Abstract
Background
Opioid pain medications are the basis for analgesia after orthopaedic injuries and procedures. However, opioids have many adverse effects, including opioid-induced androgen deficiency.
Questions/purposes
We evaluated the occurrence and affect of opioid-induced androgen deficiency on osseous union and its ability to be reversed. Additional focus was placed on its perioperative onset and its duration in an orthopaedic animal model.
Methods
A femoral osteotomy was created in 75 Sprague-Dawley rats. Postoperatively, animals were randomized into three treatment groups: control, morphine, and morphine plus testosterone. Testosterone levels were recorded preoperatively and at 48 hours, 4 weeks, and 8 weeks postoperatively. Some animals were euthanized at 4 weeks and others at 8 weeks postoperatively. Histology and micro-CT scans were used to evaluate callus. Three-point bend testing was performed to evaluate callus strength.
Results
Serum testosterone levels in the morphine group showed decreased baseline levels of 2.2 ng/mL, 1.4 ng/mL, and 1.4 ng/mL (p < 0.001), whereas the morphine plus testosterone supplementation group showed increased serum levels at 41.7 ng/mL, 11.8 ng/mL, and 19.8 ng/mL (p < 0.001) compared with control animals (3.3 ng/mL, 5.8 ng/mL, 5.2 ng/mL) at 48 hours, 4 weeks, and 8 weeks, respectively. Compared with control animals, histology and micro-CT showed an impedance of callus maturation in the two experimental groups. Morphine-treated animals showed reduction in callus strength at 8 weeks (30% of the contralateral unfractured femur strength compared with 49% seen in the control animals at 8 weeks; p = 0.048); this finding was not fully reversed by testosterone supplementation (33% of the contralateral femur strength; p = 0.171).
Conclusions
Opioid-induced androgen deficiency occurred in this orthopaedic animal model. Although we previously showed that morphine inhibits callus maturation, the current study did not show a rescue of the morphine-treated animals with testosterone supplementation in either morphologic or mechanical testing. Testosterone suppression associated with opioid administration occurred almost immediately (within 48 hours) and was suppressed continually throughout the 8-week duration of study.
Clinical Relevance
Opioid-induced androgen deficiency occurs during the perioperative orthopaedic period. Although its clinical relevance remains unknown, further evaluation is needed to determine if supplementation is warranted during the perioperative period.
Introduction
The current mainstay of analgesics administered with orthopaedic injuries and postoperative orthopaedic pain control is opioid pain medications. Greater than 80% of orthopaedic patients receive opioid medications at some point in their care [3, 20]. Unfortunately, opioid pain medications have side effects; those commonly cited include sedation and altered mentation, decreased respiratory drive, constipation, nausea, vomiting, and addiction. Opioid pain medications and their associated side effects have been shown to increase hospital cost and length of stay after orthopaedic care [23, 24]. One adverse finding seldom mentioned in the acute setting is opioid-induced androgen deficiency. Although well studied in patients receiving chronic opioids in which it increases the risk of fractures [15, 27, 29] and decreases bone mineral density [14, 18], few studies have evaluated the acute onset of androgen suppression in the postoperative setting [1, 9]. Furthermore, to our knowledge, this has not been reproduced in an orthopaedic setting with postoperative doses and durations often seen in the orthopaedic clinical setting. Daniell et al. [11] studied testosterone supplementation to mitigate some of the adverse effects associated with opioid-induced androgen deficiency in patients receiving opioids on a chronic basis. They found an improvement in function, mood, hematocrit, and an overall improvement in androgen deficiency symptoms during supplementation. To our knowledge, this type of supplementation study has not been conducted in the orthopaedic acute perioperative setting.
Findings that morphine administration inhibits fracture healing by inhibiting callus maturation in an animal model have been reported [10]. To our knowledge, the effect of opioid-induced hypogonadism on osseous healing in the acute fracture setting has not been examined. The literature shows that physiologic and supraphysiologic testosterone levels support osseous strength and healing and supplemental testosterone may improve fracture healing, particularly in hypogonadal models [5, 13, 16, 19, 21, 22, 28, 31, 32]. The testosterone suppression seen with opioid-induced androgen deficiency may be the driving mechanism behind fracture callus suppression seen with opioid administration.
The purpose of this animal-based fracture model is to address whether opioid-induced androgen deficiency occurs and its affect in an acute perioperative orthopaedic setting. Using micro-CT imaging and histology we attempted to answer whether testosterone suppression is a mechanism driving the morphologic inhibition of callus maturation and whether exogenous testosterone supplementation will reverse the inhibition and restore the biomechanical strength of the postoperative callus in the perioperative setting. Finally, we wished to determine when the onset of androgen suppression occurs and if the duration of suppression would last the 8 weeks of this study in our animal model.
Materials and Methods
Study Design
Seventy-five adult male Sprague-Dawley rats (Harlan® Laboratories, Indianapolis, IN, USA) weighing 300 g were used in this study. The study groups consisted of a subset of animals previously evaluated [10]; an additional group of animals was included in this study to specifically evaluate opioid-induced androgen deficiency and its associated osseous findings. Institutional Animal Care and Use Committee approval was obtained before study initiation. Animals were housed in groups of two to three per cage with standard 12-hour light and dark cycles observed, with ad libitum food and water consumption.
One surgeon (JC) performed all surgeries. Inhaled isoflurane anesthesia was administered per a standard rat anesthesia protocol with an induction rate of 3% to 5% and a maintenance rate of 1% to 3%. A lateral approach to the femur, with care taken to minimize disruption of the periosteum, was performed in each animal. An osteotomy was created and stabilized with a 1.5-mm nonlocking plate (Synthes, West Chester, PA, USA) using previously described methods [26]. A Stryker TPS system and Stryker Precision™ Thin blade (Stryker, Kalamazoo, MI, USA) were used to create the diaphyseal osteotomy creating an osteotomy gap of 0.38 mm, which was the kerf of the blade. The wound was closed in layers with 3.0 Monocryl® (Ethicon, Somerville, NJ, USA) and the skin closed with staples. During the procedure, 200 μL of blood was obtained for testosterone ELISA evaluation. After the procedure, the animals were transferred to a recovery area where they were monitored and allowed to awaken from anesthesia. Animal randomization occurred immediately postoperatively with the animals receiving their randomly assigned medications within the first 5 minutes of awakening from anesthesia.
There were no differences among the preoperative animal age, weight, or baseline serum testosterone levels at the time of randomization. One mortality occurred in the control group during the first 48 hours postoperatively. No other unintentional animal mortalities, wound complications, infections, or injection site complications were identified throughout the remainder of the experiment. The morphine-treated animals did not show any subjectively noticeable signs of sedation.
The 75 animals were assigned equally to one of three study groups. All animals received two doses of postoperative acetaminophen (300 mg/kg) administered 12 hours apart by oral gavage. The control group received 0.9% subcutaneous saline injections every 8 hours (100 μL/kg). The morphine group received subcutaneous morphine injections (Hospira Inc, Lake Forest, IL, USA) (5 mg/100 μL/kg) every 8 hours. This dose is the recommended postoperative analgesia dose for rats [12], not a supratherapeutic dosing regimen. The morphine plus supplemental testosterone group received the same subcutaneous morphine injections (5 mg/100 μL/kg) every 8 hours plus subcutaneous testosterone enanthate (Hikma Farmaceutica, Terrugem, Portugal) (50 mg/100 μL/kg) once every 2 weeks. These dosing regimens were continued throughout the duration of the experiment. The animals were all returned to their cages and allowed to mobilize ad libitum. Animals were weighed on a weekly basis and medications adjusted to compensate for alterations in weight.
Forty-eight hours postoperatively, 200 μL of blood was obtained for postoperative testosterone analysis and quantified by ELISA (R&D systems ELISA-KGE010, Minneapolis, MN, USA). Four weeks postoperatively, blood again was obtained from all animals and analyzed. At 4 weeks, 13 animals from each experimental group were randomly selected and euthanized, 12 from the control group to account for the postoperative mortality. At 8 weeks postoperatively the remaining 36 animals had blood drawn and were euthanized. Immediately postmortem, the bilateral femurs were disarticulated and dissected. The femurs were stripped of muscle with care taken not to disrupt periosteal callus formation. The plate was removed from the surgically treated femurs.
Serum testosterone was quantitated by ELISA as per the manufacturer’s instructions. Blood was obtained 2 hours after morphine injection to standardize postinjection testosterone levels. All samples were run in duplicate with averages used for data analysis. The data were analyzed with SoftMax® Pro 5 software (Molecular Devices, Sunnyvale, CA, USA) and a four-parameter logistic curve was used for data analysis.
The femurs underwent three-point load to failure biomechanical testing using an Instron® 8500 closed-loop servohydraulic testing machine (Instron®, Norwood, MA, USA). Cylindrical supports were placed 25 mm apart with a central loading nose directed at the osteotomy site to obtain measurements with a displacement rate of 0.1 mm/second. The specimins were kept moist with 0.9% saline and were placed horizontally with their posterior surface resting on the supports. The operative and nonoperative femurs underwent three-point load-to-failure biomechanical testing. A ratio of operative femur fracture strength to nonoperative femur fracture strength was obtained to calculate percent strength ratio to account for variables among animals. Three-point bend testing of the femurs resulted in fractures that propagated through the healing osteotomy site in all cases; there were no fractures through the screw holes of the experimental femurs. Before 3-point bend testing, micro-CT was performed using a Siemens InveonTM System micro-CT scanner (Siemens Preclinical, Knoxville, TN, USA) with a 35-μm voxel size. The micro-CT data were analyzed using Amira® software (Visage Imaging® Amira® 5.4.1; Visualization Sciences Group, Merignac, France). Callus volume was calculated with surface area three-dimensional reconstruction and quantification using Amira® software. Callus formation was quantified as intramedullary callus formation and periosteal callus formation.
Histologic studies were performed on the osteotomized femurs after three-point bend testing. The femurs were fixed in a buffered 10% formalin solution, decalcified, and embedded in a paraffin block. Serial cross-sectional cuts were taken including and adjacent to the osteotomy site. Cuts were stained with hematoxylin and eosin or trichrome stain. Two observers (JC, CS) qualitatively evaluated postmortem histologic sections in a blinded fashion. The sections were evaluated at 5x and 100x magnification to evaluate gross levels of healing, remodeling, and osteotomy consolidation. We used ImageJ software (National Institutes of Health, Bethesda, MD, USA) [25] to outline and quantify the area of immature healing callus.
Statistical Analysis
Sample size was based on previous literature to achieve a desired alpha level of 0.05 and an 80% power of detecting a 5% difference in callus maturity [8]. Brown et al. [8] showed a power analysis of 19 animals would be needed per group. We used 25 animals per group to account for potential perioperative and postoperative animal loss. Two-tailed Student’s t-tests were conducted individually comparing each experimental group with controls. T-tests were conducted comparing serum testosterone levels, three-point bend strength ratio, and callus volume. The two experimental groups were analyzed against the control group. Calculations were performed with STATA® 12.1 (StataCorp LP, College Station, TX, USA).
Results
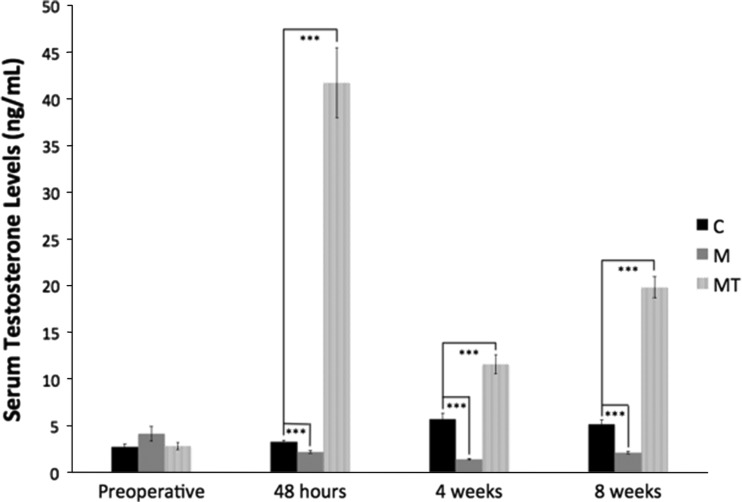
Opioid-induced androgen deficiency occurred in the rats during the perioperative period. In the morphine group, morphine administration resulted in a decrease in serum testosterone 48 hours postoperatively (3.3 ng/mL versus 2.2 ng/mL; p < 0.001), which was sustained with decreased levels at 4 weeks postoperatively (5.8 ng/mL versus 1.4 ng/mL; p < 0.001) and 8 weeks postoperatively (5.2 ng/mL versus 1.4 ng/mL; p < 0.001) compared with control animals. The morphine plus testosterone supplementation group had an increase in serum testosterone at all postoperative times (3.2 ng/mL versus 41.7 ng/mL at 48 hours, 5.8 ng/mL versus 11.8 ng/mL at 4 weeks, and 5.2 ng/mL versus 19.8 ng/mL at 8 weeks; p < 0.001) when compared with control animals (Fig. 1).
Fig. 1.
The mean serum testosterone levels (ng/mL) ± standard error measured preoperatively, 48 hours postoperatively, 4 weeks postoperatively, and 8 weeks postoperatively are shown. *** p < 0.001. C = control; M = morphine; MT = morphine + testosterone.
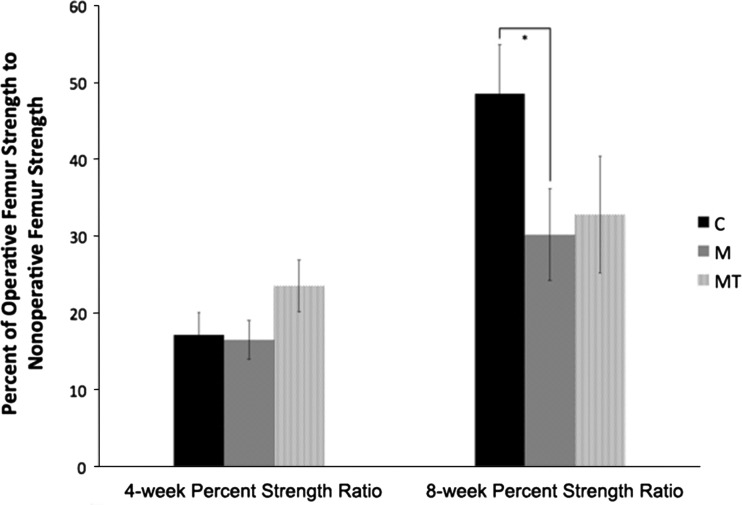
Androgen suppression does not appear to be the primary mechanism inhibiting callus strength; testosterone supplementation resulted in a negligible rectification of the inhibition seen with opioid administration. With a subset of the animals involved in this study, we previously observed morphine inhibits callus strength at 8 weeks [10]. At 4 weeks postoperatively, there was no difference comparing the control with morphine animals (17% and 16% respectively; p = 0.868) [10]. There was a trend toward increased callus strength of the morphine plus testosterone supplementation group, 24% of contralateral femur strength compared with the control group at 17% (p = 0.171). At 8 weeks postoperatively there was an expected substantial increase in callus fracture strength in the control group with continued callus maturation. This was less dramatic in both experimental groups. Comparing the control and morphine groups, there was a significant (p = 0.048) difference in final callus strength. The control animals’ operative femur strength increased to 49% of the contralateral femur compared with only 30% for the morphine-only treated animals. There was no difference at 4 weeks postoperatively in the morphine plus testosterone supplementation group (p = 0.171). Although significance was not attained, a trend for a weaker callus was observed at 8 weeks (p = 0.127) with the morphine plus testosterone supplementation group only summating to 33% of the contralateral femur strength compared with the control group at 49% (Fig. 2).
Fig. 2.
The mean ratios (± standard error) of operative femur strength to nonoperative femur strength at 4 weeks and 8 weeks postoperatively using a three-point load to failure test are shown. * p < 0.05. C = control; M = morphine; MT = morphine + testosterone.
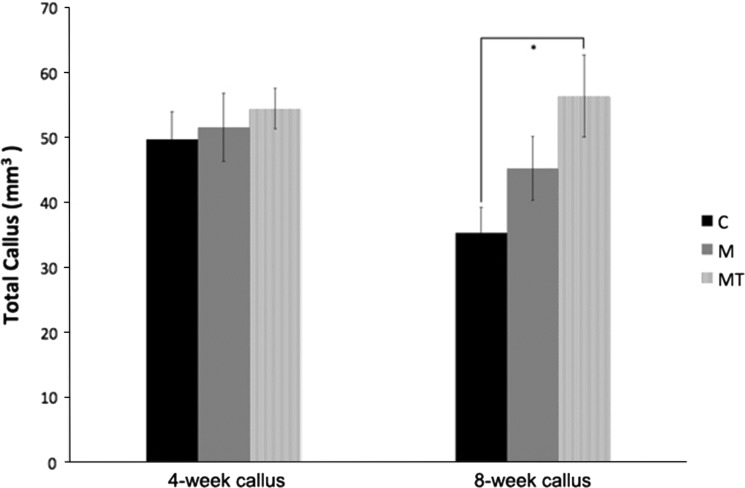
Similar to what was seen with the callus strength, although the androgen suppression occurred at all times testosterone supplementation did not appear to influence the gross callus maturity with either imaging or histology. No differences were identified in the 4-week volumetric analysis obtained through micro-CT for callus formation (49.7 mm3 versus 51.7 mm3 versus 54.4 mm3) for the control, morphine, or morphine plus testosterone supplementation groups, respectively. At 8 weeks postoperatively, there was a decrease in callus volume in the control animals, which is consistent with the expected maturation, consolidation, and remodeling of the fracture callus. Comparing the 4-week and 8-week times, the control animals had a callus reduction of 29% (p = 0.023). This amount of remodeling and callus consolidation was not seen in the animals in the morphine only group decreasing only 13% (p = 0.393); and there was a net increase in callus in the morphine plus testosterone supplementation group of only approximately 4% (p = 0.794) (Fig. 3). The net gain in callus yielded a statistical significance when the morphine plus testosterone supplementation 8-week callus was compared with the 8-week control callus (p = 0.011). Qualitative evaluation of histology sections revealed findings consistent with callus retardation in the morphine only group with negligible rescue seen in the morphine plus testosterone supplementation group (Fig. 4). Quantitative evaluation of immature callus area mirrored the volumetric data of the micro-CT. The 4-week evaluation again showed minimal difference among the groups with all being assessed as primarily immature callus with equivalent levels of fibrous tissues and areas of woven bone. The immature callus area was similar among groups and were 10.6 mm2, 9.5 mm2, and 11.6 mm2 for the control, morphine-only, and morphine plus testosterone groups respectively. At 8 weeks postoperatively, there were differences with remodeling, consolidation, and evidence of lamellar bone identified in the control group samples. The morphine only and morphine plus testosterone supplementation groups remained immature in appearance with lack of remodeling compared with the control group. The quantitative data again mirrored the micro-CT data showing a decrease in control animals to 8.2 mm2, whereas the morphine only and morphine plus testosterone groups showed no reduction of the immature callus area at 10.4 mm2 and 12.0 mm2 respectively.
Fig. 3.
The quantified micro-CT data shows the mean total callus (± standard error) at 4 weeks and 8 weeks as quantitated by Amira® software. * p < 0.05, ** p < 0.01. C = control; M = morphine; MT = morphine + testosterone.
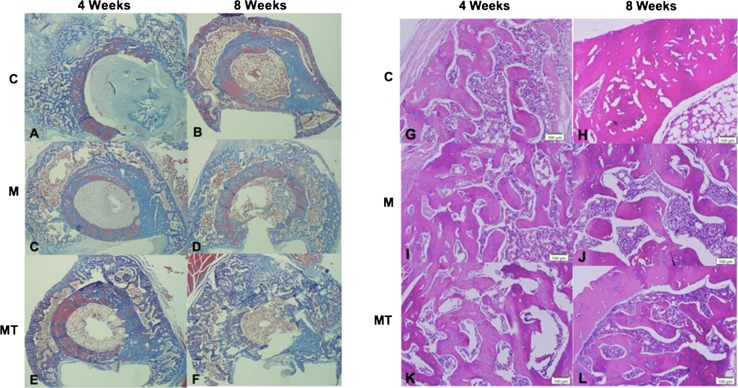
Fig. 4A–L.
Cross-sectional cuts directly adjacent to the osteotomy site at 4 weeks and 8 weeks postoperatively in the control group, the morphine treated animals and the morphine plus testosterone treated animals are shown. (A) Four weeks after osteotomy in the control animals, immature callus with areas of woven bone and fibrous tissues can be seen. (B) Eight weeks after osteotomy in the control animals, there is evidence of callus consolidation and osseous remodeling. (C) In the morphine only treated animals at 4 weeks after osteotomy, there was a similar appearance to that of the control animals at 4 weeks. There is evidence of immature callus with abundance of woven bone and fibrous tissues. (D) Animals treated with morphine only at 8 weeks after osteotomy showed limited maturation with persistence of immature appearing callus and persistence of woven bone when compared with at 4 weeks; however, there appears to be less maturation than in control animals at 8 weeks with less consolidation and remodeling of the callus. (E) In animals treated with morphine plus supplemental testosterone, this 4-week postoperative image shows an immature appearing callus similar in appearance to the that of the 4 week control and morphine only treated animals. There is a slight increase in trabecular thickness in these testosterone-supplemented animals compared with the control and morphine only groups. (F) At 8 weeks after osteotomy, persistence of immature callus with limited consolidation and remodeling and more immature appearing callus was seen in animals in the morphine plus supplemental testosterone group than for the control animals (Illustrations A–F: Stain, Trichrome; original magnification, ×5). (G) Four weeks after osteotomy, the histologic image shows immature callus with areas of immature woven bone and fibrous tissues in the control animals. (H) Eight weeks after the osteotomy, the control animals showed evidence of lamellar bone with callus remodeling and maturation. (I) A similar appearance was seen between the morphine only treated animals and the control animals at 4 weeks. There was evidence of immature callus with an abundance of woven bone and fibrous tissues, which appear similar to 4 week control animals. (J) Animals treated with morphine had limited maturation with persistence of immature appearing callus and persistence of woven bone when compared with the morphine treated animals at 4 weeks. Less maturation is evident in this image from a morphine treated animal than in the image from a control animal at 8 weeks with less consolidation and remodeling of the callus. (K) Four weeks postoperative, the image from an animal treated with morphine plus supplemental testosterone shows an immature appearing callous similar in appearance to those for the 4 week control and morphine only treated animals. There is a slight increase in trabecular thickness in these testosterone-supplemented animals compared with the control and morphine only treated groups. (L) For the morphine plus supplemental testosterone animals at 8 weeks after osteotomy, persistence of immature callus with limited consolidation and remodeling, and more immature in appearance then the control animals 8 weeks postoperatively can be seen (Illustrations G–L: Stain, Hematoxylin and eosin; original magnification, ×100).
Discussion
Testosterone is important in skeletal maintenance and skeletal repair through direct and indirect pathways via androgen receptor activation, testosterone conversion to estrogen, and its conversion to dihydrotestosterone. Opioid-induced androgen deficiency suppresses this important hormone during the critical osseous healing phase, and may be a potential modifiable risk factor for fracture healing in the orthopaedic setting. This iatrogenic testosterone suppression has been evaluated in patients receiving chronic opioids with recommendations of testosterone supplementation to limit its detrimental long-term effects (including osteoporosis and increased fracture risk); however, the acute consequences during the orthopaedic perioperative period has not been evaluated to our knowledge. The purpose of this animal-based fracture model was to address whether opioid-induced androgen deficiency occurs, its affect in an acute perioperative orthopaedic setting, and the ability to reverse these effects with supplemental testosterone.
There are several potential limitations to this study. First, we used an animal model. Additional clinically based studies will be needed to verify if our results can be correlated to clinical practice. An animal model was chosen to limit testosterone variability among subjects, standardize morphine administration for a predetermined time, and to simplify the model by having a placebo control group to compare with a treatment group and a “rescue” testosterone supplementation group in an orthopaedic setting. The duration of 8 weeks on a postoperative opioid dosing regimen is not a clinical standard of care but was used for protocol standardization. Second, the testosterone-supplemented animals attained supratherapeutic levels, which may offer an additional variable regarding why there was a lack of recovery in the supplemented group. Conversely, a previous study in which supraphysiologic testosterone enanthate dosing was investigated showed there was an appropriate rescue effect of supplementation in gonadectomized male and female rats [32]. Finally, our study may have been underpowered to show a statistical difference of the biomechanical strength of the testosterone-supplemented animals compared with the control animals, despite the trend in that direction.
Opioid-induced androgen deficiency occurred in the orthopaedic animal model we used. The animals showed a near-immediate decrease in serum testosterone levels after administration of morphine. This outcome was sustained throughout the 8-week investigational period. Previous studies have shown that synthetic alternatives such as oxycodone, methadone, fentanyl, and tramadol exhibit this hypothalamic-pituitary-gonadal suppression in animals [9] and clinically in humans [11]. The acute, subacute, and long-term effects of this observed suppression need to be clinically evaluated.
Supplemental testosterone did correct the hypogonadism seen in this orthopaedic opioid-induced androgen deficiency animal model, however it did not appear to reverse the deleterious effects of opioids on the healing fracture animal model. Previous studies have shown that testosterone supplementation improved many of the adverse effects of hypogonadism in chronic opioid users, which included reversing trabecular bone loss and increasing bone mineral density [6, 11]. In our animal study, testosterone supplementation to supratherapeutic levels did not seem to rescue the callus inhibition seen with opioid administration despite another supratherapeutic supplementation study showing positive results in hypogonadal models [32]. Brinker et al. [7] identified 84% of selected nonunions to be associated with metabolic or endocrine abnormalities. Of particular interest, 40% of the male patients with nonunions with laboratory abnormalities were identified to have low testosterone. Brinker et al. [7] did not discuss whether the patients with low testosterone were still using opioid medications; however, with a 40% prevalence of hypogonadism in the patients with nonunions, and the likelihood of patients with nonunions taking opioid pain medications, additional investigation is warranted.
There may be other potential explanations for the lack of recovery in the exogenous testosterone-supplemented animals. Aloisi et al. [2] reported that opioids alter the gene expression of aromatase and 5-alpha reductase; both are enzymes found in the bone and peripheral tissues, which modify testosterone to estrogens and dihydrotestosterone, respectively. These substrates are potent hormones that have receptors in bone and are involved in bone maintenance and metabolism, suggesting perhaps the byproducts of testosterone and not merely testosterone alone are responsible for these findings. Alternatively, the delayed maturation and associated callus weakness we identified may be independent of the observed testosterone suppression. Callus inhibition may be a direct action of opioids on the bone, the opioid effect on the brain, and the neuropeptide Y pathway expression [4] or perhaps a separate endocrine mechanism because opioids act on virtually every branch of the hypothalamic-pituitary-peripheral gland axis [30].
Given the pervasiveness of opioid analgesic use in the orthopaedic patient population, continued evaluation of the adverse effects of these medications in the orthopaedic setting is warranted. The long-term orthopaedic consequences of chronic opioid use are decreased bone mineral density [14] and an increased fracture risk [29], but the more acute and subacute effects in the orthopaedic setting have not been thoroughly studied despite their substantial use in this population. Opioid administration exceeds 80% around the time of orthopaedic injury [20] and up to 20% of patients with orthopaedic trauma continue receiving oral opioids for greater than 3 months after injury [17]. Opioid-induced androgen deficiency often is overlooked in the acute setting and is relatively unknown in orthopaedic practice. Opioid-induced androgen deficiency occurs within the first 24 to 48 hours after administration of narcotic pain medications, and the current study reproduces previous observations but in a postoperative setting. The correlation between morphine administration and callus maturation inhibition was reported [10]. In the current study, we evaluated opioid-induced androgen deficiency as being the inciting mechanism for impeding callus development and its ability to be reversed with testosterone supplementation. Sole testosterone supplementation to supratherapeutic levels did not appear to rescue the animals in the morphine plus supplemental testosterone treatment group in either callus strength or callus maturation despite elevating serum testosterone levels. Opioid-induced androgen deficiency is an underdiagnosed adverse effect associated with opioid administration during the perioperative period that warrants further investigation.
Acknowledgments
We thank Kent Bachus PhD for guidance developing this animal model and biomechanical testing in this study and Saranne Cook BS for technical support throughout this experiment.
Footnotes
Funding for this study was received from the Orthopaedic Research and Education Foundation’s (OREF)/Synthes Resident Research Project Grant and a departmental grant from the Sherman S. Coleman Resident Research Fund (JC, TFH). One of the authors (KBJ) receives career development support from the National Cancer Institute K08CA138764. Synthes Research Project Support donated surgical supplies/instruments.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research editors and board members are on file with the publication and can be viewed on request.
Clinical Orthopaedics and Related Research neither advocates nor endorses the use of any treatment, drug, or device. Readers are encouraged to always seek additional information, including FDA-approval status, of any drug or device prior to clinical use.
Each author certifies that his or her institution approved the animal protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
References
- 1.Aloisi AM, Aurilio C, Bachiocco V, Biasi G, Fiorenzani P, Pace MC, Paci V, Pari G, Passavanti G, Ravaioli L, Sindaco G, Vellucci R, Ceccarelli I. Endocrine consequences of opioid therapy. Psychoneuroendocrinology. 2009;34(suppl 1):S162–S168. doi: 10.1016/j.psyneuen.2009.05.013. [DOI] [PubMed] [Google Scholar]
- 2.Danielli B, Rovini M, Cappelli A, Anzini M, Giordano A. Aromatase and 5-alpha reductase gene expression: modulation by pain and morphine treatment in male rats. Mol Pain. 2010;6:69. doi: 10.1186/1744-8069-6-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Antonova E, Le TK, Burge R, Mershon J. Tibia shaft fractures: costly burden of nonunions. BMC Musculoskelet Disord. 2013;14:42. doi: 10.1186/1471-2474-14-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baldock PA, Driessler F, Lin S, Wong IP, Shi Y, Yulyaningsih E, Castillo L, Janmaat S, Enriquez RF, Zengin A, Kieffer BL, Schwarzer C, Eisman JA, Sainsbury A, Herzog H. The endogenous opioid dynorphin is required for normal bone homeostasis in mice. Neuropeptides. 2012;46:383–394. doi: 10.1016/j.npep.2012.08.010. [DOI] [PubMed] [Google Scholar]
- 5.Benghuzzi H, Tucci M, Tsao A, Russell G, England B, Ragab A. Stimulation of osteogenesis by means of sustained delivery of various natural androgenic hormones. Biomed Sci Instrum. 2004;40:99–104. [PubMed] [Google Scholar]
- 6.Benito M, Vasilic B, Wehrli FW, Bunker B, Wald M, Gomberg B, Wright AC, Zemel B, Cucchiara A, Snyder PJ. Effect of testosterone replacement on trabecular architecture in hypogonadal men. J Bone Miner Res. 2005;20:1785–1791. doi: 10.1359/JBMR.050606. [DOI] [PubMed] [Google Scholar]
- 7.Brinker MR, O’Connor DP, Monla YT, Earthman TP. Metabolic and endocrine abnormalities in patients with nonunions. J Orthop Trauma. 2007;21:557–570. doi: 10.1097/BOT.0b013e31814d4dc6. [DOI] [PubMed] [Google Scholar]
- 8.Brown KM, Saunders MM, Kirsch T, Donahue HJ, Reid JS. Effect of COX-2-specific inhibition on fracture-healing in the rat femur. J Bone Joint Surg Am. 2004;86:116–123. doi: 10.2106/00004623-200401000-00017. [DOI] [PubMed] [Google Scholar]
- 9.Ceccarelli I, De Padova AM, Fiorenzani P, Massafra C, Aloisi AM. Single opioid administration modifies gonadal steroids in both the CNS and plasma of male rats. Neuroscience. 2006;140:929–937. doi: 10.1016/j.neuroscience.2006.02.044. [DOI] [PubMed] [Google Scholar]
- 10.Chrastil J, Sampson C, Jones KB, Higgins TF. Postoperative opioid administration inhibits bone healing in an animal model. Clin Orthop Relat Res. 2013;471:4076–4081. doi: 10.1007/s11999-013-3232-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Daniell HW, Lentz R, Mazer NA. Open-label pilot study of testosterone patch therapy in men with opioid-induced androgen deficiency. JPain. 2006;7:200–210. doi: 10.1016/j.jpain.2005.10.009. [DOI] [PubMed] [Google Scholar]
- 12.Flecknell PA. Post-operative analgesia in rabbits and rodents. Lab Anim. 1991;20:34–37. [Google Scholar]
- 13.Frankle M, Borrelli J. The effects of testosterone propionate and methenolone enanthate on the healing of humeral osteotomies in the Wistar rat. J Invest Surg. 1990;3:93–113. doi: 10.3109/08941939009140340. [DOI] [PubMed] [Google Scholar]
- 14.Grey A, Rix-Trott K, Horne A, Gamble G, Bolland M, Reid IR. Decreased bone density in men on methadone maintenance therapy. Addiction. 2011;106:349–354. doi: 10.1111/j.1360-0443.2010.03159.x. [DOI] [PubMed] [Google Scholar]
- 15.Guo Z, Wills P, Viitanen M, Fastbom J, Winblad B. Cognitive impairment, drug use, and the risk of hip fracture in persons over 75 years old: a community-based prospective study. Am J Epidemiol. 1998;148:887–892. doi: 10.1093/oxfordjournals.aje.a009714. [DOI] [PubMed] [Google Scholar]
- 16.Gupta LP, Udupa KN. Studies on the effect of combined therapy of anabolic hormone and ascorbic acid in the treatment of fractures. Indian J Med Res. 1966;54:542–550. [PubMed] [Google Scholar]
- 17.Holman JE, Stoddard GJ, Higgins TF. Rates of prescription opiate use before and after injury in patients with orthopaedic trauma and the risk factors for prolonged opiate use. J Bone Joint Surg Am. 2013;95:1075–1080. doi: 10.2106/JBJS.L.00619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim TW, Alford DP, Malabanan A, Holick MF, Samet JH. Low bone density in patients receiving methadone maintenance treatment. Drug Alcohol Depend. 2006;85:258–262. doi: 10.1016/j.drugalcdep.2006.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koskinen EV. The influence of hormonal treatment and orchiectomy, oophorectomy and thyroidectomy on experimental fractures: a quantitative P32-autoradiographic, roentgenologic and tissue-analytic study. Acta Orthop Scand Suppl. 1965:Suppl 80:1–40. [DOI] [PubMed]
- 20.Lindenhovius AL, Helmerhorst GT, Schnellen AC, Vrahas M, Ring D, Kloen P. Differences in prescription of narcotic pain medication after operative treatment of hip and ankle fractures in the United States and The Netherlands. J Trauma. 2009;67:160–164. doi: 10.1097/TA.0b013e31818c12ee. [DOI] [PubMed] [Google Scholar]
- 21.Maus U, Andereya S, Schmidt H, Zombory G, Gravius S, Ohnsorge JA, Niedhart C. [Therapy effects of testosterone on the recovery of bone defects][ in German] Z Orthop Unfall. 2008;146:59–63. doi: 10.1055/s-2007-989436. [DOI] [PubMed] [Google Scholar]
- 22.Monteleone M, Albo G, Papalia M, Rispoli R, Scafidi G, Vernale C. Effect of the administration of an anabolic drug on the evolution of the bone callus after diaphyseal osteotomy of rabbit femur. Acta Orthop Belg. 1977;43:5–18. [PubMed] [Google Scholar]
- 23.Oderda GM, Said Q, Evans RS, Stoddard GJ, Lloyd J, Jackson K, Rublee D, Samore MH. Opioid-related adverse drug events in surgical hospitalizations: impact on costs and length of stay. Ann Pharmacother. 2007;41:400–406. doi: 10.1345/aph.1H386. [DOI] [PubMed] [Google Scholar]
- 24.Pizzi LT, Toner R, Foley K, Thomson E, Chow W, Kim M, Couto J, Royo M, Viscusi E. Relationship between potential opioid-related adverse effects and hospital length of stay in patients receiving opioids after orthopedic surgery. Pharmacotherapy. 2012;32:502–514. doi: 10.1002/j.1875-9114.2012.01101.x. [DOI] [PubMed] [Google Scholar]
- 25.Rasband WS. ImageJ. Bethesda, MD: US National Institutes of Health; 1997.
- 26.Schmidhammer R, Zandieh S, Mittermayr R, Pelinka LE, Leixnering M, Hopf R, Kroepfl A, Redl H. Assessment of bone union/5onunion in an experimental model using microcomputed technology. J Trauma. 2006;61:199–205. doi: 10.1097/01.ta.0000195987.57939.7e. [DOI] [PubMed] [Google Scholar]
- 27.Shorr RI, Griffin MR, Daugherty JR, Ray WA. Opioid analgesics and the risk of hip fracture in the elderly: codeine and propoxyphene. J Gerontol. 1992;47:M111–M1115. doi: 10.1093/geronj/47.4.M111. [DOI] [PubMed] [Google Scholar]
- 28.Tarsoly E, Jánossy J, Kosztura L. Effect of testosterone on fracture healing in hypophysectomized rats. Acta Histochem. 1979;65:25–33. doi: 10.1016/S0065-1281(79)80029-X. [DOI] [PubMed] [Google Scholar]
- 29.Vestergaard P, Rejnmark L, Mosekilde L. Fracture risk associated with the use of morphine and opiates. J Intern Med. 2006;260:76–87. doi: 10.1111/j.1365-2796.2006.01667.x. [DOI] [PubMed] [Google Scholar]
- 30.Vuong C, Van Uum SH, O’Dell LE, Lutfy K, Friedman TC. The effects of opioids and opioid analogs on animal and human endocrine systems. Endocr Rev. 2010;31:98–132. doi: 10.1210/er.2009-0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wiancko KB, Kowalewski K. Strength of callus in fractured humerus of rat treated with anti-anabolic and anabolic compounds. Acta Endocrinol (Copenh). 1961;36:310–318. doi: 10.1530/acta.0.0360310. [DOI] [PubMed] [Google Scholar]
- 32.Yarrow JF, Conover CF, Purandare AV, Bhakta AM, Zheng N, Conrad B, Altman MK, Franz SE, Wronski TJ, Borst SE. Supraphysiological testosterone enanthate administration prevents bone loss and augments bone strength in gonadectomized male and female rats. Am J Physiol Endocrinol Metab. 2008;295:E1213–E1222. doi: 10.1152/ajpendo.90640.2008. [DOI] [PubMed] [Google Scholar]