Abstract
Background
Schwannomas rarely are found in the brachial plexus, and although they are benign, they present significant challenges to surgical treatment. To our knowledge, there are few studies investigating the surgical outcomes of patients with brachial plexus tumors.
Questions/purposes
We analyzed the outcomes of 19 patients with brachial plexus schwannomas and asked: (1) How do these patients present? (2) Where are the tumors located in the brachial plexus? (3) What are the complications and neurologic results of patients after excision of the tumor?
Methods
From February 2002 to August 2012, one orthopaedic hand surgeon treated 19 patients with schwannomas of the brachial plexus. We retrospectively reviewed the medical records and MRI data of all patients. There were 11 women and eight men, with a mean age of 50.2 years (range, 32–63 years). The tumor was located on the right side in eight patients and on the left in 11 patients. We evaluated neurologic deficits preoperatively and neurologic deficits and local recurrence of tumors postoperatively. Minimum followup was 12 months (mean, 37.2 months; range, 12–90 months).
Results
The most common initial presentation was a palpable mass. The masses were located at all levels along the brachial plexus, including the root, trunk, cord, and terminal branches. The smallest mass was 1.5 × 1.5 × 0.5 cm and the largest was 11 × 10 × 6 cm. Fourteen of the 19 patients did not have any postoperative neurologic deficits. All the removed masses were proven histologically to be schwannomas. Of the five patients who had postoperative neurologic deficits, three had transient sensory deficits, one had weakness of the flexor pollicis longus and second flexor digitorum profundus, and another had weakness of the extensor pollicis longus. No recurrence was observed during the followup period.
Conclusions
Schwannomas of the brachial plexus are a potentially curable lesion with an acceptable surgical risk of injury to neurovascular structures. With precise surgical techniques, these tumors can be removed to improve symptoms with minimal morbidity.
Level of Evidence
Level IV, therapeutic study. See the Instructions for Authors for a complete description of levels of evidence.
Introduction
Tumors of the brachial plexus are relatively rare, accounting for less than 5% of all tumors in the upper extremity [12, 20]. Patients often present with a palpable mass or local or radiating upper limb pain. These tumors typically are not associated with a neurologic deficit. The rarity of these tumors combined with the complexity of the brachial plexus makes resection challenging for surgeons. Furthermore, an expanding mass arising from the brachial plexus can distort the normal neurovascular anatomy, and tumor removal or even biopsy carries a risk of producing a neurologic deficit.
The two most common brachial plexus tumors are schwannomas and neurofibromas, both of which are benign and classified as peripheral nerve sheath tumors. Primary malignant tumors of the brachial plexus are rare [6, 8]. Schwannomas are surrounded by a capsule which facilitates the surgical separation of the tumor from the normal nerve fibers, unlike neurofibromas or primary malignancies where dissection between the tumor and normal nerve fibers is difficult and surgical excision may require neurorrhaphy or nerve graft [6].
In 1886, Courvoisier [2] reported the first surgical case of a brachial plexus tumor. Surgical removal of the tumor, which probably was a schwannoma, resulted in paralysis of the deltoid and biceps muscles. Surgical removal of brachial plexus tumors with successful results has been reported in several studies [3, 7, 18]. Results of the general treatment of brachial plexus tumors were reported previously [3, 6, 8, 9, 13, 15], despite that surgical details and results differ according to the types of tumor. In contrast to neurofibromas, in which intraneural dissection with maintenance of nerve continuity is impossible because the fascicles are embedded in the tumor, the schwannoma is well encapsulated, and the fascicles of the nerve are spread over its surface. This would suggest that a schwannoma can be removed without damage to the normal nerve fascicles [17]. Therefore, we limited our analysis and study to schwannomas of the brachial plexus. To our knowledge, only one orthopaedic study [17] investigated the clinical results after surgery for schwannomas of the brachial plexus.
We reviewed patients with schwannomas of the brachial plexus who were operated on and followed up for more than 1 year. We asked: (1) How do these patients present? (2) Where are the tumors located in the brachial plexus? (3) What are the complications and neurologic results of patients after excision?
Patients and Methods
Study Cohort
This study was approved by the Seoul National University Hospital Institutional Review Board (Protocol Number: H-1304-076-482). Patients who had surgery for brachial plexus tumors between 2002 and 2012 were retrospectively reviewed. A total of 31 patients underwent surgery. Two patients were diagnosed with synovial sarcoma, four had neurofibromas, and 25 had confirmed schwannomas. Nineteen (76%) of these 25 patients had followup greater than 1 year. There were 11 women and eight men, with a mean age of 50.2 years (range, 32–63 years) (Table 1). Data obtained from the medical records included family history and physical examination findings, such as tumor size, location, mobility, local tenderness, Tinel’s sign, and neurologic deficits, including muscle power and sensory changes. All patients had preoperative MRI (Fig. 1). EMG was performed in patients presenting with radiating pain or neurologic deficits. Eight of the 19 patients had a mass on the right side and 11 on the left. The minimum followup was 12 months (mean, 37.2 months; range, 12–90 months).
Table 1.
Clinical summary
| Patient | Sex | Age (years) |
Side | Preoperative symptom | Tinel’s sign | Location | Size (cm) | Immediate postoperative status | Last followup status | Followup (months) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Female | 45 | Left | Palpable mass, tingling sensation |
+ | C6-7 roots | 2.5 × 2.0 × 1.5 | No change | Improved tingling sensation | 90 |
| 2 | Male | 48 | Right | Palpable mass | − | Upper trunk | 3.2 × 2.3 × 2.8 | No change | No pain or weakness | 85 |
| 3 | Male | 54 | Right | Palpable mass, tingling sensation |
+ | Radial nerve | 1.5 × 1.4 × 1.3 | No change | Improved tingling sensation | 78 |
| 4 | Female | 51 | Left | Palpable mass | − | Middle trunk | 2.3 × 2.0 × 2.5 | No pain or weakness | No pain or weakness | 67 |
| 5 | Female | 45 | Left | Palpable mass, tingling sensation |
+ | Lower trunk | 2.1 × 2.2 × 1.9 | No change | Improved tingling sensation | 60 |
| 6 | Male | 44 | Right | Palpable mass | − | Medial cord | 3.7 × 2.5 × 2.5 | 30% decreased sensory at fifth finger | Recovered complete | 53 |
| 7 | Male | 56 | Left | Palpable mass, tingling sensation |
+ | Median nerve | 4.2 × 3.4 × 3.0 | Worsened weakness (Grade 3) at the second FDP and FPL | Improved weakness at the second FDP and FPL (Grade 4) | 50 |
| 8 | Female | 47 | Left | Palpable mass | − | Medial cord | 3.5 × 2.5 × 0.5 | No pain or weakness | 42 | |
| 9 | Female | 63 | Left | Palpable mass, pain, weakness |
+ | C5-6 roots | 3.4 × 2.4 × 2.4 | No change, Grade 3 deltoid power |
Improved weakness at deltoid muscle (Grade 4) and pain | 39 |
| 10 | Male | 53 | Right | Palpable mass, tingling sensation |
+ | Middle trunk | 1.5 × 1.2 × 0.8 | 20% decreased sensory at first web space | Recovered completely | 30 |
| 11 | Male | 59 | Left | Palpable mass, tingling sensation |
+ | C8-T1 roots | 3.0 × 3.0 × 2.5 | No change | Improved pain and tingling sensation | 27 |
| 12 | Female | 57 | Left | Palpable mass | − | Median nerve | 3.0 × 2.5 × 2.0 | 20% decreased sensory at second finger and 10% third finger | Recovered completely | 24 |
| 13 | Female | 57 | Left | Palpable mass, Pain, tingling sensation |
+ | Radial nerve | 3.5 × 3 × 2.5 | Worsened weakness (Grade 4) at the EPL | Improved weakness (Grade 5) at the EPL | 24 |
| 14 | Female | 51 | Right | Palpable mass, tingling sensation |
+ | Middle trunk | 2.5 × 2.3 × 1.0 | No change | Improved tingling sensation | 22 |
| 15 | Female | 36 | Right | Palpable mass, tingling sensation |
+ | Middle trunk | 11 × 10 × 6 | 10% decreased sensory at first web space | Recovered completely | 20 |
| 16 | Female | 62 | Right | Palpable mass | − | Median nerve | 1.5 × 1.5 × 1.0 | No change | No pain or weakness | 18 |
| 17 | Male | 42 | Left | Palpable mass | − | Lower trunk | 2.0 × 2.0 × 1.0 | No change | No pain or weakness | 15 |
| 18 | Female | 32 | Right | Palpable mass | − | Upper trunk | 2.0 × 1.0 × 1.5 | No change | No pain or weakness | 14 |
| 19 | Male | 52 | Left | Palpable mass, tingling sensation |
+ | Middle trunk | 3.3 × 2.2 × 1.6 2.0 × 1.5 × 0.7 |
No change | Improved tingling sensation | 12 |
FDP = flexor digitorum profundus; FPL = flexor pollicis longus; EPL = extensor pollicis longus.
Fig. 1A–B.
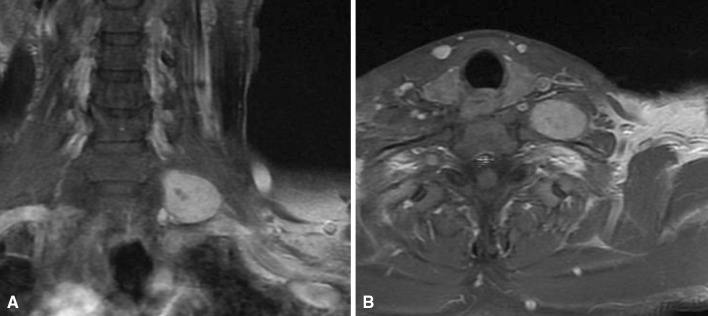
A-52-year old man presented with a palpable mass and tingling sensation in the supraclavicular area. (A) His coronal T1-weighted MR image with contrast showed a mass at the brachial plexus. (B) The axial T1-weighted MR image with contrast revealed the well-margined mass.
Surgical Procedure
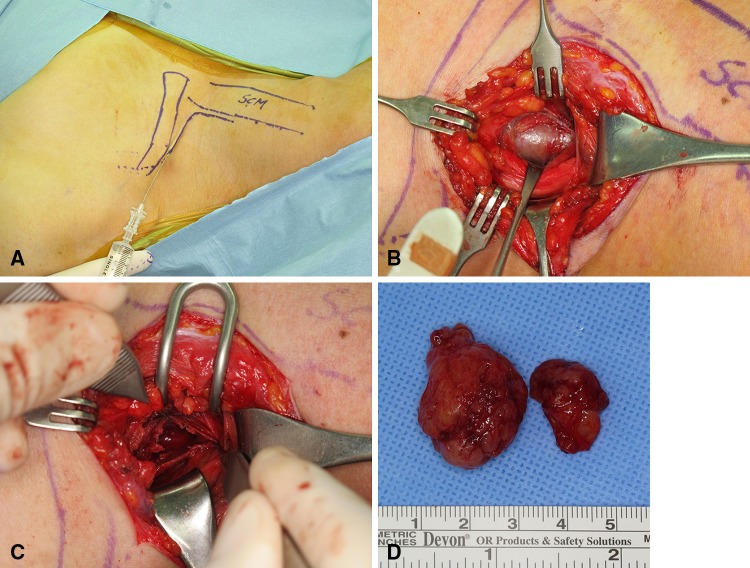
All surgical procedures were performed by one of the authors (GHB) under X4 loupe magnification using microsurgical techniques. When the mass occurred in a cord or branch level of the brachial plexus, a longitudinal incision was made over it. However, when it occurred at the root, trunk, and division level, an L-shaped incision was made on the affected neck. Before the incision was made, 1:100,000 epinephrine was injected along the line of incision for hemostasis (Fig. 2A). The vertical limb was made just lateral to the border of sternocleidomastoid muscle, and the transverse limb 1 cm above the clavicle. The platysma muscles were cut and preserved, and were sutured later to maintain contour of the neck. The omohyoid muscle was isolated and preserved. The whole brachial plexus was exposed carefully to identify the exact location of the tumor. Before making an incision on the tumor capsule, the superficial vessels on the tumor capsule were carefully cauterized with a low-power bipolar coagulator. A longitudinal incision was made on the tumor capsule and the mass was gently and bluntly dissected away from the tumor capsule (Fig. 2B). When the capsule was open and the dissection was deepened, the mass usually protruded spontaneously. On the proximal and distal ends of the mass, a small fascicle was found on which the mass arose. The tumor then could be removed in one piece without disturbing the intact nerve fascicles (Fig. 2C). This fascicle was cut to remove the mass en bloc. Sometimes fluid substance gushed out during dissection when there was cystic degeneration in the mass. When the mass was large, the tumor could not be removed in one piece and therefore was removed in pieces (Fig. 2D). All tumors were removed completely, although the larger tumors had an intralesional excision. After copious irrigation and hemostasis, a Silastic® (Dow Corning Corporation, Midland, MI, USA) Penrose drain was inserted and the wound was closed.
Fig. 2A–D.
(A) An L-shaped incision was made on the affected neck, and 1:100,000 epinephrine was injected along skin incision. (B) A longitudinal incision was made on the tumor capsule and the mass was gently and bluntly dissected away from the tumor capsule. (C) The tumor was dissected out without disturbing the intact nerve fascicle. (D) The tumor was removed in two pieces, the larger being 3.3 × 2.2 × 1.6 cm and the smaller was 2.0 × 1.5 × 0.7 cm.
Results
The most common initial presentation in all patients was a palpable mass (Table 1). Two patients had pain, one had weakness, and 10 reported a tingling sensation when their lesion was compressed.
Tumor location was variable, with three tumors at the root level, nine at the trunk level, two at the cord level, and five in the terminal branches. Tumor dimensions, as measured in biopsy specimens, ranged from 1.5 × 1.5 × 0.5 cm to 11 × 10 × 6 cm.
Of 19 patients, five reported neurologic symptoms postoperatively. Three patients had slightly decreased sensation, and two had decreased muscle power. One of the three patients who had a sensory deficit after surgery noticed decreased sensation in the ulnar nerve territory especially of the fifth finger, which showed complete recovery 10 months postoperatively. Another patient had decreased sensation of the first web space, which disappeared 3 months postoperatively. The patient who had decreased sensation at the second and third fingers showed complete improvement 1 year after excision. Two patients had motor deficits postoperatively. One of them showed Grade 3 (fair) muscle power of the second flexor digitorum profundus and flexor pollicis longus postoperatively; however, there was no sensory impairment. The power of the affected muscles slowly improved, and at 5 years’ followup, the flexor pollicis longus and the second flexor digitorum profundus power were Grade 4 (good). The other patient had decreased extensor pollicis longus muscle power Grade 4 (good), which was improved to Grade 5 (normal) 2 years postoperatively.
Discussion
Since 1970, there have been numerous studies in which treatment outcomes and therapeutic approaches in patients with tumors of the brachial plexus have been described [3–6, 8–10, 12, 14, 15, 18, 21]. Our results partly agreed with those of earlier studies.
Kehoe et al. [12] studied mostly patients with brachial plexus tumors who presented with a palpable mass, and less than ½ reported local pain or paresthesias. Huang et al. [9] reported the most common presenting symptom was pain, including local and distal pain (70%), followed by sensory loss (61%), weakness (52%), and a palpable mass (30%). In our patients, the most common presenting symptom was a palpable mass and tingling sensation when the mass was compressed.
Thirty-six percent of the tumors were located in the supraclavicular area and 64% were in the infraclavicular area in the patients of Kehoe et al. [12]. Kim et al. [13] reported 69% of schwannomas were located supraclavicularly, 17% were located infraclavicularly, and 14% were in terminal branches. In our patients, 63% of schwannomas were located in the supraclavicular area, 11% were in the infraclavicular area, and 26% were in terminal branches.
Kehoe et al. [12] reported 17% of patients had postoperative problems. Ganju et al. [6] reported 22% of patients with schwannomas had worse or new pain, whereas 30% had worse or new motor functional deficit. Huang et al. [9] reported 11% of their patients had increased pain, and 22% had worse motor function after surgery. In our results, 15 % of patients had sensory changes and 10% had motor deficits immediate postoperatively, however their deficits had improved at last followup. We think it is possible to excise schwannomas without undue neurologic consequences.
Our study has several limitations. First is the short followup. The followup for six of the 19 patients did not meet the usual criteria of being at least 24 months. Although 1-year followup was enough time to assess neurologic symptoms after surgery, it was insufficient to evaluate tumor recurrence. Second, we could not perform MRI to assess for recurrence of tumor. We evaluated by physical examination and patient’s complaints for recurrence. The brachial plexus is deeply seated in the neck and connective tissues around it are too loose to palpate the mass at an early stage. In addition, with schwannomas, a mass can take a long time to become palpable. Therefore, the recurrence rate might be increased with time. Third, this study was retrospective, thus patients were reviewed by medical records and at different times after surgery.
A technique consisting of the removal or enucleation of the schwannoma has been reported [1, 4, 11, 13, 17, 19]. However, extracapsular excision can damage the normal fascicles during dissection of the capsule. We advocate intracapsular excision with gentle dissection between the tumor capsule and normal fascicles to minimize the risk of nerve damage. The epineurial layer covering the tumor capsule should be dissected in a manner similar to peeling an onion to allow for safe removal of the tumor, which should be approached by its proximal and distal poles. In most cases, one fascicle remained at the tumor pole, and this fascicle was excised to remove the tumor. Donner et al. [5] reported that fascicles located in the tumor are usually nonfunctional; therefore, excision of these fascicles does not lead to neurologic deficits. We agree with Donner et al. [5], and all but five of our patients treated using this technique showed improvement of symptoms without a neurologic deficit. Oberle et al. [16] suggested that postoperative neurologic deficits occur mostly in patients with large tumors or long-standing symptoms, which is in agreement with our results showing that larger tumors were associated with a greater risk of neurologic deficit after surgery.
Schwannomas of the brachial plexus should be considered curable, with an acceptable surgical risk of injury to neurovascular structures in patients with symptomatic schwannomas. Neurologic injury was uncommon and relatively minor in our patients, and in most of these patients, the neurologic deficits resolved with time.
Footnotes
Each author certifies that he or she, or a member of his or her immediate family, has no funding or commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research editors and board members are on file with the publication and can be viewed on request.
Each author certifies that his or her institution approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
References
- 1.Artico M, Cervoni L, Wierzbicki V, D’Andrea V, Nucci F. Benign neural sheath tumours of major nerves: characteristics in 119 surgical cases. Acta Neurochir (Wien). 1997;139:1108–1116. doi: 10.1007/BF01410969. [DOI] [PubMed] [Google Scholar]
- 2.Courvoisier LG. Die Neurome eine Klinische Monographie. Basel, Switzerland: B Schwode; 1886. [Google Scholar]
- 3.Dart LH, Jr, MacCarty CS, Love JG, Dockerty MB. Neoplasms of the brachial plexus. Minn Med. 1970;53:959–964. [PubMed] [Google Scholar]
- 4.Das S, Ganju A, Tiel RL, Kline DG. Tumors of the brachial plexus. Neurosurg Focus. 2007;22:E26. doi: 10.3171/foc.2007.22.6.27. [DOI] [PubMed] [Google Scholar]
- 5.Donner TR, Voorhies RM, Kline DG. Neural sheath tumors of major nerves. J Neurosurg. 1994;81:362–373. doi: 10.3171/jns.1994.81.3.0362. [DOI] [PubMed] [Google Scholar]
- 6.Ganju A, Roosen N, Kline DG, Tiel RL. Outcomes in a consecutive series of 111 surgically treated plexal tumors: a review of the experience at the Louisiana State University Health Sciences Center. J Neurosurg. 2001;95:51–60. doi: 10.3171/jns.2001.95.1.0051. [DOI] [PubMed] [Google Scholar]
- 7.Godwin JT. Encapsulated neurilemoma (schwannoma) of the brachial plexus: report of eleven cases. Cancer. 1952;5:708–720. doi: 10.1002/1097-0142(195207)5:4<708::AID-CNCR2820050409>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 8.Huang JH, Samadani U, Zager EL. Brachial plexus region tumors: a review of their history, classification, surgical management, and outcomes. Neurosurg Q. 2003;13:151–161. doi: 10.1097/00013414-200309000-00001. [DOI] [Google Scholar]
- 9.Huang JH, Zaghloul K, Zager EL. Surgical management of brachial plexus region tumors. Surg Neurol. 2004;61:372–378. doi: 10.1016/j.surneu.2003.08.006. [DOI] [PubMed] [Google Scholar]
- 10.Inoue M, Kawano T, Matsumura H, Mori K, Yoshida T. Solitary benign schwannoma of the brachial plexus. Surg Neurol. 1983;20:103–108. doi: 10.1016/0090-3019(83)90458-5. [DOI] [PubMed] [Google Scholar]
- 11.Kang HJ, Shin SJ, Kang ES. Schwannomas of the upper extremity. J Hand Surg Br. 2000;25:604–607. doi: 10.1054/jhsb.2000.0472. [DOI] [PubMed] [Google Scholar]
- 12.Kehoe NJ, Reid RP, Semple JC. Solitary benign peripheral-nerve tumours: review of 32 years’ experience. J Bone Joint Surg Br. 1995;77:497–500. [PubMed] [Google Scholar]
- 13.Kim DH, Murovic JA, Tiel RL, Moes G, Kline DG. A series of 397 peripheral neural sheath tumors: 30-year experience at Louisiana State University Health Sciences Center. J Neurosurg. 2005;102:246–255. doi: 10.3171/jns.2005.102.2.0246. [DOI] [PubMed] [Google Scholar]
- 14.Leung PC. Tumours of hand. Hand. 1981;13:169–172. doi: 10.1016/S0072-968X(81)80059-9. [DOI] [PubMed] [Google Scholar]
- 15.Lusk MD, Kline DG, Garcia CA. Tumors of the brachial plexus. Neurosurgery. 1987;21:439–453. doi: 10.1227/00006123-198710000-00001. [DOI] [PubMed] [Google Scholar]
- 16.Oberle J, Kahamba J, Richter HP. Peripheral nerve schwannomas: an analysis of 16 patients. Acta Neurochir (Wien). 1997;139:949–953. doi: 10.1007/BF01411304. [DOI] [PubMed] [Google Scholar]
- 17.Park MJ, Seo KN, Kang HJ. Neurological deficit after surgical enucleation of schwannomas of the upper limb. J Bone Joint Surg Br. 2009;91:1482–1486. doi: 10.1302/0301-620X.91B11.22519. [DOI] [PubMed] [Google Scholar]
- 18.Richardson RR, Siqueira EB, Oi S, Nunez C. Neurogenic tumors of the brachial plexus: report of two cases. Neurosurgery. 1979;4:66–70. doi: 10.1227/00006123-197901000-00014. [DOI] [PubMed] [Google Scholar]
- 19.Russell SM. Preserve the nerve: microsurgical resection of peripheral nerve sheath tumors. Neurosurgery. 2007;61(3 suppl):113–117; discussion 117–118. [DOI] [PubMed]
- 20.Stack HG. Tumors of the hand. Br Med J. 1960;1:919–922. doi: 10.1136/bmj.1.5177.919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weber AL, Montandon C, Robson CD. Neurogenic tumors of the neck. Radiol Clin North Am. 2000;38:1077–1090. doi: 10.1016/S0033-8389(05)70222-0. [DOI] [PubMed] [Google Scholar]