Abstract
In the international literature, the role of Ozone (O3) in the advancement in alveolar bone healing in the absence of bone pathology was not tested before. The purpose of this study was to evaluate alveolar bone regeneration after a bone defect was created and treated with a single topical administration of O3. Alveolar bone defects were created on five healthy chacma baboons. One side of the maxilla and mandible was topically treated with a single treatment of an O3/O2 mixture (3,5–4 % O3), while the opposite sides were not treated and thus served as control. Regeneration was measured radiologically, using a standardized gray scale, as the increase in bone density in the treatment area at 3 and 6 weeks post-operative and was statistically analyzed using multivariate analysis of variance (MANOVA). There were no significant differences in densities observed between the O3/O2 mixture treatment and the control (p > 0.05). A single O3 treatment did not increase alveolar bone healing over a 3- and 6-week period in the mandible and the maxilla.
Keywords: Ozone, Alveolar bone, Regeneration
Introduction
The use of ozone (O3) in medicine and dentistry is well documented [1–6]. O3 used for medicinal purposes consists of a mixture of pure oxygen (O2 and pure O3) in a ratio of 0.05–5 % of O3 and 95–99.95 % of O2 [1] and must be prepared and used immediately as the O3 molecule is unstable [7] with a half-life of less than one minute in air [7]. O3 can be administered topically or locally as a gas or as ozogenated water, olive oil or sunflower oil [8]. In some cases the O3 is mixed with blood and the ozogenated blood then re-infused, i.e. a process called autohemotherapy [7]. O3 is an intrinsically toxic gas with hazardous outcomes when used by laymen. This has led to a general antipathy towards O3 therapy. Properly applied O3 therapy, carried out by expert physicians, however, can be very useful as an alternative to conventional medicine where the latter is found to be inadequate [9].
Research in using O3 in dentistry was mainly focused on its disinfectant properties and has been used in treating early carious lesions, in sterilization of restoration cavities before filling, in root canals and in the treatment of periodontal pockets [5] as well as to enhance epithelial wound healing in ulcerations and herpetic lesions [1]. It has also been used as a rinse for avulsed teeth and as a denture cleaner [10]. In maxillofacial and oral surgery, O3 therapy was found to be beneficial in the treatment of refractory osteomyelitis in combination with antibiotic treatment, surgical interventions and hyperbaric oxygen [6]. Complete healing of osteonecrosis of the jaw (ONJ), following bisphosphonate treatment, was achieved after a few applications of O3 in combination with antibiotic therapy [11–14]. Patients with bilateral internal derangement of the temporomandibular joints were treated with O3 gas injections in the joint, had a better clinical outcome than those who received non-steroidal anti-inflammatory drugs and muscle relaxants [15]. The biological effects that O3 has on blood cellular elements (erythrocytes, leucocytes, and platelets), and serum components (proteins, lipids, lipoproteins, glycolipids, carbohydrates, electrolytes) may be a contributing factor to its therapeutic value in patients with ONJ and or cases of multiple myeloma [16].
In a study using rats, hemostasis was achieved more rapidly when an O2 and O3 mixture was used in comparison with the use of only O2 when an injury was inflicted. A visible membrane with the characteristics of a relatively firm fibrin layer consisting of a mixed clot formed within seconds on the surface of the blood following the application of the O2 and O3 mixture [17].
As the treatment mixture utilized on the baboons contains O2 and O3, the effect of O2 on bone and wound healing needs to be considered. It is not yet certain that supplementary O2 treatment on its own enhances wound healing and bone regeneration, although hyperbaric O2 and topical O2 therapy have been described to aid wound healing. However, there are no prospective case-controlled studies and the evidence as to the efficacy thereof is presently inconsistent [18, 19]. It has been suggested that O2 is advantageous in wound healing in various ways, amongst others in the oxidative killing of bacteria, re-epithelialization, angiogenesis, and collagen synthesis [20], hence the application of O2 as a therapeutic modality to aid wound healing. The understanding of how the different states of oxygenation (hypoxia, normoxia, and hyperoxia) affect wound healing and also during different stages of wound healing [21], the role of supplemental O2 as an adjuvant therapy in wound healing remains to be clarified [18].
The paucity of evidence from the literature on the regenerative effect of O3 therapy on the bone of the mandible and maxilla motivated this study.
The purpose of this study was to evaluate and compare alveolar bone regeneration after a single topical treatment with O3, since no evidence could be found that it was investigated.
Material and Methods
Test Animals and Surgical Procedures
The study was approved by the Animal Use and Care Committee of two universities (University of Pretoria and the North-West University), and a subcommittee of the Committee for Research Ethics and Integrity of the University of Pretoria (Registration number: H019-09).
Five healthy Chacma baboons (Papio ursinus) were used. The animals were anaesthetized with intravenous ketamine hydrochloride (dose: ~10 mg/kg) to enable handling. In order to control homeostasis and pain in the surgical area, 1.8 ml (9 mg) bupivacaine with 0.5 % epinephrine 1:200,000 (as bitartrate) (Novocol Pharmaceutical of Canada, Inc. Cambridge, Ontario, Canada) was infiltrated in the area.
The premolar area on each side of the maxilla and mandible was surgically exposed. Three alveolar bone defects (3 mm deep) were created on each side of the mandible and maxilla using a 3 mm diameter trephine bur fitted onto a straight surgical hand piece connected to a surgical drilling unit. The distances between the three defects were 2 mm (Fig. 1). Twelve alveolar bone defects were created for each animal. Since there is no edentulous area in the Papio ursinus, the premolar area was selected due to the thickness of alveolar bone in this particular area. Care was taken not to involve adjacent teeth.
Fig. 1.
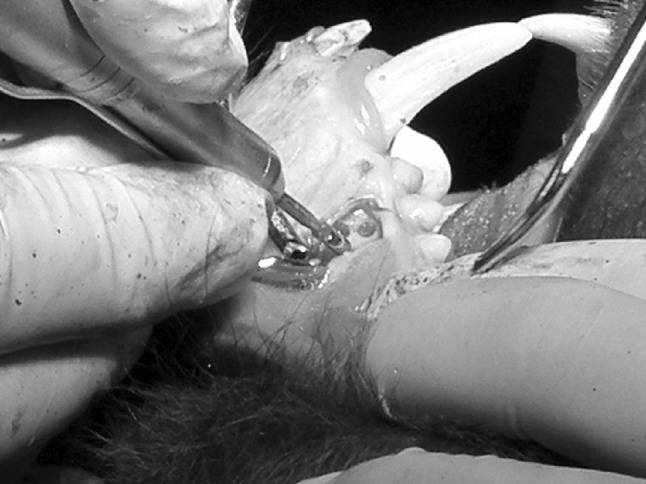
Intra-operative procedure creating bony defects in the alveolar bone of the Chacma baboon
Preparation and Application of Ozone
Ultra-pure oxygen (>99.5 % O2, BOC Special Products, Johannesburg, South Africa) was used to prepare ozone. The oxygen was passed through a dry glass-isolated alternating current corona discharge ozonizer as described [22]. The O3 generator was developed and built by the School for Physics of the North-West University (USA patent 09/914,199). A UV–Vis spectrophotometer (Pharmacia Biotech Ultrospec 3000) was used to determine the O3-concentration by measuring the absorbance of the gas mixture at 254 nm. A specially designed quartz cell was used to monitor the O3-concentration of the O3/O2-gas mixture delivered in real time during exposure to the bone defect. In order to deliver the O3 gas mixture to the bone defect, the end of a glass pipe was inserted into each site where the defect was created. O3 concentrations varied between 75 and 84 g/ml (3.5–4.0 % O3). Before the mucosal flaps were closed, each animal had a single exposure of O3 gas for 60 s to the bone defects on one side only, either left or right of the mandible and maxilla. The opposing sides served as the controls.
Radiology
Radiographs were taken pre-operatively, immediately post-operative and again after 3 and 6 weeks post-operatively from each of the quadrants (Fig. 2a–d). In order to ensure reproducibility, the following method was used:
A #21 mandibular disposable impression tray (Wright Cottrell Co., Kingsway West, West Dundee, Dundee) was cut into half to create two separate impression trays;
The sectional tray was adjusted to fit on as many teeth as possible in a particular quadrant for each animal;
A XPC-DS Digital Position System (Gendex, Lake Zurich, Illinois) bite block was secured on the tray with a commercially manufactured self-tapping screw and cyanoacrylate cement;
An aluminum step wedge, used for all the radiographs, was made out of commercially available aluminum as a 3 × 6 mm strip. Three steps were made 2 mm wide and 1 mm deep;
The aluminum step wedge was placed on the bite block, parallel to the censor and touching it. The censor was a Gendex Visualix EHD Digital intra-oral x-ray unit—size 1 (universal size) (KaVoDental, Gendex Imaging, Via A. Manzoni, 44, 20095 Cusano, Milanino) with 25.6 line pairs/mm;
Two small holes to fit the wire of a small paperclip were drilled through the aluminum and into the bite block;
Pieces of a paperclip were cut and glued into these holes. This method enabled repeated removal of the aluminum step wedge and replacement in the same position. The procedure was repeated for every bite block secured to a Dentsply-Rinn apparatus (Dentsply, Elgin, Illinois), consisting of a metal ring holder and plastic positioning ring.
Fig. 2.
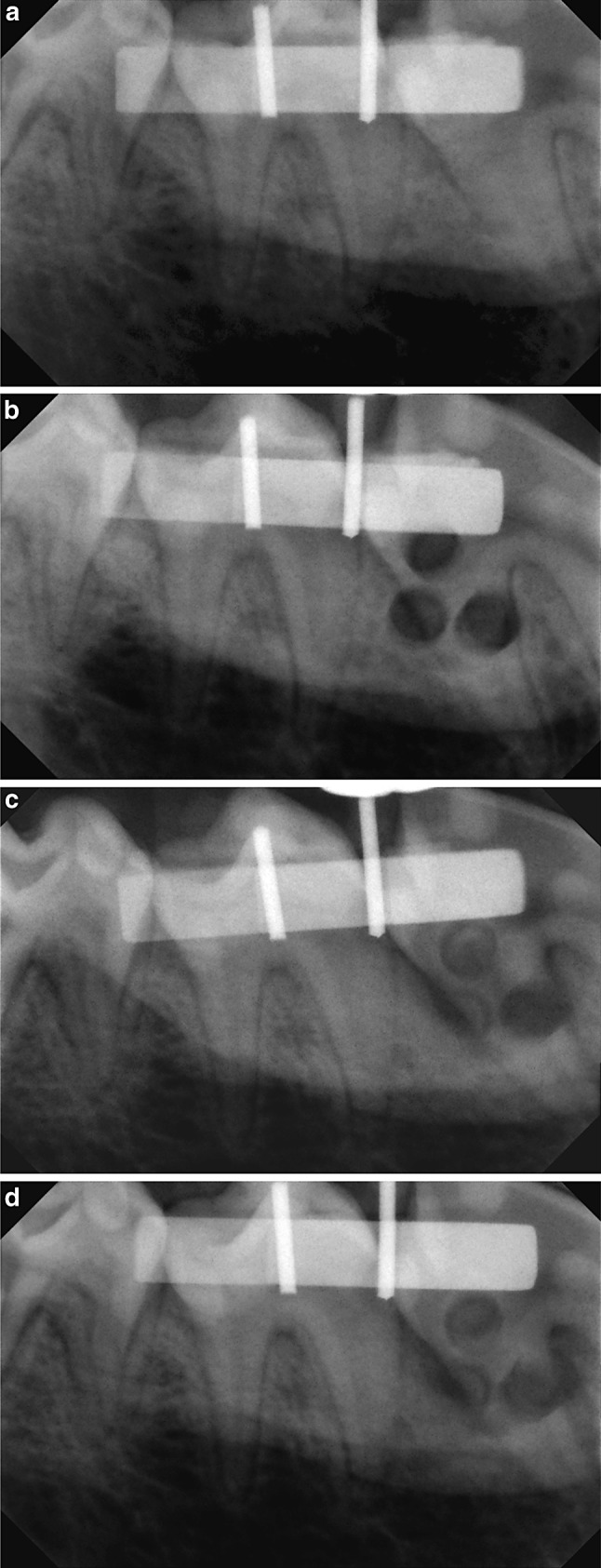
Radiographs taken pre-operatively (a), immediately post-operative (b) after 3 weeks (c) and 6 weeks post-operatively (d) from each of the quadrants
In order to acquire images, the constructed film holder was loaded with laboratory putty (Coltene/Whaledent, Switzerland), a silicone base and polysiloxane activator and positioned in the quadrant to include as much alveolar bone as possible. While the laboratory putty was still in the soft stage of the setting process, an x-ray was taken to ensure that the sensor was correctly positioned (Fig. 3). The position of the ring on the holder, and the position in the bite block, was identical for all the radiographs. The method used is fully described [23]. A Planmeca Intra Wallmount x-ray unit was used to acquire the radiographs. The radiographs were acquired at 8 mA with 63 kV and an exposure time of 0.08 s. A Toshiba D-0711 SB x-ray tube was used and the focal spot was 0.7 × 0.7 mm, the focal distance for all the images was 110 mm. Radiological analysis was done using the Gendex VixWin Pro computer software which is designed to analyze and manipulate radiographs [23].
Fig. 3.
An image of the positioning of the apparatus in the mouth of the Chacma baboon before an image was acquired
Radiological Analysis
Analysis of the radiographs was blind as none of the authors knew the identity of the animals, or which side of the mandible or maxilla was treated with O3. The code was only broken after the radiological analysis was completed. Sixteen radiographs were acquired per animal. They were imported into Adobe Photoshop (V6.0; Adobe Systems Inc., San Jose, CA) for analysis. The pre-operative radiograph was displayed on the PC screen. The lasso tool of the program was used to select an area of interest on the step wedge (Fig. 4) and also on each of the alveolar defect areas (Fig. 5) on all the radiographs that were acquired post-operatively [23]. Before the gray scale values for each biopsy site were determined, the values of the selected images on the step wedge of the four images in a quadrant were standardized using the histogram, contrast and brightness tools of the Photoshop software [24]. This was done for all four radiographs that were acquired. Therefore, the gray scale values of all radiographs were comparable. The average gray scale value for each of the three bone defects on the image was determined and recorded and the percentage change in density for the different time intervals was calculated (Table 1) [23].
Fig. 4.
Selected area of interest on aluminum step wedge
Fig. 5.
Selected area of interest on the alveolar bone defect area
Table 1.
Percentage change in density over time, as represented by gray scale values of radiographs in the ozone treated and untreated mandibular and maxillary alveolar bone
| Time | Treated | Control | p Value | |
|---|---|---|---|---|
| Maxilla | Pre-operative | 109 ± 7.02 | 87 ± 10.5 | 0.09 |
| Directly post-operative | 52 ± 8.1 | 45 ± 8.0 | 0.55 | |
| 3 weeks | 56 ± 8.0 | 49 ± 8.1 | 0.58 | |
| 6 weeks | 61 ± 7.6 | 58 ± 10.2 | 0.81 | |
| Mandible | Pre-operative | 85 ± 6.2 | 59 ± 6.4 | 0.008 |
| Directly post-operative | 41 ± 5.3 | 29 ± 7.0 | 0.18 | |
| 3 weeks | 50 ± 5.0 | 33 ± 7.3 | 0.07 | |
| 6 weeks | 55 ± 4.8 | 36 ± 7.2 | 0.06 | |
| Maxilla Mandible Combined | Pre-operative | 97 ± 5.1 | 73 ± 6.6 | 0.01 |
| Directly post-operative | 46 ± 4.9 | 37 ± 5.4 | 0.21 | |
| 3 weeks | 53 ± 4.7 | 41 ± 5.6 | 0.12 | |
| 6 weeks | 58 ± 4.4 | 47 ± 6.4 | 0.18 |
Statistical Analysis
A multivariate analysis of variance (MANOVA) was used to test whether the O3 treated biopsy areas healed faster than the control areas. Results in the text are calculated as the mean ± 1SEM.
Results
The data was analysed using multivariate analysis of variance (MANOVA) to compare the effect of the O3 treatment (treated vs. control) adjusting for the effect of pre-operative values on the measurement. The percent change in gray scale value was calculated as the decrease in density as a result of the surgical lesion and presented as a mean ± 1SEM. The rate of healing was calculated as: density at 3 or 6 weeks minus density post-biopsy divided by density pre-biopsy times 100. By using this approach, we minimized the pre-operative differences. The decrease in the directly post-operative gray scale value from the pre-operative value, as a result of the surgical defect, is about 50 % in all cases. There were no significant differences in alveolar bone density (p > 0.05) between the treated and untreated values at 3 and 6 weeks. The result is the same when the mandible and maxilla were tested separately. There were no significant differences in the gray scale values measured between the O3 treatment and the control (p > 0.05) (Table 1).
Discussion
The aim of the study was to assess alveolar bone regeneration after a single exposure for 1 min to O3 gas post-operatively, before surgical closure of the wound, directly to the surgical site. There is a lack of evidence in the literature on the effect of O3 on bone regeneration. Reports on this aspect can be found mainly in literature dealing with alternative medicine [25, 26]. Non-scientific papers promote the use of O3 gas and O3 water beneficial to bone healing, as a single treatment, after minor oral surgery. There is also insufficient evidence in the literature to evaluate the effect of O3 in oral surgery and implantology as a stand-alone therapy, without the combination thereof with other therapeutic agents or measures [2]. Most studies have focused more on the disinfectant properties of O3 in dentistry. The results obtained from this study did not indicate any advantage of using O3 to enhance alveolar bone regeneration. The fact that a single exposure to O3 was used in this study, could possibly explain the differing results obtained as compared to other studies, which used multiple exposures to O3 and which focused on the antimicrobial properties of O3 [12–14, 27]. Presently it is not practical or realistic to use multiple applications of O3 to stimulate bone regeneration in oral surgery, since surgical areas are usually closed by an oral-mucosal flap.
It is well known that O2 is required for wound healing. Wound healing is a dynamic interactive process that involves soluble mediators, blood cells, extracellular matrix, and parenchymal cells. Adequate oxygenation of any area requiring bone regeneration is therefore important in healing. Any trauma will cause a disruption in the microcirculation which can lead to anoxia at the site. Cellular metabolism is dependent on O2, specifically for the production of adenosine triphosphate (ATP) [28]. Since cellular metabolism under conditions of sub-optimal oxygenation is impaired, it provides inadequate ATP to maintain proper cellular function in the healing site [28]. Angiogenesis is also important to increase the blood supply to the regenerating tissues in order to promote wound healing. It has been reported in the literature that topical O2 therapy is beneficial to wound healing [29]. However, there is a lack of basic and clinical research to support these beneficial effects of topical O2 on wound healing [28]. In view of the role of O2 in wound healing, one can postulate that the topical application of O3 following trauma and the subsequent regeneration of tissue or bone should counteract the damaging phase of anoxia and as such should improve wound healing. In a particular study it was reported that O3 also arrests bleeding following trauma by enhancing the formation of a fibrin membrane at the site of bleeding [17]. O3 was used in several studies to assess its effect in areas of dentistry, other than bone regeneration after surgery. There are indications that O3 auto-chemotherapy combined with pharmaco-therapeutic treatment improve the regeneration of bone in post-menopausal osteoporosis after ten treatments [30]. Multiple O3 treatments in conjunction with antimicrobials, is proposed in the treatment of bisphosphonate osteonecrosis after oral surgery [16]. Ozonated water reduced post-operative infection when it was used as an irrigant and coolant where mandibular third molars were surgically removed [31]. Another study indicated that the use of topical gaseous O3 has no specific effect on alveolar osteitis and to prevent wound infection compared to chlorhexidine and control groups after mandibular impacted third molar surgery [27]. When ozonized water was used on a daily basis, it accelerated the healing of the oral mucosa following surgery with the effect being most evident during the first 2 days postoperative [32]. Although not measured specifically, this finding was confirmed by our study where accelerated mucosal healing was observed on the test side after 3 weeks after the usage of O3, as opposed to the control side. However, it did not appear that healing of alveolar bone in the maxilla or mandible after bone defects were produced, was significantly enhanced on the side that was treated with O2. This is in accordance with previous studies where the antimicrobial effect of O3 was tested in infected human root canals where the application of O3 had no improved effect in the antimicrobial properties compared to conventional treatments [33–35].
A limitation of the study was the absence of an edentulous area in the maxilla or mandible of the baboon to create a defect in the bone without involvement of teeth. Although care was taken not to disturb the roots of neighboring teeth during the surgical procedure, it appears on the radiograph that some tooth roots were involved in the defect area. Therefore, to limit the effect of a single defect area and root involvement on the results, it was decided to create three defect areas in each quadrant and use the average value in the calculation for changes in density. The alveolar bone overlying the teeth is of adequate thickness to demonstrate radiological changes in bone density over time, as demonstrated in the results. It was postulated that the influence of the granulation tissue on the changes of density, when a root was involved, will be similar in the areas of interest for all the defect areas. Furthermore, the root area is small relative to the total alveolar bone area in the defect area. It can therefore be assumed that the effect of the tissue in the root (with the defect) to the changes in density will be very small, if not negligible. The standardization of the radiographs also assisted in demonstrating changes in density of alveolar bone, regardless of tooth involvement, as the post-operative values were expressed as changes from the pre-operative value. The use of 3D images from a computed tomography scan would have been ideal to monitor changes in bone density. However, the costs involved, as well as logistical difficulties, made it principally impossible to utilize in this study. Therefore, we used standard dental radiological imaging, as it is relatively inexpensive and available in dental surgeries.
Conclusion
A single dose of O3 for 1 min immediately after alveolar bone biopsy had no significant effect on bone regeneration in the maxilla and mandible as monitored over a 6-week period through radiographic determination of bone density at the site. As such the question arises, whether there should have been a different outcome if different concentrations and multiple exposure of O3 gas were used as opposed to a single concentration and a single dose. However, no recommended standardization in concentration of O3 or the time exposure of ozone to bone could be found in the literature. Furthermore, the period of investigation was 6 weeks, which is the average period recommended for immobilization of a fracture. Although not directly applicable to this situation, it has been found that up to 70 days may be required before an extraction socket is filled with bone [36]. In addition, the clinical measurement of soft tissue healing after one exposure to O3 needs further investigation. Further studies in this regard are therefore justified in improving healing and therefore the quality of life during the post-surgical recovery period.
Acknowledgments
The personnel of the Animal Facility of the North-West University supplied the animals; Unique Dental, South Africa, supplied the radiological equipment and computer software and the Dental Research Education & Development Trust of the South African Dental Association contributed financially. Permission was granted by the Managing Editor of The South African Dental Journal for the use of figures. (Kotze MJ, et al. SADJ 67:210–214)
Conflict of interest
None.
References
- 1.Nogales CG, Ferrari PA, Kantorovich EO, Lage-Marques JL. Ozone therapy in medicine and dentistry. J Contemp Dent Pract. 2008;9:75–84. [PubMed] [Google Scholar]
- 2.Azarpazhooh A, Limeback H. The application of ozone in dentistry: a systematic review of literature. J Dent. 2008;36:104–116. doi: 10.1016/j.jdent.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 3.Lynch E. Comment on the application of ozone in dentistry: a systematic review of the literature. J Dent. 2009;37:406–410. doi: 10.1016/j.jdent.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 4.Bocci V, Lynch E, editors. Ozone: the revolution in dentistry. London: Quintessence Publishing Co.; 2004. pp. 15–22. [Google Scholar]
- 5.Bocci VA. Scientific and medical aspects of ozone therapy. State of the art. Arch Med Res. 2006;37:425–435. doi: 10.1016/j.arcmed.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 6.Stubinger S, Filippi A. The use of ozone in dentistry and maxillofacial surgery: a review. Quintessence Int. 2006;37:353–359. [PubMed] [Google Scholar]
- 7.Bocci V, Luzzi E, Corradeschi F. Oxygen-ozone therapy, a critical evaluation. Dordrecht: Kluwer Academic Publishers; 2002. p. 472. [Google Scholar]
- 8.Bocci VA. Can ozone therapy be performed if the biochemistry of the process cannot be controlled? Arch Med Res. 2007;38:584–585. doi: 10.1016/j.arcmed.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 9.Bocci V. Ozone as janus: this controversial gas can be either toxic or medically useful. Med Inflamm. 2004;13:3–11. doi: 10.1080/0962935062000197083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oizumi M, Suzuki T, Uchida M, Furuya J, Okamoto Y. In vitro testing of a denture cleaning method using ozone. J Med Dent Sci. 1998;45:135–139. [PubMed] [Google Scholar]
- 11.Ripamonti C, Maniezzo M, Ghiringhelli R, Cislaghi E, Mariani L. Medical ozone (O3) oil or gas applications heal osteonecrosis of the jaw (ONJ) in patients treated with bisphosphonates (BPs): preliminary results of a single arm study. Cancer Res. 2009;69:5046. doi: 10.1158/0008-5472.SABCS-09-5046. [DOI] [Google Scholar]
- 12.Agrillo A, Sassano P, Rinna C, Priore P, Iannetti G. Ozone therapy in extractive surgery on patients treated with bisphosphonates. J Craniofac Surg. 2007;18:1068–1070. doi: 10.1097/SCS.0b013e3181572609. [DOI] [PubMed] [Google Scholar]
- 13.Agrillo A, Ungari C, Filiaci F, Priore P, Iannetti G. Ozone therapy in the treatment of avascular bisphosphonate-related jaw osteonecrosis. J Craniofac Surg. 2007;18:1071–1075. doi: 10.1097/scs.0b013e31857261f. [DOI] [PubMed] [Google Scholar]
- 14.Ripamonti C, Cislaghi E, Massimo LM. Efficacy and safety of medical ozone (O3) delivered in oil suspension applications for the treatment of osteonecrosis of the jaw in patients with bone metastases treated with bisphosphonates: preliminary results of a phase I–II study. Oral Oncol. 2011;47:185–190. doi: 10.1016/j.oraloncology.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 15.Daif ET. Role of intra-articular ozone gas injection in the management of internal derangement of the temporomandibular joint. Oral Surg Oral Med Oral Pathol Oral Radiol. 2012;113:e10–e14. doi: 10.1016/j.tripleo.2011.08.006. [DOI] [PubMed] [Google Scholar]
- 16.Petrucci MT, Gallucci C, Agrillo A, Mustazza MA, Robin FO. Role of ozone therapy in the treatment of osteonecrosis of the jaws in multiple myeloma patients. Haematologica. 2007;92:1289–1290. doi: 10.3324/haematol.11096. [DOI] [PubMed] [Google Scholar]
- 17.Kashperskii YuP, Adamyan AA, Makarov VA, Glyantsev SP, Zhukov VA. Study of the hemostatic properties of gaseous ozone. Eksp Biol Med. 1995;120:62–65. [PubMed] [Google Scholar]
- 18.Rodriqurz PG, Felix FN, Woodley DT, Shim EK. The role of oxygen in wound healing: a review of the literature. Dermatol Surg. 2008;34:1159–1169. doi: 10.1111/j.1524-4725.2008.34254.x. [DOI] [PubMed] [Google Scholar]
- 19.Schrem S, Szeimies RM, Prantl L, Karrer S, Landthaler M, Babilas P. Oxygen in acute and chronic wound healing. Br J Dermatol. 2010;163:257–268. doi: 10.1111/j.1365-2133.2010.09804.x. [DOI] [PubMed] [Google Scholar]
- 20.Bishop A. Role of oxygen in wound healing. J Wound Care. 2008;17:399–402. doi: 10.12968/jowc.2008.17.9.30937. [DOI] [PubMed] [Google Scholar]
- 21.Tandara AA, Mustoe TA. Oxygen in wound healing-more than a nutrient. World J Surg. 2004;28:294–300. doi: 10.1007/s00268-003-7400-2. [DOI] [PubMed] [Google Scholar]
- 22.Du Plessis LH, van der Westhuizen FH, Kotzé HF. The effect of blood ozonation on mitochondrial function and apoptosis of peripheral blood mononuclear cells in the presence and absence of plasma antioxidants. Afr J Biotechnol. 2007;6:1763–1769. [Google Scholar]
- 23.Kotze MJ, Bütow K-W, Kotze HF. A radiological method to evaluate alveolar bone regeneration in the Chacma baboon (Papio ursinus) SADJ. 2012;67:210–214. [PubMed] [Google Scholar]
- 24.Bittar-Cortez JA, Passeri LA, Bóscolo FN, Haiter-Neto F. Comparison of hard tissue density changes around implants assessed in digitized conventional radiographs and subtraction images. Clin Oral Implants Res. 2006;17:560–564. doi: 10.1111/j.1600-0501.2006.01256.x. [DOI] [PubMed] [Google Scholar]
- 25.Breiner MA (2007) Ozone in dentistry. In: Whole body news update Feb 2007. KSB Promotions, Michigan
- 26.Frascino AVM, Juliana N, Andrea M, Zindel MC. Bone repair of monocortical wounds after ozonized water irrigation. Barcelona: IADR; 2010. [Google Scholar]
- 27.Burdurlu Ç, Delibasi Ç, Denize E, Arslan A. Evaluation of effects of topical ozone and chlorhexidine application on alveolar osteitis and wound infection following mandibular impacted third molar surgery. Turkiye Klinikleri J Dental Sci. 2011;17:17–23. [Google Scholar]
- 28.Broussard CL. Hyperbaric oxygenation and wound healing. J Vasc Nurs. 2004;22:42–48. doi: 10.1016/j.jvn.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 29.Fries RB, Wallace WA, Roy S, Kuppusamy P, Bergdall V, Gordillo GM, Melvin WS, Sen CK. Dermal excisional wound healing in pigs following treatment with topically applied pure oxygen. Mutat Res. 2005;579:172–181. doi: 10.1016/j.mrfmmm.2005.02.023. [DOI] [PubMed] [Google Scholar]
- 30.Riva-Sanseverino E. The influence of ozone therapy on the remineralization of bone tissue in osteoporosis. Ozonachrichten. 1987;6:75–82. [Google Scholar]
- 31.Phillipi A. The use of ozonized water as a cooling and irrigating medium during osteotomy procedures. Community Dent Oral Epidemiol. 1999;10:619–922. [Google Scholar]
- 32.Filippi A. The influence of ozonised water on the epithelial wound healing process in oral cavity (in German) Dtsch Zahnarztl. 2001;56:104–108. [Google Scholar]
- 33.Estrela C, Estrela CRA, Decurcio DA, Hollanda ACB, SilvaJ A. Antimicrobial efficacy of ozonated water, gaseous ozone, sodium hypochlorite and chlorhexidine in infected human root canals. Intern Endod J. 2007;40:85–93. doi: 10.1111/j.1365-2591.2006.01185.x. [DOI] [PubMed] [Google Scholar]
- 34.Hems RS, Gulabivala K, Ng YL, Ready D, Spratt DA. An in vitro evaluation of the ability of ozone to kill a strain of Enterococcus faecalis. Intern Endod J. 2005;38:22–29. doi: 10.1111/j.1365-2591.2004.00891.x. [DOI] [PubMed] [Google Scholar]
- 35.Nagayoshi M, Kitamura C, Fukuizumi T, Nishihara T, Terashita M. Antimicrobial effect of ozonated water on bacteria invading dentinal tubules. J Endod. 2004;30:778–781. doi: 10.1097/00004770-200411000-00007. [DOI] [PubMed] [Google Scholar]
- 36.Aguirre JI, Altman MK, Vanegas SM, Franz SE, Bassit ACF. Effects of alendronate on bone healing after tooth extraction in rats. Oral Dis. 2010;16:674–685. doi: 10.1111/j.1601-0825.2010.01677.x. [DOI] [PubMed] [Google Scholar]