Abstract
Background
Type 1 Modic changes are characterized by edema, vascularization, and inflammation, which lead to intervertebral disc degeneration. Macrophage migration inhibitory factor (MIF) is a proinflammatory cytokine closely related to the inflammatory cytokines detected in degenerative intervertebral disc tissues. However, the existence and role of MIF and its receptor CD74 in intervertebral disc degeneration have not been elucidated.
Questions/purposes
We asked whether (1) MIF and its receptor CD74 are expressed in cartilage end plates with Type 1 Modic changes, (2) MIF is associated with cartilage end plate degeneration, (3) the MIF antagonist (S, R)-3(4-hydroxyphenyl)-4, 5-dihydro-5-isoxazole acetic acid methyl ester (ISO-1) suppresses MIF-induced inflammatory cytokine release, and (4) inflammatory cytokines are released by cartilage end plate chondrocytes via CD74 by activating the CD74 antibody (CD74Ab).
Methods
We examined MIF and CD74 expression by human cartilage end plate chondrocytes and tissues with Type 1 Modic changes from eight patients using immunocytofluorescence and immunohistochemistry. MIF production by the chondrocytes was assessed by ELISA and PCR. We compared cytokine release by chondrocytes treated with MIF in the presence or absence of exogenous ISO-1 by ELISA. Cytokine release by chondrocytes after treatment with CD74Ab was determined by ELISA.
Results
MIF was expressed in degenerated human cartilage end plate tissues and chondrocytes. Lipopolysaccharide and tumor necrosis factor α (TNF-α) upregulated MIF expression and increased MIF secretion in chondrocytes in a dose-dependent manner. MIF increased the secretion of IL-6, IL-8, and prostaglandin E2 (PGE2) in a dose-dependent manner. ISO-1 reduced the secretion of IL-6, IL-8, and PGE2. CD74Ab activated CD74 and induced release of inflammatory cytokines.
Conclusions
Chondrocytes in cartilage end plate with Type 1 Modic changes express MIF and its receptor CD74. MIF might promote the inflammatory response through CD74. MIF-induced cytokine release appears to be suppressed by ISO-1, and CD74Ab could induce cytokine release.
Clinical Relevance
The MIF/CD74 pathway may represent a crucial target for treating disc degeneration since inhibiting the function of MIF with its antagonist ISO-1 can reduce MIF-induced inflammation and exert potent therapeutic effects.
Introduction
Cartilage end plates play an important role in transporting nutrients to the nucleus pulposus. The structural change of the cartilage end plate is the key factor initiating and enhancing disc degeneration [25]. A Modic change is a common phenomenon on the vertebral end plates and correlates with clinical symptoms. It can be divided into three types of changes (Types 1, 2, and 3 Modic changes), based on abnormalities in the vertebral end plates and adjacent bone marrow observed on MR images [28]. Histologic studies have shown that Type 1 Modic changes are characterized by edema, vascularization, and inflammation, which might be the potential mechanisms underlying low back pain [3, 26]. Research has shown that cartilage end plate chondrocytes can express and secrete proinflammatory cytokines and that these cytokines are involved in the pathogenesis of cartilage end plate degeneration [29]. Ohtori et al. [29] found that tumor necrosis factor-α (TNF-α) is discovered in the cartilage end plates of patients and control subjects, and the expression of these proinflammatory cytokines was significantly greater in patients with Type 1 Modic changes compared with expression in patients with Type 2 Modic changes and control subjects.
Macrophage migration inhibitory factor (MIF) is a multifunctional cytokine that plays an important role in many pathophysiologic responses in vivo [6, 23, 24]. As an important proinflammatory cytokine, MIF can counteract glucocorticoid signaling by activating immune or inflammatory cells and promoting inflammatory cytokine release [9]. Moreover, MIF has been shown to induce various pathologic events, such as acute respiratory distress syndrome [15], arthritis [22], glomerulonephritis [20], and angiogenesis [12, 30]. Moreover, Type 1 Modic changes of cartilage end plates are characterized by an inflammatory reaction, but to our knowledge, the relationship between MIF and cartilage end plates with Type 1 Modic changes has not been investigated. MIF is closely associated with proinflammatory cytokines detected in degenerative cartilaginous tissues and the intervertebral disc, such as TNF-α, IL-1β, IL-6, IL-8, and interferon γ (IFN-γ). Crock [13] suggested that toxic chemicals produced by the nucleus pulposus can diffuse through the cartilage end plate, and these proinflammatory cytokine signals might exert potent autocrine and paracrine effects on cell activation and inflammatory response during cartilage end plate injury and degeneration. We found that MIF expressed in the nucleus pulposus can inhibit the migration of cartilage end plate-derived stem cells by reacting with CD74 [33]. However, the potential inflammatory role of MIF in cartilage end plate degeneration remains unclear, and inhibiting the function of MIF might counter inflammation response and exert potential therapeutic effects. CD74 functions as a receptor for MIF and is highly expressed in inflammatory disorders accompanied with MIF expression [27]. CD74 is important for MIF to mediate its biologic activities in vivo, and the upregulation of cell surface receptor CD74 is a step toward mediating MIF-CD74 signal transduction [27]. MIF binds to CD74 and activates signal transduction, leading to the production of proinflammatory cytokines [7].We also showed that CD74 inhibition might enhance the homing capability of cartilage end plate-derived stem cells toward to disc lesions [33]. However, the potential inflammatory role of CD74 in cartilage end plate degeneration remains unknown, and it might be involved in the pathogenesis of cartilage end plate inflammation. We therefore explored the role of MIF in cartilage end plate degeneration. We asked whether (1) MIF and MIF receptor CD74 are expressed in cartilage end plates with Type 1 Modic changes, (2) MIF is associated with cartilage end plate degeneration, (3) the MIF antagonist (S, R)-3(4-hydroxyphenyl)-4, 5-dihydro-5-isoxazole acetic acid methyl ester (ISO-1) suppresses MIF-induced inflammatory cytokine release, and (4) inflammatory cytokines are released by cartilage end plate chondrocytes via CD74 by activating CD74 antibody (CD74Ab).
Patients and Methods
Patients and Samples
Human degenerated disc samples were obtained from five male and three female patients (mean age, 52.5 years; range, 36–62 years) undergoing multilevel posterior discectomy and fusion for degenerative lumber disease at Xinqiao Hospital (Table 1). A Modic classification system for cartilage end plate degeneration was used. The mean intraobserver kappa value is 0.91and 0.94, and the mean interobserver kappa value is 0.92. Degenerated cartilage end plates were harvested by two experienced spine surgeons (JW, CL), according to a protocol described previously [17], and washed in sterile phosphate buffered saline (PBS; 0.1 mol/L, pH 7.4). Patients with autoimmune and infectious diseases such as rheumatoid arthritis, inflammatory bowel disease, and septic shock were excluded. We obtained institutional review board approval from the Third Military Medical University and documented informed consent in each case.
Table 1.
Disc grade, level, and procurement indication and patient demographics
| Procurement site | Disc level | Modic type | Gender | Age (years) |
|---|---|---|---|---|
| Spondylolisthesis | L4-L5 | I | F | 63 |
| Spondylolisthesis | L4-L5 | I | M | 49 |
| Vertebral instability | L4-L5 | I | M | 53 |
| Discogenic low back pain | L4-L5 | I | M | 62 |
| Discogenic low back pain | L5-S1 | I | M | 47 |
| Discogenic low back pain | L5-S1 | I | F | 45 |
| Spinal stenosis | L5-S1 | I | F | 41 |
| Spinal stenosis | L4-L5 | I | M | 60 |
Cartilage End Plate Expression of MIF and CD74
We used immunohistochemistry and immunocytofluorescence to determine the expression of MIF and CD74 in cartilage end plates with Type 1 Modic changes at the tissue and cell levels, respectively. MIF and CD74 were localized by immunohistochemistry in eight intervertebral disc samples using a previously described protocol [33]. Four-micrometer paraffin sections were dewaxed, hydrated, and washed with distilled water. Then, sections were blocked for endogenous peroxidase with 3% hydrogen peroxide. After antigen retrieval in citrate buffer, samples were washed three times in PBS with 0.1% Triton X™ (Dow Chemical Co, Midland, MI, USA) and treated with PBS with 0.1% Triton X™ and 1% bovine serum albumin. Five sections for MIF staining and five for CD74 staining were obtained from each of the eight patients. The sections were washed three times with PBS, followed by incubation with primary monoclonal rabbit anti-human CD74 antibody (1:100; Epitomics, Burlingame, CA, USA) or polyclonal rabbit anti-human MIF antibody (1:100; Santa Cruz Biotechnology, Santa Cruz, CA, USA) overnight at 4° C. The sections then were washed, followed by incubation with peroxidase-conjugated anti-rabbit immunoglobulin G (IgG) second antibody (1:500; ZSGB-BIO, Beijing, China) for 30 minutes at room temperature. Final reaction products with 3, 3′-diaminobenzidine and hematoxylin counterstaining were observed by light microscopy (BX60; Olympus, Tokyo, Japan). For negative controls, the primary antibodies (anti-CD74 and anti-MIF) were omitted.
Cartilage End Plate Degeneration and MIF
To answer our second question, ELISA and reverse-transcriptase (RT)-PCR were used to determine the effect of proinflammatory cytokines on the cartilage end plate chondrocyte-derived MIF release. We then tested the effect of recombinant MIF (r-MIF) on IL-6, IL-8, prostaglandin E2 (PGE2), TNF-α, and IL-1β secretion by cartilage end plate chondrocytes.
Surgically explanted cartilage end plate tissue was kept in a culture medium with sterile PBS. The cartilage end plate was examined carefully to remove the nucleus pulposus, annulus fibrosus, and granulation and ligament tissue using an ophthalmic surgery set under a dissecting microscope (magnification, ×4). Then, the cartilage end plate tissues were minced into 1-mm3 morsels. As we did in an earlier related experiment [33], cells were isolated in Dulbecco’s Minimum Essential Medium (DMEM)/F12 with 0.2% Type II collagenase (Sigma-Aldrich, St Louis, MO, USA) at 37° C for 12 hours. Suspended cells were filtered through a 70-μm filter to minimize cell aggregates. The suspension was transferred to sterile 15-mL polypropylene culture tubes, and all tubes were centrifuged at 100 g for 5 minutes. The suspension solution was discarded and pellets resuspended in DMEM/F12 with 10% fetal calf serum; 1% penicillin-streptomycin (Life Technologies, Grand Island, NY, USA) was added to eliminate bacteria. Total cell numbers in tissue samples ranged from 1.5 × 104 to 5.0 × 104 cells. The cells in culture bottles were cultured in 5% CO2 at 37° C. The culture medium was replaced at 3-day intervals for the first three subcultures to eliminate macrophages or other leukocytes.
At the fourth passage, cells were plated on a cell culture dish (NEST Biotech Co, Ltd, Shanghai, China). First, when confluence was obtained, the cells were fixed in 100% methanol and treated with 0.1% Triton X™ and 1% bovine serum albumin. Cells were stained with primary monoclonal rabbit anti-human CD74 antibody (1:100; Epitomics) or polyclonal rabbit anti-human MIF antibody (1:100; Santa Cruz Biotechnology) overnight at 4° C. Three samples were used for each type of staining, each repeated three times. The cells were washed three times in PBS with 0.1% Triton X™ and then stained with the second antibody using fluorescein-5-isothiocyanate (FITC)-conjugated goat anti-rabbit IgG (1:500; ZSGB-BIO) for 1 hour at room temperature. Next, the cell nuclei were stained with 4′, 6-diamidino-2-phenylindole (DAPI) (0.1 μg/mL; Sigma-Aldrich). For negative controls, the primary antibodies were omitted. The cell culture dish was examined with a laser scanning confocal microscope.
MIF, IL-1β, TNF-α, IL-6, IL-8, and PGE2 concentrations in the conditioned medium were assayed using a commercially available ELISA kit (r-MIF; R&D Systems, Minneapolis, MN, USA) in accordance with the manufacturer’s recommendations. Absorbance was measured at 450 nm using a microplate reader.
Suppression of MIF-induced Inflammatory Cytokine Release
We compared the r-MIF-induced inflammatory cytokine expression in the presence and absence of exogenous MIF antagonist ISO-1. MIF and ISO-1 were added to the DMEM/F12 before ELISA. ISO-1 was added 30 minutes before the assay where indicated. Absorbance was measured at 450 nm using a microplate reader. Three independent experiments with triplicates were performed in each experiment; the results were expressed as mean ± 95% CI.
Activation of CD74 Antibody
We tested the effect of CD74Ab on inflammatory cytokine release by cartilage end plate chondrocytes. In some experiments, CD74-activating antibody (CD74Ab; Santa Cruz Biotechnology) was added to the DMEM/F12 before ELISA where indicated. The CD74Ab was dialyzed against sodium azide. The above protocol was described by Can et al. [11]. Absorbance was measured at 450 nm using a microplate reader. Three independent experiments with triplicates were performed in each experiment; the results were expressed as mean ± 95% CI.
Total RNA was extracted from macrophages using a commercially available RNase kit (Qiagen GmbH, Hilden, Germany) in accordance with the manufacturer’s instructions. cDNA was obtained from the total RNA using RNase-free DNase (Qiagen). Purified RNA was quantified using a spectrophotometer (Beckman, Fullerton, CA, USA) measured at 260 and 280 nm. Total RNA was reverse-transcribed into cDNA and amplified by PCR using the ThermoScript™ RT-PCR system (Life Technologies) (Table 2). mRNA levels were normalized to glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA levels. The above protocol was described previously [10, 11]. Three independent experiments with triplicates were performed in each experiment; the results were expressed as mean ± 95% CI.
Table 2.
Primers used for RT-PCR analysis
| Target gene | Primer sequence |
|---|---|
| MIF | 5′-GCGCGTGCGTCTGTGCC-3′ 5′-GACCACGTGCACCGCGATGTA- 3′ |
| GAPDH | 5′-TGGGGTGATGCTGGTGCTGAGT-3′ 5′-AGGTTTCTCCAGGCGGCATGTC-3′ |
RT = reverse transcriptase; MIF = macrophage migration inhibitory factor; GAPDH = glyceraldehyde-3-phosphate dehydrogenase.
Statistical Analysis
Data are expressed as mean ± 95% CI of three independent experiments. Data were analyzed using one-way ANOVA comparisons of factors varying between groups. Significance was set at p < 0.05. We performed statistical analyses with SPSS® for Windows® 19.0 (IBM Corp, Armonk, NY, USA).
Results
Cartilage End Plate Expression of MIF and CD74
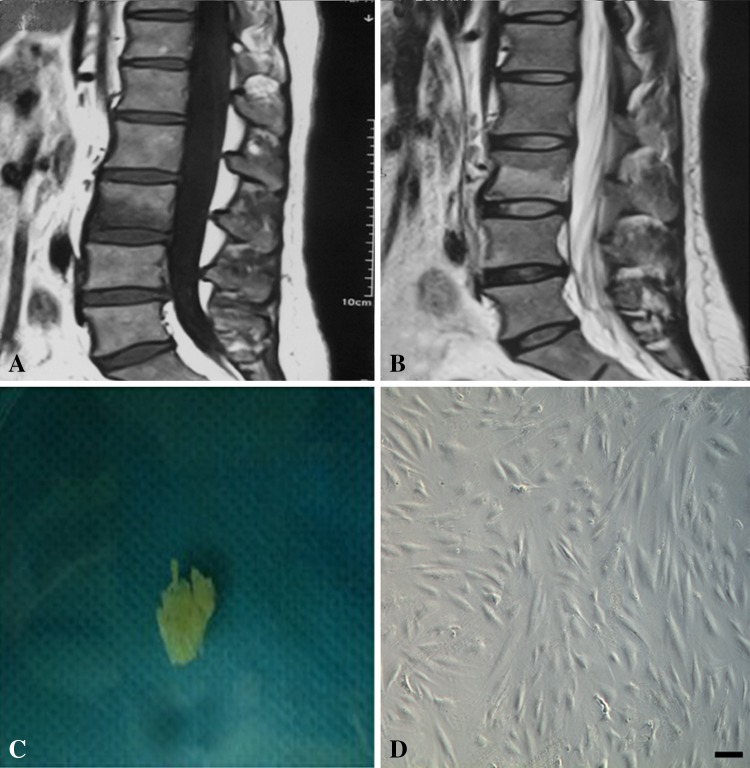
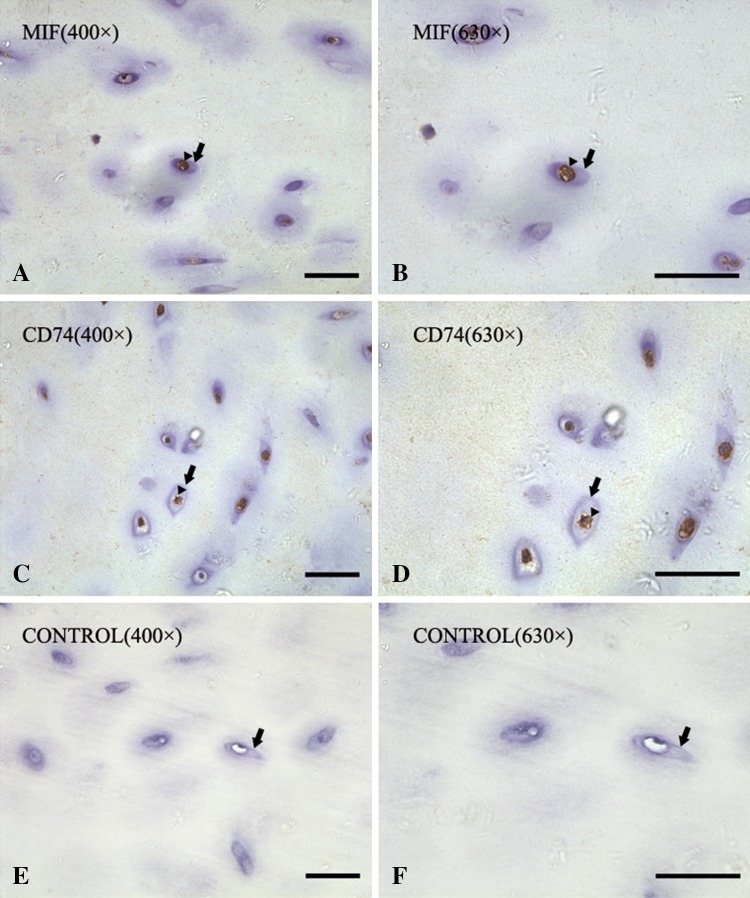
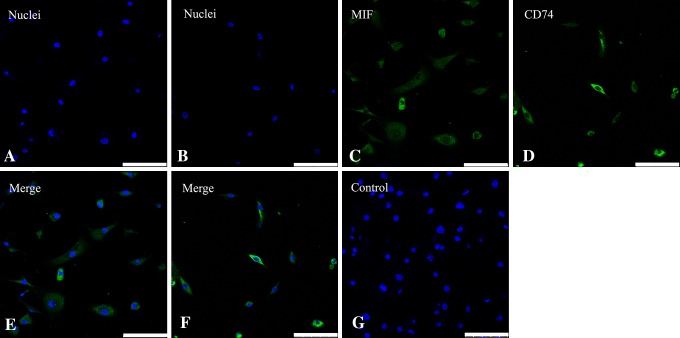
Staining showed MIF and CD74 appeared diffusely throughout human cartilage end plate specimens with Type 1 Modic changes. Type 1 Modic changes had decreased signal intensity on T1-weighted MR images (Fig. 1A) and increased signal intensity on T2-weighted images (Fig. 1B). The cartilage end plate is a white and translucent horizontal layer of hyaline cartilage (Fig. 1C). Adherent spindle-shaped cells can be seen on the plate (Fig. 1D). Immunohistochemical staining showed that MIF was stained on the cytoplasm and process of the chondrocytes at ×400 (Fig. 2A) and ×630 (Fig. 2B). CD74 also was found on the membranes of chondrocytes at ×400 (Fig. 2C) and ×630 (Fig. 2D). There was no staining in negative control samples at ×400 (Fig. 2E) and ×630 (Fig. 2F). Immunofluorescence showed that MIF and CD74 immunoreactivity appeared in cartilage end plate chondrocytes. Nuclei were stained with DAPI (blue) for MIF staining (Fig. 3A) and CD74 staining (Fig. 3B). Chondrocytes were subjected to immunofluorescence stain with FITC-conjugated MIF antibody (green) (Fig. 3C) and CD74 antibody (Fig. 3D). The merge image was observed for MIF (Fig. 3E) and CD74 (Fig. 3F), and no immunoreactivity was found in the IgG controls (Fig. 3G).
Fig. 1A–D.
Type 1 Modic changes have (A) decreased signal intensity on T1-weighted MR images and (B) increased signal intensity on T2-weighted images. Bone marrow edema is shown in Type 1 Modic changes, and there is mild enhancement of the disc. (C) A photograph shows the morphologic features of the cartilage end plate tissue. The cartilage end plate is a white, translucent horizontal layer of hyaline cartilage and is flatter, less malleable, and calcified. (D) The morphologic features of the chondrocytes are shown. They are spindle- and polygonal-shaped adherent cells. Bar = 100 μm.
Fig. 2A–F.
Immunohistochemistry staining for MIF is shown in paraffin-embedded sections of degenerate human cartilage end plate tissues from patients with Type 1 Modic changes observed on MRI. The black arrowheads show the immunoreactive chondrocytes and the black arrows show the cartilage lacuna edge in all images. MIF-immunoreactive chondrocytes are shown at (A) ×400 and (B) ×630 magnification. CD74-immunoreactive chondrocytes are shown at (C) ×400 and (D) ×630 magnification. The absence of staining in negative controls is shown at (E) ×400 and (F) ×630. Bar = 50 μm.
Fig. 3A–G.
Images of immunostaining with anti-MIF antibody and CD74 antibody in cartilage end plate chondrocytes cultured in monolayer are shown. Nuclei were stained with (A) DAPI (blue) for MIF staining and (B) CD74 staining. Immunoreactivity (green) for (C) MIF and (D) CD74 are clearly identified in cultured cartilage end plate chondrocytes. The double staining of DAPI and immunoreactive chondrocytes for (E) MIF and (F) CD74 are shown in the merge images. (G) No immunoreactivity was seen in the cells of the negative controls. Bar = 100 μm.
Cartilage End plate Degeneration and MIF
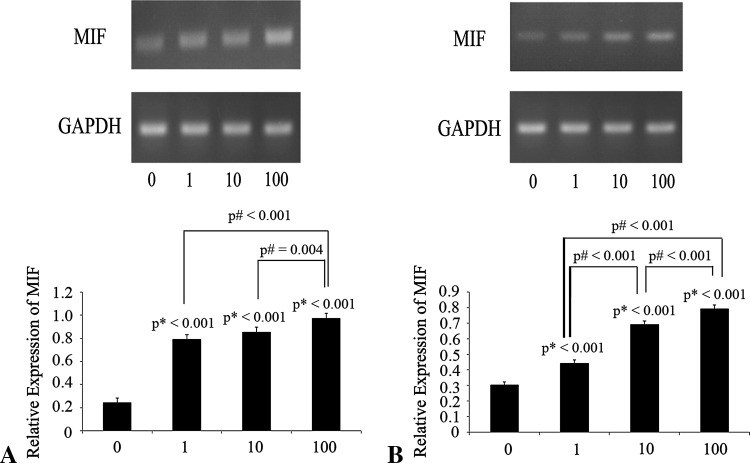
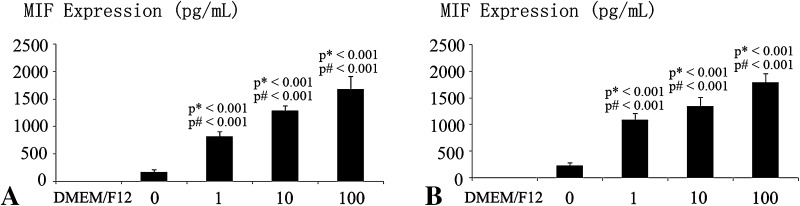
MIF might be involved in the cartilage end plate degeneration by enhancing the expression and secretion of IL-6, IL-8 and PGE2. TNF-α and lipopolysaccharide increased MIF mRNA expression in a dose-dependent manner (Fig. 4). TNF-α and lipopolysaccharide induced a dose-dependent increase in MIF in DMEM/F12. DMEM/F12 was used as a control (Fig. 5).
Fig. 4A–B.
The effects of proinflammatory cytokines on MIF mRNA expression by human cartilage endplate chondrocytes cultured in monolayer are shown. (A) When human cartilage end plate chondrocytes were treated with various concentrations of TNF-α (0, 1, 10, and 100 ng/mL) for 6 hours, TNF-α increased MIF mRNA levels in the cultured cells. (B) When human cartilage end plate chondrocytes were treated with various concentrations of lipopolysaccharide (0, 1, 10, and 100 ng/mL) for 6 hours, lipopolysaccharide increased MIF mRNA levels in the cultured cells. Total RNA was extracted and reverse-transcribed by RT-PCR. The expression level of MIF was normalized to GAPDH. Data are presented as mean ± SD and expressed as a percentage of control. p* = probability value as compared with the 0 ng/mL group; p# = probability value between groups.
Fig. 5A–B.
The graphs show the effects of proinflammatory cytokines on MIF secretion by human cartilage end plate chondrocytes cultured in monolayer. (A) When the cartilage end plate chondrocytes were treated with various concentrations of TNF-α (0, 1, 10, and 100 ng/mL) for 12 hours, TNF-α increased MIF expression in a dose-dependent manner as assessed by ELISA. (B) When the cartilage end plate chondrocytes were treated with various concentrations of lipopolysaccharide (0, 1, 10, and 100 ng/mL) for 12 hours, lipopolysaccharide increased MIF expression in a dose-dependent manner as assessed by ELISA. Data are presented as mean ± SD. p* = probability value as compared with the DMEM/F12 group; p# = probability value as compared with the 0 ng/mL group.
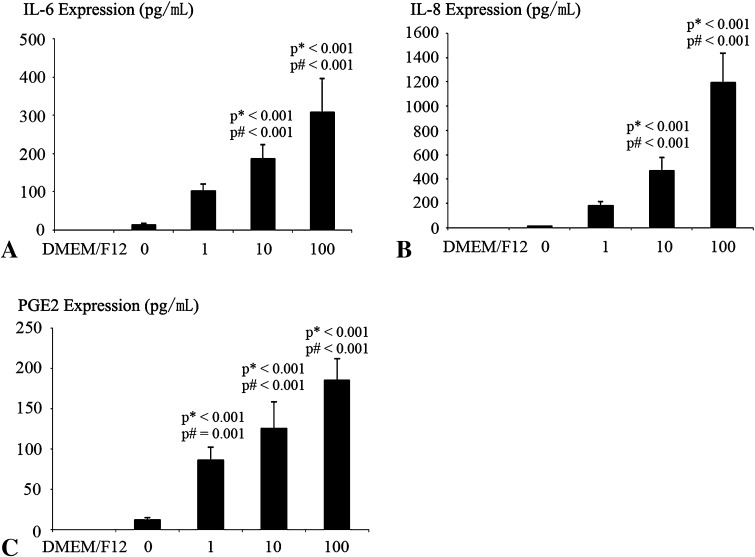
Detectable quantities of proinflammatory cytokines IL-6, IL-8, PGE2, IL-1β, and TNF-α were produced without stimulus. MIF induced a dose-dependent increase of IL-6, IL-8, and PGE2 release. IL-8 increased more than IL-6 and PGE2 in response to MIF (Fig. 6). Although there were TNF-α and IL-1β secretions in the culture medium, MIF did not exert an effect on TNF-α and IL-1β secretion.
Fig. 6A–C.
The effects of MIF on proinflammatory cytokines expressed in cartilage end plate chondrocytes are shown. For dose-dependent evaluation of protein secretion, cells were exposed to 0, 1, 10, and 100 ng/mL MIF for 12 hours and to DMEM/F12 as a negative control. The secretion of IL-6, IL-8, and PGE2 in the culture medium was assessed by ELISA. There were marked dose-dependent increases in (A) IL-6, (B) IL-8, and (C) PGE2 protein secretions. Data are presented as mean ± SD. p* = probability value as compared with the DMEM/F12 group; p# = probability value as compared with the 0 ng/mL group.
Suppression of MIF-induced Inflammatory Cytokine Release
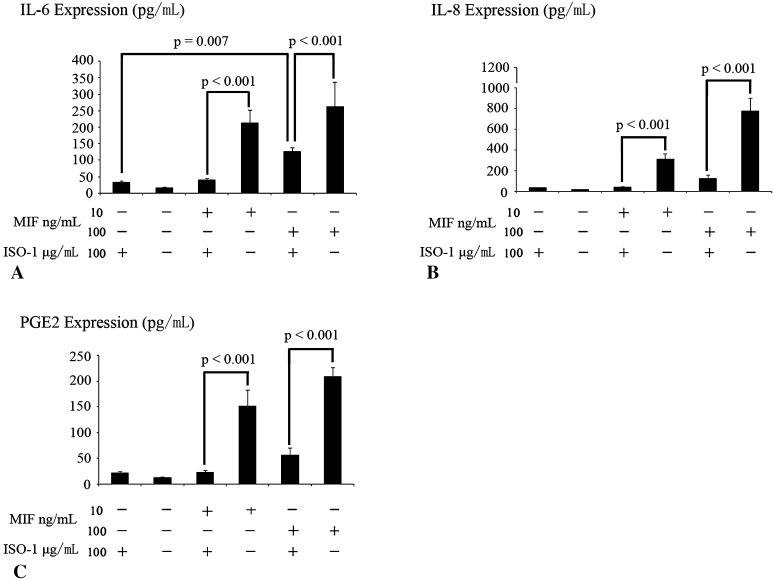
ISO-1 could be used to suppress the MIF-induced secretion of IL-6, IL-8, and PGE2.
A comparable upregulatory effect was observed as MIF-induced release of IL-6, IL-8, and PGE2 at 10 and 100 ng/mL for 12 hours; however, 100 μg ISO-1 significantly downregulated IL-6, IL-8, and PGE2 secretion in response to 10 and 100 ng/mL MIF (Fig. 7). ISO-1 did not induce inflammatory cytokine release and did not exert an effect on MIF-induced TNF-α secretion (95% CI, DMEM/F12: 22.06–26.32 pg/mL; ISO-1: 24.04–32.42 pg/mL, p = 0.11; and IL-1β, 95% CI, DMEM/F12: 21.88–26.63 pg/mL; ISO-1: 23.76–29.8 pg/mL, p = 0.23).
Fig. 7A–C.
The effects of ISO-1 on MIF-induced proinflammatory cytokine secretion are shown. Preincubation of 10 or 100 ng/mL MIF with 100 μg/mL ISO-1 inhibited MIF-induced (A) IL-6, (B) IL-8, and (C) PGE2 protein secretion. Data are presented as mean ± SD. * = statistical significance of p < 0.05 as compared with the corresponding MIF-treated cells preincubated with ISO-1; # = p < 0.05 as compared with the cartilage end plate chondrocytes with ISO-1. There was no difference between cartilage end plate chondrocytes with or without ISO-1.
Activation of CD74 Antibody
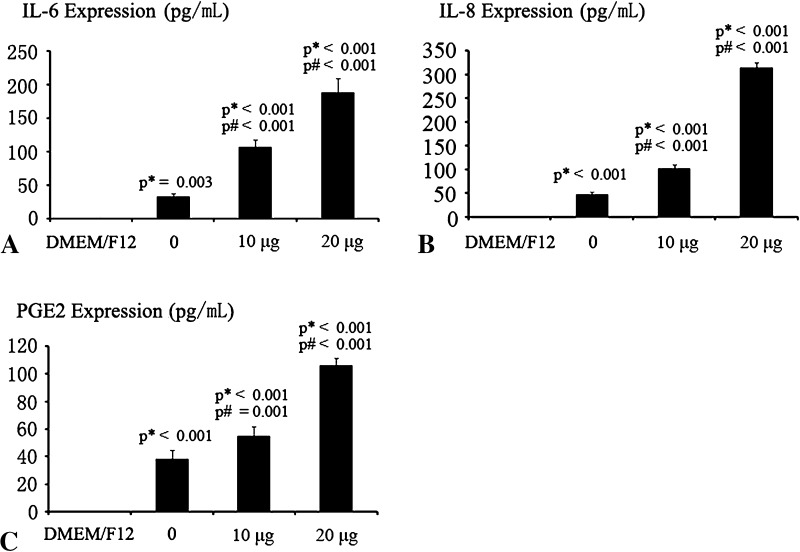
The activation of MIF receptor CD74 could induce the secretion of inflammatory cytokines IL-6, IL-8, and PGE2. CD74Ab induced a dose-dependent increase in IL-6, IL-8, and PGE2 in the culture medium. No MIF was detected in the culture medium alone. IL-8 (95% CI, 300.54–325.39 pg/mL) increased more than IL-6 (95% CI, 167.42–209.11 pg/mL) in response to 20 μg CD74Ab, and IL-6 increased more than PGE2 (95% CI, 100.41–111.19 pg/mL) in response to 20 μg CD74Ab (Fig. 8). However, CD74Ab did not exert an effect on TNF-α (95% CI, DMEM/F12:17.72–22.7 pg/mL, 20 μg CD74Ab:16.56–20.49 pg/mL) (p = 0.36) and IL-1β secretion (95% CI, DMEM/F12: 18.42–23.03 pg/mL, 20 μg CD74Ab: 20.08–22.9 pg/mL) (p = 0.6).
Fig. 8A–C.
The effects of CD74Ab on proinflammatory cytokines expressed in cartilage end plate chondrocytes are shown. For dose-dependent evaluation of protein secretion, cells were exposed to 0, 10, and 20 μg/mL CD74Ab for 12 hours and to DMEM/F12 as negative control. The secretion of IL-6, IL-8, and PGE2 in the culture medium was assessed by ELISA. There are marked dose-dependent increases in (A) IL-6, (B) IL-8, and (C) PGE2 protein secretions. Data are presented as mean ± SD. p* = probability value as compared with the DMEM/F12 group; p# = probability as compared with the 0 ng/mL group.
Discussion
MIF reportedly promotes inflammatory cytokine release through the CD74 receptor, and it is closely related to the inflammatory cytokines expressed in degenerated intervertebral disc tissues [6, 23, 24, 32]. However, the inflammatory role of MIF and CD74 in cartilage end plate largely remains unclear. The potential clinical implications of our study might be beneficial for therapeutic intervention in disc degenerative disorders. We therefore asked whether (1) MIF and MIF receptor CD74 are expressed in cartilage end plates with Type 1 Modic changes, (2) MIF is associated with cartilage end plate degeneration, (3) the MIF antagonist ISO-1 suppresses MIF-induced inflammatory cytokine release, and (4) inflammatory cytokines are released by cartilage end plate chondrocytes via CD74 by CD74Ab.
We acknowledge limitations to our study. First, although we examined the mechanism of cartilage end plate inflammation in patients with Type 1 Modic changes, MIF and CD74 play a crucial role in initiating and promoting inflammatory response in intervertebral disc degeneration, and targeting MIF by ISO-1 in intervertebral disc disorders can reduce inflammation. However, this being a laboratory-based in vitro study, the effect of ISO-1 on the body systems remains largely unknown. In vivo studies involving cartilage end plate with Type 1 Modic changes from animals are needed for future research regarding the safety and efficacy of ISO-1 as an intervention in inflammatory and degenerative disease. Nevertheless, the data reported here provide a theoretical basis for further research. Second, cells in degenerated intervertebral disc tissues are composed of heterogeneous cell types, such as macrophages [31], and the Passage 0 culture is not pure, although macrophages are suspension cells that can be removed by culture medium replacement. However, the cartilage end plate chondrocytes are under the stimulus of macrophage-induced MIF at a different level, which might affect accuracy of results. Chondrocytes from nondegenerated cartilage end plates are needed for negative control in future studies. Nevertheless, we subjected cartilage end plate chondrocyte culture from the same patient to each experiment at the fourth passage of culture in our study, and this study represents overall trends of data despite discrepancies between groups. Third, we did not include patients with normal and other types of Modic changes in cartilage end plates in our study. A previous study showed that Type 1 Modic changes are an ongoing active degenerative process which are featured with fissured end plates with adjacent vascular granulation tissue adjacent in the bone marrow [26]. Cartilage end plates with Type 1 Modic changes are much more commonly associated with severe inflammation response than that with Type 2 Modic changes [29]. However, the current study is a pilot study and is based on the phenomenon that inflammation is more closely related to cartilage end plates with Type 1 Modic changes than cartilage end plates with normal and other types of Modic changes. Cartilage end plates with normal and other types of Modic changes should be included to further elucidate the pathophysiologic mechanism of MIF in disc degeneration. Nevertheless, this is a different study question that merits a separate dedicated research effort. MIF is an important proinflammatory cytokine involved in many inflammatory and autoimmune disorders in vivo via CD74. The upregulation of cell surface receptor CD74 is a step toward mediating MIF-CD74 signal transduction [27]. Veillat et al. [32] reported that MIF can induce human ectopic endometrial cells to secrete cell growth factor (VEGF), IL-8, and monocyte chemotactic protein-1(MCP-1) via CD44, CD74, and MAPK signaling pathway. Similar to their study, we found that MIF and CD74 are highly expressed in the cartilage end plate with Type I Modic changes. MIF might bind to CD74 and activate signal transduction, leading to the production of inflammatory cytokines. However, further study is required to determine the interaction mechanisms between MIF and CD74 in disc degeneration. Inflammatory cytokines such as IL-6, IL-8, PGE2, TNF-α, and IL-1β are closely related to disc degeneration and discogenic back pain. Studies have been done to elucidate their role in degenerative disease. Kim et al. [18, 19] showed that TNF-α and IL-1β are derived predominantly from macrophagelike cells, while IL-8, IL-6, and nitric oxide can be secreted by intervertebral disc cells. Le Maitre et al. [21] reported that TNF-α and IL-1β are expressed by cells in degenerate intervertebral disc tissues and may be associated with pathogenesis of intervertebral disc degeneration. However, Burke et al. [8] found that there is no TNF-α or IL-1β secretion by intervertebral disc tissues after stimulation with lipopolysaccharide. We found that IL-6, IL-8, and PGE2 are increased after stimulation with MIF, although TNF-α and IL-1β do not increase. Taken together, these data support the notion that IL-6, IL-8, and PGE2 are produced mainly by cartilage end plate chondrocytes, whereas TNF-α and IL-1β are secreted predominantly from infiltrating cells in the degenerate intervertebral disc tissues, not native cartilage end plate chondrocytes. Furthermore, we found that IL-8 increased more than IL-6 and PGE2 in response to inflammatory stimulus, and IL-8 showed a similar trend as those reported by Kim et al. [18]. IL-8 is a chemoattractant for lymphocytes and neutrophils [4, 5], present in significant concentrations in intervertebral disc tissues [1]. Therefore, our data suggest that IL-8 may play an important role in MIF-induced inflammation and warrants further investigation.
ISO-1, a specific antagonist of MIF, is capable of reducing MIF elevations found in inflammatory disease to normal levels by turning off the auto-crine and paracrine loops, allowing the immune system to reset [2]. ISO-1 has been shown to be effective in inhibiting MIF in many of the disease models tested, such as inflammatory and autoimmune disorders and infectious disease [2].
Cvetkovic et al. [14] showed that administration of ISO-1 significantly inhibited hyperglycemia and insulitis, decreased pancreatic MIF staining, and also reduced inflammatory cytokine production in autoimmune diabetes. Veillat et al. [32] found that ISO-1 can be used to suppress MIF-induced VEGF, IL-8, and MCP-1. Similar to their findings, our data suggest that ISO-1 not only induces the secretion of inflammatory cytokines (Fig. 7), and but also neutralizes the proinflammatory activity of MIF, which would be beneficial for control of inflammatory activity and degradation of the extracellular matrix induced by inflammatory cytokines.
CD74 is an integral membrane protein which acts as a MIF receptor and regulates functions of the major histocompatibility complex Class II molecules; thus, many biologic activities of MIF are via activation of the CD74 receptor. CD74 immunostaining is increased in inflammatory diseases, and MIF binding to CD74 induces nuclear factor κ-light-chain-enhancer of activated B cells (NF-κB) and the secretion of inflammatory cytokines [7]. CD74 can be stimulated by CD74Ab or MIF to increase the DNA and protein synthesis in the B cells through the NF-κB signal pathway [16]. We found that CD74 activation with CD74Ab leads to the secretion of the inflammatory cytokines IL-6, IL-8, and PGE2 in a dose-dependent manner. It seems that MIF secreted by cartilage end plate chondrocytes might interact with CD74 on the chondrocytes and subsequent cellular responses via the autocrine and paracrine patterns in the degenerate cartilage end plate tissues.
Our observations suggest MIF promotes inflammatory cytokine release from cartilage end plate chondrocytes with Type 1 Modic changes through the CD74 receptor, which would shed light on targeting the MIF/CD74 signaling pathway for cartilage end plate degeneration treatment. Combined treatment with a MIF antagonist ISO-1 could decrease the inflammatory response in cartilage end plates. Because inflammatory cytokines expressed in intervertebral disc tissues are closely related to the cartilage end plate degeneration, it is an alternative way to achieve a satisfactory therapeutic effect through reducing inflammation by ISO-1. However, in vivo study is needed to test the efficiency and safety of ISO-1. Our observations provide compelling evidence that the effectiveness of anti-MIF therapies in chondrocytes from patients with Type 1 Modic changes and potential therapies targeted at inhibiting MIF would be effective in the prevention of intervetebral disc degeneration, and the treatment of discogenic pain.
Acknowledgments
We thank Jian Wang Prof and Changqing Li Prof (Orthopaedic Department, Xinqiao Hospital, Third Military Medical University, Chongqing, China) for identification and acquirement of cartilage end plates with Type 1 Modic changes. We also thank Dawei Zhang PhD (Orthopaedic Department, Xinqiao Hospital) for help with molecular experiments. This work was also supported by the National Natural Science Foundation of China (81271982).
Footnotes
Each author certifies that he or she, or a member of his or her immediate family, has no commercial associations (eg, stock ownership, consultancies, patent/licensing arrangements, equity interest, etc) that might represent a conflict of interest related to the submitted article.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research editors and board members are on file with the publication and can be viewed on request.
Each author certifies that his or her institution approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
This work was performed at Xinqiao Hospital, Third Military Medical University, Chongqing, China and Wuhan General Hospital of Guangzhou Military Area Command, Wuhan, China.
References
- 1.Ahn SH, Cho YW, Ahn MW, Jang SH, Sohn YK, Kim HS. (2002) mRNA expression of cytokines and chemokines in herniated lumbar intervertebral discs. Spine (Phila Pa 1976). 27:911–917. [DOI] [PubMed]
- 2.Al-Abed Y, VanPatten S. MIF as a disease target: ISO-1 as a proof-of-concept therapeutic. Future Med Chem. 2011;3:45–63. doi: 10.4155/fmc.10.281. [DOI] [PubMed] [Google Scholar]
- 3.Albert HB, Kjaer P, Jensen TS, Sorensen J, Bendix T, Manniche C. Modic changes, possible causes and relation to low back pain. Med Hypotheses. 2008;70:361–368. doi: 10.1016/j.mehy.2007.05.014. [DOI] [PubMed] [Google Scholar]
- 4.Baggiolini M, Clark-Lewis I. Interleukin-8, a chemotactic and inflammatory cytokine. FEBS Lett. 1992;307:97–101. doi: 10.1016/0014-5793(92)80909-Z. [DOI] [PubMed] [Google Scholar]
- 5.Baggiolini M, Dewald B, Moser B. Interleukin-8 and related chemotactic cytokines-CXC and CC chemokines. Adv Immunol. 1994;55:97–179. doi: 10.1016/S0065-2776(08)60509-X. [DOI] [PubMed] [Google Scholar]
- 6.Baugh JA, Bucala R. Macrophage migration inhibitory factor. Crit Care Med. 2002;30(1 suppl):S27–S35. doi: 10.1097/00003246-200201001-00004. [DOI] [PubMed] [Google Scholar]
- 7.Beswick EJ, Reyes VE. CD74 in antigen presentation, inflammation, and cancers of the gastrointestinal tract. World J Gastroenterol. 2009;15:2855–2861. doi: 10.3748/wjg.15.2855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burke JG, Watson RW, Conhyea D, McCormack D, Dowling FE, Walsh MG, Fitzpatrick JM. Human nucleus pulposis can respond to a pro-inflammatory stimulus. Spine (Phila Pa 1976). 2003;28:2685–2693. [DOI] [PubMed]
- 9.Calandra T, Bernhagen J, Metz CN, Spiegel LA, Bacher M, Donnelly T, Cerami A, Bucala R. MIF as a glucocorticoid-induced modulator of cytokine production. Nature. 1995;377:68–71. doi: 10.1038/377068a0. [DOI] [PubMed] [Google Scholar]
- 10.Calandra T, Bernhagen J, Mitchell RA, Bucala R. The macrophage is an important and previously unrecognized source of macrophage migration inhibitory factor. J Exp Med. 1994;179:1895–1902. doi: 10.1084/jem.179.6.1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cao W, Morin M, Sengers V, Metz C, Roger T, Maheux R, Akoum A. Tumour necrosis factor-α up-regulates macrophage migration inhibitory factor expression in endometrial stromal cells via the nuclear transcription factor NF-κB. Hum Reprod. 2006;21:421–428. doi: 10.1093/humrep/dei315. [DOI] [PubMed] [Google Scholar]
- 12.Cheng RJ, Deng WG, Niu CB, Li YY, Fu Y. Expression of macrophage migration inhibitory factor and CD74 in cervical squamous cell carcinoma. Int J Gynecol Cancer. 2011;21:1004–1012. doi: 10.1097/IGC.0b013e31821c45b7. [DOI] [PubMed] [Google Scholar]
- 13.Crock HV. Internal disc disruption: a challenge to disc prolapse fifty years on. Spine (Phila Pa 1976). 1986;11:650–653. [PubMed]
- 14.Cvetkovic I, Al-Abed Y, Miljkovic D, Maksimovic-Ivanic D, Roth J, Bacher M, Lan HY, Nicoletti F, Stosic-Grujicic S. Critical role of macrophage migration inhibitory factor activity in experimental autoimmune diabetes. Endocrinology. 2005;146:2942–2951. doi: 10.1210/en.2004-1393. [DOI] [PubMed] [Google Scholar]
- 15.Donnelly SC, Haslett C, Reid PT, Grant IS, Wallace WA, Metz CN, Bruce LJ, Bucala R. Regulatory role for macrophage migration inhibitory factor in acute respiratory distress syndrome. Nat Med. 1997;3:320–323. doi: 10.1038/nm0397-320. [DOI] [PubMed] [Google Scholar]
- 16.Gore Y, Starlets D, Maharshak N, Becker-Herman S, Kaneyuki U, Leng L, Bucala R, Shachar I. Macrophage migration inhibitory factor induces B cell survival by activation of a CD74-CD44 receptor complex. J Biol Chem. 2008;283:2784–2792. doi: 10.1074/jbc.M703265200. [DOI] [PubMed] [Google Scholar]
- 17.Kim H, Lee JU, Moon SH, Kim HC, Kwon UH, Seol NH, Kim HJ, Park JO, Chun HJ, Kwon IK. Zonal responsiveness of the human intervertebral disc to bone morphogenetic protein-2. Spine (Phila Pa 1976). 2009;34:1834–1838. [DOI] [PubMed]
- 18.Kim JH, Studer RK, Sowa GA, Vo NV, Kang JD. Activated macrophage-like THP-1 cells modulate anulus fibrosus cell production of inflammatory mediators in response to cytokines. Spine (Phila Pa 1976). 2008;33:2253–2259. [DOI] [PubMed]
- 19.Kim JH, Studer RK, Vo NV, Sowa GA, Kang JD. p38 MAPK inhibition selectively mitigates inflammatory mediators and VEGF production in AF cells co-cultured with activated macrophage-like THP-1 cells. Osteoarthritis Cartilage. 2009;17:1662–1669. doi: 10.1016/j.joca.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 20.Lan HY, Bacher M, Yang N, Mu W, Nikolic-Paterson DJ, Metz C, Meinhardt A, Bucala R, Atkins RC. The pathogenic role of macrophage migration inhibitory factor in immunologically induced kidney disease in the rat. J Exp Med. 1997;185:1455–1465. doi: 10.1084/jem.185.8.1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Le Maitre CL, Hoyland JA, Freemont AJ. Catabolic cytokine expression in degenerate and herniated human intervertebral discs: IL-1beta and TNFalpha expression profile. Arthritis Res Ther. 2007;9:R77. doi: 10.1186/ar2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leech M, Metz C, Bucala R, Morand EF. Regulation of macrophage migration inhibitory factor by endogenous glucocorticoids in rat adjuvant-induced arthritis. Arthritis Rheum. 2000;43:827–833. doi: 10.1002/1529-0131(200004)43:4<827::AID-ANR13>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 23.Leng L, Bucala R. Macrophage migration inhibitory factor. Crit Care Med. 2005;33(12 suppl):S475–S477. doi: 10.1097/01.CCM.0000191278.04636.D8. [DOI] [PubMed] [Google Scholar]
- 24.Lolis E, Bucala R. Macrophage migration inhibitory factor. Expert Opin Ther Targets. 2003;7:153–164. doi: 10.1517/14728222.7.2.153. [DOI] [PubMed] [Google Scholar]
- 25.Loreto C, Musumeci G, Castorina A, Loreto C, Martinez G. Degenerative disc disease of herniated intervertebral discs is associated with extracellular matrix remodeling, vimentin-positive cells and cell death. Ann Anat. 2011;193:156–162. doi: 10.1016/j.aanat.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 26.Ma XL, Ma JX, Wang T, Tian P, Han C. Possible role of autoimmune reaction in Modic Type I changes. Med Hypotheses. 2011;76:692–694. doi: 10.1016/j.mehy.2011.01.035. [DOI] [PubMed] [Google Scholar]
- 27.Meyer-Siegler KL, Iczkowski KA, Leng L, Bucala R, Vera PL. Inhibition of macrophage migration inhibitory factor or its receptor (CD74) attenuates growth and invasion of DU-145 prostate cancer cells. J Immunol. 2006;177:8730–8739. doi: 10.4049/jimmunol.177.12.8730. [DOI] [PubMed] [Google Scholar]
- 28.Modic MT, Steinberg PM, Ross JS, Masaryk TJ, Carter JR. Degenerative disk disease: assessment of changes in vertebral body marrow with MR imaging. Radiology. 1988;166:193–199. doi: 10.1148/radiology.166.1.3336678. [DOI] [PubMed] [Google Scholar]
- 29.Ohtori S, Inoue G, Ito T, Koshi T, Ozawa T, Doya H, Saito T, Moriya H, Takahashi K. Tumor necrosis factor-immunoreactive cells and PGP 9.5-immunoreactive nerve fibers in vertebral endplates of patients with discogenic low back pain and Modic Type 1 or Type 2 changes on MRI. Spine (Phila Pa 1976). 2006;31:1026–1031. [DOI] [PubMed]
- 30.Shun CT, Lin JT, Huang SP, Lin MT, Wu MS. Expression of macrophage migration inhibitory factor is associated with enhanced angiogenesis and advanced stage in gastric carcinomas. World J Gastroenterol. 2005;11:3767–3771. doi: 10.3748/wjg.v11.i24.3767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Takahashi H, Suguro T, Okazima Y, Motegi M, Okada Y, Kakiuchi T. Inflammatory cytokines in the herniated disc of the lumbar spine. Spine (Phila Pa 1976). 1996;21:218–224. [DOI] [PubMed]
- 32.Veillat V, Carli C, Metz CN, Al-Abed Y, Naccache PH, Akoum A. Macrophage migration inhibitory factor elicits an angiogenic phenotype in human ectopic endometrial cells and triggers the production of major angiogenic factors via CD44, CD74, and MAPK signaling pathways. J Clin Endocrinol Metab. 2010;95:E403–E412. doi: 10.1210/jc.2010-0417. [DOI] [PubMed] [Google Scholar]
- 33.Xiong CJ, Huang B, Zhou Y, Cun YP, Liu LT, Wang J, Li CQ, Pan Y, Wang H. Macrophage migration inhibitory factor inhibits the migration of cartilage end plate-derived stem cells by reacting with CD74. PloS One. 2012;7:e43984. doi: 10.1371/journal.pone.0043984. [DOI] [PMC free article] [PubMed] [Google Scholar]