Abstract
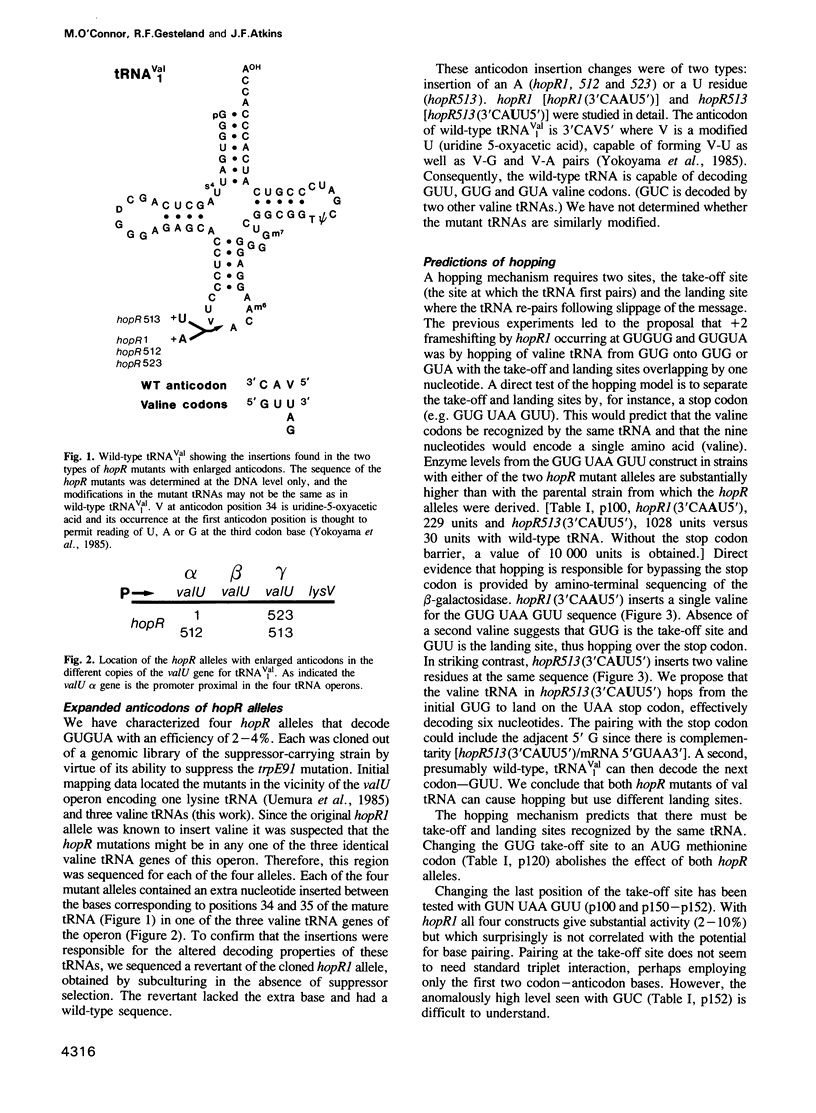
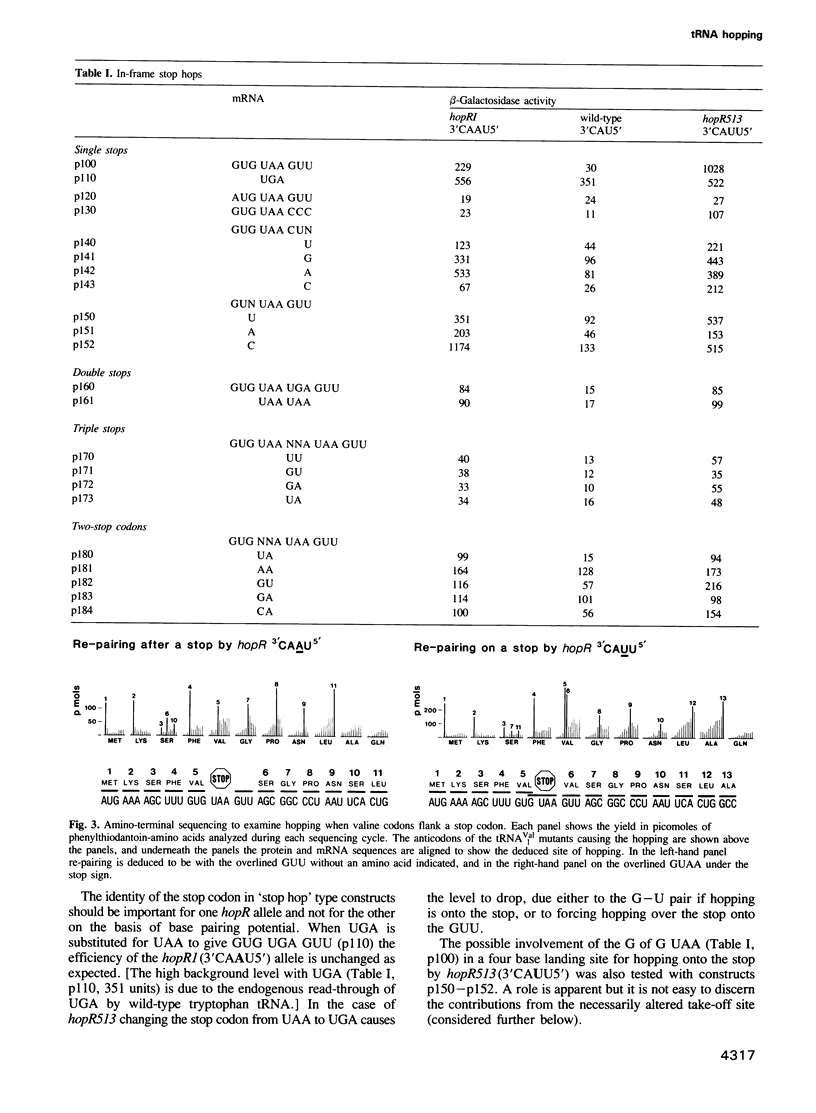
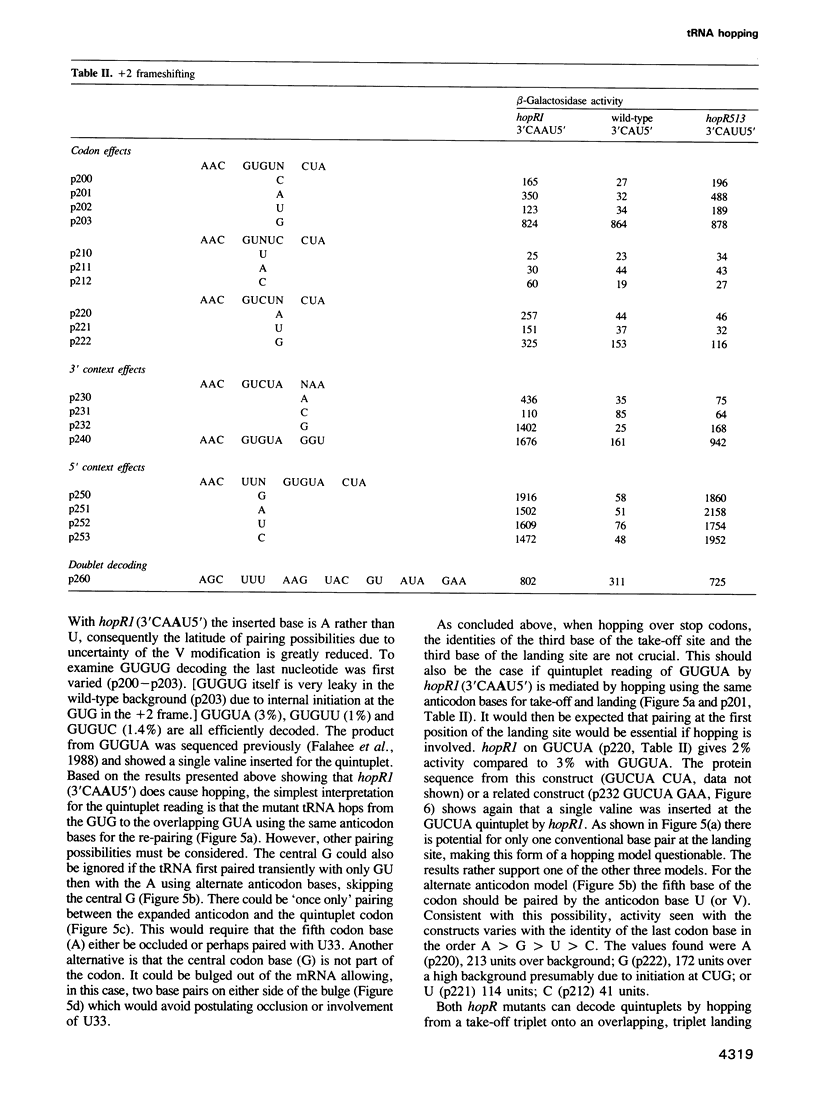
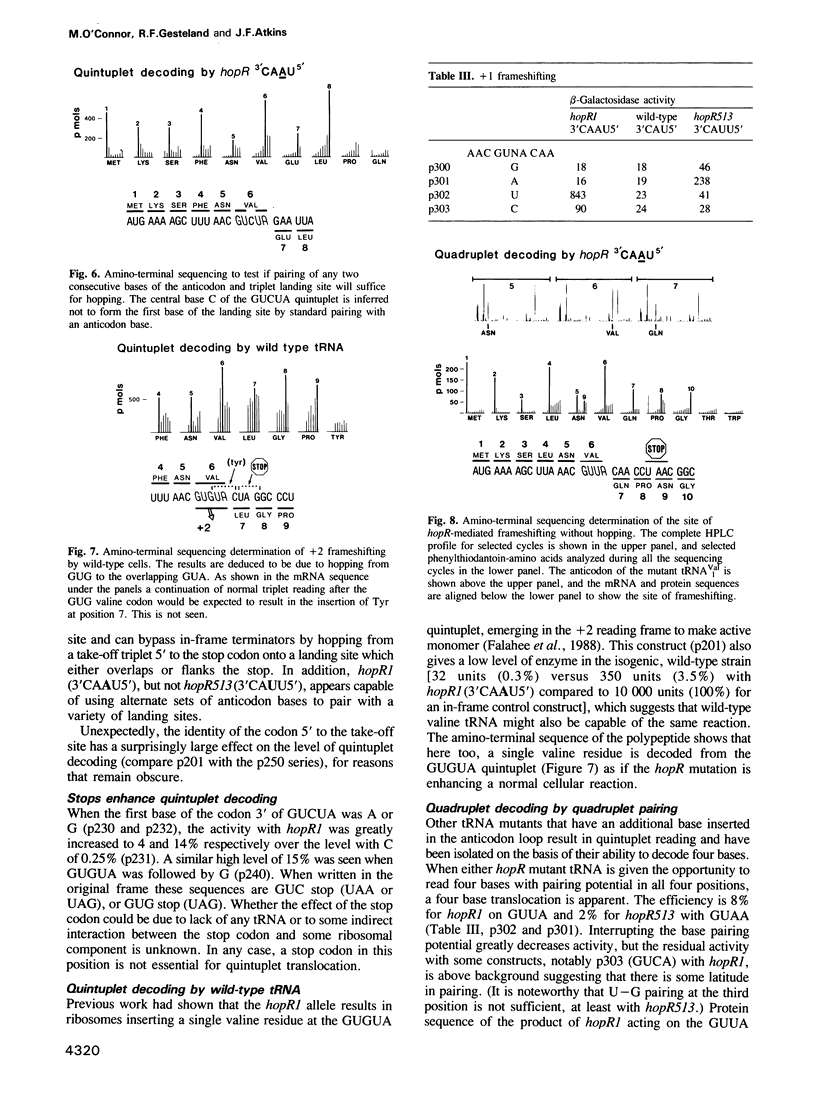
At a low level wild-type tRNA(1Val) inserts a single amino acid (valine) for the five nucleotide sequence GUGUA which has overlapping valine codons. Mutants of tRNA(1Val) with an insertion of A or U between positions 34 and 35 of their anticodons have enhanced reading of the quintuplet sequences. We propose that this decoding occurs by a hopping mechanism rather than by quintuplet pairing. Such hopping involves disengagement of the paired codon and anticodon with the mRNA slipping two (or more) bases along the ribosomal--peptidyl tRNA complex and subsequently re-pairing at a second codon--the landing site. The mutant with the anticodon sequence 3'CAAU5' 'hops' over the stop codon in the mRNA sequence GUG UAA GUU with the insertion of a single amino acid (valine). In contrast, in reading the same sequence, the mutant with the anticodon 3'CAUU5' hops onto the stop with the insertion of two valine residues. It is likely that in some instances of hopping alternate anticodon bases are used for the initial pairing and at the landing site.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atkins J. F., Nichols B. P., Thompson S. The nucleotide sequence of the first externally suppressible--1 frameshift mutant, and of some nearby leaky frameshift mutants. EMBO J. 1983;2(8):1345–1350. doi: 10.1002/j.1460-2075.1983.tb01590.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergemann K., Nierhaus K. H. Codon-anticodon interaction at the ribosomal P site improves the accuracy of the decoding process. Biochem Int. 1984 Jan;8(1):121–126. [PubMed] [Google Scholar]
- Chen E. Y., Seeburg P. H. Supercoil sequencing: a fast and simple method for sequencing plasmid DNA. DNA. 1985 Apr;4(2):165–170. doi: 10.1089/dna.1985.4.165. [DOI] [PubMed] [Google Scholar]
- Chopra I., Shales S. W., Ward J. M., Wallace L. J. Reduced expression of Tn 10-mediated tetracycline resistance in Escherichia coli containing more than one copy of the transposon. J Gen Microbiol. 1981 Sep;126(1):45–54. doi: 10.1099/00221287-126-1-45. [DOI] [PubMed] [Google Scholar]
- Falahee M. B., Weiss R. B., O'Connor M., Doonan S., Gesteland R. F., Atkins J. F. Mutants of translational components that alter reading frame by two steps forward or one step back. J Biol Chem. 1988 Dec 5;263(34):18099–18103. [PubMed] [Google Scholar]
- Huang W. M., Ao S. Z., Casjens S., Orlandi R., Zeikus R., Weiss R., Winge D., Fang M. A persistent untranslated sequence within bacteriophage T4 DNA topoisomerase gene 60. Science. 1988 Feb 26;239(4843):1005–1012. doi: 10.1126/science.2830666. [DOI] [PubMed] [Google Scholar]
- Isberg R. R., Falkow S. A single genetic locus encoded by Yersinia pseudotuberculosis permits invasion of cultured animal cells by Escherichia coli K-12. Nature. 1985 Sep 19;317(6034):262–264. doi: 10.1038/317262a0. [DOI] [PubMed] [Google Scholar]
- Jacks T., Madhani H. D., Masiarz F. R., Varmus H. E. Signals for ribosomal frameshifting in the Rous sarcoma virus gag-pol region. Cell. 1988 Nov 4;55(3):447–458. doi: 10.1016/0092-8674(88)90031-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M., Tzagoloff A. Assembly of the mitochondrial membrane system: sequences of yeast mitochondrial valine and an unusual threonine tRNA gene. Cell. 1979 Sep;18(1):47–53. doi: 10.1016/0092-8674(79)90352-0. [DOI] [PubMed] [Google Scholar]
- Messing J., Gronenborn B., Müller-Hill B., Hans Hopschneider P. Filamentous coliphage M13 as a cloning vehicle: insertion of a HindII fragment of the lac regulatory region in M13 replicative form in vitro. Proc Natl Acad Sci U S A. 1977 Sep;74(9):3642–3646. doi: 10.1073/pnas.74.9.3642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murgola E. J., Prather N. E., Mims B. H., Pagel F. T., Hijazi K. A. Anticodon shift in tRNA: a novel mechanism in missense and nonsense suppression. Proc Natl Acad Sci U S A. 1983 Aug;80(16):4936–4939. doi: 10.1073/pnas.80.16.4936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Mahony D. J., Mims B. H., Thompson S., Murgola E. J., Atkins J. F. Glycine tRNA mutants with normal anticodon loop size cause -1 frameshifting. Proc Natl Acad Sci U S A. 1989 Oct;86(20):7979–7983. doi: 10.1073/pnas.86.20.7979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ofengand J., Liou R. Correct codon--anticodon base pairing at the 5'-anticodon position blocks covalent cross-linking between transfer ribonucleic acid and 16S RNA at the ribosomal P site. Biochemistry. 1981 Feb 3;20(3):552–559. doi: 10.1021/bi00506a017. [DOI] [PubMed] [Google Scholar]
- Rheinberger H. J., Sternbach H., Nierhaus K. H. Three tRNA binding sites on Escherichia coli ribosomes. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5310–5314. doi: 10.1073/pnas.78.9.5310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarabhai A., Brenner S. A mutant which reinitiates the polypeptide chain after chain termination. J Mol Biol. 1967 Jul 14;27(1):145–162. doi: 10.1016/0022-2836(67)90357-9. [DOI] [PubMed] [Google Scholar]
- Stoker N. G., Fairweather N. F., Spratt B. G. Versatile low-copy-number plasmid vectors for cloning in Escherichia coli. Gene. 1982 Jun;18(3):335–341. doi: 10.1016/0378-1119(82)90172-x. [DOI] [PubMed] [Google Scholar]
- Sumner-Smith M., Hottinger H., Willis I., Koch T. L., Arentzen R., Söll D. The sup8 tRNALeu gene of Schizosaccharomyces pombe has an unusual intervening sequence and reduced pairing in the anticodon stem. Mol Gen Genet. 1984;197(3):447–452. doi: 10.1007/BF00329941. [DOI] [PubMed] [Google Scholar]
- Tucker S. D., Murgola E. J., Pagel F. T. Missense and nonsense suppressors can correct frameshift mutations. Biochimie. 1989 Jun;71(6):729–739. doi: 10.1016/0300-9084(89)90089-8. [DOI] [PubMed] [Google Scholar]
- Uemura H., Thorbjarnardóttir S., Gamulin V., Yano J., Andrésson O. S., Söll D., Eggertsson G. supN ochre suppressor gene in Escherichia coli codes for tRNALys. J Bacteriol. 1985 Sep;163(3):1288–1289. doi: 10.1128/jb.163.3.1288-1289.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WATSON J. D. THE SYNTHESIS OF PROTEINS UPON RIBOSOMES. Bull Soc Chim Biol (Paris) 1964;46:1399–1425. [PubMed] [Google Scholar]
- Ward J. M., Grinsted J. Physical and genetic analysis of the Inc-W group plasmids R388, Sa, and R7K. Plasmid. 1982 May;7(3):239–250. doi: 10.1016/0147-619x(82)90005-1. [DOI] [PubMed] [Google Scholar]
- Weiss R. B., Dunn D. M., Atkins J. F., Gesteland R. F. Slippery runs, shifty stops, backward steps, and forward hops: -2, -1, +1, +2, +5, and +6 ribosomal frameshifting. Cold Spring Harb Symp Quant Biol. 1987;52:687–693. doi: 10.1101/sqb.1987.052.01.078. [DOI] [PubMed] [Google Scholar]
- Weiss R. B., Dunn D. M., Dahlberg A. E., Atkins J. F., Gesteland R. F. Reading frame switch caused by base-pair formation between the 3' end of 16S rRNA and the mRNA during elongation of protein synthesis in Escherichia coli. EMBO J. 1988 May;7(5):1503–1507. doi: 10.1002/j.1460-2075.1988.tb02969.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoyama S., Watanabe T., Murao K., Ishikura H., Yamaizumi Z., Nishimura S., Miyazawa T. Molecular mechanism of codon recognition by tRNA species with modified uridine in the first position of the anticodon. Proc Natl Acad Sci U S A. 1985 Aug;82(15):4905–4909. doi: 10.1073/pnas.82.15.4905. [DOI] [PMC free article] [PubMed] [Google Scholar]



