Abstract
Background
Disc space narrowing, osteophytes, and disc degeneration are common and increase with aging. Few animal models are appropriate for the study of spontaneous age-related cervical disc degeneration.
Questions/purposes
We used the sand rat, a member of the gerbil family with well-recognized age-related lumbar disc degeneration, to determine whether spontaneous cervical disc degeneration differed from lumbar degeneration when evaluated by (1) radiologic and (2) histologic measures. Animals 2 to 25 months of age were used in these analyses.
Methods
Cervical and lumbar discs of 99 sand rats were analyzed with radiology, and cervical discs of 67 sand rats were studied with histology. Lateral digital radiographs of cervical and lumbar spines were scored for presence or absence of wedging, disc space narrowing, osteophytes, end plate calcification, and irregular disc margins at C2-C3 through C6-C7 and T12-L1 through L7-S1. Percentages for presence were calculated and statistically analyzed for younger (range, 2-11.9 months old) versus older (range, 12.0-25 months old) animals.
Results
Cervical discs in younger animals exhibited a greater proportion of irregular margins compared with lumbar sites (94% versus 83%; p = 0.02; 95% CI for difference, 2.7, 19.0%). In older animals, cervical discs showed a greater proportion of osteophytes than did lumbar discs (7% versus 0%; p < 0.0001). The incidence of disc space narrowing was greater in cervical versus lumbar sites (99% versus 90%; p = 0.0008). Cervical spine sites which contained osteophytes morphologically showed irregular disc margins and revealed an extrusion of herniated disc material in the osteophytes.
Conclusions
Radiologic and morphologic studies confirmed age-related disc degeneration in the cervical spine of the sand rat.
Clinical Relevance
Clinical cervical aging studies have shown that 14% of asymptomatic subjects younger than 40 years have abnormal MRI scans with an increase to 50% by 50 years old. We studied an economic rodent model for cervical age-related spontaneous disc.
Introduction
Animal models are important tools in many types of orthopaedic spine research. The proceedings summary of the 1995 National Institutes of Health workshop on back pain emphasized the importance of this costly healthcare problem and recommended areas of future research, including the importance of animal models for the study of disc degeneration. In this work, Krag commented that “… the usefulness of a particular model derives not from the extent to which it mimics reality (because that cannot be known in advance), but rather from the extent to which it facilitates the formulation and subsequent testing of hypotheses that lead to an improved understanding of that reality” [13].
Basic science studies of disc degeneration are complicated because in most species, disc degeneration must be induced with either surgical injury such as needle puncture (rabbit and other animal models [4, 11]) or chemonucleolysis [13, 16]. Needle puncture models have produced important data, but this method is now known to result in cell death, decreased dynamic modulus, and increased creep of the disc during short-term loading [12]. Needle injury also has been clinically associated with accelerated adjacent segment degeneration [18]. Sobajima et al. [27] commented that the classic stab model causes immediate herniation of the nucleus pulposus and thus cannot mimic slower changes in the nucleus or annulus associated with onset and progression of disc degeneration. It also is not appropriate as a model to test therapies that focus on early degeneration. Therefore, non-injury models, such as the spontaneous age-related lumbar disc degeneration in the sand rat, have great research value.
The sand rat (Psammomys obesus obesus), a member of the gerbil family, has age-related spontaneous lumbar disc degeneration develop. This model of lumbar disc degeneration first came to attention in the scientific literature in the 1980s [1, 17, 22–26, 32]. These early studies documented histologic and radiologic changes in animals 2, 3, 6, and 18 to 30 months of age and illustrated the histologic presence of granular debris in the nucleus, small annular tears, herniations into and through cartilage end plates, and ligament calcification. In the sand rat and in the human, the most advanced disc degenerative changes involve the lumbar and thoracolumbar spinal regions.
Human and sand rat lumbar degenerative features share many common features, including disc space narrowing, wedging, end plate calcification, irregular disc margins, and subchondral sclerosis [5, 9]. This model was used successfully in analyses of radiologic changes in the lumbar spine in cross-sectional and prospective animal populations [9, 28, 31], analyses of vascularization of the end plate [8], and experimental autologous disc cell implantation [10].
Clinical studies of the aging cervical spine have shown that 14% of asymptomatic subjects younger than 40 years have abnormal MRI scans with an increase to 50% by 50 years old [2, 6, 7]. Disc space narrowing, osteophytes, and disc degeneration are common and increase with age. Relatively few animal models have been used in analyses of cervical disc degeneration. Panjabi [20] reviewed animal models used in cervical spine biomechanical research, noting previous work in the canine [3] and goat [21, 30], and Yingling et al. [29] used the porcine cervical spine to model human lumbar spine.
In the current study, we used the sand rat, a member of the gerbil family with well-recognized age-related lumbar disc degeneration, and asked whether spontaneous cervical disc degeneration differed from lumbar degeneration as determined by (1) radiologic and (2) histologic measures. Animals 2 to 25 months old were analyzed.
Methods
Animal Model
Animal studies were performed after approval by the Institutional Animal Care and Use Committee. Psammomys obesus obesus specimens were obtained from our colony of animals of known ages. One to two animals were housed per cage in a dedicated housing room. Animals were provided tap water and Purina sand rat diet #5L09 (Ralston-Purina, Richmond, IN, USA) ad libitum; this special low-calorie diet is a formulation with 2.42 kcal/g metabolizable energy, which prevents the development of diabetes seen with high calorie ad libitum feeding. Animals were identified by IMI-10000 Implantable Micro Identification chips (BioMedic Data Systems, Inc, Maywood, NJ, USA).
Radiologic Analyses
Study Population
Cervical and lumbar discs of 99 sand rats (48 males and 51 females) 2.1 to 24.7 months old were radiologically evaluated. One author (RP), blinded to the ages of the animals, scored digital radiographic images for the following outcome measures: presence (scored as 1) or absence (scored as 0) of wedging, disc space narrowing, osteophytes, end plate calcification, and irregular disc margins at C2-C3 through C6-C7 and T12-L1 through L7-S1, and percentages were calculated for the presence or absence of these features. When there was poor imaging or bone overlap at a cervical or lumbar site, the respective feature for that site was not scored.
Histologic and Morphologic Studies
At euthanization, cervical spines were removed, trimmed of adherent tissue, fixed in 10% neutral buffered formalin, and decalcified and embedded in paraffin. Four-micron thick midsagittal sections were cut and sections stained with Masson-trichrome, dehydrated, cleared in xylene, and mounted using resinous mounting media. Morphologic studies were performed on 35 specimens from sand rats 2 to 11.9 months old and 32 specimens from sand rats older than 12 months. Histologic studies included qualitative study of sections prepared with routine stains (such as hematoxylin and eosin) and Masson-trichrome staining for assessment of disc and bone features.
Statistical Methods
Standard statistical analyses were performed using calculation of means ± SD. Radiologic data on presence or absence of wedging, disc space narrowing, osteophytes, end plate calcification, and irregular disc margins were statistically analyzed in younger (2-11.9 months old; n = 28) versus older animals (older than 12 months, n = 63) using the chi-square test or Fisher’s exact test with SAS Version 9.2 (SAS Institute Inc, Cary, NC, USA). A p value of 0.05 or less was considered statistically significant. Radiologic data were collected on all scorable cervical and lumbar discs in the same animal and a final data set formulated, which contained all cervical and lumbar data for animals of all ages.
Results
Radiologic Findings
Radiologic assessment showed that wedging was common in cervical and lumbar sites in both age groups (Table 1). Disc space narrowing differed in older animals for cervical versus lumbar sites. The incidence of narrowing was significantly greater in cervical sites compared with lumbar sites (98.7% versus 90.2%; p = 0.0008) (Table 2). In older animals, cervical discs showed a significantly greater proportion of osteophytes than did lumbar discs (6.5% versus 0%, respectively; p < 0.0001) (Table 3). In older animals cervical discs showed a significantly greater proportion of osteophytes than did lumbar discs (6.5% versus 0%, respectively; p < 0.0001). End plate calcification was present in both age groups in all cervical and lumbar discs. Cervical discs in younger animals exhibited a significantly greater proportion of irregular margins compared with lumbar sites (93.9% versus 83.1%, respectively; p = 0.02) (Table 4). Disc space narrowing and irregular disc margins were much less evident in younger compared to older animals (Fig. 1).
Table 1.
Analysis of the radiologic feature of wedging.
| Spinal site | Percentage present | Fraction present | Percentage present | Fraction present | p value, younger versus older |
|---|---|---|---|---|---|
| Age 2–11.9 months | Age ≥ 12 months | ||||
| Cervical | |||||
| C2-C3 | 82.4% | 14/17 | 100% | 32/32 | |
| C3-C4 | 100% | 17/17 | 100% | 32/32 | |
| C4-C5 | 88.2% | 15/17 | 90.6% | 29/32 | |
| C5-C6 | 87.5% | 14/16 | 93.6% | 29/31 | |
| C6-C7 | 73.3% | 11/15 | 80.8% | 21/26 | |
| Overall cervical | 86.6% | 71/82 | 93.5% | 143/153 | 0.078 |
| Lumbar | |||||
| T12-L1 | 82.4% | 14/17 | 100% | 32/32 | |
| L1-L2 | 82.4% | 14/17 | 75.0% | 24/32 | |
| L2-L3 | 58.8% | 10/17 | 68.8% | 22/32 | |
| L3-L4 | 82.4% | 14/17 | 75.0% | 24/32 | |
| L4-L5 | 94.1% | 16/17 | 90.6% | 29/32 | |
| L5-L6 | 100% | 17/17 | 100% | 32/32 | |
| L6-L7 | 100% | 17/17 | 100% | 31/31 | |
| L7-S1 | 100% | 17/17 | 96.8% | 30/31 | |
| Overall lumbar | 87.5% | 119/136 | 88.2% | 224/254 | 0.842 |
| p value, analyses of overall data, cervical versus lumbar | 0.845 | 0.830 | |||
Table 2.
Analysis of the radiologic feature of disc space narrowing.
| Spinal site | Percentage present | Fraction present | Percentage present | Fraction present | p value, younger versus older |
|---|---|---|---|---|---|
| Age 2–11.9 months | Age ≥ 12 months | ||||
| Cervical | |||||
| C2-C3 | 88.2% | 15/17 | 100% | 32/32 | |
| C3-C4 | 100% | 17/17 | 100% | 32/32 | |
| C4-C5 | 100% | 17/17 | 96.9% | 31/32 | |
| C5-C6 | 100% | 16/16 | 100% | 31/31 | |
| C6-C7 | 86.7% | 13/15 | 96.2% | 25/26 | |
| Overall cervical | 95.1% | 78/82 | 98.7% | 151/153 | 0.098 |
| Lumbar | |||||
| T12-L1 | 88.2% | 15/17 | 90.6% | 29/32 | |
| L1-L2 | 88.2% | 15/17 | 81.3% | 26/32 | |
| L2-L3 | 64.7% | 11/17 | 81.3% | 26/32 | |
| L3-L4 | 82.4% | 14/17 | 81.3% | 26/32 | |
| L4-L5 | 94/1% | 16/17 | 90.6& | 29/32 | |
| L5-L6 | 100% | 17/17 | 100% | 32/32 | |
| L6-L7 | 100% | 17/17 | 100% | 31/31 | |
| L7-S1 | 100% | 17/17 | 96.8% | 30/31 | |
| Overall lumbar | 89.7% | 122/136 | 90.2% | 229/254 | 0.887 |
| p value, analyses of overall data, cervical versus lumbar | 0.159 | 0.0008 | |||
Table 3.
Analysis of the radiologic feature of osteophytes.
| Spinal site | Percentage present | Fraction present | Percentage present | Fraction present | p value, younger versus older |
|---|---|---|---|---|---|
| Age 2–11.9 months | Age ≥ 12 months | ||||
| Cervical | |||||
| C2-C3 | 0 | 0/17 | 0% | 0/32 | |
| C3-C4 | 5.9% | 1/17 | 9.4% | 3/32 | |
| C4-C5 | 0% | 0/17 | 12.5% | 4/32 | |
| C5-C6 | 0% | 0/16 | 6.5% | 2/31 | |
| C6-C7 | 0% | 0/15 | 3.9% | 1/26 | |
| Overall cervical | 1.2% | 1/82 | 6.5% | 10/154 | 0.103 |
| Lumbar | |||||
| T12-L1 | 0% | 0/17 | 0% | 0/32 | |
| L1-L2 | 0% | 0/17 | 0% | 0/32 | |
| L2-L3 | 0% | 0/17 | 0% | 0/32 | |
| L3-L4 | 0% | 0/17 | 0% | 0/32 | |
| L4-L5 | 0% | 0/17 | 0% | 0/32 | |
| L5-L6 | 0% | 0/17 | 0% | 0/32 | |
| L6-L7 | 0% | 0/17 | 0% | 0/31 | |
| L7-S1 | 0% | 0/17 | 0% | 0/31 | |
| Overall lumbar | 0% | 0/136 | 0% | 0/254 | 1.0 |
| p value, analyses of overall data, cervical versus lumbar | 0.379 | < 0.0001 | |||
Table 4.
Analysis of the radiologic feature of irregular disc margins.
| Spinal site | Percentage present | Fraction present | Percentage present | Fraction present | p value, young versus old |
|---|---|---|---|---|---|
| Age 2–11.9 months | Age ≥ 12 months | ||||
| Cervical | |||||
| C2-C3 | 82.4% | 14/17 | 87.5% | 28/32 | |
| C3-C4 | 100% | 17/17 | 93.8% | 30/32 | |
| C4-C5 | 94.1% | 16/17 | 96.7% | 31/32 | |
| C5-C6 | 100% | 16/16 | 93.6% | 29/31 | |
| C6-C7 | 93.3% | 14/15 | 80.8% | 21/26 | |
| Overall cervical | 93.9% | 77/82 | 90.8% | 139/153 | 0.413 |
| Lumbar | |||||
| T12-L1 | 76.5% | 13/17 | 93.8% | 30/32 | 0.200 |
| L1-L2 | 64.7% | 11/17 | 75.0% | 24/32 | |
| L2-L3 | 71.9% | 9/17 | 71.9% | 23/32 | |
| L3-L4 | 76.5% | 13/17 | 75.0% | 24/32 | |
| L4-L5 | 94.1% | 16/17 | 93.8% | 30/32 | |
| L5-L6 | 100% | 17/17 | 96.9% | 31/32 | |
| L6-L7 | 100% | 17/17 | 96.8% | 30/31 | |
| L7-S1 | 100% | 17/17 | 100% | 31/31 | |
| Overall lumbar | 83.1% | 113/136 | 87.8% | 223/254 | |
| p value, analyses of overall data, cervical versus lumbar | 0.021 | 0.341 | |||
Fig. 1A–C.
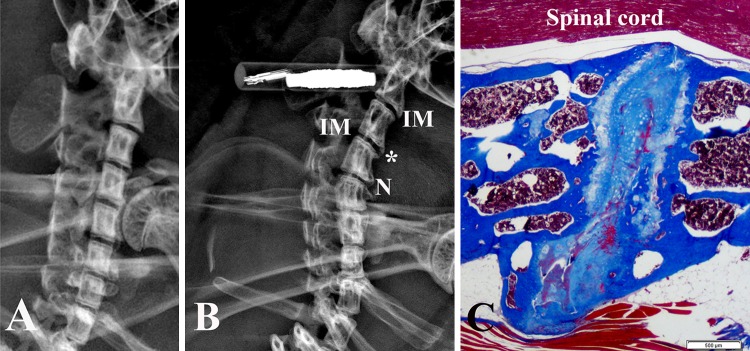
Representative radiologic sagittal views of cervical spines are shown for (A) a 2.1-month-old sand rat and (B) a 22.7-month-old animal. Prominent irregular disc margins (IM) and disc space narrowing (N) can be seen in the older animal. *an osteophyte that is shown in histologic section in Illustration C. The white bar to the left of the spine is an animal ID chip. (C) A histologic section through the osteophyte region of the older animal shows the presence of extruded herniated disc material (Stain, Masson-trichome; bar = 500 μm).
Morphologic Findings
In cervical discs levels in younger animals, the nucleus pulposus often was observed to still contain notochordal cells (Fig. 2), a finding consistent with previous observations in lumbar disc sites [1]. Histologic evidence of cervical disc degeneration was present in the older animals as reflected in prominent wedging, narrowing (Fig. 2), and osteophyte formation (Fig. 1). With progressive aging, the nucleus in cervical discs became narrowed, and was increasingly difficult to identify in the oldest animals (Fig. 2). Cervical discs with osteophytes often revealed an extrusion of herniated disc material in osteophyte regions (Fig. 1). Representative morphologic images of all discs in a complete cervical spine section of an 18-month-old animal are shown (Fig. 3).
Fig. 2A–D.
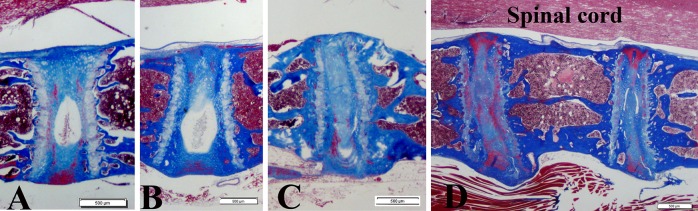
Representative midsagittal histologic images of cervical discs are shown from (A) a 3.9-month-old animal with normal morphologic features, (B) an 11.6-month-old animal with prominent wedging, (C) an 11.9-month-old animal with disc space narrowing, and (D) advanced disc narrowing and degeneration with osteophyte formation and possible disc herniation in the osteophyte body in an 18-month-old animal (Stain, Masson-trichrome; bars = 500 μm).
Fig. 3A–C.
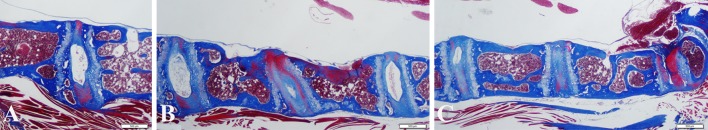
Histologic images are shown for (A) the C7-T1 level, (B) C6-C7, C5-C6, and C4-C-5 levels, (left to right, respectively), and (C) C3-C4, C2-C3, and C1-C2 levels (left to right, respectively) from an 18-month-old animal (Stain, Masson-trichrome; bar = 500 μm).
Discussion
There are few animal models of cervical spine degeneration. The objective of our study was to determine whether cervical disc degeneration differed from lumbar degeneration using radiologic and histologic analyses of the spine in the sand rat, a small animal model of age-related, spontaneous lumbar disc degeneration. Animals 2 to greater than 12 months old were studied. Radiologic and morphologic studies confirmed age-related disc degeneration in the cervical spine of the sand rat.
Challenges and potential experimental limitations of cervical spine studies in the sand rat were the small size of this spine segment in very young animals and the expert care that was needed when dissecting the highest cervical levels from the skull. Attention to detail also was needed when sectioning the paraffin blocks to obtain good midsagittal dissection for histologic examination. Careful positioning was needed to obtain good radiologic images without the presence of other overlapping bones. When there was poor imaging or bone overlap at a cervical or lumbar site, the respective feature for that site was not scored. Our study has numerous strengths, including analysis of cervical and lumbar sites in the same animal, and histologic analyses of morphologic features of the cervical spine, complementary to our digital radiologic imaging, with histologic views of the midsagittal cervical spine. The sand rat is a quadruped, thus it does not reproduce biomechanical forces as seen in the human biped. In addition, this cervical model would be difficult to use for therapeutic investigations such as stem cell injections or other therapeutic testing.
Radiologic documentation of the sand rat cervical spine showed disc space narrowing, wedging, irregular disc margins, and osteophyte formation—features which mimic documented clinical degenerative features present in the aging human cervical spine [5, 9]. Our data identified a significantly higher incidence of irregular disc margins in the cervical spines of younger animals compared with lumbar sites; this suggests that disc degeneration may be proceeding at different rates in cervical and lumbar sites and points to the need for future study of cervical disc changes in this interesting and important animal model.
Histologic studies complemented the radiologic features and showed cellular and tissue changes in the nucleus related to cervical disc space narrowing, prominent wedging, and osteophyte formation. Morphologic examination of cervical discs with osteophytes revealed the novel finding of the presence of extruded herniated disc material in osteophyte bodies.
Literature on the clinical disc contains relatively few studies that assessed the association between cervical and lumbar disc changes and progression in healthy subjects [14, 15, 19]. Cervical spines of asymptomatic healthy subjects examined by Boden et al. [2] exhibited abnormal MRI scans in 14% of subjects younger than 40 years; the percentage increased to 50% in subjects 50 years old. In general, clinical studies show that stenosis in cervical or lumbar sites would positively predict stenosis in other spine regions 15% to 32% of the time. Okada et al. [19] suggested that individual factors (possibly genetic) may stimulate degeneration in cervical and lumbar discs simultaneously. Okada et al. [19] also found that cervical spine disc degeneration was more prevalent in patients with lumbar disc herniation than in healthy subjects, with the suggestion of the importance of attention to cervical discs in patients with lumbar herniations.
The data presented here provide validation of an economic, small rodent model with spontaneous, age-related degeneration of the cervical spine. Cervical changes were assessed versus features of lumbar changes present in the same animal. A significantly higher incidence of irregular disc margins in the cervical spines of young animals was found versus lumbar sites; this suggests that disc degeneration may be proceeding at different rates in cervical and lumbar sites. Future sand rat studies designed to provide detailed quantitative analyses of bone features in micro-CT cervical spine models and immunohistochemical analyses of matrix features in cervical discs would be a useful contribution to the literature. Since the sand rat is a member of the gerbil family, lack of specific antibodies for extracellular matrix analyses may be continue to be challenging in future work.
Acknowledgments
We thank the Brooks-Carolinas Back Pain Research Endowment for general laboratory support, Natalia Zinchenko for assistance with digital radiographs, and Karen C. Fay, Juanita Jolly, Cliff Williams, and Kim Mihalko DVM, for expert animal care and management of our sand rat colony.
Footnotes
Authors (HEG and ENH) have received general laboratory support from the Brooks-Carolinas Back Pain Research Endowment (Charlotte, NC, USA).
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research editors and board members are on file with the publication and can be viewed on request.
Each author certifies that his or her institution approved the animal protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
This work was performed at Carolinas Medical Center, Charlotte, NC, USA.
References
- 1.Adler JH, Schoenbaum M, Silberberg R. Early onset of disk degeneration and spondylosis in sand rats (Psammomys obesus obesus) Vet Pathol. 1983;20:13–22. doi: 10.1177/030098588302000102. [DOI] [PubMed] [Google Scholar]
- 2.Boden SD, McCowin PR, Davis DO, Dina TS, Mark AS, Wiesel S. Abnormal magnetic-resonance scans of the cervical spine in asymptomatic subjects: a prospective investigation. J Bone Joint Surg Am. 1990;72:1178–1184. [PubMed] [Google Scholar]
- 3.Crisco JJ, Panjabi MM, Wang E, Price MA, Pelker RR. The injured canine cervical spine after six months of healing: an in vitro three-dimensional study. Spine. 1990;15:1047–1052. doi: 10.1097/00007632-199015100-00012. [DOI] [PubMed] [Google Scholar]
- 4.Elliott DM, Yerramalli CS, Beckstein JC, Boxberger JI, Johannessen W, Vresilovic EJ. The effect of relative needle diameter in puncture and sham injection animal models of degeneration. Spine. 2008;33:588–596. doi: 10.1097/BRS.0b013e318166e0a2. [DOI] [PubMed] [Google Scholar]
- 5.Fraser RD, Bleasel JF, Moskowitz RW. Spinal degeneration: Pathogenesis and Medical Management. In: Ducker TB, Hadler NM, Kostuik JP, Weinstein JN, Whitecloud TS, eds. The Adult Spine. Principles and Practices. 2nd ed. Philadelphia, PA: Lippincott-Raven; 1997:735–759.
- 6.Gore DR. Radiological evaluation of the degenerative cervical spine. In: Clark CR, editor. The Cervical Spine. 3. Philadelphia, PA: Lippincott-Raven; 1998. pp. 765–778. [Google Scholar]
- 7.Gore DR, Sepic SB. Anterior discectomy and fusion for pai(Phila Pa 1976)nful cervical disc disease: a report of 50 patients with an average follow-up of 21 years. Spine (Phila Pa 1976) 1998;23:2047–2051. doi: 10.1097/00007632-199810010-00002. [DOI] [PubMed] [Google Scholar]
- 8.Gruber HE, Ashraf N, Kilburn J, Williams C, Norton HJ, Gordon BE, Hanley EN., Jr Vertebral endplate architecture and vascularization: application of micro-computerized tomography, a vascular tracer, and immunocytochemistry in analyses of disc degeneration in the aging sand rat. Spine (Phila Pa 1976) 2005;30:2593–2600. doi: 10.1097/01.brs.0000187877.30149.83. [DOI] [PubMed] [Google Scholar]
- 9.Gruber HE, Johnson T, Norton HJ, Hanley EN., Jr The sand rat model for disc degeneration: radiologic characterization of age-related changes: cross-sectional and prospective analyses. Spine (Phila Pa 1976) 2002;27:230–234. doi: 10.1097/00007632-200202010-00004. [DOI] [PubMed] [Google Scholar]
- 10.Gruber HE, Johnson TL, Leslie K, Ingram JA, Martin D, Hoelscher G, Banks D, Phieffer L, Coldham G, Hanley EN., Jr Autologous intervertebral disc cell implantation: a model using Psammomys obesus, the sand rat. Spine (Phila Pa 1976. 2002;27:1626–1633. doi: 10.1097/00007632-200208010-00007. [DOI] [PubMed] [Google Scholar]
- 11.Kim KS, Yoon ST, Li J, Park JS, Hutton WC. Disc degeneration in the rabbit: a biochemical and radiological comparison between four disc injury models. Spine (Phila Pa 1976) 2005;30:33–37. doi: 10.1097/01.brs.0000174559.13749.83. [DOI] [PubMed] [Google Scholar]
- 12.Korecki CL, Costi JJ, Iatridis J. Needle puncture injury affects intervertebral disc mechanics and biology in an organ culture model. Spine (Phila Pa 1976) 2008;33:235–241. doi: 10.1097/BRS.0b013e3181624504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Krag M. Animal models for human disk degeneration. In: Weinstein JN, Gordon SL, editors. Low Back Pain: A Scientific and Clinical Overview. 1. Rosemont, IL: American Academy of Orthopaedic Surgeons; 1996. pp. 479–492. [Google Scholar]
- 14.Lee MJ, Garcia R, Cassinelli EH, Furey C, Riew KD. Tandem stenosis: a cadaveric study in osseous morphology. Spine J. 2008;8:1003–1006. doi: 10.1016/j.spinee.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 15.Master DL, Eubanks JD, Ahn NU. Prevalence of concurrent lumbar and cervical arthrosis: an anatomic study of cadaveric specimens. Spine (Phila Pa 1976). 2009;34:E272–E275. [DOI] [PubMed]
- 16.Matsuzaki H, Wakabayashi K. Animal models for reconstruction of vertebral column and intervertebral disc. In: An YH, Friedman RJ, editors. Animal Models in Orthopaedic Research. 1. Boca Raton, FL: CRC Press; 1999. pp. 539–547. [Google Scholar]
- 17.Moskowitz RW, Ziv I, Denko CW, Boja B, Jones PK, Adler JH. Spondylosis in sand rats: a model of intervertebral disc degeneration and hyperostosis. J Orthop Res. 1990;8:401–411. doi: 10.1002/jor.1100080312. [DOI] [PubMed] [Google Scholar]
- 18.Nassr A, Lee JT, Bashir RS, Rihn JA, Eck JC, Kang JD, Lim MR. Does incorrect level needle localization during anterior cervical discectomy and fusion lead to accelerated disc degeneration? Spine (Phila Pa 1976) 2009;34:189–192. doi: 10.1097/BRS.0b013e3181913872. [DOI] [PubMed] [Google Scholar]
- 19.Okada E, Matsumoto M, Fujiwara H, Toyama Y. Disc degeneration of cervical spine on MRI in pateints with lumbar disc herniation: comparison study with asymptomatic volunteers. Eur Spine J. 2011;20:585–591. doi: 10.1007/s00586-010-1644-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Panjabi MM. Cervical spine models for biomechanical research. Spine (Phila Pa 1976) 1998;23:2684–2699. doi: 10.1097/00007632-199812150-00007. [DOI] [PubMed] [Google Scholar]
- 21.Pintar FA, Maiman DJ, Hollowell JP, Yoganandan N, Droese KW, Reinartz JM, Cuddy B. Fusion rate and biomechanical stiffness of hydroxylapatite versus autogenous bone grafts for anterior discectomy: an in vivo animal study. Spine (Phila Pa 1976). 1994;19:2524–2528. [DOI] [PubMed]
- 22.Silberberg R. Histologic and morphometric observations on vertebral bone of aging sand rats. Spine (Phila Pa 1976). 1988;13:202–208. [DOI] [PubMed]
- 23.Silberberg R. The vertebral column of diabetic sand rats (Psammomys obesus obesus) Expt Cell Biol. 1988;56:217–220. doi: 10.1159/000163483. [DOI] [PubMed] [Google Scholar]
- 24.Silberberg R, Adler JH. Comparison of truncal and caudal lesions in the vertebral column of the sand rat (Psammomys obesus obesus) Isr J Med Sci. 1983;19:1064–1071. [PubMed] [Google Scholar]
- 25.Silberberg R, Aufdermaur M, Adler JH. Degeneration of the intervertebral disks and spondylosis in aging sand rats. Arch Pathol Lab Med. 1979;103:231–235. [PubMed] [Google Scholar]
- 26.Silberberg R, Meier-Ruge W, Odermatt B. Age-related changes in fibronectin in annulus fibrosus of the sand rat (Psammomys obesus obesus) Expl Cell Biol. 1989;57:233–237. doi: 10.1159/000163532. [DOI] [PubMed] [Google Scholar]
- 27.Sobajima S, Kompel JF, Kim JS, Wallach CJ, Robertson DD, Vogt MT, Kang JD, Gilbertson LG. (2005) A slowly progressive and reproducible animal model of intervertebral disc degeneration characterized by MRI, X-ray, and histology. Spine (Phila Pa 1976) 2005;30:15–24. doi: 10.1097/00007632-200511150-00023. [DOI] [PubMed] [Google Scholar]
- 28.Wilson C, Brown D, Najarian K, Hanley EN, Jr, Gruber HE. Computer aided vertebral visualization and analysis: a methodology using the sand rat, a small animal model of disc degeneration. BMC Musculoskelet Disord. 2003;4:4. doi: 10.1186/1471-2474-4-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yingling VR, Callaghan JP, McGill SM. The porcine cervical spine as a model of the human lumbar spine: an anatomical, geometric, and functional comparison. J Spinal Disord. 1999;12:415–423. doi: 10.1097/00002517-199912050-00012. [DOI] [PubMed] [Google Scholar]
- 30.Zdeblick TA, Abitbol J-J, Kunz DN, McCabe RP, Garfin S. Cervical stability after sequential capsule resection. Spine (Phila Pa 1976) 1993;18:2005–2008. doi: 10.1097/00007632-199310001-00013. [DOI] [PubMed] [Google Scholar]
- 31.Ziran BH, Pineda S, Pokharna H, Esteki A, Mansour JM, Moskowitz RW. Biomechanical, radiologic, and histopathologic correlations in the pathogenesis of experimental intervertebral disc disease. Spine (Phila Pa 1976) 1994;19:2159–2163. doi: 10.1097/00007632-199410000-00005. [DOI] [PubMed] [Google Scholar]
- 32.Ziv I, Moskowitz RW, Kraise I, Adler JH, Maroudas A. Physicochemical properties of the aging and diabetic sand rat intervertebral disc. J Orthop Res. 1992;10:205–210. doi: 10.1002/jor.1100100207. [DOI] [PubMed] [Google Scholar]


