Abstract
Background
The direct anterior approach for THA offers some advantages, but is associated with a significant learning curve. Some of the technical difficulties can be addressed by the use of intraoperative fluoroscopy which may improve the accuracy of acetabular component placement.
Questions/purposes
The purposes of this study were to determine if (1) there is decreased variability of acetabular cup inclination and anteversion with the direct anterior approach using fluoroscopic guidance as compared with the posterior approach THA without radiographic guidance; (2) if there is a learning curve associated with achieving accuracy with the direct anterior approach THA. We also wanted (3) to assess the frequency of complications including dislocation with the anterior approach, which initially had a learning curve, and the posterior approach.
Methods
This retrospective, comparative study of 825 THAs (372 posterior THAs without fluoroscopic guidance and 453 direct anterior THAs, performed by one surgeon, focused on a radiographic analysis to determine cup inclination and anteversion on standardized pelvic radiographs using specialized software. The first 100 direct anterior THAs performed while transitioning from the posterior approach to the direct anterior approach were included in the learning curve group. During this learning curve period, the direct anterior approach was used for all patients except those with conversion of previously fixed intertrochanteric or femoral neck fractures to THAs, gluteus medius tears, and obese patients with an immobile abdominal pannus (100 of 127 THAs). Variability of the acetabular component was compared among the posterior group, learning curve group, and direct anterior group.
Results
Variances for cup inclination and anteversion were significantly lower in the direct anterior group (19 and 16 respectively, p < 0.01) as compared with the posterior group (50 and 79 respectively).Target inclination and anteversion were achieved better in the direct anterior group (98% and 97% respectively) as compared with the posterior group (86% and 77% respectively) (p < 0.01, OR for inclination = 9.1, 95% CI, 3.5 to 23.4; OR for anteversion = 8, 95% CI, 4 to 16). In the learning curve group, target anteversion achieved (91% of cases) was marginally lower than that of the direct anterior group (p = 0.03; OR = 2.9, 95% CI, 1.1 to 7.3) and target inclination (95%) was similar (p = 0.13). There was one posterior dislocation in the posterior group, two anterior dislocations in the learning curve group, and none in the direct anterior group.
Conclusions
Use of fluoroscopy with the patient in the supine position during direct anterior THA enables intraoperative assessment of cup orientation resulting in decreased variability of acetabular cup anteversion. However, there is a learning curve associated with achieving this accuracy. We could not discern whether this difference was the result of the approach or the use of fluoroscopy in the direct anterior group.
Level of Evidence
Level III, therapeutic study. See the Instructions for Authors for a complete description of levels of evidence.
Introduction
Acetabular cup inclination and anteversion are of paramount importance for the long-term success of THA. It is intimately associated with stability with reports in literature describing a higher incidence of dislocation in cases where cup position was not in the “safe zones” [6, 10, 15]. Similarly, ROM of the prosthetic joint and prosthetic impingement is closely related to cup position [4]. Acetabular cup malposition also is associated with increased polyethylene wear and periprosthetic osteolysis [7, 12]. A freehand technique for socket implantation relies on internal pelvic landmarks and/or relationship between fixed bony landmarks such as the anterior superior iliac spines and pubic symphysis with the surgical table [10]. However, it has been shown that achieving target cup position with this technique can be difficult even for experienced surgeons [5, 9, 24]. The freehand technique has a broad range of cup orientations with a higher proportion of outliers [10]. The need to improve this accuracy and precision of component positioning has led to the development of techniques such as navigation and robotic-guided THA systems.
The direct anterior approach for THA has gained considerable interest among orthopaedic surgeons because of the relative muscle-sparing nature of this approach and decreased rates of dislocation [14, 25]. There is some clinical data to support that it offers early recovery as compared with the lateral, anterolateral, and posterior approaches with no long-term clinical differences [1, 8, 21, 22]. However, it has been shown to have a significant learning curve [3, 26]. Fluoroscopic guidance can be used to improve component positioning during THA. It also can be used to make reliable adjustments to pelvic tilt with the patient in the supine position. Matta et al. [14], who described their results with the direct anterior approach using fluoroscopy, reported that cup position is more reliable with this technique with target cup positions achieved in the majority of patients.
The aim of our study was to compare the variability of acetabular position with THA performed by one surgeon (JAR) using the direct anterior approach with fluoroscopy with that performed using the conventional posterior approach without fluoroscopy. We specifically asked if (1) direct anterior THA involving use of fluoroscopy significantly decreases the variability for acetabular component anteversion and inclination, and (2) if there is a learning curve associated with the use and interpretation of fluoroscopic images for assessment of cup position. We also wanted (3) to assess the frequency of complications including dislocation with the anterior approach, which initially involved a learning curve, and the posterior approach.
Patients and Methods
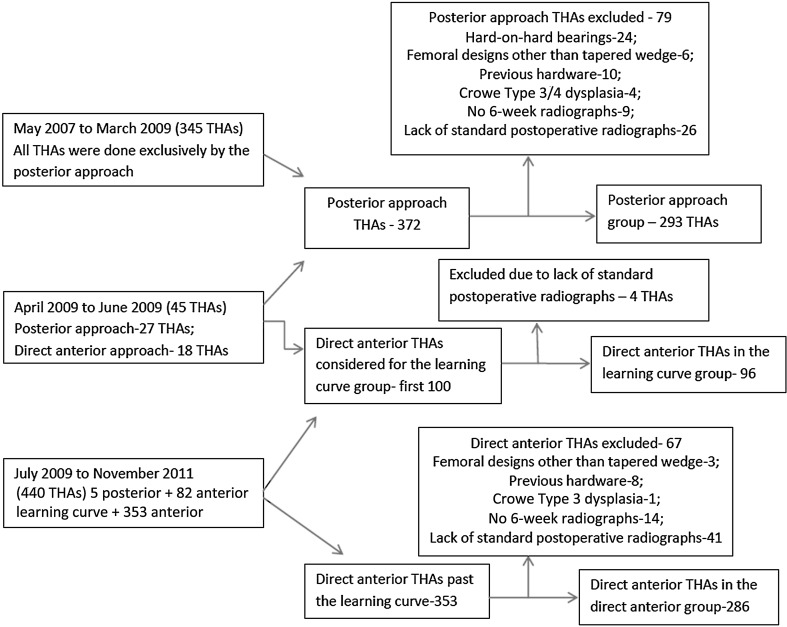
A prospectively maintained database of THAs performed by the senior surgeon (JAR) at one center from May 2006 to November 2011 was reviewed for this retrospective comparative study. All posterior THAs were done between May 2007 and June 2009 and all direct anterior THAs were done from April 2009 to November 2011. The posterior approach was initially the only approach used by the surgeon in more than 2000 THAs before May 2007 (Fig. 1). During the learning curve phase, the surgeon used the posterior approach (27 patients) for conversion of previously fixed intertrochanteric or femoral neck fractures to THA and for obese patients with an immobile abdominal pannus. Subsequently, the direct anterior approach was used for all but five patients (three with previous intertrochanteric fractures with hardware and two with preoperatively identified associated large gluteus medius tears). All patients who underwent primary THAs, unilateral or bilateral, with a cementless hemispheric acetabular design and cementless tapered wedge femoral component were included in the study. Patients with a diagnosis of Crowe Type 3 or 4 dysplasia, previous hardware, or bearing surfaces other than metal-on-polyethylene or ceramic-on-polyethylene were excluded from the study (Fig. 1). The method used for radiographic analysis of cup anteversion is difficult to perform and less reliable with metal-on-metal or ceramic-on-metal bearings because it relies on the decreased radiodensity of the face of the acetabular cup projected on radiographs [10]. Additionally, patients who did not have standardized postoperative standing radiographs were excluded from the radiographic analysis because these radiographs were required to perform that analysis (Fig. 1). This study was approved by the institutional review board.
Fig. 1.
A flow chart shows the number of THAs done during the study period and the exclusion criteria in the direct anterior, posterior, and learning curve goups.
Of 372 posterior THAs performed in 352 patients from May 2007 to June 2009, 293 were available for study in the posterior group based on the inclusion and exclusion criteria (Fig. 1). Of 453 direct anterior THAs in 424 patients, the first 100 THAs were performed in patients included in the learning curve group. Ninety-six of these patients fulfilled our criteria for inclusion. From the remaining 353 patients who had direct anterior THAs, 286 comprised the direct anterior group for the purpose of our analysis. There were 126 males and 167 females in the posterior group with a mean age of 60.6 years (± 11) and a mean BMI of 25.9 kg/m2 (± 4). The direct anterior group consisted of 130 males and 156 females with a mean age of 61.8 years (± 12) and a mean BMI of 26.4 kg/m2 (± 5). The learning curve group consisted of 41 males and 55 females with mean age of 63.4 years (± 10) and mean BMI of 25.2 kg/m2 (± 3). The majority of the operations were done for osteoarthritis of the hip (Table 1). There were no significant differences among the groups in these demographic features.
Table 1.
Demographic features of study groups
| Group | Age (years) | Sex | BMI (kg/m2) | Preoperative diagnosis |
|---|---|---|---|---|
| Posterior approach group | 60.6 (± 11) | 126 males; 167 females | 25.9 (± 4) | OA-274;AVN-9;posttraumatic OA-10 |
| Learning curve group | 63.4 (± 10) | 41 males; 55 females | 25.2 (± 3) | OA-86;AVN-7;posttraumatic OA-3 |
| Direct anterior approach group | 61.8 (± 12) | 130 males; 156 females | 26.4 (± 5) | OA-269;AVN-6;posttraumatic OA-11 |
| p values | 0.31 | 0.80 | 0.45 | 0.33 |
OA = osteoarthritis; AVN = avascular necrosis.
All posterior THAs were performed with a uniform technique as described by Ranawat and Maynard [18]. The patients were positioned in the lateral decubitus position on a special table devised with attachments for fixing the padded posts to stabilize the pelvis posteriorly (sacrum) and anteriorly (pubic symphysis and rami). The patients were positioned by two surgical assistants but supervised by the senior author (JAR). Internal landmarks used for assessment of cup position included the anterior (pubic) and posterior (ischial) walls and superior rim of the native acetabulum, with the transverse acetabular ligament used as a secondary confirmation landmark. Anteversion also was verified with respect to the angle between the trial handle and a line approximately parallel to the anterior pelvic plane (highest point of the iliac crest to the greater trochanter). The target anteversion range for cup position was 10o to 30o and target inclination range was 30o to 50o. After the appropriate cup position was identified during a trial, the direction of the introducer was marked on the thigh of the patient with a marking pen to improve reproducibility of the final cup position. Stability of the THA was assessed using the combined anteversion test (coplanar test) of Ranawat et al. [19] and resistance to dislocation with flexion, adduction, and internal rotation. In addition, anterior capsular tightness was assessed for anterior stability by extending the hip to neutral, flexing the knee to 90o and externally rotating the hip simultaneously while judging the distance between the posterior border of the greater trochanter and the ischium with the index finger interposed. If the greater trochanter did not touch the interposed finger, it would indicate a tight anterior capsule. If it crushed the index finger, it would indicate a loose anterior capsule. Limb length discrepancy was assessed using the Steinmann pin technique [20]. The external rotators and posterior capsule were repaired as a single flap with the help of trochanteric drill-hole sutures [19].
Direct anterior THAs were performed with the patient in the supine position according to the technique described by Lovell [13], with the use of a standard operating table, a table-mounted femoral elevator (Omni-Tract Surgical, St Paul, MN, USA), selective soft tissue releases (posterosuperior hip capsule over the saddle of the femoral neck, conjoined and piriformis tendons) based on mobility of the femur, and the use of fluoroscopy in every case. The senior author (JAR) performed capsulectomies in the initial 100 direct anterior THAs and a capsulotomy thereafter. The first fluoroscopic image is an AP pelvis radiograph used to compare pelvic inclination with that of the preoperative standing AP pelvis radiograph. The pelvic tilt then was corrected if required by changing the inclination of the table or adjusting the inclination of the C-arm beam in the appropriate direction (sagittal, coronal, and axial planes) to make it similar to that observed on the preoperative standing radiograph. Subsequently, fluoroscopy was used for assessment of acetabular reaming to determine if the medial bone and superior subchondral bone were reamed to the appropriate depth as per preoperative templating (Fig. 2). A fluoroscopic image then was taken during and after final cup placement to assess the inclination and anteversion (by examining the shape of the lateral edge of the cup, whether it is acute or obtuse and the width of the face of the ellipse of the cup) (Fig. 3) and to assess if optimal acetabular-bone interface contact had been achieved. The target anteversion range in the learning curve group was similar to that of the posterior approach group (range, 10o–30o) but was lower for the direct anterior group (5o–25o).The target inclination range was similar in all groups (range, 30o–50o). Subsequently, fluoroscopic images were taken to confirm the femoral component size, canal fill, hip offset, and limb length as compared with the opposite hip. The surgeon started to perform anterior stability assessment using provocative testing involving external rotation in neutral hip extension and in 30° hip extension after the initial 100 direct anterior THAs.
Fig. 2.
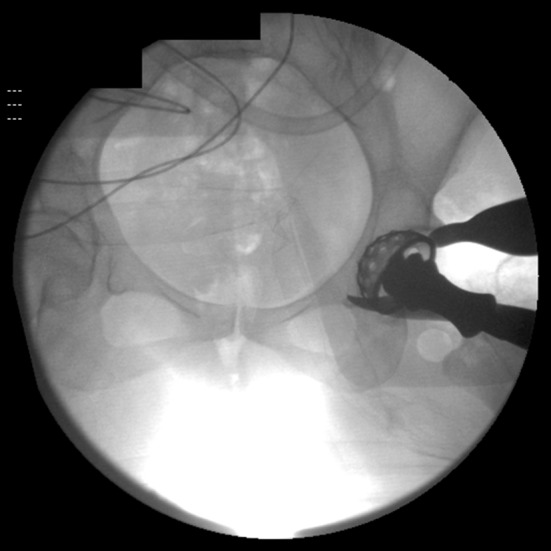
This fluoroscopic image allows assessment of reaming of the acetabular cup.
Fig. 3A–B.
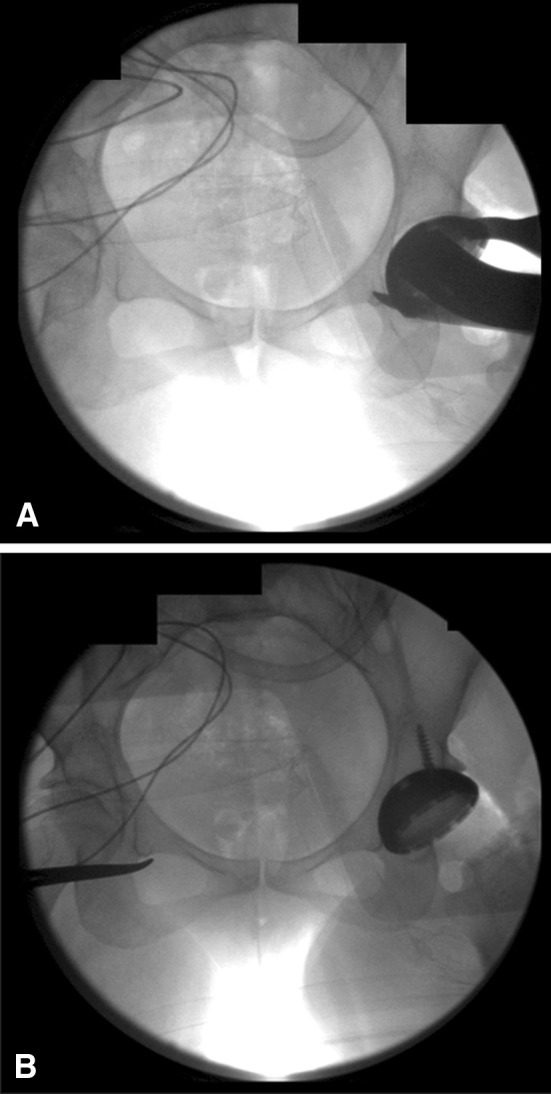
The fluoroscopic images show the acetabular component (A) during and (B) after final cup placement.
A radiographic analysis was performed on 6-week postoperative AP pelvis radiographs taken with the patients in the standing position. Standard preoperative AP radiographs were taken with patients in the standing position with hips in neutral position, the radiation beam centered on the pubic symphysis, and film-focus distance approximately 120 cm. Postoperative AP radiographs were taken in a similar fashion. However, postoperative radiographs where the pelvic tilt and rotation were markedly different from those of preoperative radiographs as measured by position of the tip of the coccyx and pubic symphysis and symmetry of the obturator foramina were excluded to eliminate variability arising from this. A picture archiving and communications system (PACS) software (Fujifilm, Stanford, CT, USA) was used to make the measurements. Acetabular inclination was measured as the angle between the interteardrop line and the long axis of the acetabular cup face. Acetabular anteversion was determined as per the technique described by Liaw et al. [11] with a mean degree of error of 0.8o (SD, 0.8o). It has been shown to be a valid and reliable method for radiographic analysis of cup anteversion [16]. For calculation, three lines were drawn parallel to the major diameter of the ellipse represented by the rim of the acetabular cup face; two lines along the edges of the rim and the third line tangential to the superomedial border of the cup. Distances between these lines were calculated and put in a trigonometric formula to calculate acetabular anteversion. The analysis was done by two independent observers (PAR, SB) in a blinded fashion. Intraclass correlation coefficients were calculated to measure interobserver reliability of the radiographic method for calculation of inclination (0.92; 95% CI, 0.87–0.96) and anteversion (0.91; 95% CI, 0.85–0.95) showing a good degree of agreement.
For data analysis, means and SDs for acetabular inclination and anteversion in each group were calculated. Variances (square of the SDs) were used to define the variability of the outcome measure. They were compared using the F test to determine if they were statistically different. The proportion of THAs which were within the target ranges for abduction and anteversion in all groups were compared with p values and effect sizes (OR) with CIs calculated. Means of inclination and anteversion and demographic features also were compared and effect sizes (Cohen’s d) were calculated. An independent t-test (two-tailed) was used for normally distributed continuous data and the Mann-Whitney U test was used for nonparametric data. Chi-square and Fisher’s exact test were used for comparing categorical data. A p value of 0.05 was set as the level of statistical significance. Statistical analysis was performed using SPSS software (Version 16; Chicago, IL, USA).
Results
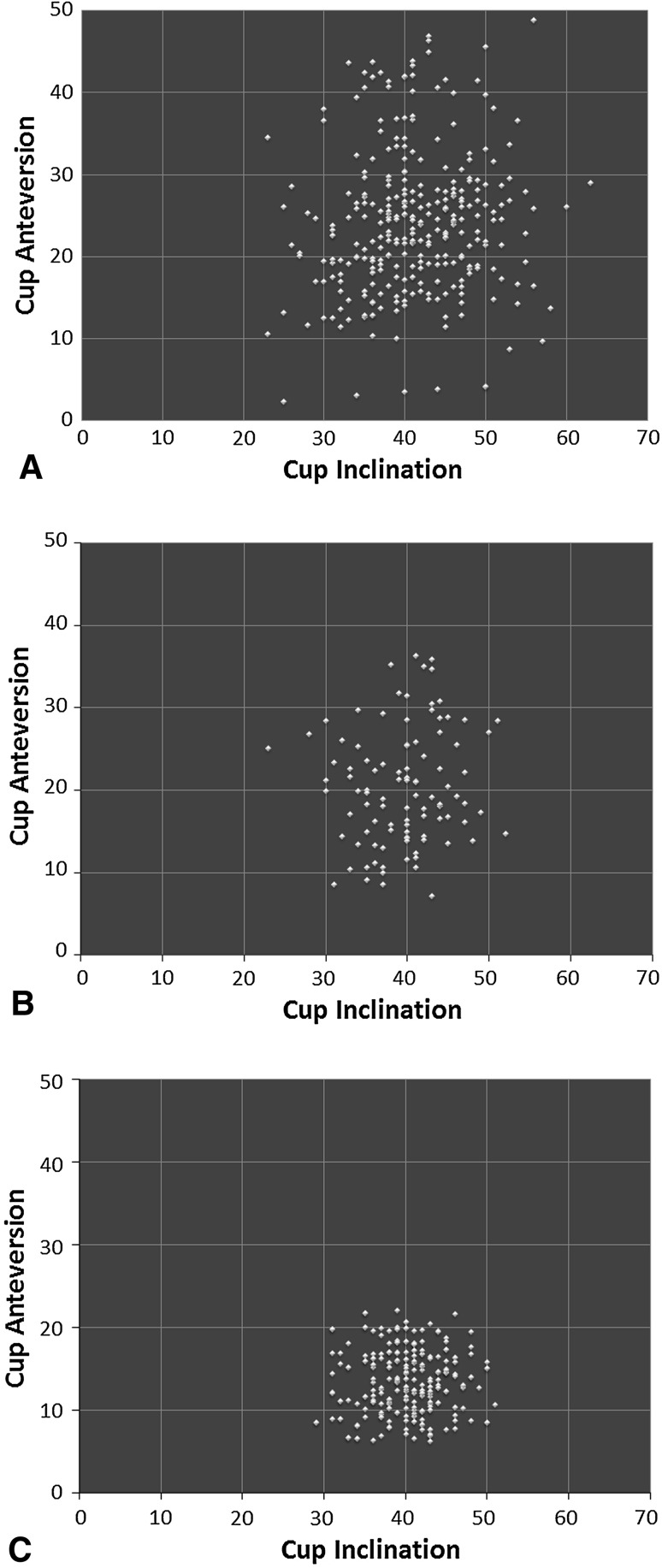
Variances were lower for cup inclination and anteversion in the direct anterior group (19 and 16 respectively) as compared with the posterior group (50 and 79 respectively) (Table 2). As per the F test for equality of variances, these differences were statistically significant (p values for inclination and anteversion were < 0.001). Target inclination and anteversion were achieved better in the direct anterior group (98% and 97% respectively) as compared with the posterior group (86% and 77% respectively) (p < 0.01, OR for inclination = 9.1, 95% CI, 3.5 to 23.4; OR for anteversion = 8, 95% CI, 4 to 16). This is depicted in the scatterplot (Fig. 4) representing cup positions for all patients in both groups with anteversion on the Y-axis and inclination on the X-axis. The degree of dispersion is lower in the direct anterior group compared with the posterior group.
Table 2.
Mean, standard deviation, and variances for cup inclination and anteversion
| Approach | Acetabular inclination | Acetabular anteversion | ||||
|---|---|---|---|---|---|---|
| Mean | SD | Variance† | Mean | SD | Variance† | |
| Posterior approach | 41.2 | 7.0 | 50 | 24.0 | 8.7 | 79 |
| Learning curve group (direct anterior) | 39.3 | 5.1 | 26 | 20.2 | 6.3 | 48 |
| Direct anterior approach group | 40.4 | 4.4 | 19 | 13.3 | 4.0 | 16 |
| Posterior approach versus direct anterior approach p values | 0.1 | < 0.01* | < 0.01* ǂd = 1.58 (1.4–1.8) | < 0.01* | ||
| Posterior approach versus learning curve p values | 0.07 | < 0.01* | < 0.01* d = 0.47 (0.2–0.7) | < 0.01* | ||
| Learning curve versus direct anterior approach p values | 0.08 | 0.04* | < 0.01* d = 1.47 (1.2–1.7) | < 0.01* | ||
†p values per F test for equality of variances; *significant values; ǂCohen’s d effect size (with 95% CI).
Fig. 4A–C.
The scatterplots show decreasing dispersion (thus decreased variability) across the (A) posterior group, (B) direct anterior learning approach group, and (C) direct anterior group with the highest dispersion in the posterior approach group and lowest in the direct anterior approach group.
In the first 100 procedures with the direct anterior approach (learning curve group), the variation in anteversion (48) was marginally higher than the remaining procedures using that approach (16), and marginally lower than the procedures in which the posterior approach (79) was used. Target anteversion was achieved in 91% of cases compared with 77% in posterior group (p = 0.004; OR = 2.8; 95% CI, 1.3 to 5.9) and 97% in the direct anterior group (p = 0.03; OR = 2.9; 95% CI, 1.1 to 7.3). Target inclination achieved in the learning curve group (95%) was marginally better than for the posterior group (86%; p = 0.02; OR = 2.9; 95% CI, 1.1 to 7.7) but similar to that of the direct anterior group (98%; p = 0.13).
Mean followups in the posterior, learning curve, and direct anterior groups were 30 months (range, 1–66 months), 22 months (range, 1–32 months), and 16 months (range, 1–26 months) respectively. Recurrent posterior dislocation in the posterior group required revision to a constrained liner in one patient. There were two single anterior dislocations in the learning curve group which required revision in the form of liner exchange to an anterior lipped liner and increased neck length. There were no dislocations in the subsequent direct anterior cases. Mean operative time was higher in the learning curve group (103 ± 18 minutes) as compared with the posterior (84 ± 14 minutes; p < 0.01) and direct anterior groups (90 ± 15 minutes; p < 0.01). There were two displaced greater trochanter fractures in the learning curve group, one undisplaced greater trochanter fracture in the direct anterior group, and none in the posterior group. There were four intraoperative femoral fractures in the posterior group, two in the learning curve group, and three in the direct anterior group which were fixed with cables. There were no femoral perforations during broaching or ankle fractures in the study cohort.
Mean cup inclination values were statistically similar in all the groups (posterior, 41.2°; learning curve, 39.3°; direct anterior, 40.4°) (Table 2). Mean anteversion values were highest in the posterior group (24°) and lowest in the direct anterior group (13.3°) corresponding to the change in target anteversion following the anterior dislocations in the learning curve group (20° mean anteversion).
Discussion
Acetabular component position is one of the key elements of a successful THA. The direct anterior approach to THA has been gaining popularity as a result of the proposed minimally invasive nature of this approach, faster and earlier recovery [1, 8, 22], and the reported lower incidence of dislocation after this technique [14, 25]. Our study shows improvement in the variability of acetabular cup position after use of direct anterior approach THAs with fluoroscopy by a surgeon experienced in posterior approach THAs, however, there is a learning curve associated with the use of fluoroscopy to achieve improved accuracy in acetabular component positioning.
There are some limitations of our study. All biases associated with a retrospective, nonrandomized study influence the interpretation of our outcomes. There were specific selection criteria for the direct anterior approach especially in the learning curve group. We excluded a substantial number of patients owing to the lack of standard radiographs which might influence the interpretation of our radiographic results. Although radiographic assessment was done in a blinded fashion, clinical assessment of complications was done in a nonblinded fashion. The radiographic method of analysis is less accurate than CT for assessment of acetabular anteversion. However, the technique we used was valid and reliable [16]. It also made possible the retrospective assessment of a large number of patients, which is difficult with a CT-based study. In addition, we could not determine combined anteversion as femoral anteversion values could not be calculated on plain radiographs and thus we could not determine its influence on our results. The senior surgeon (JAR) implants the femoral components in lower anteversion with the direct anterior approach as compared with the posterior approach for optimum anterior stability. Although accuracy of component positioning was better in the direct anterior group, there was just one dislocation in the posterior group and two anterior dislocations in the learning curve phase with the limitation of our variable followups. Therefore, the clinical significance of decreased variability in the anterior group is debatable. Moreover, the long-term effects of precise component positioning on wear and implant survival cannot be determined by our study.
No documentation of fluoroscopy time was available in the patient records; therefore, the increase in surgical time resulting from use of fluoroscopy cannot be discussed. In addition, the use of fluoroscopy entails the risk of increased radiation exposure for patients and the surgical team, which needs to be understood when using fluoroscopy with THA. We also compared the direct anterior approach with fluoroscopic guidance with the posterior approach without such guidance. It is possible that the differences in acetabular position we observed had more to do with the use of fluoroscopy than the approach per se, but we cannot conclusively differentiate the influence of these two variables with our study design. Finally, this study reflects the experience of one high-volume arthroplasty surgeon at an academic center; therefore, the reproducibility of these results at different centers involving surgeons of varying volumes and experience will need to be studied.
Our study showed there was a significant improvement in the variability of cup positioning by one surgeon who has performed more than 2000 posterior THAs and transitioned to using the direct anterior as the preferred approach. Other than the assessment of component position intraoperatively, another important factor contributing to this improvement in variability is the ability to make adjustments in pelvic tilt using fluoroscopy with the patient in the supine position. Pelvic tilt can be variable with the patient in the lateral decubitus position [27]. Nishikubo et al. [17] showed that the accuracy of component positioning can be improved by preoperative correction of errors in pelvic tilt. They described a technique of correcting pelvic tilt with posterior THAs (lateral decubitus position) by manipulating the operating table and use of fluoroscopy, which improved acetabular component positioning. The variability attributable to the use of internal landmarks for cup positioning in the posterior group also could be a factor influencing our results. We observed decreased variances for both cup anteversion and inclination. Matta et al. [14] reported a mean abduction angle of 42° (SD, 4°) and mean anteversion of 19.4° (SD, 5.2°) in their series of 458 THAs performed through the direct anterior approach and using fluoroscopy. Ninety-six percent of hips were placed in the target range of abduction (35°–50°) and 93% were placed in the target range of anteversion (10°–25°). The SDs they reported [14] are similar to those of our study. Barrett et al. [1] reported a significantly lower SD for cup anteversion (20.1° ± 5.9°) with the direct anterior approach compared with the posterior approach (25.8° ± 8.1°) but no significant difference in the SD for cup inclination (47.1° ± 6.1° direct anterior and 42.4° ± 7.6° posterior), probably because of a smaller sample size.
The variances for cup inclination and anteversion were higher in the initial 100 direct anterior THAs implying that there is a learning curve associated with the interpretation and use of fluoroscopy to guide acetabular cup position. Woolson et al. [26], in a study of direct anterior approach THAs performed by low-volume, community practice surgeons, reported a significantly higher number of acetabular component abduction outliers (21% outside 30°–50º) despite the use of fluoroscopy. However, in our study, the variances in the learning curve group were slightly lower than that of the posterior group indicating that, even in this learning phase, the variability is better than a freehand technique for cup implantation by a high-volume arthroplasty surgeon.
The surgical time was significantly higher during the learning curve phase and the incidence of greater trochanter fractures was greater while transitioning to the direct anterior approach. In our study, these fractures occurred while taking the neck cut. We believe that ensuring the leg is in neutral position, appropriate neck-cut technique with retractors medially and at the lateral saddle of the neck is important in avoiding this complication. Some authors have described a high rate of femoral perforations in the learning curve phase [3, 23, 26]. There were no femoral perforations in our series which suggests the importance of adequate femoral exposure with release of the superior capsule with or without release of rotators (conjoint +/− piriformis) before commencement of femoral preparation.
There were two anterior dislocations in the learning curve group. The target anteversion during this phase of the anterior approach was similar to that of the posterior approach. Subsequent to these dislocations and introduction of provocative anterior stability testing with external rotation in extension and hyperextension, the target anteversion was lowered. This change is reflected in the mean anteversion values in the three groups. This also explains lower mean anteversion values with the direct anterior approach in our study as compared with those of Matta et al. [14] and Barrett et al. [1]. Matta et al. [14] reported three dislocations in their series, two of which were anterior. Biedermann et al. [2] in their study on anterior dislocations after standard, anterolateral, transgluteal THAs, found a higher mean anteversion (17°) and higher mean abduction (48°) in patients with anterior dislocation. However, the clinical affect of acetabular orientation on anterior dislocation after direct anterior THA is unclear and probably would require a study of large sample size to evaluate all factors associated with it.
The direct anterior approach THA performed with the use of fluoroscopy improves the variability of acetabular component placement (cup inclination and anteversion) as compared with the posterior approach THA without fluoroscopy. There is a learning curve associated with achieving a high level of accuracy. Elucidation of the effect of this improved accuracy with fluoroscopic-guided direct anterior THA on implant survivorship and patient function will require long-term studies.
Footnotes
Each author certifies that he or she, or a member of his or her immediate family, has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research editors and board members are on file with the publication and can be viewed on request.
Each author certifies that his or her institution approved the human protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
References
- 1.Barrett WP, Turner SE, Leopold JP. Prospective randomized study of direct anterior vs postero-lateral approach for total hip arthroplasty. J Arthroplasty. 2013;28:1634–1638. doi: 10.1016/j.arth.2013.01.034. [DOI] [PubMed] [Google Scholar]
- 2.Biedermann R, Tonin A, Krismer M, Rachbauer F, Eibl G, Stöckl B. Reducing the risk of dislocation after total hip arthroplasty: the effect of orientation of the acetabular component. J Bone Joint Surg Br. 2005;87:762–769. doi: 10.1302/0301-620X.87B6.14745. [DOI] [PubMed] [Google Scholar]
- 3.De Geest T, Vansintjan P, De Loore G. Direct anterior total hip arthroplasty: complications and early outcome in a series of 300 cases. Acta Orthop Belg. 2013;79:166–173. [PubMed] [Google Scholar]
- 4.D’Lima DD, Urquhart AG, Buehler KO, Walker RH, Colwell CW., Jr The effect of the orientation of the acetabular and femoral components on the range of motion of the hip at different head-neck ratios. J Bone Joint Surg Am. 2000;82:315–321. doi: 10.2106/00004623-200003000-00003. [DOI] [PubMed] [Google Scholar]
- 5.Eddine TA, Migaud H, Chantelot C, Cotten A, Fontaine C, Duquennoy A. Variations of pelvic anteversion in the lying and standing positions: analysis of 24 control subjects and implications for CT measurement of position of a prosthetic cup. Surg Radiol Anat. 2001;23:105–110. doi: 10.1007/s00276-001-0105-z. [DOI] [PubMed] [Google Scholar]
- 6.Jolles BM, Zangger P, Leyvraz PF. Factors predisposing to dislocation after primary total hip arthroplasty: a multivariate analysis. J Arthroplasty. 2002;17:282–288. doi: 10.1054/arth.2002.30286. [DOI] [PubMed] [Google Scholar]
- 7.Kennedy JG, Rogers WB, Soffe KE, Sullivan RJ, Griffen DG, Sheehan LJ. Effect of acetabular component orientation on recurrent dislocation, pelvic osteolysis, polyethylene wear, and component migration. J Arthroplasty. 1998;13:530–534. doi: 10.1016/S0883-5403(98)90052-3. [DOI] [PubMed] [Google Scholar]
- 8.Klausmeier V, Lugade V, Jewett BA, Collis DK, Chou LS. Is there faster recovery with an anterior or anterolateral THA? A pilot study. Clin Orthop Relat Res. 2010;468:533–541. doi: 10.1007/s11999-009-1075-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leenders T, Vandevelde D, Mahieu G, Nuyts R. Reduction in variability of acetabular cup abduction using computer assisted surgery: a prospective and randomized study. Comput Aided Surg. 2002;7:99–106. doi: 10.3109/10929080209146021. [DOI] [PubMed] [Google Scholar]
- 10.Lewinnek GE, Lewis JL, Tarr R, Compere CL, Zimmerman JR. Dislocations after total hip-replacement arthroplasties. J Bone Joint Surg Am. 1978;60:217–220. [PubMed] [Google Scholar]
- 11.Liaw CK, Yang RS, Hou SM, Wu TY, Fuh CS. Measurement of the acetabular cup anteversion on simulated radiographs. J Arthroplasty. 2009;24:468–474. doi: 10.1016/j.arth.2007.10.029. [DOI] [PubMed] [Google Scholar]
- 12.Little NJ, Busch CA, Gallagher JA, Rorabeck CH, Bourne RB. Acetabular polyethylene wear and acetabular inclination and femoral offset. Clin Orthop Relat Res. 2009;467:2895–2900. doi: 10.1007/s11999-009-0845-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lovell TP. Single-incision direct anterior approach for total hip arthroplasty using a standard operating table. J Arthroplasty. 2008;23(7 suppl):64–68. doi: 10.1016/j.arth.2008.06.027. [DOI] [PubMed] [Google Scholar]
- 14.Matta JM, Shahrdar C, Ferguson T. Single-incision anterior approach for total hip arthroplasty on an orthopaedic table. Clin Orthop Relat Res. 2005;441:115–124. doi: 10.1097/01.blo.0000194309.70518.cb. [DOI] [PubMed] [Google Scholar]
- 15.McCollum DE, Gray WJ. Dislocation after total hip arthroplasty: causes and prevention. Clin Orthop Relat Res. 1990;261:159–170. [PubMed] [Google Scholar]
- 16.Nho JH, Lee YK, Kim HJ, Ha YC, Suh YS, Koo KH. Reliability and validity of measuring version of the acetabular component. J Bone Joint Surg Br. 2012;94:32–36. doi: 10.1302/0301-620X.94B1.27621. [DOI] [PubMed] [Google Scholar]
- 17.Nishikubo Y, Fujioka M, Ueshima K, Saito M, Kubo T. Preoperative fluoroscopic imaging reduces variability of acetabular component positioning. J Arthroplasty. 2011;26:1088–1094. doi: 10.1016/j.arth.2011.05.011. [DOI] [PubMed] [Google Scholar]
- 18.Ranawat CS, Maynard MJ. Modern techniques of cemented total hip arthroplasty. Techniques Orthop. 1991;6:17–25. doi: 10.1097/00013611-199109000-00004. [DOI] [Google Scholar]
- 19.Ranawat CS, Ranawat AS, Rasquinha VJ. Mastering the art of cemented femoral stem fixation. J Arthroplasty. 2004;19(4 suppl 1):85–91. doi: 10.1016/j.arth.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 20.Ranawat CS, Rao RR, Rodriguez JA, Bhende HS. Correction of limb-length inequality during total hip arthroplasty. J Arthroplasty. 2001;16:715–720. doi: 10.1054/arth.2001.24442. [DOI] [PubMed] [Google Scholar]
- 21.Restrepo C, Parvizi J, Pour AE, Hozack WJ. Prospective randomized study of two surgical approaches for total hip arthroplasty. J Arthroplasty. 2010;25:671–679. doi: 10.1016/j.arth.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 22.Rodriguez JA, Deshmukh AJ, Rathod PA, Greiz ML, Deshmane PP, Hepinstall MS, Ranawat AS. Does the direct anterior approach in THA offer faster rehabilitation and comparable safety to the posterior approach? Clin Orthop Relat Res. 2013;472:455–463. doi: 10.1007/s11999-013-3231-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rüdiger HA, Betz M, Zingg PO, McManus J, Dora CF. Outcome after proximal femoral fractures during primary total hip replacement by the direct anterior approach. Arch Orthop Trauma Surg. 2013;133:569–573. doi: 10.1007/s00402-013-1697-6. [DOI] [PubMed] [Google Scholar]
- 24.Saxler G, Marx A, Vandevelde D, Langlotz U, Tannast M, Wiese M, Michaelis U, Kemper G, Grützner PA, Steffen R, von Knoch M, Holland-Letz T, Bernsmann K. The accuracy of free-hand cup positioning: a CT based measurement of cup placement in 105 total hip arthroplasties. Int Orthop. 2004;28:198–201. doi: 10.1007/s00264-004-0542-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Siguier T, Siguier M, Brumpt B. Mini-incision anterior approach does not increase dislocation rate: a study of 1037 total hip replacements. Clin Orthop Relat Res. 2004;426:164–173. doi: 10.1097/01.blo.0000136651.21191.9f. [DOI] [PubMed] [Google Scholar]
- 26.Woolson ST, Pouliot MA, Huddleston JI. Primary total hip arthroplasty using an anterior approach and a fracture table: short-term results from a community hospital. J Arthroplasty. 2009;24:999–1005. doi: 10.1016/j.arth.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 27.Zhu J, Wan Z, Dorr LD. Quantification of pelvic tilt in total hip arthroplasty. Clin Orthop Relat Res. 2010;468:571–575. doi: 10.1007/s11999-009-1064-7. [DOI] [PMC free article] [PubMed] [Google Scholar]