy in Ludwig’s angina. J Oral Maxillofac Surg. 1985;43:436–439. doi: 10.1016/S0278-2391(85)80051-3. [DOI] [PubMed] [Google Scholar]
- 10.Peterson LJ. Contemporary management of deep infections of the neck. J Oral Maxillofac Surg. 1993;51:226–231. doi: 10.1016/S0278-2391(10)80162-4. [DOI] [PubMed] [Google Scholar]
- 11.Kuriyama T, Karasawa T, Nakagawa K, Saiki Y, Yamamoto E, Nakamura S. Bacteriologic features and antimicrobial susceptibility in isolates from orofacial odontogenic infections. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2000;90:600–608. doi: 10.1067/moe.2000.109639. [DOI] [PubMed] [Google Scholar]
- 12.Storoe W, Haug RH, Lillich TT. The changing face of odontogenic infections. J Oral Maxillofac Surg. 2001;59:739–748. doi: 10.1053/joms.2001.24285. [DOI] [PubMed] [Google Scholar]
- 13.Al-Belasy FA, Hairam AR. The efficacy of azithromycin in the treatment of acute infraorbital space infection. J Oral Maxillofac Surg. 2003;61:310–316. doi: 10.1053/joms.2003.50063. [DOI] [PubMed] [Google Scholar]
- 14.Sixou JL, Magaud C, Gougeon AJ, Cormier M, Bonnaure-Mallet M, Rennies M. Microbiology of mandibular third molar pericoronitis: incidence of β-lactamase-producing bacteria. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2003;95:855–859. doi: 10.1067/moe.2003.238. [DOI] [PubMed] [Google Scholar]
- 15.Umeda M, Minamikawa T, Komatsubara H, Shibuya Y, Yokoo S, Komori T, et al. Necrotizing fascitis caused by dental infection: a retrospective analysis of 9 cases and a review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2003;95:283–290. doi: 10.1067/moe.2003.85. [DOI] [PubMed] [Google Scholar]
- 16.Wang LF, Kuo WR, Tsai SM, Huang KJ. Characterizations of life- threatening deep cervical space infections: a review of one hundred ninety-six cases. Am J Otolaryngol. 2003;24:111–117. doi: 10.1053/ajot.2003.31. [DOI] [PubMed] [Google Scholar]
- 17.Biller JA, Murr AH. The importance of etiology on the clinical course of neck abscesses. Otolaryngol Head Neck Surg. 2004;131:388–391. doi: 10.1016/j.otohns.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 18.Patel SM, Mo JH, Walker MT, Adley B, Noskin GA. Epidural abscess and osteomyelitis due to actinobacillus actinomycetemcomitans. Diagn Microbiol Infect Dis. 2004;50:283–285. doi: 10.1016/j.diagmicrobio.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 19.Stefanopoulos PK, Kolokotronis AE. The clinical significance of anaerobic bacteria in acute orofacial odontogenic infections. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2004;98:398–408. doi: 10.1016/j.tripleo.2004.01.016. [DOI] [PubMed] [Google Scholar]
- 20.Ohshima A, Ariji Y, Goto M, Izumi M, Naitoh M, Kurita K. Anatomic considerations for the spread of odontogenic infection originating from the pericoronitis of impacted mandibular third molar: computed tomographic analyses. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2004;98:589–597. doi: 10.1016/j.tripleo.2004.07.012. [DOI] [PubMed] [Google Scholar]
- 21.Lida Y, Honda K, Suzuki T, Matsukawa S, Kawal T, et al. Brain abscess in which porphyromonas gingivalis was detected in cerebrospinal fluid. British. J Oral Maxillofac Surg. 2004;42:180. doi: 10.1016/S0266-4356(03)00190-6. [DOI] [PubMed] [Google Scholar]
- 22.Mihos P, Potaris K, Gakidis I, Papadakis D, Rallis G. Management of descending necrotizing mediastinitis. J Oral Maxillofac Surg. 2004;62:966–972. doi: 10.1016/j.joms.2003.08.039. [DOI] [PubMed] [Google Scholar]
- 23.Huang TT, Tseng FY, Liu TC, Hsu CJ, Chen YS. Deep neck infection in diabetic patients: comparison of clinical picture and outcomes with nondiabetic patients. Otolaryngol Head Neck Surg. 2005;132:943–947. doi: 10.1016/j.otohns.2005.01.035. [DOI] [PubMed] [Google Scholar]
- 24.Scolozzi Lombardi T, Edney T, Jaques B. Enteric bacteria osteomyelitis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2005;99:E42–E46. doi: 10.1016/j.tripleo.2005.02.077. [DOI] [PubMed] [Google Scholar]
- 25.Wang J, Ahani A, Pogrel MA. A five year retrospective study of odontogenic maxillofacial infections in a large urban public hospital. Int J Oral Maxillofac Surg. 2005;34:646–649. doi: 10.1016/j.ijom.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 26.Akst LM, Albani BJ, Strome M. Subacute infratemporal fossa cellulitis with subsequent abscess formation in an immunocompromised patient. Am J Otolaryngol. 2005;26:35–38. doi: 10.1016/j.amjoto.2004.06.015. [DOI] [PubMed] [Google Scholar]
- 27.Thomas Flynn R. Severe odontogenic infections, part 1: prospective report I. J Oral Maxillofac Surg. 2006;64:1093–1103. doi: 10.1016/j.joms.2006.03.015. [DOI] [PubMed] [Google Scholar]
- 28.Gordon NC, Connelly S. Management of head and neck infections in the immunocompromised patient. Oral Maxillofac Surg Clin N Am. 2003;15:103–110. doi: 10.1016/S1042-3699(02)00079-1. [DOI] [PubMed] [Google Scholar]
- 29.Anthony RJ. Microbiology and antibiotic sensitivities of head and neck space infections of odontogenic origin. J Oral Maxillofac Surg. 2006;64:1377–1380. doi: 10.1016/j.joms.2006.05.023. [DOI] [PubMed] [Google Scholar]
- 30.Thomas Flynn R. Severe odontogenic infections, Part 2: prospective report II. J Oral Maxillofac Surg. 2006;64:1104–1113. doi: 10.1016/j.joms.2006.03.031. [DOI] [PubMed] [Google Scholar]